Highlights
-
•
Observational data on interleukin-6 receptor inhibitors (IL6ri) for COVID-19 disease are reported.
-
•
IL6ri therapy was found to be associated with improved COVID-19 outcomes.
-
•
The treatment benefit was greatest when therapy was initiated early during the disease course.
-
•
IL6ri therapy appears to be superior to remdesivir and dexamethasone.
Keywords: COVID-19, Tocilizumab, Sarilumab, Interleukin-6 inhibitors, Cytokine release syndrome
Abstract
Objective
The aim of this observational study was to determine the optimal timing of interleukin-6 receptor inhibitor (IL6ri) administration for coronavirus disease 2019 (COVID-19).
Methods
Patients with COVID-19 were given an IL6ri (sarilumab or tocilizumab) based on iteratively reviewed guidelines. IL6ri were initially reserved for critically ill patients, but after review, treatment was liberalized to patients with lower oxygen requirements. Patients were divided into two groups: those requiring ≤45% fraction of inspired oxygen (FiO2) (termed stage IIB) and those requiring >45% FiO2 (termed stage III) at the time of IL6ri administration. The main outcomes were all-cause mortality, discharge alive from hospital, and extubation.
Results
A total of 255 COVID-19 patients were treated with IL6ri (149 stage IIB and 106 stage III). Patients treated in stage IIB had lower mortality than those treated in stage III (adjusted hazard ratio (aHR) 0.24, 95% confidence interval (CI) 0.08–0.74). Overall, 218 (85.5%) patients were discharged alive. Patients treated in stage IIB were more likely to be discharged (aHR 1.43, 95% CI 1.06–1.93) and were less likely to be intubated (aHR 0.43, 95% CI 0.24–0.79).
Conclusions
IL6ri administration prior to >45% FiO2 requirement was associated with improved COVID-19 outcomes. This can guide clinical management pending results from randomized controlled trials.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first discovered in Wuhan, China in December 2019 and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 (Huang et al., 2020). While the majority of patients infected with SARS-CoV-2 have mild to moderate symptoms, approximately 10% of infected Americans are hospitalized (CDC COVID-19 Response Team, 2020). Recent natural history observations from the USA have shown that approximately 33% of hospitalized patients require mechanical ventilation (Goyal et al., 2020). In the USA and UK, around 45–50% of COVID-19 patients requiring admission to the intensive care unit (ICU) have died (Audit and Centre, 2020; Bhatraju et al., 2020).
Other pathogenic coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), can induce a cytokine release syndrome (CRS), which may confer extensive morbidity (Channappanavar and Perlman, 2017, Moore and June, 2020). CRS is characterized by elevated serum levels of various proinflammatory cytokines, such as interleukin (IL)-6. In CRS with COVID-19, other inflammatory markers, such as C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and fibrinogen are strongly associated with IL-6 plasma levels (Lee et al., 2014, Liu et al., 2020). COVID-19 patients with markedly elevated IL-6 and CRP are more likely to have worse disease and greater mortality (Chen et al., 2020, Herold et al., 2020, Ruan et al., 2020). This has led to an interest in exploring IL-6 inhibition as a therapeutic modality for severe cases of COVID-19.
Tocilizumab and sarilumab are humanized monoclonal antibodies that target solble and membrane-bound IL-6 receptors, and inhibit downstream IL-6 effects (Moore and June, 2020, Raimondo et al., 2017). IL-6 inhibitors (IL6ri) have been approved by the US Food and Drug Administration for the treatment of CRS in patients undergoing chimeric antigen receptor T-cell therapy. Results from an open-label, uncontrolled case series (n = 21) showed that tocilizumab administration led to rapid improvement in symptoms and radiographic abnormalities among patients with severe COVID-19 (Xu et al., 2020). More recent larger observational studies have further confirmed the benefits of IL6ri therapy in patients with COVID-19 (Guaraldi et al., 2020, Price et al., 2020). However, results from randomized clinical trials are not yet available to guide evidence-based clinical management during this pandemic.
Although multiple observational studies have demonstrated a benefit with IL6ri therapy, the optimal timing for IL6ri use remains unclear. If given too early, these drugs have the potential of blunting the necessary antiviral response (Guaraldi et al., 2020, Jego et al., 2003). If given too late, after cytokine-mediated tissue injury has already taken place, these drugs may be ineffective. A multidisciplinary group of physicians and pharmacists instituted off-label use of tocilizumab and sarilumab and iteratively reviewed clinical outcomes to optimize the timing of IL6ri use. This article reports our clinical experience with the use of IL6ri for patients with COVID-19 disease with hypoxemia.
