Abstract
Ligusticum chuanxiong (LC) is a Chinese materia medica which is widely used in clinical settings to treat headaches, blood extravasation, and arthritis. Recent studies demonstrate that LC possesses versatile pharmacological functions, including antiatherosclerosis, antimigraine, antiaging, and anticancer properties. Moreover, LC also shows protective effects in the progression of different diseases that damage somatic cells. Oxidative stress and inflammation, which can induce somatic cell apoptosis, are the main factors associated with an abundance of diseases, whose progresses can be reversed by LC. In order to comprehensively review the molecular mechanisms associated with the protective effects of LC, we collected and integrated all its related studies on the anti-inflammatory, antioxidant, and antiapoptotic effects. The results show that LC could exhibit the mentioned biological activities by modulating several signaling pathways, specifically the NF-κB, Nrf2, protein kinase, and caspase-3 pathways. In future investigations, the pharmacokinetic properties of bioactive compounds in LC and the signaling pathway modulation of LC could be focused.
1. Introduction
Emerging evidence demonstrates that oxidative stress and inflammation are two predominant factors inducing severe organ damage which could result in digestive diseases, cardiovascular diseases, and diabetes [1, 2]. Generally, the basal level of ROS is essential for cell survival and growth since it plays a crucial role in the modulation of several signaling pathways [3]. For instance, the phosphatidylinositol 3-kinases/protein kinase B (PI3K/Akt) pathway is one of the best-known ROS-regulated pathways controlling cellular proliferation. While a low level of ROS is important for several physiological processes [4], its overproduction by peroxisome and mitochondria could induce oxidative stress in the human body [5, 6]. This in turn can lead to cell apoptosis by damaging its organelle and chromatin [7] as well as trigger immune response and inflammation due to the degeneration of biomacromolecules such as DNA and proteins [8]. The enhanced inflammatory response due to oxidative stress is closely associated with increased risk of cardiovascular diseases such as atherosclerosis and hypertension [9]. Therefore, scientists have taken a significant step forward to search for suitable candidates that can protect against oxidative stress and inflammation.
As natural products are found to be imperative sources of medicinal agents that exhibit anti-inflammatory and antioxidant activities [10, 11], a diverse group of Chinese materia medica have also generated a great deal of interest [12–14]. According to traditional Chinese medicine theories and recent studies, Ligusticum chuanxiong (LC) possesses a protective effect which ameliorates the lesions of various diseases induced by inflammation, oxidative stress, and apoptosis [15–17]. It is a deciduous plant belonging to the Umbelliferae family and is predominantly distributed in the Sichuan Province in southwest China. Most of the effective components of this traditional herb come from phenolic acids, phthalide lactones, polysaccharides, steroids, volatile oils, and alkaloids [16, 18–20]. Traditionally, LC is often used as medicine to treat headache, blood extravasation, and arthritis [21]. A recent study by Liu and coworkers reported that LC shows protective activity against myocardial ischemia [22] while another paper published by Wang suggested that LC could reduce isoproterenol-induced myocardial ischemia injury in rat models [23]. In addition, LC is also found to possess antineoplastic potential on HeLa cells [24]. Its protective effect on D-galactose-induced liver and kidney injury is potentially linked with alleviation of oxidative stress and inflammatory response [25]. These functions proposed the possible use of LC as a treatment for a range of oxidative stress- and inflammation-associated diseases which include but not limited to atherosclerosis, migraine, and hepatic fibrosis [26–28].
Herein, this review aims to highlight the protective effects of LC and its associated molecular mechanisms with a special focus on its anti-inflammatory, antioxidant, and antiapoptotic effects. The references and articles presented have been collected and analyzed through several online databases, including ScienceDirect, China National Knowledge Infrastructure (CNKI), Springer, and Wiley.
2. Molecular Mechanisms of LC's Anti-Inflammatory, Antioxidant, and Antiapoptotic Effects
Over the past decades, tremendous efforts have been made in unraveling the protective effect and the molecular mechanisms of LC in diseases that are closely linked to oxidative stress and inflammation. In this section, the anti-inflammatory, antioxidant, and antiapoptotic properties of LC are discussed. The associated molecular mechanisms and signaling pathways are studied in depth as well (Table 1), and the corresponding schemes are summarized in Figures 1–3.
Table 1.
Protective effects of LC.
| Drug | Dose | Pathway | Model | Ref |
|---|---|---|---|---|
| Anti-inflammatory | ||||
| Ligustrazine | 50, 100, 200 mg/kg | NLRP3/NF-κB↓ | CCl4-treated rats | [29] |
| Ligustilide | — | Protein kinase/NF-κB↓ | ApoE−/− mice | [15] |
| Ligustrazine | — | NF-κB↓ | Aβ25-35-treated microglial cells | [30] |
| Water extraction | — | TLR4/NF-κB↓ | MACO rat | [31] |
| Ligustrazine | 200 mg/kg | NF-κB↓, IL-10↓ | Spinal cord injury rats | [32] |
|
| ||||
| Antioxidant | ||||
| Polysaccharides | 2.95 mg/mL, 8 mg/mL | — | DPPH | [11] |
| Polysaccharides | 0.05–1.5 mg/mL | — | DPPH and H2O2 | [33] |
| Ethanol extraction | 600, 1200 mg/kg | Nrf2↑ | Isoproterenol-treated rats | [34] |
| Water extraction | 600, 1200 mg/kg | Nrf2↑ | Isoproterenol-treated rats | [34] |
| Ferulic acid | — | Keap/Nrf2/HO-1↑ | 2′,7′-Dichlorofluorescein diacetate-treated H9C2 cells | [35] |
| Polysaccharides | 10–50 mg/kg | PCG-1α/Nrf2↑ | Rat model | [28] |
|
| ||||
| Antiapoptosis | ||||
| Butanol part of ethanol extraction | 1–100 μM | PKA/CREB↑ | MTT-treated PC12 cells | [36] |
| Butanol part of ethanol extraction | 1–100 μM | Erk↑ | MTT-treated PC12 cells | [37] |
| Water extraction | — | TLR4↓ | MACO rats | [38] |
| Water extraction | — | Caspase-3↓ | MACO rats | [38] |
| Total phenolic acid | 0.2 mg/mL | Bax/Bcl-2↓ | MTT-treated human umbilical vein endothelial cells | [39] |
| Ethanol extraction | — | GAP-43↑ | Microsphere-induced cerebral embolism rats | [40] |
| Water extraction | — | CHP proteins↓ | MACO rats | [41] |
Figure 1.
