Abstract
Activation of GABAA receptors causes in immature neurons a functionally relevant decrease in the intracellular Cl− concentration ([Cl−]i), a process termed ionic plasticity. Amount and duration of ionic plasticity depends on kinetic properties of [Cl−]i homeostasis. In order to characterize the capacity of Cl− accumulation and to quantify the effect of persistent GABAergic activity on [Cl−]i, we performed gramicidin-perforated patch-clamp recordings from CA3 pyramidal neurons of immature (postnatal day 4–7) rat hippocampal slices. These experiments revealed that inhibition of NKCC1 decreased [Cl−]i toward passive distribution with a time constant of 381 s. In contrast, active Cl− accumulation occurred with a time constant of 155 s, corresponding to a rate of 15.4 µM/s. Inhibition of phasic GABAergic activity had no significant effect on steady state [Cl−]i. Inhibition of tonic GABAergic currents induced a significant [Cl−]i increase by 1.6 mM, while activation of tonic extrasynaptic GABAA receptors with THIP significantly reduced [Cl−]i.. Simulations of neuronal [Cl−]i homeostasis supported the observation, that basal levels of synaptic GABAergic activation do not affect [Cl−]i. In summary, these results indicate that active Cl−-uptake in immature hippocampal neurons is sufficient to maintain stable [Cl−]i at basal levels of phasic and to some extent also to compensate tonic GABAergic activity.
Subject terms: Computational biology and bioinformatics, Developmental biology, Neuroscience
Introduction
GABA (γ-amino butyric acid) is a major inhibitory neurotransmitter in the central nervous system of mammals1 and is involved in the regulation of excitation, control of motor output or sensory integration, generation of oscillatory activity, neuronal assembly formation, and neuronal plasticity2. The responses to GABA are mediated via metabotropic GABAB receptors and ionotropic GABAA and GABAC receptors. GABAA/C receptors represent ligand-gated anion-channels with a high permeability for Cl− ions, while HCO3− ions contribute only partially to the ionic currents1. During early neuronal development GABAA receptor-mediated responses are depolarizing, due to a high [Cl−]i3. This high [Cl−]i is maintained by the activity of the isoform 1 of the Na+-dependent K+, Cl− Cotransporter (NKCC1), which mediates an uptake of Cl−4–8. The depolarizing GABAergic responses in the immature hippocampus have been associated with the generation of spontaneous oscillatory activity transients that are essential for brain development9,10, but also to the higher incidence and pharmacological refractoriness of epileptic seizures in the immature CNS11–14.
A variety of studies have demonstrated that the Cl−-fluxes through GABAA receptors influence [Cl−]i on a shorter time scale and thus temporarily affect the amplitude of subsequent GABAergic responses15–19. This process is termed ionic plasticity5,20. Such activity-dependent [Cl−]i transients have been implicated in a variety of pathophysiological processes21–23, but they also underlie physiological functions, e.g. in the developing spinal cord where transient activity-dependent collapses of the Cl− gradient generate slow oscillatory activity7,24. The size of activity-dependent Cl− transients is on the first instance determined by the relation between Cl− influx and the capacity of Cl− extrusion systems5,25. Several computational studies revealed that, in addition to a variety of morphological and/or electrophysiological properties26–28, the capacity of the transmembrane Cl−-transport is the main factor determining the spatiotemporal dynamics as well as the final amount of activity-dependent alterations in [Cl−]i23,26,27,29–31. Therefore, a quantification of the transport capacity of Cl−-extrusion or -uptake is necessary for a better understanding of GABAergic and glycinergic function during early development.
However, to our knowledge a detailed investigation of the kinetic properties of Cl−-transport has only been published for Cajal-Retzius cells4 and immature spinal-cord motoneurons7 as well as for mature cultured hippocampal23 and thalamic neurons32. Whereas in mature hippocampal neurons the [Cl−]i relaxation upon a massive [Cl−]i increase occurs rather fast within ~ 30s23, in immature neurons a rather inefficient Cl−-transport was observed4,7. In these neurons the recovery after [Cl−]i depletion requires ~ 10–20 min4,7. Accordingly, it has been shown for both, Cajal-Retzius cells and immature motoneurons, that physiological levels of neuronal activity can cause functionally relevant changes in [Cl−]i19,33. Another striking example for activity-dependent changes in [Cl−]i has been described in the immature hippocampus, where the massive GABAergic synaptic drive during giant depolarizing potentials, a network phenomenon essential for the development of hippocampal connectivity34, causes massive alterations in [Cl−]i17. However, while several studies have already reported that the high [Cl−]i of immature hippocampal neurons is maintained by NKCC114,35,36, to our knowledge no information about the kinetic properties of Cl−-transport in immature hippocampal neurons is available yet.
Therefore, we performed gramicidin-perforated patch-clamp recordings from visually identified CA3 pyramidal neurons of immature (postnatal day [P] 4–7) rat hippocampus to quantify the active and passive transport rates for Cl− in immature hippocampal neurons. In addition, we analyzed whether baseline levels of synaptic and extrasynaptic GABAergic activity can influence [Cl−]i. Since the massive [Cl−]i transients caused by GDPs would impair the analysis of the kinetic properties of [Cl−]i homeostasis, we performed these experiments in coronal hippocampal slices, as in these slices only a fraction of connectivity is maintained37 and therefore they fail to generate GDPs. Our experiments revealed that the rate of transmembrane Cl−-transport is rather low in immature CA3 neurons. Nevertheless, these transport rates are sufficient to maintain a stable [Cl−]i during baseline synaptic GABAergic activity, while the physiological levels of tonic GABAergic currents provoke a slight shift in [Cl−]i.