Methods
Study population, setting, and data collection
Physicians from the departments of adult and pediatric infectious diseases, rheumatology, and pulmonary/critical care, as well as clinical pharmacy specialists, collaborated in an institutional treatment panel that continuously reviewed the emerging COVID-19 treatment data and instituted off-label use of IL6ri under the WHO monitored emergency use of unregistered and investigational interventions framework (WHO, 2016). The infectious diseases consult team notified members of the treatment panel regarding patients with suspected or confirmed COVID-19 infection who had progressive hypoxemic respiratory failure during their hospitalization at Boston Medical Center (BMC). BMC is a large safety net hospital that primarily serves socio-economically disadvantaged patients with a high rate of comorbid medical conditions. The treatment panel responded with recommendations for or against treatment with IL6ri within 30 min. If approved, verbal assent was obtained from patients or their healthcare proxies prior to IL6ri administration. Iterative reviews were done to evaluate the impact and update treatment guidelines.
Initially, the treatment panel reserved IL6ri for patients with confirmed COVID-19 infection or with a highly suspicious clinical presentation who were being considered for intubation with fraction of inspired oxygen (FiO2) needs >45% and elevated inflammation, as evidenced by one or more plasma markers (e.g., CRP >100 mg/l, ferritin >700 ng/ml, or LDH >450 U/l). Patients with confirmed or suspected bacterial infections were excluded. Tocilizumab was administered as a single 8 mg/kg intravenous infusion. A review of the initial experience found limited improvement in oxygen requirement; the panel therefore recommended changing the criteria to include patients with worsening respiratory status defined as FiO2 requirement between 27% and 33% or with an alveolar-arterial gradient >50 mmHg and with elevated plasma inflammatory markers (classified as CRP >100 mg/l or LDH >450 U/l). After April 8, 2020, all potential IL6ri candidates were actively identified by repeatedly monitoring these parameters in confirmed and suspected COVID-19 patients multiple times during the day and night. Additionally, tocilizumab was reduced to a single dose of 400 mg. Due to a limited tocilizumab stock, we also employed sarilumab, another IL6ri, which has the same mechanism of action as tocilizumab, at a 200 mg single dose. Patients who failed to defervesce within 12–24 h were re-dosed.
At BMC, the treatment panel also recommended the treatment of all patients with hydroxychloroquine (400 mg twice daily for 1 day, then 200 mg twice daily for 4 days) and azithromycin (500 mg on the first day, then 250 mg daily for 4 days). On April 10, colchicine was also made available as an initial treatment. The use of these medicines was discontinued on April 23, 2020 because, based on an internal review of clinical data, they were found to be ineffective in reducing rates of ICU transfer, intubation, and mortality after controlling for the use of biological agents (Sagar et al., unpublished data). Studies from external sources also corroborated these internal findings (Geleris et al., 2020).
This report provides data on the first 255 consecutive SARS-CoV-2 PCR-confirmed patients treated with IL6ri during the period March 17 to April 30, 2020. Demographic and clinical information were obtained from a chart review. Follow-up was censored on May 25, 2020, by which time all patients had reached at least one of three clinical endpoints: (1) discharge, (2) intubation, or (3) death. No imputations were made for missing data.
All activities associated with this project were approved by the Boston University Medical Center Institutional Review Board (IRB).
Statistical analysis
A conceptual framework has been proposed for COVID-19 disease progression although there are no biomarkers, vital sign abnormalities, and oxygen requirements that underpin this staging (Siddiqi and Mehra, 2020). Patients were divided into two groups based on whether they required ≤45% FiO2 (termed as stage IIB) or >45% FiO2 (classified as stage III) prior to IL6ri administration. The FiO2 division is similar to that proposed previously for CRS (Lee et al., 2014).