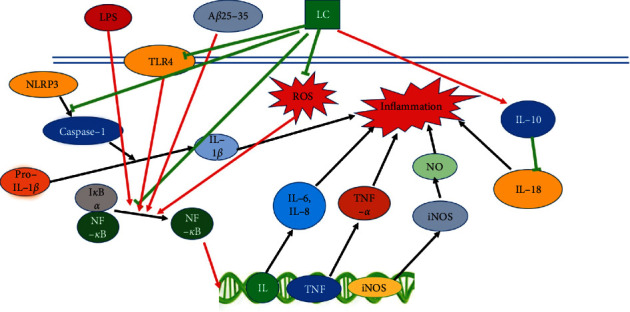
The molecular mechanism involves in the protective effects of LC.
Figure 2.
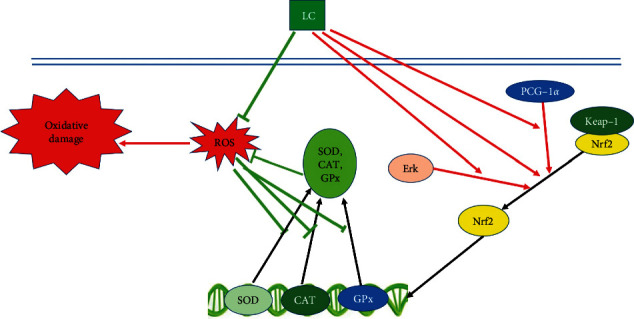
The antioxidant mechanism of LC.
Figure 3.
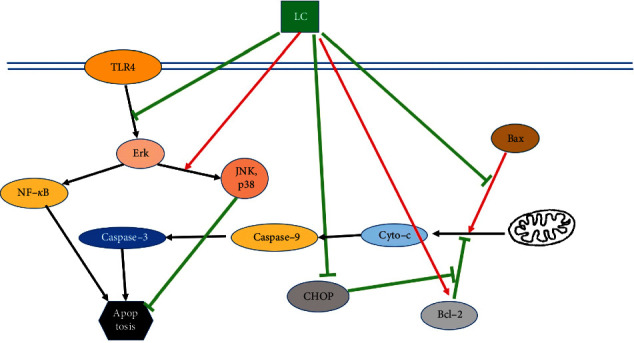
The antiapoptotic mechanism of LC.
2.1. Anti-Inflammatory Actions
Inflammation is a complex biological response of the immune system that is triggered by harmful endogenous and/or exogenous stimuli. While inflammation serves as a vital survival mechanism to fight against illness or injury, systemic chronic inflammation has been shown to be associated with increased risk of developing a variety of common chronic diseases [42] such as Alzheimer's disease [43] and diabetic nephropathy [44, 45]. The inflammation-induced somatic cell apoptosis might result in severe lesions in organs, promoting the disease progression. Following the discovery that LC exhibits anti-inflammatory action, the molecular mechanisms and signaling pathways of how LC modulates inflammatory response becomes a high-value target for the discovery of new leads that can slow down such disease progression.
In recent studies, LC has been shown to possess anti-inflammatory properties via suppression of proinflammatory cytokines [25, 29]. Usually, lipopolysaccharides (LPS) act as an endotoxin to induce the production of many proinflammatory cytokines in both in vitro and in vivo assays to study the anti-inflammatory properties of an active component. Several studies reported that LC plays a role in the suppression of LPS-induced nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) production in RAW 264.7 cells and BV-2 cells [15, 30, 31, 46]. Or et al. also suggested that LC prohibits the activation of BV-2 cells induced by LPS. Upon further investigations, they discovered that senkyunolide A and ligustilide are the active anti-inflammatory components in LC, and these two lactones are able to suppress the secretion of TNF-α directly and reduce inducible nitric oxide synthase (iNOS) activity [15]. Meanwhile, another study by Liu et al. showed that LC inhibits degeneration of inhibitor κB-α (IκBα) by blocking the NF-κB/p38 pathway and suppressing the expression of several proinflammatory cytokines in RAW 264.7 cells [32].
In addition to proinflammatory cytokines, a high level of reactive oxygen species (ROS) is also noxious for living organisms because it might damage proteins and lipids and also activate the NF-κB pathway which might lead to inflammation. In a study by Mahmoud et al., ferulic acid has been found to block the NF-κB pathway which is activated by ROS [47]. Moreover, Hu et al. proposed that senkyunolide I, another bioactive compound in LC, is able to reduce the ROS-induced production of TNF-α, IL-1β, IL-6, and IFN-γ in BV-2 cells [29].
In 2015, Wu et al. studied the anti-inflammatory responses that LC has on platelet-derived growth factor- (PDGF-) induced hepatic stellate cells from a CCl4-treated rat model. They found that ligustrazine efficiently suppresses the production of caspase-1 which is induced by nucleotide-binding oligomerization domain-like receptor 3 (NLRP3) in hepatic stellate cells, as well as reduces the levels of IL-1β and IL-18 through inhibition of the NLRP3/NF-κB pathway [48].
Moreover, the protein kinase/NF-κB is another pathway which might induce inflammation. Lei et al. found that in the ApoE−/− mouse model, phthalides in LC inhibit the expression of Akt and via downregulation of the NF-κB signaling pathway, and ligustilide is able to suppress the expression of activator protein-1 (AP-1) which is a promoter of CD137. Through these two pathways, secretion of many proinflammatory cytokines can be suppressed [28].
