Abstract
The new virus, SARS-CoV-2, has probably affected millions of people world-wide since December 2019 and killed thousand. We have designed a model and used it to quantify the effect of local protective measures on the SARS-CoV-2 epidemic, assess their effectiveness and adapt health service strategies in Toulouse, France.
Keywords: SARS-CoV-2, Protective measures, Statistical model, Public health
Dear Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in December 2019 and spread, mainly by sustained human-to-human transmission, so rapidly that it threatened to saturate health services1. This led the governments of several countries to employ strict lockdown to slow the progression of the pandemic at the start of 20202 , 3. The SARS-CoV-2 epidemic has resumed in European countries, including France, since the end of the summer. As a result, several large cities have taken measures to limit the transmission of the virus4. A recent study published in this journal assessed the impact of different strategies including lockdown to contain the spread of the SARS-CoV-2 in the London population during the first phase of epidemic in March 20205. In a complementary way, we evaluated here the effectiveness of each individual public health measure chosen as alternatives to a new strict lockdown, on the basis of the real SARS-CoV-2 dynamics. modeling the global evolution of the epidemic in France has become very complicated since the strategies used differ between regions and cities4. But their effectiveness could be assessed by quantifying the local impact of protective measures against SARS-CoV-2. These could then be adapted for use in other contexts.
Our statistical model is based on a diffusion and transmission coefficient that varies with an individual's age, the likelihood of contagion, and a reduction coefficient that accounts for the impact of public health measures on virus transmission in the French city of Toulouse. We have used models to measure how the dynamics of the SARS-CoV-2 infection is influenced by each individual public health measure.
Consider the variables . is defined as the age class variable:
On day for a given age class is the real number of healthy people and is the estimated number. and are the real and the estimated numbers of contagious carriers infected for days . and are the real and estimated numbers of people in a given age class who are immunized or have died. We assume that the risk of reinfection by SARS-CoV-2 after a first infection is negligible.
is defined as the number of days a person is contagious. is the number of healthy people who a contagious person contacts and infects. We assume that the contagion coefficient varies over time and peaks when the virus load is maximal: 7 days after the start of infection6. is the total population at the start of the epidemic phase, is the real multiplier for the rate at which the epidemic spreads according to the protective measures applied , is the estimated coefficient. and are equal to 1 when no protective measures are in use.
is given by:
On the transition from day to day , we have:
| (1) |
| (2) |
| (3) |
The model is a discretized version of a Susceptible Infectious and Recovered (SIR)-type model7
We have set based on current data in our area.8
We estimated the initial model settings using data collected by the Toulouse Virology Laboratory. is corrected by fitting the predicted data to the real data as:
The data obtained by the Toulouse Virology Laboratory using serological tests showed that the real seroprevalence in the Toulouse urban area was around 1.5% at the end of lockdown (May 11, 2020). The population of the Toulouse urban area is around 1 million, which implies a number of immune individuals close to 15,000 in mid-May. The number of SARS-CoV-2 cases gradually increased from July 21, 2020. Date corresponds to October 6, 2020.