Results
Steady-state distribution of [Cl−]i in immature CA3 pyramidal cells
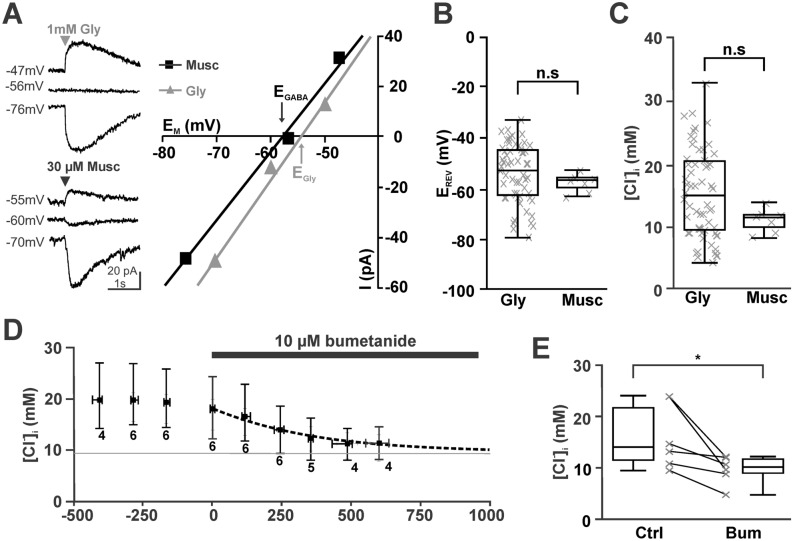
In this study we recorded in total from 121 CA3 pyramidal cells under gramicidin-perforated patch-clamp conditions. We estimated the reversal potential of GABAergic (EGABA) and glycinergic (EGly) currents from short (2–10 ms) puffs of 30 µM muscimol or 0.2–1 mM glycine applied focally to the soma of the pyramidal cells (Fig. 1A). The use of glycine pulses was necessary for the determination of [Cl−]i in part of the following experiments, as in these experiments gabazine or picrotoxin were used to eliminate phasic and tonic GABAergic currents. At a holding potential of − 70 mV these cells showed an EGABA of − 57 [-55.6, -60.0] mV (n = 7) or an EGly of − 52 [-44.6, -62.7] mV (n = 58, Fig. 1B). Both values were not significantly different (p = 0.315, Mann–Whitney). Using published values for the HCO3− permeability of GABA (0.18) or glycine (0.11) receptors38 and the estimated extra- and intracellular HCO3− concentrations, these values correspond to a [Cl−]i of 11.5 [9.8, 11.8] mM (n = 7) and 14.8 [9.4, 20.3] mM (n = 58), respectively (Fig. 1C). These [Cl−]i values were also not significantly different (p = 0.117, Mann–Whitney).
Figure 1.
Inhibition of NKCC1 with bumetanide leads to a slow [Cl−]i decrease. (A) Determination of reversal potentials for GABAergic (black) and glycinergic (gray) currents measured under gramicidin-perforated conditions. The left traces represent typical current traces upon focal application of 1 mM glycine (upper traces) and 30 µM muscimol (lower traces). Only 3 agonist applications at 3 different holding potentials were performed to minimize the effects of agonist–evoked Cl− currents on [Cl−]i. (B) Box plot diagrams illustrating that glycinergic and GABAergic reversal potentials are comparable. (C) The [Cl−]i calculated from the reversal potentials revealed comparable values for both agonists. (D) Bath application of 10 µM bumetanide induced an exponential decrease of [Cl−]i (dashed line) towards the passive distribution (gray line). Data points represent median ± interquartile range, number of experiments are indicated below the error bars. E: Statistical analysis demonstrating the reliable decrease of [Cl−]i in all experiments.
In order to determine the kinetics of passive Cl−-efflux and to confirm that this high [Cl−]i was maintained by the activity of the NKCC114,35, we first analyzed the effect of the NKCC1 inhibitor bumetanide on [Cl−]i. Bath application of 10 µM bumetanide resulted in a significant (p = 0.028, Wilcoxon) decline of [Cl−]i from 14.5 [11.5, 21.6] mM (n = 6) to 10.2 [9.0, 11.8] mM (n = 6) within ~ 10 min (Fig. 1D,E). This decline in the [Cl−]i could be fitted by a monoexponential function using a τ of 381 s (Fig. 1D). From this function we estimated a maximal passive Cl− efflux of 15.5 µM/s at a [Cl−]i of ~ 15 mM. The [Cl−]i of 10.2 [9.0, 11.8] mM (n = 6) obtained in the presence of bumetanide is in the range of the passive Cl− distribution of 9.1 mM. In summary, this result confirms that NKCC1 substantially contribute to the active Cl− accumulation and is counteracting a passive Cl− efflux5,8.
Estimation of the capacity of NKCC1 mediated Cl− uptake
Since the kinetics of [Cl−] transport is one major factor influencing ionic plasticity17,26–28,39,40, we next determined the kinetic properties of Cl− uptake after an artificial [Cl−]i reduction. To quantify the kinetics of the [Cl−]i reuptake we decreased [Cl−]i by 25 pulses of either 1–3 mM glycine or 30 µM muscimol applied with a frequency of 0.5 Hz to voltage-clamped neurons (Fig. 2A). This procedure significantly (p = 0.002, Wilcoxon) reduced the [Cl−]i by 3.2 [2.6, 3.8] mM (n = 12) from 11.4 [10.3, 11.8] mM to 7.9 [7.7, 8.4] mM (Fig. 2B). As neither the amount of the [Cl−]i decrease (p = 0.808, Mann–Whitney) nor the time constants of [Cl−]i recovery (p = 0.291, Mann–Whitney) were significantly different between glycine- and muscimol-application experiments, the data was pooled. The subsequent recovery of [Cl−]i was monitored by determining EREV with a small number of test pulses given at intervals of ~ 100 s, to avoid substantial Cl− fluxes by these test pulses4. These experiments showed that [Cl−]i returned to the resting values within ~ 10 min (Fig. 2C). This increase in [Cl−]i could be described with a monoexponential function using a time constant τ of 155 s. At a [Cl−]i of 9.1 mM, which represents a passive distribution at − 70 mV holding potential and thus eliminates passive fluxes, the active Cl− uptake rate amounted to 15.4 µM/s. In summary, these results indicate that NKCC1-mediated Cl− uptake is sufficient to maintain [Cl−]i at the observed values, but that this transport process is rather slow in immature CA3 pyramidal neurons.
Figure 2.
Slow recovery of [Cl−]i after depletion. (A) Typical current traces upon application of 1 mM glycine at holding potentials of − 43, − 53 and − 73 mV. The thin traces represent representative current traces during the unloading protocol. Note the obvious shift in EREV after the unloading protocol, which reversed after 300 s. (B) Statistical analysis demonstrating the reliable decrease of [Cl−]i in all experiments upon repetitive stimulation. (C) Analysis of [Cl−]i before and after the stimulation protocol revealed that [Cl−]i recovered with an exponential time course (dashed line) towards the baseline [Cl−]i. Data points represent median ± interquartiles, number of experiments are indicated below the error bars. (D) Typical current traces upon application of 30 µM muscimol. Note the obvious shift in EREV after repetitive muscimol application under current-clamp conditions. (E) Analysis of [Cl−]i before and after repetitive muscimol application revealed that [Cl−]i was significantly reduced after massive GABAergic stimulation.