Besides, Kim et al. reported that ligustrazine, an alkaloid in LC, significantly inhibits the production of TNF-α, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) in microglial cells which are activated by Aβ25-35, a stimulus in the brain that can activate inflammatory response in microglial cells, through the interdiction of the NF-κB pathway [33].
In the development of intracerebral hemorrhages, the expression of Toll-like receptor 4 (TLR4) is enhanced, and this could lead to an increase in the levels of TNF-α, IL-6, and IL-1β through the TLR4/NF-κB pathway, followed by aggravation of brain lesions. Fortunately, recent investigations have revealed that the use of LC could significantly suppress the level of proinflammatory cytokines induced by TLR4 [49, 50].
In addition, the study published by Hu et al. reported ligustrazine (200 mg/kg) could reduce inflammation in a rat model with spinal cord injury by stimulating the secretion of IL-10 that is able to neutralize IL-18, a proinflammatory cytokine [35].
The studies mentioned above demonstrated that LC could inhibit the release of various proinflammatory cytokines which is activated by different stimuli in diverse cell lines, providing evidence that LC possesses anti-inflammatory action.
2.2. Antioxidant Actions
Oxidative stress is one of the main mechanisms that leads to damage of cell structure and inducement of many maladies. Inside cells, low or moderate concentration of ROS is necessary for cell functioning, but high concentration might result in oxidative stress and damage of biomacromolecules and finally leads to cell apoptosis and organ lesion [3]. Therefore, the modulation of ROS helps to decrease oxidative stress and limit cell damage [51, 52]. Recent studies have shown that LC possesses excellent ROS scavenging ability and has the potential to stimulate the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [34, 53, 54]. In this section, we reviewed recent studies on the antioxidant potentials of LC and its underlying mechanisms.
According to recent studies, polysaccharides in LC are able to scavenge ROS in an in vitro model. As polysaccharides are strong reducers, oxidative stress induced by diverse oxidative reagents, including 1,1-diphenyl-2-picrylhydrazyl (DPPH), H2O2, and 2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), could be attenuated by them in LC [16, 34, 53]. Among the three different polysaccharides in LC isolated by Hu et al., two of them show a high to moderate DPPH scavenging effect with IC50 values at 2.95 mg/mL and 8 mg/mL, respectively [16]. Similarly, Liu et al. also isolated polysaccharides from LC and assayed their antioxidant abilities. In their experiment, polysaccharides in LC can dose-dependently (0.05–1.5 mg/mL) scavenge diverse oxidative reagents. Notably, when H2O2 is used as the oxidative reagent, the scavenging ability of these polysaccharides is close to butylated hydroxytoluene, which is a compound with a strong reducing capacity [53]. However, as these experiments are carried out in vitro, the membrane permeability of these polysaccharides remains unclear, and this could be a perspective for future research. Moreover, ferulic acid can also scavenge ROS because it possesses the bulky conjugation system with a side chain [36, 37], which might be another component that contributes to the ROS scavenging ability of LC.
The modulation of antioxidant enzymes is another common pathway responsible for the antioxidant properties of LC. Under oxidative stress, the activity of antioxidant enzymes usually declines and results in an increase in ROS [38]. Recent studies demonstrate that LC can promote the expression of antioxidant enzymes, including SOD, CAT, and GPx, in somatic cells [25, 40, 54]. Through the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, organisms are able to protect against oxidative stress. In a study by Wang et al., water or ethanol extract of LC (600 mg/kg and 1200 mg/kg) can ameliorate the oxidative stress in the isoproterenol-treated rat model. They further analyzed the variations of SOD, Nrf2, and HO-1 in cardiac muscle tissue, and the results show that the levels of these proteins increased in the LC-treated group. This suggests that LC can activate the Nrf2 pathway to alleviate oxidative stress. Notably, the level of SOD is higher in the ethanol extract group, which implies that the main component responsible for the activation of the Nrf2 pathway could be phthalide lactones [41]. Moreover, according to a study by Gong et al., ferulic acid can increase the progress of Nrf2 which segregates from Keap-1 and combines with the promoter ARE in the cell nucleus to enhance the expression of heme oxygenase-1 (HO-1) in oxidation-sensitive dye 2′,7′-dichlorofluorescein diacetate-treated H9C2 cells [36]. In addition, peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) is another upstream signal of the Nrf2 pathway. Huang et al. found that, through the PCG-1α/Nrf2 pathway, polysaccharides (10–50 mg/kg) in LC upregulate the levels of antioxidants SOD1, SOD2, CAT, glutathione S-transferase P-1, and glutathione S-transferase M-1, which all play roles as antioxidants [34]. Meanwhile, LC is found to increase the phosphorylation of Erk1/2 which could ultimately increase the phosphorylation of Nrf2 [39].
2.3. Antiapoptotic Actions
Oxidative stress and high level of inflammatory cytokines are linked to an increase in cell apoptosis, which could lead to various organ injuries. Two pathways to control cell apoptosis include suppression of inflammation and oxidative stress, as well as modulation of apoptotic factors [11, 32, 55].
Recent studies proposed that LC shows antiapoptotic actions by modulating protein kinase pathways. In an investigation from Lin et al., the butanol part of ethanol-extracted LC (1–100 μM) can increase the viability of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide- (MTT-) treated PC12 cells, but this function diminishes at a concentration of 300 μM [56]. The mechanism is that LC increases the level of cAMP response element-binding protein (CREB) which possesses antiapoptotic effect through the protein kinase A (PKA) pathway [56]. In their further investigations, they found that LC can enhance the level of Erk, another downstream signal of PKA that is responsible for inhibition of apoptosis. They also found that LC can increase the level of phosphorylated c-Jun NH2-terminal kinase (JNK) and phosphorylated p38 protein belonging to the MAPK family which possesses antiapoptotic ability [57]. However, in another study conducted by Gu et al., Erk acts as a proapoptosis factor in the TLR4/NK-κB pathway. In this experiment, water extraction of LC is able to reduce the content of cerebral water in MACO rat model owing to the antiapoptotic action of LC through inhibition of TLR4-activated Erk [49].