Descriptive statistics were employed to summarize the data; median and interquartile range (IQR), or mean and standard deviation values are reported, as appropriate. Differences in categorical variables between the two groups were assessed using the Chi-square test or Fisher’s exact test. The Mann–Whitney U-test was used to estimate the significance of differences in the continuous variables between the two groups. Differences between the stage IIB and III groups with respect to mortality, discharge, and extubation were described with the use of Kaplan–Meier analysis and Cox proportional hazards models. Comorbidities and demographic factors in which there were differences between stage IIB and stage III patients with a p < 0.1 were included in the Cox model. To allow for differences in our experience with administering IL6ri and providing supportive care, we also adjusted for the period of care in all analyses. The entire treatment from March 17 to April 30 was divided into two periods in which prior to and after April 8 were classified as the early and late period, respectively. April 8 was selected as the cut-off for the early period, as it was the halfway point and several logistical interventions to expedite the administration of IL6ri were implemented.
To compare mortality in this cohort to that observed in other cohorts around the world, a numeric approach was employed. We used the properties of the binomial distribution to generate probability density functions around mortality from this cohort, as well as for the intervention and control groups from the published dexamethasone and remdesivir randomized clinical trials, which both provided 28-day mortality data (Horby et al., 2020, Wang et al., 2020). We sampled with replacement from our cohort and each of the randomized controlled trial (RCT) cohorts. We completed 10 000 Monte Carlo simulations in which we sampled with replacement from our cohort and each of the four RCT groups (remdesivir RCT control, remdesivir RCT intervention, dexamethasone RCT control, dexamethasone RCT intervention) and reported the number of patients alive after 10 000 simulations, as well as the percentage of times a specific treatment was associated with the lowest mortality.
These analyses were performed with Prism version 8.4.1 (GraphPad Software, San Diego, CA, USA), IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA), and TreeAge Pro version 20.1.0 (TreeAge, Williamstown, MA, USA).
Results
Demographic and clinical characteristics of the patients
There were 255 SARS-CoV-2 confirmed patients who received tocilizumab or sarilumab, with 106 treated in stage IIB and 149 treated in stage III. The median age of the 255 patients was 59 years (IQR 47–70 years) and they were predominantly male (63.1%) (Table 1 ). Common comorbidities in this cohort included hypertension (49.0%), obesity (defined as a body mass index (BMI) >30 kg/m2) (52.9%), diabetes (31.0%), and asthma (29%). There were no significant differences in demographic data or comorbidities between the stage IIB and stage III groups. As compared to stage IIB patients, stage III patients trended towards a greater prevalence of obesity (48.2% vs 59.4%, p = 0.098) and a higher CRP (157 mg/l vs 133 mg/l, p = 0.064) (Table 1, Table 2 ).
Table 1.
Baseline demographic and clinical characteristics of patients receiving tocilizumab.
| All IL6ri (n = 255) | Stage IIB (n = 149) | Stage III (n = 106) | p-Valuea | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, median (IQR) | 59 (47–70) | 57 (45–68) | 61 (50–72) | 0.095b |
| Sex, male, n (%) | 161 (63.1%) | 90 (60.4%) | 71 (67.0%) | 0.295 |
| Comorbidities, n (%) | ||||
| Obesity | 135 (52.9%) | 72 (48.3%) | 63 (59.4%) | 0.098 |
| COPD | 15 (5.9%) | 7 (4.7%) | 8 (7.5%) | 0.421 |
| Asthma | 29 (11.4%) | 19 (12.8%) | 10 (9.4%) | 0.433 |
| CHF | 5 (2.0%) | 3 (2.0%) | 2 (1.9%) | >0.999 |
| CAD | 7 (2.7%) | 3 (2.0%) | 4 (3.8%) | 0.454 |
| Diabetes | 79 (31.0%) | 55 (36.9%) | 24 (32.1%) | 0.505 |
| Hypertension | 125 (49.0%) | 76 (51.0%) | 49 (46.2%) | 0.525 |
| CKD | 12 (4.7%) | 7 (4.7%) | 5 (4.7%) | >0.999 |
| HIV | 3 (1.2%) | 3 (2.0%) | 0 (0%) | 0.268 |
| Systemic cancer | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Disease severity, n (%) | ||||
| ICU care | 105 (41.2%) | 35 (23.5%) | 70 (66.0%) | <0.0001 |
| Intubation | 68 (26.7%) | 22 (14.8%) | 46 (43.4%) | <0.0001 |
| Period of care, n (%) | ||||
| After April 8, 2020 | 161 (63.1%) | 92 (61.7%) | 69 (65.1%) | 0.601 |
Abbreviations: IL6ri, interleukin-6 inhibitors; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CAD, coronary artery disease; CKD, chronic kidney disease; HIV, human immunodeficiency virus infection; ICU, intensive care unit.