Effect of spontaneous phasic GABAergic activity
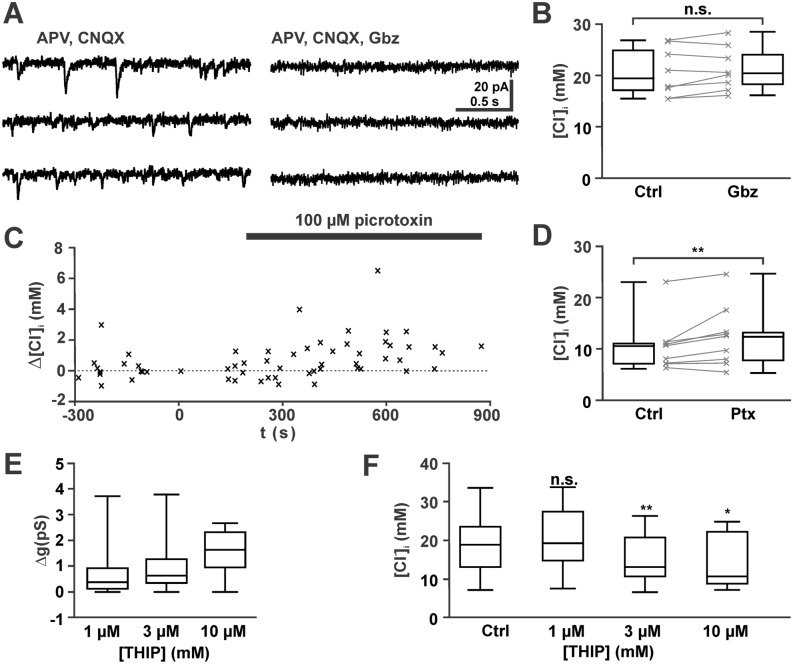
Given the low capacity of NKCC1-mediated Cl−-uptake, we next investigate whether GABAergic activity can override [Cl−]i homeostatic processes and thus influence the steady-state [Cl−]i levels. In accordance with previous publications that demonstrated substantial [Cl−]i changes upon frequent activation of GABAA receptors15,16, we observed that the repetitive application of 25 muscimol (30 µM) pulses with a frequency of 0.5 Hz led to a significant (p = 0.003, Wilcoxon) decrease in [Cl−]i by 3.1 [2.2, 3.7] mM (n = 11) (Fig. 2D,E).
Since this result indicates that GABAergic activity has the potential to contribute to [Cl−]i homeostasis, we next investigated whether the observed levels of spontaneous synaptic (phasic) GABAergic activity or the tonic GABAergic conductance influence the resting [Cl−]i of pyramidal cells. For this purpose, GABAergic current are isolated by bath application of the glutamatergic antagonists CNQX (30 µM) and APV (20 µM). In line with previous studies reporting a moderate frequency of GABAA mediated synaptic events in the immature hippocampus13,41,42, the frequency of pharmacologically isolated GABAergic PSCs in the present study was 2.03 [1.28, 2.03] Hz (n = 6 cells with 1531 events), with a median amplitude of 8.5 [7.3, 14.8] pA (corresponding to a peak conductance of 122 [105.4, 213.6] pS). These GABAergic PSCs were completely suppressed in the presence of 1 µM gabazine (Fig. 3A), which at this concentration selectively blocks synaptic GABAA receptors13. To unravel whether an inhibition of synaptic GABAergic activity influences [Cl−]i, we determined [Cl−]i before and after synaptic GABAergic activity was inhibited for 5—16 min under current clamp conditions. These experiments revealed that after a complete blockade of spontaneous GABAergic inputs, [Cl−]i was non-significantly (p = 0.161, Wilcoxon test) altered by 0.7 [-0.5, 1.6] mM (n = 8) from 18.3 [15.8, 23.8] mM to 19.2 [17.1, 23.0] mM (Fig. 3B). Note that the tendency (p = 0.07, Mann–Whitney U-test) to higher basal [Cl−]i in these neurons, as compared to all recordings, led to a higher driving force for Cl− ions and would thus result in even higher activity-dependent [Cl−]i changes. We conclude from these results that the capacity of NKCC1-mediated Cl− uptake in immature CA3 pyramidal neurons is sufficient to cope with the Cl−-influx caused by spontaneous GABAergic synaptic inputs.
Figure 3.
No effect of phasic (synaptic) and mild effect of tonic GABAergic activity on [Cl−]i. (A) Typical current traces illustrating that pharmacologically isolated GABAergic PSCs were completely suppressed by 1 µM gabazine (Gbz). (B) Statistical analysis illustrating that a complete suppression of GABAergic PSCs has no significant effect on [Cl−]i.. (C) Shift in [Cl−]i upon bath application of 100 µM picrotoxin (Ptx) under voltage-clamp conditions. Ptx inhibits both tonic (extrasynaptic) as well as phasic (synaptic) GABAergic currents. Each symbol represents an individual data point from n = 9 experiments. The [Cl−]i was related to the [Cl−]i in the last measurement before Ptx application. Note the tendency toward an increased [Cl−]i in the presence of Ptx. (D) Statistical analyses of these experiments revealed an increased [Cl−]i after the onset of Ptx application. (E) Bath application of THIP dose-dependently increased the membrane conductance. THIP enhances tonic (extrasynaptic) GABAergic conductance. F: Statistical analysis illustrating that THIP induced a dose-dependent decrease in [Cl−]i.
Tonic currents mediated by extrasynaptic GABA receptors contribute substantially to passive [Cl−]i fluxes in immature neurons 4, as such tonic currents mediate a larger charge transfer43. Since in the immature hippocampus extrasynaptic receptors substantially contribute to the excitability9,13,41, we also investigated whether tonic GABAergic currents influence [Cl−]i. For this purpose, we blocked tonic and phasic GABAergic currents with picrotoxin13. Bath application of 100 µM picrotoxin unravelled a tonic GABAergic conductance of 0.9 [0.49, 1.01] pS (n = 9). The continuous application of 100 µM PTX for 5–12 min led under voltage-clamp conditions to a slight, but significant (p = 0.008, Wilcoxon test) increase in [Cl−]i by 1.6 [0.7, 1.9] mM (n = 9) from 10.8 [7.3, 11.3] mM to 12.7 [8.1, 13.5] mM (Fig. 3C,D).
In line with this observation, enhancement of tonic conductance using THIP led to a small decrease in [Cl−]i. To avoid the induction of epileptiform activity by THIP13, these experiments are performed in the continuous presence of the glutamatergic antagonists CNQX (30 µM) and APV (20 µM). Bath application of 1 µM THIP activated a median membrane conductance of 0.28 [0.03, 0.81] pS (n = 14), which increased to 0.37 [0.09, 1.01] pS (n = 13) and 0.67 [0.02, 1.35] pS (n = 12) in the presences of 3 µM and 10 µM THIP, respectively (Fig. 3E). These enhanced tonic currents led to a significant (p = 0.033, Mann–Whitney U-tests) decrease in [Cl−]i (Fig. 3F). Upon a constant THIP application for 4 – 12 min [Cl−]i decreased in the presence of 3 µM THIP by 3.0 [1.8, 6.9] mM (n = 13, p = 0.009, Wilcoxon test) and in 10 µM THIP by 5.0 [0.5, 10.3] mM (n = 12, p = 0.015, Wilcoxon test). In the presence of 1 µM THIP no significant (p = 0.638, Wilcoxon test) [Cl−]i alteration (0.09 [-3.4, 4.4] mM, n = 14) was observed. In summary, these results suggest that basal levels of tonic GABAergic activity can induce Cl− fluxes that influence mildly the resting [Cl−]i.