Caspase family is another category of proteins related to apoptosis. Gu et al. reported that water-extracted LC inhibits the production of caspase-3 and caspase-12 in the MCAO rat model [49]. Furthermore, another experiment conducted by Gu et al. demonstrates that total phenolic acids in LC (0.2 mg/mL) could downregulate Bax/Bcl-2 which can cause the activation of caspase-3 in MTT-treated human umbilical vein endothelial cells [58]. LC could also inhibit the activation of caspase-3 directly, increasing the viability of MTT-treated PC12 cells [59].
The modulations of other proteins which are crucial for apoptosis are also researched. In an experiment conducted by Wang et al., ethanol-extracted LC could significantly increase the level of growth-associated protein-43 (GAP-43) which is related to the growth of hippocampal in a microsphere-induced cerebral embolism rat model. As a result, the recovery of neurons significantly increases [60]. Another study found that unfolded protein in the endoplasmic reticulum is increased in an MCAO rat model, which in turn induces endoplasmic reticulum stress and enhances the level of CHOP protein which can inhibit the function of Bcl-2 and result in apoptosis of neurons. Nevertheless, this increase in CHOP protein can be suppressed by water-extracted LC [61].
Through the modulations of caspases, the Bcl-2 family proteins, and other related protein kinases, LC can play a significant role in the protection of cells in diseases which involve drastic somatic cell apoptosis such as stroke and neurodegenerative diseases.
3. Protective Effect of LC
With its diverse anti-inflammatory, antioxidant, and antiapoptotic properties, LC demonstrates protective effect in various diseases (Table 2).
Table 2.
Diseases ameliorated by LC.
| Disease | Drug | Dosage (mg/kg) | Model | Ref |
|---|---|---|---|---|
| Atherosclerosis | Total lactone | 30, 60 | ApoE−/− mice | [56] |
| Cerebral trauma | Ligustrazine | 20 | Controlled cortical impact mice | [49] |
| Migraine | Senkyunolide I | 16, 32 | Nitroglycerin-treated rats | [58] |
| Diabetic nephropathy | Ethanol extraction | 25, 50, 100 | Streptozotocin-treated mice | [24] |
| Intervertebral disc degeneration | Ligustilide | 10 | Mice treated by surgery | [42] |
| Oxidative damage on renal | Ligustrazine | 80 | Gentamicin-treated mice | [59] |
| Ischemic cerebral stroke | Senkyunolide I | 36, 72 | Ischemia-reperfusion injured rats | [53] |
| Cognitive impairment | Ligustilide | — | D-galactose-treated mice | [60] |
Through anti-inflammatory action, LC can protect an organism from multiple lesions. Atherosclerosis is shown to have a strong correlation with oxidized lipid deposition, and it has been suggested to induce the production of proinflammatory cytokines such as TNF-α. Through the NF-κB pathway, the levels of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 increase in human umbilical vein endothelial cells and result in the formation of plaques [28, 62]. In a study by Xiao et al., total lactones in LC (30 and 60 mg/kg, 3 months) can suppress the serum lipid level in an ApoE−/− rat model with high-fat diet, suggesting that LC has a potential on the medical treatment of atherosclerosis. They further found that LC can dose-dependently (3.125–25 μg/mL) inhibit the levels of intercellular adhesion molecule-1 and vascular cellular adhesion molecule-1 via inhibition of the NF-κB pathway [62]. Furthermore, Lei et al. reported that the treatment of atherosclerosis with phthalides in LC in the ApoE−/− mouse model is related to the inhibition of NF-κB and AP-1 expressions and as well as suppression of CD137 level which is considered as a biomarker of atherosclerosis [28].
Furthermore, Han et al. found that phthalides in LC play a protective role against damage associated with intracerebral hemorrhaging. Through the inhibition of the TLR4/NF-κB pathway, the secretion of TNF-α, IL-6, and IL-1β decreases, and the injury to the brain due to intracerebral hemorrhage can be attenuated [50, 51]. In the progression of cerebral trauma, the permeability of the blood-brain barrier decreases due to the increase of TNF-α and IL-1β. Wang et al. reported that ligustrazine (20 mg/kg) can decrease the level of TNF-α and IL-1β in the plasma of a rat model [63]. Additionally, through the NOS inhibition and the monoamine neurotransmitter modulation, senkyunolide I (16 and 32 mg/kg) showed treatment potential for migraine rats [64].
LC also shows protective effects on other organs during the inflammatory progress. Diabetes induces injury to the kidney by stimulating inflammatory factors. Yang et al. reported that ethanol-extracted LC (25, 50, and 100 mg/kg) has a therapeutic effect in streptozotocin-induced diabetic nephropathy mice through the inhibition of the NF-κB pathway, which subsequently induces the production of TNF-α [65]. Intervertebral disc degeneration is another disease induced by inflammatory damage on organs. In a study by Wang et al., ligustilide (10 mg/kg) suppresses the level of IL-1β, downregulates the expression of cyclooxygenase-2, iNOS, TNF-α, and IL-6, and upregulates IL-10 and transforming growth factor-β (TGF-β), leading to an antiapoptotic function in the nucleus pulposus cells of the intervertebral disc [46].