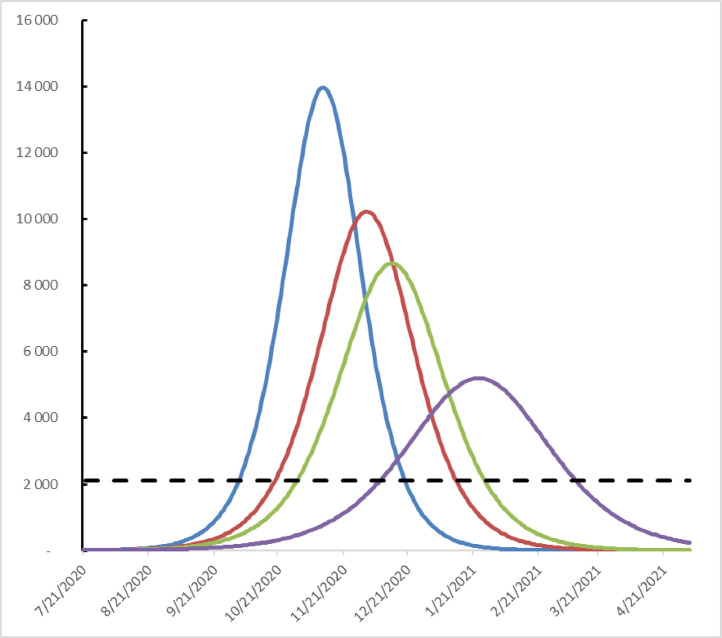
We estimated to be 82% at the end of July 2020, with no specific local protective measures in place. The epidemic is evolving as shown by the blue curve, with a peak of nearly 14,000 new cases on November 11 (Fig. 1 ). But the local authorities decided to make the wearing of masks compulsory in certain areas of the city on August 5. This reduced the virus coefficient of transmission (75%). The epidemic then evolved as shown by the red curve with a maximum of new cases approaching the 10,000 expected on December 2 (Fig. 1). Mask wearing was made compulsory throughout Toulouse on August 21, and the estimated value of became 72%. The epidemic therefore evolved as shown by the green curve with a peak around 8500 new cases on December 13 (Fig. 1). Finally, some public spaces, such as bars, were placed under curfew and certain sports structures were added to the measures already in force on September 26. This resulted in a value of 65% and the epidemic evolved as indicated by the purple curve with a peak approaching 5000 new cases on January 22, 2021 (Fig. 1). Making compulsory the mask wearing and restricting access to some spaces open to the public reduced the maximum number of new infected cases per day by more than 75% and delayed the infected peak by approximately 2 months (from blue curve to purple curve). But these measures did not make it possible to avoid the saturation of the intensive care units in Toulouse (2100 new cases per day: black dotted line), which led the government authorities to add a global curfew on October 16. Its impact on the spread of the SARS-CoV-2 remains to be assessed.
Fig. 1.
Dynamics of SARS-CoV-2 infection per day from July 21, 2020 to May 5, 2021 according to the decisions on the protective measures .blue: no specific local measure red: masks are mandatory in some urban areas, green: masks are mandatory in the all Toulouse urban area, purple: closure of some public spaces and compulsory masks. The black dotted line corresponds to the new infections threshold at which the intensive care units could be saturated.
We believe that this provides a basis for estimating the effect of specific restrictions on the dynamics of the SARS-CoV-2 epidemic. Its application could well indicate how local strategies can be tailored to avoid saturation of health services.
Acknowledgements
The English text was edited by Dr Owen Parkes.
References
- 1.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020 doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimeglio C., Loubes J.M., Deporte B., Dubois M., Latour J., Mansuy J.M., Izopet J. The SARS-CoV-2 seroprevalence is the key factor for deconfinement in France. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwan N.A., Burgess R.A., Ashworth S. Scientific consensus on the COVID-19 pandemic: we need to act now. Lancet. 2020 doi: 10.1016/S0140-6736(20)32153-X. S0140-6736(20)32153-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restrictions and requirements in metropolitan France. Available at https://www.gouvernement.fr/en/coronavirus-covid-19 (2020).
- 5.Goscé L., Phillips P.A., Spinola P., Gupta D.R.K., Abubakar P.I. Modelling SARS-COV2 spread in London: approaches to lift the lockdown. J Infect. 2020;81(2):260–265. doi: 10.1016/j.jinf.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M.G. et al. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Res Crit Care Med. 2020 doi: 10.1164/rccm.202003-0524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kermack W.O., McKendrick A.G. A contribution to the mathematical theory of epidemics. Proc R Soc Lond. 1927;A 115:700–721. [Google Scholar]
- 8.Epidemiological report on COVID-19 (October 2020). Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-8-octobre-2020 (2020).