Differences between stage IIB and stage III patients were calculated using Fisher’s exact test.
Calculated using the Mann–Whitney U-test.
Table 2.
Clinical features one day prior to IL-6 inhibitor administration.
| Characteristica | All IL6ri (n = 255) | Stage IIB (n = 149) | Stage III (n = 106) | p-Valueb |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| FiO2 (%) | 36 (33–39) | 29 (28–30) | 47 (40–53) | <0.001 |
| Maximum temperature (°C) | 38.5 (38.4–38.6) | 38.5 (38.3–38.7) | 38.5 (38.4–38.7) | 0.130 |
| C-reactive protein (mg/l) | 142 (132–153) | 133 (121–147) | 157 (137–176) | 0.064 |
| Lactate dehydrogenase (U/l) | 453 (426–480) | 429 (403–454) | 492 (433–549) | 0.270 |
| D-dimer (ng/ml) | 829 (334–1324) | 1054 (237–1871) | 501 (348–654) | 0.519 |
| Ferritin (ng/ml) | 1343 (906–1781) | 1278 (968–1587) | 1491 (412–2570) | 0.386 |
| Absolute lymphocyte count (×109/l) | 1.5 (0.8–2.2) | 1.7 (0.5–2.9) | 1.1 (1.0–1.2) | 0.487 |
| Aspartate aminotransferase (IU/l) | 52 (47–56) | 52 (47–58) | 57 (48–66) | 0.467 |
| Alanine aminotransferase (IU/l) | 43 (38–48) | 44 (38–51) | 42 (34–50) | 0.696 |
| Oxygen modality on day of IL6ri therapy | All IL6-inhibitor (n = 255) | Stage IIB (n = 149) | Stage III (n = 106) | p-Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Mechanical ventilation | 17 (6.7%) | 1 (0.7%) | 16 (15.1%) | <0.001c |
| Nasal cannula | 142 (55.7%) | 142 (95.3%) | 0 (0.0%) | |
| Nasal pendant | 52 (20.4%) | 5 (3.3%) | 47 (44.3%) | |
| Non-rebreather | 43(16.9%) | 0 (0.0%) | 43 (40.6%) | |
| Room air | 1 (0.4%) | 1 (0.7%) | 0 (0.0%) |
IL-6, interleukin-6; IL6ri, interleukin-6 inhibitors; CI, confidence interval; FiO2, maximum fraction of inspired oxygen.
FiO2, temperature, and laboratory values were recorded for the 24-h period prior to IL6ri.
The p-values represent differences between the stage IIB and stage III groups calculated using the Mann–Whitney U-test.
Value calculated by Fisher’s exact test (Fisher’s exact only for mechanical ventilation or all different ones?).
Clinical features and trajectory prior to IL6ri
Twenty-four hours prior to IL6ri administration, patients in the stage IIB group had a lower mean FiO2 requirement compared to the stage III group (Table 2), but there was otherwise no significant difference in maximum recorded temperature (Tmax), CRP, ferritin, LDH, D-dimer, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). At the time of IL6ri treatment, most patients in the stage IIB group were receiving oxygen through a nasal cannula (142/149, 95.3%), while most patients in the stage III group were on a nasal pendant (47/106, 44.3%) or non-rebreather mask (43/106, 40.6%). Only one patient in the stage IIB group was ventilated; 16 were ventilated in the stage III group. Thus, the stage IIB and stage III group had comparable inflammation but different oxygen requirements prior to and on the day of IL6ri administration.