Oxidative stress often causes injury to diverse organs by damaging biomacromolecules, which leads to apoptosis of somatic cells. Sue et al. reported that ligustrazine (80 mg/kg) can induce the expression of HO-1 in gentamicin-treated mice by which oxidative damage on renal can be ameliorated [66]. The central nervous system can also be affected by oxidative stress. For instance, Hu et al. found that senkyunolide I (36 and 72 mg/kg) can alleviate damage to the cerebrum caused by oxidative stress in ischemic stroke. In this progress, LC can increase the level of HO-1 and SOD through activation of the p-Erk/Nrf2 pathway [39]. In addition, ligustilide has been shown to decrease D-galactose-induced ROS production which could activate caspase-3 and cause neurotoxicity that may result in neuron apoptosis and cognitive impairment, respectively [67].
This evidence demonstrates that LC possesses protective effect in the progress of various diseases induced by an inflammatory response and oxidative damage. However, there are also many other diseases that can be alleviated by the protective effect of LC, but the mechanisms are still unclear. For instance, Zou et al. reported that LC can be used to treat hypertension [68] and Liu et al. reported the medical effect of LC on myocardial ischemia [22]. It is valuable to explore their underlying mechanisms in the future.
4. Conclusion and Future Perspectives
As a Chinese materia medica, LC is used clinically for various diseases, including migraine, rheumatism, and amenorrhea. Pharmacological investigations demonstrate that LC possesses antiatherosclerosis, anticancer, antioxidant, antiaging, and antihypertensive properties. Furthermore, molecular mechanistic studies show that LC can modulate a diverse array of cytokines, which allows LC to possess anti-inflammatory, antioxidant, and antiapoptotic effects. Through the blocking of the NF-κB pathway and upstream signals, LC can suppress the expression of proinflammatory cytokines, by which the progression of inflammation could be inhibited. The levels of various antioxidant enzymes could also be enhanced via activation of the Nrf2 pathway. LC also possesses strong scavenging ability that directly interacts with ROS. Additionally, through the modulation of caspase-3, Bax family, and MAPK family and their upstream signals in different cell types, LC controls apoptosis. Also, one pathway might coordinate or neutralize another pathway. For example, ROS activates the NF-κB pathway which might result in inflammation, while scavenging ROS can ameliorate oxidative stress and inflammation. Moreover, LC can activate Erk under a serum-deprived environment and show antiapoptotic function, and it can also prohibit TLR4-induced Erk in MCAO rats. Interestingly, the experimental model of the former study is PC12 cells, which is a category of neuron cells. It is valuable to ascertain the interaction between LC and Erk protein in the neuron in future investigations. Generally, via the three pathways discussed, LC shows protective effects as an anti-inflammatory, an antioxidant, and a modulator of apoptosis during the progression of various diseases.
However, there are still research gaps to be fulfilled in order to comprehensively understand the mechanism of the protective functions of LC. We propose the following two suggestions for future investigations of LC:
Most of the mechanisms mentioned above occurred intracellularly, and the pathways of how LC enters into cells are important for the understanding of the interaction of LC with the plasma membrane.
Via the synergism of antioxidant, anti-inflammatory, and antiapoptotic effects, LC showed medical treatment's potential to ischemic stroke; therefore, in order to validate the bioactive compounds in LC as drug, further study on its blood-brain barrier permeability and pharmacokinetic properties should be carried out.
Acknowledgments
This study was financially supported by the 12th innovation activity plan of undergraduates in theShanghai University of Traditional Chinese Medicine (No. 2019SHUTCM060), the Natural Science Foundation of Shanghai (No. 19ZR1457700), 2020 New Agricultural Science Research and Reform Practice Project of the Ministry of Education, and 2020 Key Undergraduate Education Reform Project of Shanghai Colleges.
Abbreviation
- Aβ:
Amyloid-β peptide
- Akt:
Protein kinase B
- AP-1:
Activator protein 1
- CAT:
Catalase
- CNKI:
China National Knowledge Infrastructure
- CREB:
cAMP response element-binding protein
- DPPH:
1,1-Diphenyl-2-picrylhydrazyl
- Erk1/2:
Extracellular signal-regulated kinase 1/2
- GAP-43:
Growth-associated protein-43
- GPx:
Glutathione peroxidase
- HO-1:
Heme oxygenase 1
- HSC:
Hepatic stellate cells
- IκBα:
Inhibitor kappa B alpha
- IKK:
Inhibitor of NF-κB kinase
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- JNK:
c-Jun NH2-terminal kinase
- LC:
Ligusticum chuanxiong
- LPS:
Lipopolysaccharides
- MAPK:
Mitogen-activated protein kinase
- MCAO:
Middle cerebral artery occlusion
- MCP-1:
Monotype chemoattractant protein-1
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MyD88:
Myeloid differentiation factor 88
- NF-κB:
Nuclear factor-kappa B
- NLRP3:
Nucleotide-binding oligomerization domain-like receptor 3
- NO:
Nitric oxide
- Nrf2:
Nuclear factor erythroid 2-related factor 2
- PC12:
Pheochromocytoma 12
- PDGF:
Platelet-derived growth factor
- PI3K:
phosphatidylinositol 3-kinase
- p-Erk:
Phosphorylated Erk
- PGC-1α:
Peroxisome proliferator-activated receptor-gamma coactivator 1α
- PKA:
Protein kinase A
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- TGF-β:
Transforming growth factor-β
- TLR4:
Toll-like receptor 4
- TKA1:
Tyrosine kinase activator protein 1
- TNF-α:
Tumor necrosis factor-alpha
- TRAF6:
TNF receptor-associated factor 6.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Cabello-Verrugio C., Simon F., Trollet C., Santibañez J. F. Oxidative stress in disease and aging: mechanisms and therapies 2016. Oxidative Medicine and Cellular Longevity. 2017;2017:1. doi: 10.1155/2017/4310469.4310469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neteamaie R., Plantinga T., Veerdonk F., Smit J., Netea M. Modulation of inflammation by autophagy: consequences for human disease. Autophagy. 2016;12(2):245–260. doi: 10.1080/15548627.2015.1071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Circu M., Aw T. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology and Medicine. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendran P., Nandakumar N., Rengarajan T., et al. Antioxidants and human diseases. Clinica Chimica Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Collett G., Campbell F. Curcumin induces C-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25(11):2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- 6.Corpas F., Barroso J., Rio L. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends in Plant Science. 2001;6(4):145–150. doi: 10.1016/s1360-1385(01)01898-2. [DOI] [PubMed] [Google Scholar]
- 7.Jiao R., Liu Y., Gao H., Jia X., So K. The antioxidant and antitumor properties of plant polysaccharides. The American Journal of Chinese Medicine. 2016;44(3):463–488. doi: 10.1142/s0192415x16500269. [DOI] [PubMed] [Google Scholar]
- 8.Russ T. C., Stamatakis E., Hamer M., Starr J. M., Kivimaki M., Batty G. D. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ. 2012;345:p. e4933. doi: 10.1136/bmj.e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S., Buttar H., Chintameneni M., Kaur G. Prevention of cardiovascular diseases with anti-inflammatory and anti-oxidant nutraceuticals and herbal products: an overview of pre-clinical and clinical studies. Recent Patents on Inflammation & Allergy Drug Discovery. 2018;12(2):145–157. doi: 10.2174/1872213x12666180815144803. [DOI] [PubMed] [Google Scholar]
- 10.Azab A., Nassar A., Azab A. Anti-inflammatory activity of natural products. Molecules. 2016;21(10):p. 1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H., Yih K., Yang C., Huang K. Antioxidant activity and major chemical component analyses of twenty-six commercially available essential oils. Journal of Food and Drug Analysis. 2017;25(4):881–889. doi: 10.1016/j.jfda.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Xu L., Lao Y., Zhang H., Xu H. Natural products targeting EGFR signaling pathways as potential anticancer drugs. Current Protein & Peptide Science. 2018;19(4):380–388. doi: 10.2174/1389203718666170106104211. [DOI] [PubMed] [Google Scholar]
- 13.Pu W., Sun L., Gao X., et al. Targeting tumor-associated macrophages by anti-tumor Chinese materia medica. Chinese Journal of Integrative Medicine. 2017;23(10):723–732. doi: 10.1007/s11655-017-2974-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S., Li X., Yang X. Drug-likeness prediction of chemical constituents isolated from Chinese materia medica ciwujia. Journal of Ethnopharmacology. 2017;198:131–138. doi: 10.1016/j.jep.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Or T. C., Yang C. L., Law A. H., et al. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology. 2011;60(6):823–831. doi: 10.1016/j.neuropharm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Hu J., Jia X., Fang X., et al. Ultrasonic extraction, antioxidant and anticancer activities of novel polysaccharides from chuanxiong rhizome. International Journal of Biological Macromolecules. 2016;85:277–284. doi: 10.1016/j.ijbiomac.2015.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Ng C., Shiu H., et al. Neuroprotective effect of da chuanxiong formula against cognitive and motor deficits in a rat controlled cortical impact model of traumatic brain injury. Journal of Ethnopharmacology. 2018;217:11–22. doi: 10.1016/j.jep.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Chen J., Wu H., et al. Identification of key constituents in volatile oil of Ligusticum chuanxiong based on data mining approaches. Pharmaceutical Biology. 2011;49(5):445–455. doi: 10.3109/13880209.2010.523426. [DOI] [PubMed] [Google Scholar]
- 19.Zschocke S., Klaiber I., Bauer R., Vogler B. HPLC-coupled spectroscopic techniques (uv, ms, nmr) for the structure elucidation of phthalides in Ligusticum chuanxiong. Molecular Diversity. 2005;9(1):33–39. doi: 10.1007/s11030-005-1305-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Ding M., Liu D. Phenolic acids analysis in Ligusticum chuanxiong using HPLC. Journal of Chromatographic Science. 2005;43(8):389–393. doi: 10.1093/chromsci/43.8.389. [DOI] [PubMed] [Google Scholar]
- 21.Chinese Pharmacopoeia Commission. The Chinese Pharmacopoeia, Volume I. Beijing, China: China Pharmaceutical Science and Technology Press; 2020. [Google Scholar]
- 22.Liu X., Li X., Ji S., Cui X., Li M. Screening of bioactive ingredients in Ligusticum chuanxiong hort for protection against myocardial ischemia. Cellular Physiology and Biochemistry. 2016;40(3-4):770–780. doi: 10.1159/000453137. [DOI] [PubMed] [Google Scholar]
- 23.Wang G., Dai G., Song J., et al. Lactone component from Ligusticum chuanxiong alleviates myocardial ischemia injury through inhibiting autophagy. Frontiers in Pharmacology. 2018;9:p. 301. doi: 10.3389/fphar.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong L., Yu Z., Bao Y., et al. Screening and analysis of an antineoplastic compound in rhizoma chuanxiong by means of in vitro metabolism and HPLC-MS. Analytical and Bioanalytical Chemistry. 2006;386(2):264–274. doi: 10.1007/s00216-006-0621-0. [DOI] [PubMed] [Google Scholar]
- 25.Mo Z., Liu Y., Li C., et al. Protective effect of SFE-CO2 of Ligusticum chuanxiong hort against D-galactose-induced injury in the mouse liver and kidney. Rejuvenation Research. 2017;20(3):231–243. doi: 10.1089/rej.2016.1870. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y. J., Zhang Y. H., Wang L., et al. Levistolide a induces apoptosis via ROS-mediated er stress pathway in colon cancer cells. Nature Medicine. 2017;42:929–938. doi: 10.1159/000478647. [DOI] [PubMed] [Google Scholar]