Estimation of activity dependent [Cl−]i alterations using compartmental modeling
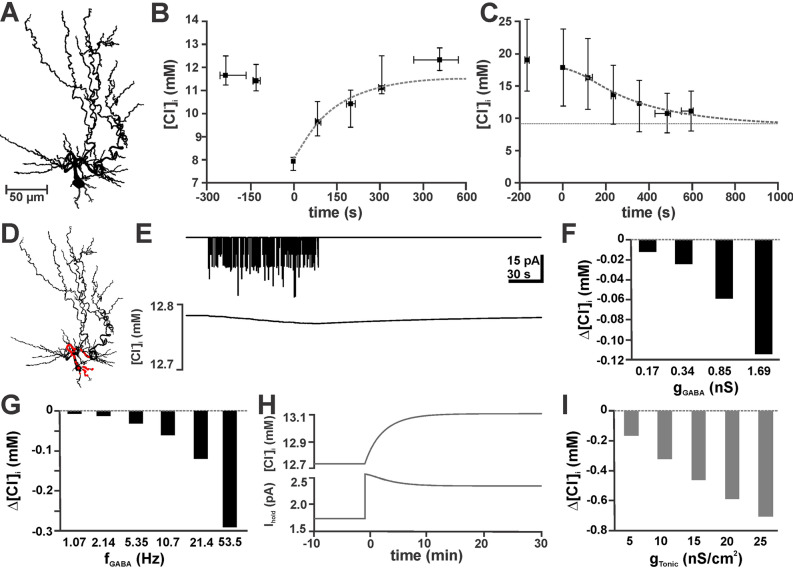
Finally, we used a data-driven biophysical model of Cl− dynamics to estimate whether Cl− fluxes due to synaptic (phasic) and extrasynaptic (tonic) activation of GABAA channels, transmembrane Cl− transport and Cl− diffusion are able to account for the observed stability of [Cl−]i. To set the geometry of this computational model we employed the 3D-reconstructed morphology of a young CA3 pyramidal cell (Fig. 4A). The diffusion of Cl− inside the dendritic tree was simulated using deterministic compartmental diffusion modeling implemented in NEURON40,44 (see Methods). To simulate tonic GABAergic currents, we added a tonic conductance of 8.75 nS/cm2, which allowed the model to replicate the passive Cl− fluxes observed in immature CA3 neurons (Fig. 4B). Next we implemented an active Cl− accumulation process with a τCl of 78.5 s and a target [Cl−]i ([Cl−]i0) of 13.3 mM, which allowed the modeled neurons to replicate the experimentally determined kinetics of [Cl−]i relaxation and the steady-state [Cl−]i (Fig. 4C).
Figure 4.
A biophysically realistic compartmental model of active Cl−uptake and diffusion confirmed limited alterations in [Cl−]i due to phasic and tonic GABAergic currents. (A) Morphology of the reconstructed CA3 pyramidal cell used for numerical simulations. (B) Fit of the experimentally observed [Cl−]i relaxation after Cl− depletion with adequate parameter settings (τ = 78.5 s; [Cl−]i0 = 13.3 mM) for a simulated active Cl− accumulation process (dashed line). (C) Simulation of the experimentally observed [Cl−]i decline after elimination of active Cl− accumulation (black symbols) by implementation of a passive tonic Cl− conductance at a density of 8.75 nS/cm2 (dashed line) in a simulated neuron lacking active Cl−-uptake. (D) Representation of the reconstructed neuron in the NEURON model. The locations of GABAergic synapses are indicated by red dots. (E) Holding current (upper panel) and [Cl−]i (lower panel) upon repetitive synaptic stimulation (gGABA 0.169 nS at 2.14 Hz for 100 s). (F) [Cl−]i changes induced by 100 s of random synaptic activity with different gGABA. (G) [Cl−]i changes induced by 100 s of random synaptic activity with a gGABA of 0.169 nS at different frequencies. Note that, in line with experiments, physiological values (0.169 nS at 2.14 Hz) induced only marginal [Cl−]i changes. (H) [Cl−]i and holding-current (Ihold) upon elimination of the basal tonic GABAergic conductance of 8.75 nS/cm2 at t = 0. (I) [Cl−]i changes induced by the addition of a tonic GABAergic component to the basal tonic current.
To simulate phasic GABAergic activity, we modeled stochastic activation of 107 GABAA synapses located randomly in the soma and perisomatic dendrites45 (Fig. 4D). The initial values for the conductance of GABAA synapses (gGABA = 169 pS) and the frequency of GABAergic synaptic currents (2.14 Hz) were based on the mean values of the experimental data. In accordance with the patch-clamp observation, addition of physiological levels of GABAergic synaptic inputs had only a marginal effect on [Cl−]i. After 100 s of continuous GABAergic activity at 2.14 Hz [Cl−]i decreased by only 0.012 mM (Fig. 4E,F). Augmenting gGABA enhanced the synaptically evoked [Cl−]i decline (Fig. 4F), which however remained small (0.114 mM) even if gGABA was increased to 1.69 nS. Increasing the frequency of GABAergic synaptic inputs from 2.14 to 5.35 Hz had only a marginal effect of the [Cl−]i change (0.031 mM, Fig. 4G), but at frequencies of ~ 20 and ~ 50 Hz [Cl−]i changes by 0.12 mM and 0.29 mM, respectively, were induced (Fig. 4G). In summary, these results indicate that basal levels of synaptic activity had no major effect in [Cl−]i.
To analyze the influence of tonic GABAergic currents, we omitted the tonic GABAergic conductance of 8.75 nS/cm2, which changed the holding current (Ihold) by 0.8 pA and led to a [Cl−]i increase by 0.32 mM (Fig. 4H). Enhancing tonic GABAergic currents by adding multiples of 5 nS/cm2 to the basal tonic conductance induced a dose-dependent decrease in [Cl−]i that, however, remained below 0.8 mM (Fig. 4I). These additional tonic conductances between 5 and 25 nS/cm2 induced a linear decrease in the inward current between − 0.3 and − 1.28 pA, respectively.
In summary, these simulations with a realistic computational model of Cl− dynamics support the observation, that NKCC1-mediated Cl− transport is sufficient to maintain [Cl−]i at basal levels of phasic GABAergic activity, while tonic currents have a mild effect on steady-state [Cl−]i.
Discussion
To comprehend the physiological role of depolarizing GABAergic responses in the immature hippocampus under conditions that dynamically challenge [Cl−]i homeostasis, information about the kinetic properties of [Cl−]i homeostasis is required39. The main findings of the present study can be summarized as follows: (i) NKCC1 is the main Cl− transporter in immature CA3 pyramidal cells and mediates an active Cl−-accumulation with a rate of 15.4 µM/s. (ii) Physiological levels of tonic GABAergic activation induce a slight decrease in [Cl−]i by 1.6 mM. (iii) In contrast, spontaneous phasic GABAergic PSCs do not significantly affect [Cl−]i. We conclude from these results, that the [Cl−]i of immature CA3 pyramidal neurons represents a steady-state equilibrium between active NKCC1-mediated Cl−-accumulation and passive Cl−-efflux, mainly via tonic GABAergic currents. The capacity of the NKCC1-mediated Cl− uptake in CA3 pyramidal cells is, however, sufficient to maintain a high [Cl−]i under basal levels of GABAergic activity.