Treatment outcomes
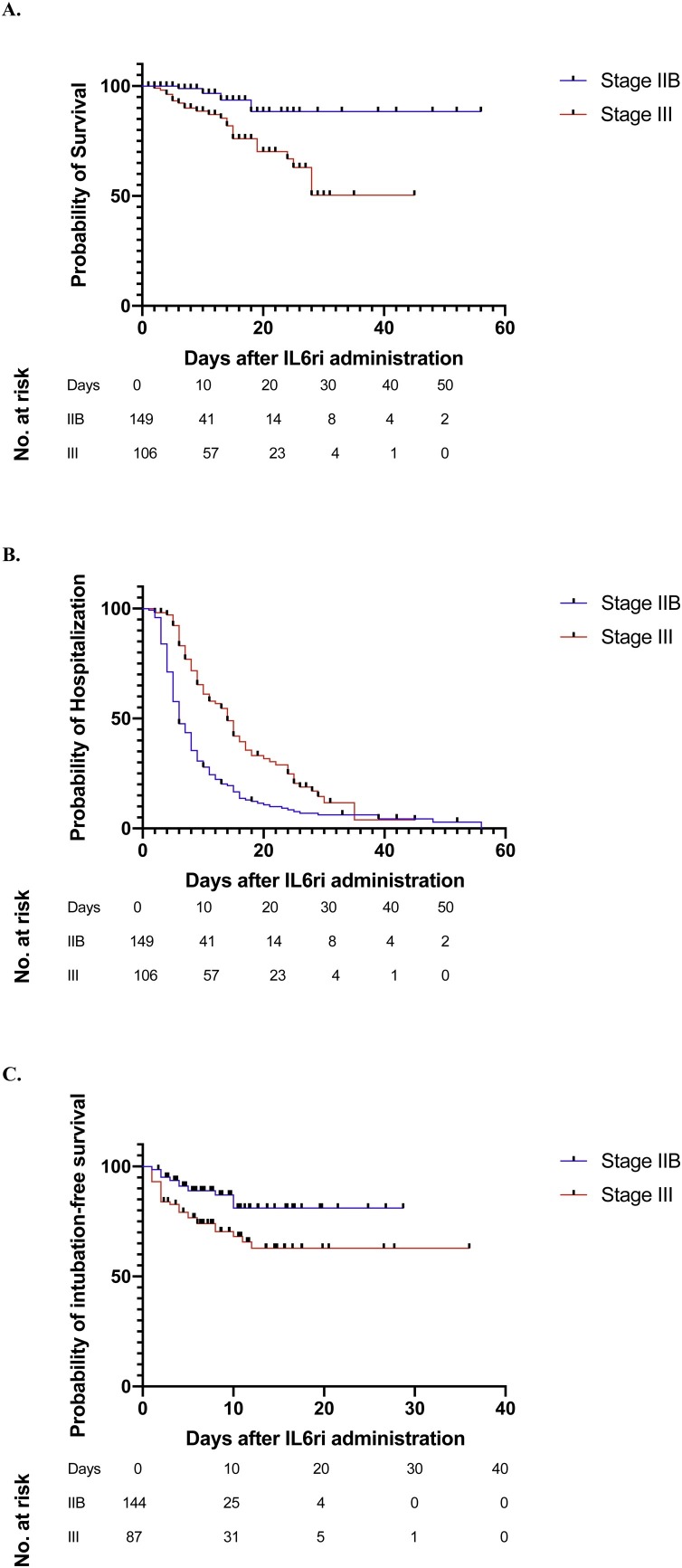
At the time data were censored, 10 (3.9%) patients – four in stage IIB and six in stage III – remained in the hospital, with a minimum of 25 days of follow-up after IL6ri administration. Of the 255 patients, 28 (10.9%) died, including four (2.7%) patients in the stage IIB and 24 (22.6%) patients in the stage III group (Table 3 ). The rate of death was lower among the stage IIB patients when compared to the stage III patients after adjusting for age, obesity, period of care, CRP, need for mechanical ventilation, and ICU care prior to IL6ri administration (adjusted hazard ratio (aHR) for the stage IIB group as compared to the stage III group: 0.20, 95% confidence interval (CI) 0.07–0.60) (Figure 1 A).
Table 3.
Clinical outcomes of patients treated with IL6-inhibitors.