- 27.Yuan X., Han B., Feng Z. M., et al. Chemical constituents of Ligusticum chuanxiong and their anti-inflammation and hepatoprotective activities. Bioorganic Chemistry. 2020;101 doi: 10.1016/j.bioorg.2020.104016. [DOI] [PubMed] [Google Scholar]
- 28.Lei W., Deng Y.-F., Hu X.-Y., Nib J.-N., Jiang M., Bai G. Phthalides, senkyunolide A and ligustilide, show immunomodulatory effect in improving atherosclerosis, through inhibiting AP-1 and NF-κB expression. Biomedicine & Pharmacotherapy. 2019;117 doi: 10.1016/j.biopha.2019.109074. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y., Wang Y., Liang S., Jiang J., Yang Y., Zhang P. Senkyunolide I attenuates oxygen-glucose deprivation/reoxygenation-induced inflammation in microglial cells. Behavioural Brain Research. 2016;1649:123–131. doi: 10.1016/j.brainres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z.-K., Lu C.-F., Zhang H.-T., et al. Anti-inflammatory ligustilides from Ligusticum chuanxiong. Fitoterapia. 2013;91:21–27. doi: 10.1016/j.fitote.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Han B, Feng Z., Jiang J., Yang Y., Zhang P. Bioactive thionic compounds and aromatic glycosides from Ligusticum chuanxiong. Acta Pharmaceutica Sinica B. 2018;8(5):818–824. doi: 10.1016/j.apsb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z. K., Ng Y.-F., Shiu X.-Y., Ni J.-N., Jiang M., Bai G. A traditional Chinese formula composed of Chuanxiong Rhizoma and Gastrodiae Rhizoma (Da Chuanxiong Formula) suppresses inflammatory response in LPS -induced RAW 264.7 cells through inhibition of NF-κB pathway. Journal of Ethnopharmacology. 2017;196:20–28. doi: 10.1016/j.jep.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.-Z., Kim J.-H., Lee Z.-M., Li J.-H., Li X.-M., Lu H.-B. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid β and interferon-γ in rat brain microglia. Journal of the Neurological Sciences. 2014;740(1-2):504–511. doi: 10.1016/j.ejphar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Huang C., Cao X., Chen X., et al. A pectic polysaccharide from Ligusticum chuanxiong promotes intestine antioxidant defense in aged mice. Carbohyd Polymers. 2017;174:915–922. doi: 10.1016/j.carbpol.2017.06.122. [DOI] [PubMed] [Google Scholar]
- 35.Hu J.-Z., Huang J.-H., Xiao Z.-M., et al. Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. Journal of the Neurological Sciences. 2013;324(1-2):94–99. doi: 10.1016/j.jns.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Gong G., Huang V., Wang H., et al. Ferulic acid orchestrates anti-oxidative properties of danggui buxue tang, an ancient herbal decoction: elucidation by chemical knock-out approach. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0165486.e0165486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C.-M., Tang Y.-Q., Liu X.-L., et al. Effects of ferulic acid on antioxidant activity in angelicae sinensis radix, chuanxiong rhizoma, and their combination. Chinese Journal of Natural Medicines. 2015;13(6):401–408. doi: 10.1016/s1875-5364(15)30032-7. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y., Li X., Liu T., et al. Senkyunolide H protects against MPP+-induced apoptosis via the ROS-mediated mitogen-activated protein kinase pathway in PC12 cells. Environmental Toxicology and Pharmacology. 2019;65:73–81. doi: 10.1016/j.etap.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y., Duan M., Liang S., Wang Y., Feng Y. Senkyunolide I protects rat brain against focal cerebral ischemia–reperfusion injury by up-regulating p-erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Research. 2015;1605:39–48. doi: 10.1016/j.brainres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Gim S.-A., Sung J.-H., Dong F.-A., au M.-O., au P.-O. Ethanolic extract of rhizome of Ligusticum chuanxiong Hort. (chuanxiong) enhances endothelium-dependent vascular reactivity in ovariectomized rats fed with high-fat diet. Food Function. 2014;5(10):2475–2485. doi: 10.1039/c4fo00211c. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y.-L., Liu Y.-C., Hou C.-L., Lai W.-L., Lin Y.-R., Huang N.-K. Prevention effect of Ligusticum chuanxiong extraction against oxidative stress injury induced by myocardial ischemia through activation of Nrf2 signaling pathwa. China Journal of Chinese Materia Medica. 2017;42(24):4834–4840. doi: 10.19540/j.cnki.cjcmm.20171010.001. [DOI] [PubMed] [Google Scholar]
- 42.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinneya J. W., Bemillerb S. M., Murtishawa A. S., Leisganga A. M., Salazara A. M., Lambb B. T. Inflammation as a central mechanism in alzheimer’s disease. Brain Research. 2018;1649:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duran-Salgado M. B., Rubio-Guerra A. F. Diabetic nephropathy and inflammation. World Journal of Diabetes. 2014;5(3):p. 393. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matoba K., Takeda X.-Q., Nagai Y., et al. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. International Journal of Molecular Sciences. 2019;91(14):p. 3393. doi: 10.3390/ijms20143393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K., Chen T., Ying X., et al. Ligustilide alleviated IL-1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. International Immunopharmacology. 2019;69:398–407. doi: 10.1016/j.intimp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud A. M., Hussein S.-O., Eltwab S. M., et al. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food and Function. 2019;10(8):4593–4607. doi: 10.1039/c9fo00114j. [DOI] [PubMed] [Google Scholar]
- 48.Wu X., Zhang F., Xiong X., Zhu Y., Zhao M., Duan J.-a. Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway. IUBMB Life. 2015;67(4):312–321. doi: 10.1002/iub.1348. [DOI] [PubMed] [Google Scholar]