In the present study we used relative HCO3− permeability ratios of 0.18 for GABAA and of 0.11 for glycine receptors, which were determined by Bormann et al. in cultured spinal cord neurons38. While published permeability ratios for GABAA (0.44) and glycine receptors (0.40) in hippocampal neurons are available46, using these values resulted in unrealistically low [Cl−]i values (ranging from 0.4 to 30 mM). Therefore we prefer to use the values provided by Bormann et al.38. Importantly, the qualitative results of our study will not be altered, if the higher relative HCO3− permeability ratios are used.
The first outcome of this study is that inhibition of NKCC1 with 10 µM bumetanide induced a decline in [Cl−]i towards the passive distribution, which supports the fact that this transporter constitutes the main Cl− uptake mechanism in immature CA3 pyramidal cells14,35,47. Bumetanide has been reported to also inhibit other targets, like KCC2 or aquaporins48,49, however, at slightly higher effective doses of 55 µM and 100 µM, respectively. An inhibition of these transporters will have no effect on the time constant of the [Cl−]i decay upon bumetanide application, but our experiments could not exclude a minor contribution of the [Cl−]i extruder KCC2 to steady-state [Cl−]i levels. The [Cl−]i decline observed in this experiment upon pharmacological inhibition of NKCC1 represents basal Cl− fluxes, which can be mediated via different types of voltage-, volume-, or ligand-gated Cl− channels50. Next, we determined the capacity of the NKCC1-mediated Cl−-uptake system by quantifying the rate of Cl−-accumulation after [Cl−]i depletion. Because the [Cl−]i recovery depends only on the properties of the uptake systems and should be independent of the agonists used for the induction of the [Cl−]i depletion and since the time constants of [Cl−]i recovery are not significantly different between glycine- and muscimol-application, data of muscimol- and glycine application experiments were pooled. In these experiments we determined an uptake rate of 15.4 µM/s. This value was comparable to the [Cl−]i uptake rate determined previously in immature cortical neurons4. In more mature neurons inhibition of transmembrane Cl− transport with furosemide induced a positive shift in EGABA, indicating that in slightly older neurons the activity of KCC2 is required to establish a low [Cl−]i51.
The maximal rate for passive Cl− efflux during the [Cl−]i decline amounted to ~ 15.5 µM/s and is thus in the range of the Cl−-uptake. However, while the effective rate of Cl−-uptake was determined at 9.1 mM, which is the passive [Cl−]i-distribution and thus omits any passive Cl−-fluxes, the rate of Cl−-efflux was determined at an arbitrary [Cl−]i value of 15 mM. Please note, that both fluxes depend on the [Cl−]i-gradient across the membrane, with the passive fluxes disappearing at 9.1 mM, while the NKCC1-mediated uptake has a considerably lesser dependency of the [Cl−]i gradient5. Thus the combination of these oppositely directed Cl-fluxes resulted in a steady-state equilibrium39, which is maintained at a [Cl−]i substantially higher than the passive distribution and thus supports depolarizing GABAergic responses. On the other hand, one should keep in mind that the present study only revealed the properties of the [Cl−]i homeostasis in the soma. Variation in the functional expression of active Cl−-transport and passive Cl−-fluxes may result in different capacities of [Cl−]i homeostasis in distinct compartments.
Further experiments of the present study revealed, that inhibition of phasic, synaptic GABAergic inputs did not affect [Cl−]i. In agreement with this lack of an effect of phasic GABAergic inputs on [Cl−]i, the compartmental simulations in NEURON revealed no major shifts in [Cl−]i upon realistic background activation of synaptic GABAergic inputs. In addition, the simulations demonstrated that moderate enhancement of either frequency or conductance of GABAergic synaptic inputs induced only small changes in [Cl−]i. Only at frequencies > 10 Hz substantial [Cl−]i changes were induced. This observation is in contrast to Cajal-Retzius cells, where already moderate, physiological levels of synaptic GABAergic activity (3.6 ± 0.6 Hz) mediate substantial [Cl−]i alterations19, and to neurons from juvenile hippocampal slice cultures, where ongoing phasic activity is required to allow dynamic changes in EGABA51. However, in the present simulations [Cl−]i was determined in the soma, to match the modelling results with the experimental design of the gramicidin perforated patch experiments. Within the dendritic compartment these levels of GABAergic synaptic activity will induce substantial GABAergic [Cl−]i transients17.
In addition, the present study demonstrated that inhibition of both, phasic and tonic GABAergic currents with picrotoxin13 evoked a small, but significant [Cl−]i increase. In line with this observation, the pharmacological induction of extrasynaptic GABAergic currents with THIP led to a substantial reduction in [Cl−]i. This observation could be replicated in the NEURON simulations by the addition of tonic Cl− conductances. Our experiments suggest that the substantial tonic current found in immature hippocampal neurons52, contributes to steady state [Cl−]i. The dissimilar effects of phasic and tonic GABAergic currents directly reflect the different levels of charge transfer mediated by the short phasic responses (ca. 0.015 pC/s at 2.13 Hz and a gGABA of 169 pS) in contrast to the persistent but small amplitude tonic GABAergic currents (ca. 0.46 pC/s at 8.75 nS/cm2)43.
A variety of studies in the adult brain have shown that excessive GABAergic stimulation can induce Cl−-fluxes that exceed the capacity of active Cl− transport and consequently increase [Cl−]i and alter GABAergic responses15,16,18,25,40,51,53. The low capacity of the NKCC1-mediated Cl− uptake makes immature neurons particularly susceptible to such effects. On the other hand, spontaneous, highly correlated activity transients are typical for developing neuronal systems54. These correlated activity transients are characterized by the synchronous activation of glutamatergic and GABAergic activity55,56. The resulting massive GABAergic inputs may exceed the capacity of transmembrane Cl− transport and induce substantial [Cl−]i changes. The depolarizing glutamatergic inputs during correlated network activity can even augment the GABAergic Cl− fluxes and thus aggravate the [Cl−]i alterations57. Indeed it has been demonstrated that such physiological bursts of activity can led to substantial [Cl−]i shifts in the spinal cord33,58, neocortex19, and immature hippocampus17. Whereas in the adult system such changes will decrease GABAergic inhibition and thus contribute to the establishment of hyperexcitable states and reduced pharmacological responsiveness18, in immature neurons the activity-dependent [Cl−]i reduction will decrease the excitatory potential of GABAA receptor-mediated responses. And since shunting inhibition remains constant55,59, this activity-dependent [Cl−]i decrease will augment the inhibitory potential of GABAergic responses. Therefore, the low capacity of Cl− export in immature neurons may be an adaptation to prevent hyper-excitability mediated by depolarizing GABA responses, as has been originally suggested by Ben-Ari3. In addition, at least for the immature spinal cord it has been demonstrated that such activity-dependent [Cl−]i transients can determine the frequency of spontaneous activity transients by temporarily reducing the excitatory effect of GABA58. Thus it is tempting to speculate that recurrent alterations in [Cl−]i may also contribute to slow oscillatory phenomena in other regions of the developing nervous system.