| All IL6ri (n = 255) | Stage IIB (n = 149) | Stage III (n = 106) | |||
|---|---|---|---|---|---|
| Clinical outcomes | n (%) | n (%) | n (%) | OR (95% CI) | aHR (95% CI) |
| Died | 28 (10.9%) | 4 (2.7%) | 24 (22.6%) | 0.09 (0.03–0.27)a | 0.20 (0.07–0.60)b |
| Discharged alive | 218 (85.5%) | 141 (94.6%) | 77 (72.6%) | 6.64 (2.86–14.24)a | 2.04 (1.53–2.73)b |
| Intubated 24 h post IL6ri | 44/231 (19.0%) | 17/144 (11.8%) | 27/87 (31.0%) | 0.30 (0.16–0.60)a | 0.39 (0.20–0.74)c |
| Hospitalized, ventilated | 1 (0.4%) | 0 (0%) | 1 (0.9%) | – | – |
| Hospitalized, non-ventilated | 9 (3.5%) | 4 (2.7%) | 5 (13.2%) | – | – |
| Safety | n (%) | n (%) | n (%) | OR (95% CI) | aHR (95% CI) |
| Secondary infection | 34 (5.0%) | 18 (12.1%) | 16 (15.1%) | 0.77 (0.39–1.58)a | – |
| Duration of illness | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | p-Value | aHR (95% CI) |
| Length of stay (days)d | 12.9 (11.7–14.1) | 11.3 (9.7–13.0) | 15.0 (13.3–16.8) | <0.001e | – |
IL-6, interleukin-6; IL6ri, interleukin-6 inhibitors; OR, odds ratio; CI, confidence interval; aHR, adjusted hazard ratio; ICU, intensive care unit.
OR derived from univariate analysis of IL6ri in stage IIB as compared to stage III using Fisher’s exact test.
aHR derived from Cox proportional hazards model, which controlled for age, obesity, C-reactive protein, period of care, ICU care, and mechanical ventilation.
aHR derived from Cox proportional hazards model, which controlled for age, obesity, C-reactive protein, and period of care.
Length of stay refers to the time between the date of admission and date of discharge.
Calculated using the Mann–Whitney U-test.
Figure 1.
Survival curves for patients treated with interleukin-6 inhibitors (IL6ri) in stage IIB and in stage III. Shown here are (A) differences in time to mortality after IL6ri dose, (B) differences in time to discharge after IL6ri, and (C) differences in time to intubation after IL6ri administration. The number of patients at risk for each outcome at different time-points are displayed below the survival curves.
Overall, 218 (85.5%) patients were discharged alive. The rate of discharge was higher among the stage IIB group compared to the stage III group after adjusting for age, obesity, period of care, CRP, need for mechanical ventilation, and ICU care prior to drug administration (aHR 2.04, 95% CI 1.53–2.73) (Figure 1B). The mean total length of stay was 12.9 days (95% CI 11.7–14.1 days). Patients treated in stage IIB had a shorter mean length of stay (mean 11.3, 95% CI 9.7–13.0 days) compared to stage III patients (mean 15.0, 95% CI 13.3–16.8 days), p < 0.001 (Table 3).
A total of 68 (26.7%) patients required mechanical ventilation; of these, 44 were intubated more than 24 h after receiving IL6ri. The rate of intubation at more than 24 h after IL6ri administration was lower in the stage IIB group when compared to the stage III group after accounting for age, obesity, period of care, and CRP (aHR for the stage IIB group as compared to the stage III group: 0.20, 95% CI 0.07–0.60) (Figure 1C).
Comparison of IL6ri treatment to randomized trial outcomes
On comparison of the cohort of IL6ri recipients at BMC to patients in the remdesivir and dexamethasone RCTs, patient age, the sex distribution, and comorbidities were similar (Supplementary Material Table S1). However, whereas 99.6% of BMC patients were getting oxygen supplementation prior to IL6ri, rates of oxygen supplementation were lower in the remdesivir intervention (81.6%), remdesivir control (83.3%), dexamethasone intervention (76.2%), and dexamethasone control (76.3%) groups.