- 49.Gu J., Su S., Guo J., Wakamatsu K. Anti-inflammatory and anti-apoptotic effects of the combination of Ligusticum chuanxiong and radix paeoniae against focal cerebral ischaemia via TLR4/MyD88/MAPK/NF-κB signalling pathway in MCAO rats. Journal of Pharmacy & Pharmacology. 2018;70(2):268–277. doi: 10.1111/jphp.12841. [DOI] [PubMed] [Google Scholar]
- 50.Han L., Liu D., Zeng Q.-K., et al. The neuroprotective effects and probable mechanisms of ligustilide and its degradative products on intracerebral hemorrhage in mice. International Immunopharmacology. 2018;63:43–57. doi: 10.1016/j.intimp.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 51.Ito S., Agata M., Okochi K., Wakamatsu K., Yang S.-M., Kong F.-L. The potent pro-oxidant activity of rhododendrol-eumelanin is enhanced by ultraviolet a radiation. Pigment Cell & Melanoma Research. 2018;31(4):523–528. doi: 10.1111/pcmr.12696. [DOI] [PubMed] [Google Scholar]
- 52.Guo H. Comparisons of combined oxidant capacity and redox-weighted oxidant capacity in their association with increasing levels of FeNO. Chemosphere. 2018;211:584–590. doi: 10.1016/j.chemosphere.2018.07.191. [DOI] [PubMed] [Google Scholar]
- 53.Liu J., Zheng S., Fan Q., Yuan J.-C., Yang S.-M., Kong F.-L. Optimisation of high-pressure ultrasonic-assisted extraction and antioxidant capacity of polysaccharides from the rhizome of Ligusticum chuanxiong. International Journal of Blological Macromolecules. 2015;76:80–85. doi: 10.1016/j.ijbiomac.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 54.Hou Y. Z., Zhao G. R., Yang J., et al. Protective effect of Ligusticum chuanxiong and angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Science. 2004;75(14):1775–1786. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Gim S.-A., Sung J.-H., Shah F.-A., Kim M.-O., Koh P.-O. Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model. Laboratory Animal Research. 2013;29(2):63–69. doi: 10.5625/lar.2013.29.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Y.-L., Lee Y.-C., Huang C.-L., Lai W.-L., Lin Y.-R., Huang N.-K. Ligusticum chuanxiong prevents rat pheochromocytoma cells from serum deprivation-induced apoptosis through a protein kinase A-dependent pathway. Journal of Ethnopharmacology. 2007;109(3):428–434. doi: 10.1016/j.jep.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Lin Y.-L., Wang G.-J., Huang C.-L., et al. Ligusticum chuanxiong as a potential neuroprotectant for preventing serum deprivation-induced apoptosis in rat pheochromocytoma cells: functional roles of mitogen-activated protein kinases. Journal of Ethnopharmacology. 2009;122(3):417–423. doi: 10.1016/j.jep.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Gu J., Feng L., Yuan J., et al. Effect of different composition structures of total paeony glycoside component and total phenolic acid component of chuanxiong rhizome on human umbilical vein endothelial cells with hypoxic injury. China Journal of Chinese Materia Medica. 2015;40(5):920–926. [PubMed] [Google Scholar]
- 59.Jia R. R., Gou Y. L., Ho L. S., Ng C.‐P., Tan N. H., Chan H. C. Anti‐apoptotic activity of Bak Foong Pills and its ingredients on 6‐hydroxydopamine‐induced neurotoxicity in PC12 cells. Cell Biology Intertional. 2005;29(10):835–842. doi: 10.1016/j.cellbi.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 60.Wang M., Yao M., Liu J., et al. Ligusticum chuanxiong exerts neuroprotection by promoting adult neurogenesis and inhibiting inflammation in the hippocampus of ME cerebral ischemia rats. Journal of Ethnopharmacology. 2020;249:p. 112385. doi: 10.1016/j.jep.2019.112385. [DOI] [PubMed] [Google Scholar]
- 61.Gu J., Chen J., Yang N., et al. Combination of Ligusticum chuanxiong and Radix Paeoniae ameliorate focal cerebral ischemic in MCAO rats via endoplasmic reticulum stress-dependent apoptotic signaling pathway. Journal of Ethnopharmacology. 2016;187:313–324. doi: 10.1016/j.jep.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 62.Xiao W.-J., Wang Y.-R., Li L., et al. Lactones from Ligusticum chuanxiong hort. Reduces atherosclerotic lesions in ApoE-deficient mice via inhibiting over expression of NF-kB-Dependent adhesion molecules. Fitoterapia. 2014;227:240–246. doi: 10.1016/j.fitote.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Wang A., Zhu G., Qian P., Tao Z. Tetramethylpyrazine reduces blood-brain barrier permeability associated with enhancement of peripheral cholinergic anti-inflammatory effects for treating traumatic brain injury. Experimental and Therapeutic Medicine. 2017;14(3):2392–2400. doi: 10.3892/etm.2017.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J.-J., Liang S., Xu Y.-P., et al. Effect and mechanism of senkyunolide I as an anti‐migraine compound from Ligusticum chuanxiong. Journal of Pharmacy and Pharmacology. 2011;63(2):261–266. doi: 10.1111/j.2042-7158.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 65.Yang W., Li Y., Gao H., et al. Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin-induced diabetic nephropathy in mice. Journal of Ethnopharmacology. 2018;227:166–175. doi: 10.1016/j.jep.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 66.Sue Y.-M., Cheng C.-F., Chang C.-C., Chou Y., Chen C.-H., Juan S.-H. Antioxidation and anti-inflammation by haem oxygenase-1 contribute to protection by tetramethylpyrazine against gentamicin-induced apoptosis in murine renal tubular cells. Nephrology Dialysis Transplantation. 2009;24(3):769–777. doi: 10.1093/ndt/gfn545. [DOI] [PubMed] [Google Scholar]
- 67.Li J., Zhu Q., Lu Y., et al. Ligustilide prevents cognitive impairment and attenuates neurotoxicity in D-galactose induced aging mice brain. Brain Research. 2015;1595:19–28. doi: 10.1016/j.brainres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Zou Z.-d., Liu N., Guo P., et al. Analysis on clinical treatment in hypertension by traditional Chinese medicine for 10 Years in beijing. China Journal of Chinese Materia Medica. 2007;32(15):1569–1572. [PubMed] [Google Scholar]