In summary, the results of our present study demonstrate that the capacity of Cl− accumulation is limited in immature hippocampal CA3 pyramidal neurons. This finding, in combination with a quantification in immature cortical neurons4 and the observation of activity-related [Cl−]i transients in other immature tissues19,33, suggests that an unstable [Cl−]i homeostasis may be an innate feature of immature neurons.
Methods
Slice preparation
All experiments were conducted in accordance with EU directive 86/609/EEC for the use of animals in research and the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the local ethical committee (Landesuntersuchungsanstalt RLP, Koblenz, Germany). All efforts were made to minimize the number of animals and their suffering. Wistar rat pups of P4-7 were obtained from the local breeding facility and were deeply anesthetized with enflurane (Ethrane, Abbot Laboratories, Wiesbaden, Germany). After decapitation, the brains were quickly removed and immersed for 2–3 min in ice-cold standard artificial cerebrospinal fluid (ACSF, composition see below). Coronal slices (400 µm thickness) including the hippocampus were cut on a vibratome (HR2, Sigmann Elektronik, Hüffenhardt, Germany). The slices were stored in an incubation chamber filled with oxygenated ACSF at room temperature before they were transferred to the recording chamber.
Data acquisition and analysis
Gramicidin-perforated whole-cell patch-clamp recordings were performed as described previously4,13 at 31 ± 1 °C in a submerged-type recording chamber attached to the fixed stage of a microscope (BX51 WI, Olympus). Pyramidal neurons in stratum pyramidale of the CA3 region were identified by their location and morphological appearance in infrared differential interference contrast image. Patch-pipettes (5–12 MΩ) were pulled from borosilicate glass capillaries (2.0 mm outside, 1.16 mm inside diameter, Science Products, Hofheim, Germany) on a vertical puller (PP-830, Narishige) and filled with pipette solution containing 130 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 Na2-ATP, 0.5 Na-GTP (pH adjusted to 7.4 with KOH and osmolarity to 306 mOsm with sucrose). For gramicidin-perforated patch-clamp recordings 10–50 µg/ml gramicidin D (Sigma, St Louis, MO, USA) was added from a stock solution (1–2 mg/ml in DMSO) on the day of experiment. Experiments were omitted from analysis, when an instantaneous or constant shift in the muscimol/glycine reversal potential towards positive values, determined by the high [Cl−] of the pipette solution, occurred, as they indicate insufficient perforated-patch conditions.
Signals were recorded with a discontinuous voltage-clamp/current-clamp amplifier (SEC05L, NPI, Tamm, Germany), low-pass filtered at 3 kHz and stored and analyzed using an ITC-1600 AD/DA board (HEKA) and TIDA software. Input resistance and capacitance were determined from a series of hyperpolarizing current steps. The apparent cell surface was estimated using a specific capacitance of 2 µF/cm2. Spontaneous postsynaptic currents (sPSCs) were detected and analysed from whole-cell patch-clamp recordings according to their amplitude and shape by appropriate settings using Minianalysis Software (Synaptosoft, Fort Lee, NJ).
The [Cl−]i was determined from the reversal potentials of GABAergic and glycinergic currents recorded under voltage-clamp conditions using the Goldman–Hodgkin–Katz equation:
For the calculation of [Cl−]i from EGABA we used a [Cl−]e of 133.5 mM, an extracellular HCO3− concentration ([HCO3−]e) of 24 mM, a [HCO3−]i of 14.1 mM, and published values for the HCO3− permeability of GABA (0.18) or glycine (0.11) receptors38. GABAergic and glycinergic currents were evoked by brief (2–10 ms) pulses of 30 µM muscimol or 0.2–1 mM glycine from a patch pipette positioned close to the soma via a custom built pressure application system (Lee, Westbrook, CT) at a pressure of 0.5 bar. The use of glycine pulses was necessary to allow the determination of [Cl−]i in the presence of gabazine or picrotoxin, which eliminate GABAergic currents13.
All values were given as median ± interquartile range, in the panels median ± interquartile range was used for time-dependent plots, while summarized results were shown as box and whisker plots (minimum, first quartile, median, third quartile, maximum). For statistical analysis of unpaired data Mann–Whitney U-tests and for paired data Wilcoxon signed-rank test were used (Systat 11, Point Richmond, CA). Significance was assigned at levels of 0.05 (*), 0.01 (**) and 0.001 (***).
Morphological reconstruction
For morphological reconstruction, 18 CA3 pyramidal cells were filled with biocytin under whole-cell conditions. From this 18 stained neurons, two typical cells were used for quantitative somatodendritic reconstruction after visual inspection. For this purpose, 0.5–1% biocytin (Sigma, Taufkirchen, Germany) was added to the pipette solution. After filling of the cells, slices were fixed for at least 24 h in 4% paraformaldehyde. Subsequently they were rinsed and incubated 60 min with 0.5% H2O2 and 0.8% Triton-X100 to inhibit endogenous peroxidases. After overnight incubation with an avidin-coupled peroxidase (ABC kit, Vectorlabs, Burlingame, CA, USA), slices were pre-incubated in 0.5 mM diaminobenzidine and subsequently developed in diaminobenzidine and 0.015% H2O2. The slices were finally rinsed, dehydrated slowly through alcohol and propylenoxide, and embedded in Durcupan (Fluka, Buchs, Switzerland). Reconstruction and morphological analysis of the biocytin-labelled neurons were performed using the 60 × oil-immersion objective (NA 1.4) of a Nikon Eclipse 80i (Nikon, Germany) attached to a computer system (Neurolucida; MBF Bioscience Europe). Data was corrected for tissue shrinkage after importing to the NEURON environment. For this purpose we used the values suggested by Staiger et al.60 and expanded the x-/y-dimensions by 12.5% and the z-dimension by 50%.
Compartmental modeling
The reconstructed CA3 pyramidal cell (see above) was imported into the NEURON simulation program (neuron.yale.edu). The following passive parameters were used: Ra (specific axial resistance) = 35.4 Ωcm; gpas (passive specific membrane conductance) = 17.05 nS/cm2; Epas = -74.05 mV, Cm (specific membrane capacitance) = 1 µF/cm2. In addition, a tonic leak Cl− conductance:
with a conductance gtonic of 8.75 nS/cm2 was inserted. Implementing these parameters in the reconstructed morphology resulted in a resting membrane potential of − 70 mV and an input resistance of 306 MΩ.