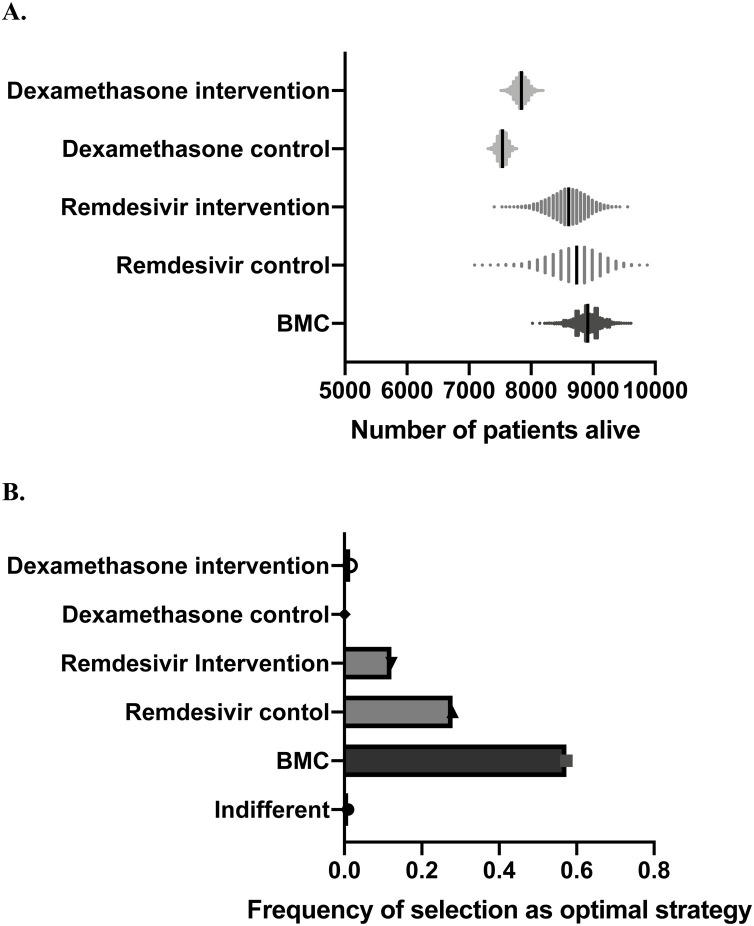
On performing 10 000 Monte Carlo simulations in which we sampled with replacement from the probability density functions around morality in each cohort, mean survival was 89.0% in this cohort, compared to 87.0% in the remdesivir control arm, 86.0% in the remdesivir intervention arm, 75.4% in the dexamethasone control arm, and 78.4% in the dexamethasone intervention arm. Survival was highest in the BMC cohort in 57.3% of the simulations (Figure 2 ).
Figure 2.
(A) Patients alive after 10 000 Monte Carlo simulations at Boston Medical Center (BMC) and in the two arms of the remdesivir and dexamethasone trials respectively. (B) Frequency of being selected as optimal strategy.
Adverse events
Of the 255 patients, 34 (13.3%) had secondary bacterial infections at ≥48 h after receiving an IL6ri medication. Four (11.8%) of these patients ultimately died. Secondary infections were not associated with an increased risk of mortality (odds ratio (OR) 1.09, 95% CI 0.36–3.38) or decreased rate of discharge (OR 0.76, 95% CI 0.29–1.99).
Discussion
It was found that although IL6ri was reserved for some of the most severely ill patients at BMC, the observed mortality rate (10.9%) was comparable to the overall mortality reported in New York City (9.7–10.8%) including patients with both moderate and severe disease (Goyal et al., 2020, Richardson et al., 2020). Indeed, the mortality of 2.7% observed among the 149 patients treated in stage IIB is among the lowest reported for patients who would be classified as having severe disease based on the level of their plasma inflammatory markers (Chen et al., 2020, Herold et al., 2020, Ruan et al., 2020). The IL6ri recipients at BMC had considerably higher supplementary oxygen requirements, indicating more advanced disease, than patients in the remdesivir and dexamethasone trials, and would have been expected to have a higher mortality rate. However, sampling-with-replacement analysis demonstrated that IL6ri recipients had a lower mortality rate than patients in the intervention and control groups of those trials. Furthermore, the mortality rate (22.9%) for the 105 (41.1%) BMC patients who required ICU care was considerably lower than the published 45–50% mortality in other ICU cohorts (Audit and Centre, 2020; Bhatraju et al., 2020). The majority of patients (85.5%) were also discharged alive, which is higher than the reported rate with standard of care (36–66%) over a similar time of follow-up (Goyal et al., 2020, Richardson et al., 2020). In aggregate, IL6ri use was associated with decreased mortality, decreased rate of intubation, higher likelihood of being discharged alive, and shorter length of stay.