Cl− diffusion and uptake were calculated by standard compartmental diffusion modeling40,44. To simulate intracellular Cl− dynamics, we adapted our previously published model40. Longitudinal Cl− diffusion along dendrites was modeled as the exchange of Cl− between adjacent compartments. For radial diffusion, the volume was discretized into a series of 4 concentric shells around a cylindrical core and Cl− was allowed to flow between adjacent shells61. The free diffusion coefficient of Cl− inside neurons (DCl) was set to 2 µm2/ms53. To simulate Cl− uptake, a pump mechanism for transmembrane Cl− transport was included. Cl−transport was modeled as exponential recovery of [Cl−]i to its target [Cl−]i ([Cl−]i0) with a time constant τCl.
The pump mechanism approximates an NKCC1-like Cl− transport mechanism.
The impact of GABAergic Cl− currents on [Cl−]i was calculated as:
with F = 96,485 C/mol (Faraday constant). GABAA synapses were simulated as a postsynaptic parallel Cl− and HCO3− conductance with exponential rise and exponential decay40:
where P is a fractional ionic conductance that was used to split the GABAA conductance (gGABA) into Cl− and HCO3− conductance. ECl and EHCO3 were calculated from Nernst equation. The GABAA conductance was modeled using a two-term exponential function, using values of rise time (0.5 ms) and decay time (37 ms)17. Parameters used in our simulations were as follows: [Cl−]o = 133.5 mM, [HCO3−]i = 14.1 mM, [HCO3−]o = 24 mM, temperature = 31 °C, PGABA = 0.18, and PGly = 0.1138. The GABAA inputs (107 synapses, peak conductance 0.169 nS)17 were activated stochastically (Poisson) with a frequency of 0.02 Hz, corresponding to a main PSC frequency of 2.14, except where noted. Source codes of all models are available at ModelDB (https://modeldb.yale.edu/266811; password is “hippocampus”).
Solutions and drugs
The bathing solution consisted of 126 NaCl, 26 NaHCO3, 1.25 NaH2PO5, 1 MgCl2, 2 CaCl2, 2.5 KCl, 10 glucose (pH 7.4, osmolarity 306 mOsm) and was equilibrated with 95% O2 / 5% CO2 at least 1 h before use. GABA (γ-amino butyric acid), 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (gabazine, SR-95531), picrotoxin (PTX), 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP), glycine and bumetanide were purchased from Sigma, and DL-2-Amino-5-phosphonopentanoic acid (APV), 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), muscimol and tetrodotoxin (TTX), from Biotrend (Cologne, Germany). TTX and glycine were dissolved in distilled water and picrotoxin, gabazine, muscimol, bumetanide, CNQX and APV in dimethylsulfoxide (DMSO). All substances were added to the solutions shortly before the experiment. The DMSO concentration of the final solution never exceeded 0.2%.
Acknowledgements
The authors thank Beate Krumm, for excellent technical assistance. RC was graduate student of the Neuroscience Graduate School at the University of Mainz. AL received a stipend from the FTN and was a graduate student in the TrandsMed program. The work was supported by supported by a BMBF grant to PJ (No. 031L0229) and DFG grants to WK (KI 835/3) and HJL (SFB 1080-A01).
Author contributions
W.K., and P.J. conceptualized the study, S.N.K. and R.C. performed the electrophysiological recordings, J.F.S. provided the neuronal reconstruction, N.M., A.L., P.J. and W.K. programmed and analyzed the in-silico modelling, W.K. and H.J.L. wrote the manuscript. All authors have read and agreed on the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
All authors declare that they have no competing financial, professional or personal interests that might have influenced the objectives, performance or presentation of the study described in this manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog. Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 2.Swanson OK, Maffei A. From hiring to firing: activation of inhibitory neurons and their recruitment in behavior. Front. Mol. Neurosci. 2019;12:1–9. doi: 10.3389/fnmol.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 4.Achilles K, et al. Kinetic properties of Cl− uptake mediated by Na+-dependent K+-2Cl− cotransport in immature rat neocortical neurons. J. Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Islas C, Chub N, Wenner P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J. Neurophysiol. 2009;101:507–518. doi: 10.1152/jn.90986.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M, Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 2015;9:1–14. doi: 10.3389/fncel.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J. Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirischuk S, et al. Modulation of neocortical development by early neuronal activity: Physiology and pathophysiology. Front. Cell. Neurosci. 2017;11:1–21. doi: 10.3389/fncel.2017.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann. Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 12.Mazarati A, Shin D, Sankar R. Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia. 2009;50:2117–2122. doi: 10.1111/j.1528-1167.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolbaev SN, Sharopov S, Dierkes PW, Luhmann HJ, Kilb W. Phasic GABAA-receptor activation is required to suppress epileptiform activity in the CA3 region of the immature rat hippocampus. Epilepsia. 2012;53:888–896. doi: 10.1111/j.1528-1167.2012.03442.x. [DOI] [PubMed] [Google Scholar]
- 14.Dzhala VI, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 15.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J. Neurophysiol. 1989;61:501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- 16.Isomura Y, et al. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J. Neurophysiol. 2003;90:2752–2756. doi: 10.1152/jn.00142.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi A, Jedlicka P, Luhmann HJ, Kilb W. Giant depolarizing potentials trigger transient changes in the intracellular Cl− concentration in CA3 pyramidal neurons of the immature mouse hippocampus. Front. Cell. Neurosci. 2018;12:420. doi: 10.3389/fncel.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Neurobiology of disease pyramidal cells accumulate chloride at seizure onset. Neurobiol. Dis. 2012;47:358–366. doi: 10.1016/j.nbd.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolbaev SN, Luhmann HJ, Kilb W. Activity-dependent scaling of GABAergic excitation by dynamic Cl− changes in Cajal–Retzius cells. Pflugers Arch. Eur. J. Physiol. 2011;461:557–565. doi: 10.1007/s00424-011-0935-4. [DOI] [PubMed] [Google Scholar]
- 20.Raimondo JV, Markram H, Akerman CJ. Short-term ionic plasticity at GABAergic synapses. Front. Synaptic Neurosci. 2012;4:5. doi: 10.3389/fnsyn.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaila K, Ruusuvuori E, Seja P, Voipio J, Puskarjov M. GABA actions and ionic plasticity in epilepsy. Curr. Opin. Neurobiol. 2014;26:34–41. doi: 10.1016/j.conb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Wright R, Raimondo JV, Akerman CJ. Spatial and temporal dynamics in the ionic driving force for GABA(A) Receptors. Neural Plast. 2011;2011:728395. doi: 10.1155/2011/728395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currin CB, Trevelyan AJ, Akerman CJ, Raimondo JV. Chloride dynamics alter the input-output properties of neurons. PLoS Comput. Biol. 2020;16:1–22. doi: 10.1371/journal.pcbi.1007932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chub N, Mentis GZ, O’Donovan MJ. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J. Neurophysiol. 2006;95:323–330. doi: 10.1152/jn.00162.2005. [DOI] [PubMed] [Google Scholar]