The greatest benefit with IL6ri use was observed in patients who received the drug in stage IIB, prior to critical respiratory decompensation. It is possible that the stage IIB treatment group as compared to the stage III group was healthier and less likely to have a morbid outcome. However, there were no significant differences in pre-existing comorbidities (Table 1). Indeed, in univariate analyses, age >70 years, coronary artery disease (CAD), and hypertension (HTN) were associated with an increased risk of mortality and decreased likelihood of being discharged alive. (Supplementary Material Table S2). While stage III patients as compared to stage IIB patients tended to be older, the two groups had similar proportions of individuals with CAD and HTN. These similarities suggest that the two groups had relatively equivalent risk for COVID-19 disease progression (Chen et al., 2020, Herold et al., 2020, Ruan et al., 2020). However, the patients in stage III had progressed further along the COVID-19 disease spectrum, as indicated by the difference in FiO2 and trend towards higher CRP when compared to stage IIB patients (Siddiqi and Mehra, 2020). It is speculated that cytokine-mediated damage has already occurred by the time the patient develops severe hypoxemic respiratory failure, similar to the conclusions from laboratory-based studies (Channappanavar and Perlman, 2017, Moore and June, 2020). Furthermore, stage IIB patients had significantly higher CRP and LDH as compared to the presumably healthier patient population at our hospital, who never qualified for biologicals based on the protocol and who were discharged alive (Supplementary Material Figure S1). CRP and LDH have been found to be good predictors of mortality (Yan et al., 2020). Thus, stage IIB patients had comorbidities and a level of inflammation associated with poor COVID-19 outcomes. The improved outcomes in the stage IIB patients as compared to the stage III patients suggests that IL6ri intervention should occur prior to the onset of critical illness for maximum benefit.
Interpretation of this case series is limited by the lack of a control group, the small size of the cohort, missing data given the pragmatic design, and the open-label, non-randomized use of IL6ri. The results might also have been influenced by factors such as increased clinical experience towards the end of the observation period and differences in thresholds for hospitalization and discharge. In the absence of a control group, clinically significant observations were made, but definitive conclusions could not be made from these data. Findings from ongoing randomized clinical trials will provide a more definitive understanding of the effectiveness and safety of IL6ri for treating severe COVID-19 disease.
The study finding that prompt IL6ri treatment prior to the onset of critical disease is associated with reduced mortality from severe COVID-19 disease could be used to guide current clinical management while the medical community awaits more definitive results from RCTs.
Funding
This work was supported by National Institutes of Health grants K24-AI145661 and 5T32AI052074-13. The funding sources had no role in the study design, analysis, or reporting.
Ethical approval
All activities associated with this project were approved by the Boston University Medical Center IRB.
Conflict of interest
The authors have no competing interests to report.
Acknowledgements
Boston Medical Center Covid-19 Treatment Panel: Sacha Al Hassan, Archana Asundi, Elizabeth D. Barnett, Joshua A. Barocas, Alison L. Blackman, Eleanor Broadbent, Ingrid Y. Camelo, Diana F. Clarke, Dylan J. Clemens, Robyn Cohen, Ezra Cohen, Muhammad Dhanani, Rachel L. Epstein, Marisol A. Figueira, Leah Harvey, Finn J. Hawkins, Andrew J. Henderson, Natasha S. Hochberg, Robin R. Ingalls, Raagini Jawa, Ghulam K. Khan, Simeon Kimmel, Eugene Y. Kissin, Rotem Lapidot, Philip A. Lederer, Jai G. Marathe, Alison L. Nelson, Tuhina Neogi, Stephen I. Pelton, Ellen Rubin, Vishakha Sabharwal, Ahmed Saeed, Bhavesh B. Shah, Amy B. Triche, Allan J. Walkey
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.07.023.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audit ICN, Centre R . Intensive Care National Audit & Research Centre; London: 2020. ICNARC report on COVID-19 in critical care.https://www.icnarc.org/DataServices/Attachments/Download/c5a62b13-6486-ea11-9125-00505601089b [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang J., Yang Y., Ma H., Li Z., Zhang Z. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv. 2020;2020 doi: 10.15252/emmm.202012421. 03.01.20029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Johannes H., Bergwelt-Baildon M., Klein M. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. J Allergy Clin Immunol. 2020;146(1):128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo M.G., Biggioggero M., Crotti C., Becciolini A., Favalli E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Dev Ther. 2017;11:1593–1603. doi: 10.2147/DDDT.S100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest. 2020;S0012-3692(20):31670–31676. doi: 10.1016/j.chest.2020.06.006. S0012-3692(20):31670-31676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):E474–E484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G., Palucka A.K., Blanck J.-P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2016. Managing ethical issues in infectious disease outbreaks. [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting Characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhang H.-T., Goncalves J., Xiao Y., Wang M., Guo Y. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2(5):283–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.