- 25.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl− and HCO3− transport. J. Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi A, Jedlicka P, Luhmann HJ, Kilb W. Interactions between membrane resistance, GABA-A receptor properties, bicarbonate dynamics and Cl−-transport shape activity-dependent changes of intracellular Cl− concentration. Int. J. Mol. Sci. 2019;20:1–22. doi: 10.3390/ijms20061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyon N, et al. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. Plos Comput. Biol. 2011;7:e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohapatra N, et al. Spines slow down dendritic chloride diffusion and affect short-term ionic plasticity of GABAergic inhibition. Sci. Rep. 2016;6:23196. doi: 10.1038/srep23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyon N, Prescott SA, Koninck YD. Mild KCC2 Hypofunction causes inconspicuous chloride dysregulation that degrades neural coding. Front. Cell. Neurosci. 2016;9:1–16. doi: 10.3389/fncel.2015.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchin A, Chizhov A, Huberfeld G, Miles R, Gutkin BS. Reduced efficacy of the KCC2 cotransporter promotes epileptic oscillations in a subiculum network model. J. Neurosci. 2016;36:11619–11633. doi: 10.1523/JNEUROSCI.4228-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Düsterwald KM, et al. Biophysical models reveal the relative importance of transporter proteins and impermeant anions in chloride homeostasis. Elife. 2018;7:1–30. doi: 10.7554/eLife.39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt T, et al. Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus. Sci. Rep. 2018;8:13929. doi: 10.1038/s41598-018-31762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Islas C, Chub N, Garcia-Bereguiain MA, Wenner P. GABAergic synaptic scaling in embryonic motoneurons is mediated by a shift in the chloride reversal potential. J. Neurosci. 2010;30:13016–13020. doi: 10.1523/JNEUROSCI.1659-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griguoli M, Cherubini E. Early correlated network activity in the hippocampus: its putative role in shaping neuronal circuits. Front. Cell. Neurosci. 2017;11:255. doi: 10.3389/fncel.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sipila ST, Schuchmann S, Voipio J, Yamada J, Kaila K. The cation-chloride cotransporter NKCC1 promotes sharp waves in the neonatal rat hippocampus. J. Physiol. 2006;573:765–773. doi: 10.1113/jphysiol.2006.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen R, et al. Activation of glycine receptors modulates spontaneous epileptiform activity in the immature rat hippocampus. J. Physiol. 2014;592:2153–2168. doi: 10.1113/jphysiol.2014.271700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong G, Metheny H, Johnson BN, Cohen AS. A comparison of different slicing planes in preservation of major hippocampal pathway fibers in the mouse. Front. Neuroanat. 2017;11:1–17. doi: 10.3389/fnana.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyon N, Vinay L, Prescott SA, De Koninck Y. Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron. 2016;89:1157–1172. doi: 10.1016/j.neuron.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Jedlicka P, Deller T, Gutkin BS, Backus KH. Activity-dependent intracellular chloride accumulation and diffusion controls GABAA receptor-mediated synaptic transmission. Hippocampus. 2011;21:885–898. doi: 10.1002/hipo.20804. [DOI] [PubMed] [Google Scholar]
- 41.Marchionni I, Omrani A, Cherubini E. In the developing rat hippocampus a tonic GABA A—mediated conductance selectively enhances the glutamatergic drive of principal cells. J. Physiol. 2007;581:515–528. doi: 10.1113/jphysiol.2006.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABAA mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48:96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 43.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Mohapatra N, Santamaria F, Jedlicka P. Modeling the effects of neuronal morphology on dendritic chloride diffusion and GABAergic inhibition. BMC Neurosci. 2014;6:23196. doi: 10.1038/srep23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papp E, Leinekugel X, Henze DA, Lee J, Buzsáki G. The apical shaft of CA1 pyramidal cells is under GABAergic interneuronal control. Neuroscience. 2001;102:715–721. doi: 10.1016/S0306-4522(00)00584-4. [DOI] [PubMed] [Google Scholar]
- 46.Fatima-Shad K, Barry PH. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc. R. Soc. B Biol. Sci. 1993;253:69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- 47.Sipilä ST, et al. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J. Neurosci. 2009;29:6982–6988. doi: 10.1523/JNEUROSCI.0443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: Implications for [K+](o) regulation. Am. J. Physiol. Cell Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 49.Migliati E, et al. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009;76:105–112. doi: 10.1124/mol.108.053744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jentsch TJ, Pusch M, Rehfeldt A, Steinmeyer K. The ClC family of voltage-gated chloride channels: structure and function. Ann. N.Y. Acad. Sci. 1993;707:285–293. doi: 10.1111/j.1749-6632.1993.tb38059.x. [DOI] [PubMed] [Google Scholar]
- 51.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on E(Cl)—in hippocampal CA3 neurons. J. Neurophysiol. 1989;61:512–523. doi: 10.1152/jn.1989.61.3.512. [DOI] [PubMed] [Google Scholar]
- 52.Sipilä ST, Voipio J, Kaila K. GAT-1 acts to limit a tonic GABAA current in rat CA3 pyramidal neurons at birth. Eur. J. Neurosci. 2007;25:717–722. doi: 10.1111/j.1460-9568.2007.05342.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuner T, Augustine GJ. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal neurons. Neuron. 2000;27:447–459. doi: 10.1016/S0896-6273(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 54.Luhmann HJ, et al. Spontaneous neuronal activity in developing neocortical networks: From single cells to large-scale interactions. Front. Neural Circuits. 2016;10:40. doi: 10.3389/fncir.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalilov I, Minlebaev M, Mukhtarov M, Khazipov R. Dynamic changes from depolarizing to hyperpolarizing GABAergic actions during giant depolarizing potentials in the neonatal rat hippocampus. J. Neurosci. 2015;35:12635–12642. doi: 10.1523/JNEUROSCI.1922-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khazipov R, et al. Early development of neuronal activity in the primate hippocampus in utero. J. Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halbhuber L, Achtner C, Luhmann HJ, Sinning A, Kilb W. Coincident activation of glutamate receptors enhances GABAA receptor-induced ionic plasticity of the intracellular Cl− concentration in dissociated neuronal cultures. Front. Cell. Neurosci. 2019;13:1–15. doi: 10.3389/fncel.2019.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J. Neurophysiol. 2001;85:2166–2176. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- 59.Kolbaev SN, Achilles K, Luhmann HJ, Kilb W. Effect of depolarizing GABAA-mediated membrane responses on excitability of cajal-retzius cells in the immature rat neocortex. J. Neurophysiol. 2011;106:2034–2044. doi: 10.1152/jn.00699.2010. [DOI] [PubMed] [Google Scholar]
- 60.Staiger JF, Bojak I, Miceli S, Schubert D. A gradual depth-dependent change in connectivity features of supragranular pyramidal cells in rat barrel cortex. Brain Struct. Funct. 2015;220:1317–1337. doi: 10.1007/s00429-014-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hines ML, Carnevale NT. Expanding NEURON’s repertoire of mechanisms with NMODL. Neural Comput. 2000;12:995–1007. doi: 10.1162/089976600300015475. [DOI] [PubMed] [Google Scholar]