Key Points
Question
Does adding vein of Marshall ethanol infusion to the catheter ablation procedure reduce the recurrence of atrial fibrillation in patients with persistent atrial fibrillation?
Findings
In this randomized clinical trial that included 343 patients with persistent atrial fibrillation, catheter ablation with vein of Marshall ethanol infusion, compared with catheter ablation alone, resulted in freedom from atrial fibrillation or prolonged atrial tachycardia in 49% vs 38% at both 6 and 12 months, a difference that was statistically significant.
Meaning
In patients with persistent atrial fibrillation, treatment with combined catheter ablation and vein of Marshall ethanol infusion had better outcomes compared with catheter ablation alone.
Abstract
Importance
Catheter ablation of persistent atrial fibrillation (AF) has limited success. Procedural strategies beyond pulmonary vein isolation have failed to consistently improve results. The vein of Marshall contains innervation and AF triggers that can be ablated by retrograde ethanol infusion.
Objective
To determine whether vein of Marshall ethanol infusion could improve ablation results in persistent AF when added to catheter ablation.
Design, Setting, and Participants
The Vein of Marshall Ethanol for Untreated Persistent AF (VENUS) trial was an investigator-initiated, National Institutes of Health–funded, randomized, single-blinded trial conducted in 12 centers in the United States. Patients (N = 350) with persistent AF referred for first ablation were enrolled from October 2013 through June 2018. Follow-up concluded in June 2019.
Interventions
Patients were randomly assigned to catheter ablation alone (n = 158) or catheter ablation combined with vein of Marshall ethanol infusion (n = 185) in a 1:1.15 ratio to accommodate for 15% technical vein of Marshall ethanol infusion failures.
Main Outcomes and Measures
The primary outcome was freedom from AF or atrial tachycardia for longer than 30 seconds after a single procedure, without antiarrhythmic drugs, at both 6 and 12 months. Outcome assessment was blinded to randomization treatment. There were 12 secondary outcomes, including AF burden, freedom from AF after multiple procedures, perimitral block, and others.
Results
Of the 343 randomized patients (mean [SD] age, 66.5 [9.7] years; 261 men), 316 (92.1%) completed the trial. Vein of Marshall ethanol was successfully delivered in 155 of 185 patients. At 6 and 12 months, the proportion of patients with freedom from AF/atrial tachycardia after a single procedure was 49.2% (91/185) in the catheter ablation combined with vein of Marshall ethanol infusion group compared with 38% (60/158) in the catheter ablation alone group (difference, 11.2% [95% CI, 0.8%-21.7%]; P = .04). Of the 12 secondary outcomes, 9 were not significantly different, but AF burden (zero burden in 78.3% vs 67.9%; difference, 10.4% [95% CI, 2.9%-17.9%]; P = .01), freedom from AF after multiple procedures (65.2% vs 53.8%; difference, 11.4% [95% CI, 0.6%-22.2%]; P = .04), and success achieving perimitral block (80.6% vs 51.3%; difference, 29.3% [95% CI, 19.3%-39.3%]; P < .001) were significantly improved in vein of Marshall–treated patients. Adverse events were similar between groups.
Conclusions and Relevance
Among patients with persistent AF, addition of vein of Marshall ethanol infusion to catheter ablation, compared with catheter ablation alone, increased the likelihood of remaining free of AF or atrial tachycardia at 6 and 12 months. Further research is needed to assess longer-term efficacy.
Trial Registration
ClinicalTrials.gov Identifier: NCT01898221
This randomized trial compares the effect of transcatheter pulmonary vein isolation during catheter ablation with vs without ethanol infusion into the vein of Marshall, an embryologic remnant of the left superior vena cava implicated in atrial fibrillation pathogenesis, on freedom from atrial arrhythmia at 12 months.
Introduction
Catheter ablation is an established treatment for atrial fibrillation (AF), particularly drug-refractory paroxysmal AF.1 The procedural strategy is pulmonary vein isolation, aimed at isolating ectopic beats that trigger AF paroxysms.2 For persistent AF, results of pulmonary vein isolation are suboptimal. Recurrent AF and atrial tachycardias often lead to repeat procedures. Strategies to systematically expand the lesion sets to include linear lesions or ablate areas of complex electrograms have failed to consistently improve outcomes.3,4
The vein of Marshall, an embryological remnant of the left superior vena cava, has been implicated in the pathogenesis of AF, as a source of AF triggers,5 and a tract for parasympathetic6 and sympathetic7 innervations that modulate electrophysiological properties of atrial tissue and contribute to AF maintenance.8 Additionally, the vein of Marshall is located within the mitral isthmus, critical for perimitral atrial tachycardia.9 Retrograde balloon cannulation and ethanol infusion in the vein of Marshall create a local ablation,10 eliminate AF triggers11and vein of Marshall innervation,12 facilitate mitral isthmus ablation,9 and have shown potential in preliminary studies.13
The Vein of Marshall Ethanol for Unablated Persistent AF (VENUS) trial tested the hypothesis that adding vein of Marshall ethanol infusion to de novo catheter ablation of persistent AF could lead to increased chances of maintaining normal rhythm.
Methods
Trial Design and Oversight
This was a multicenter, randomized clinical trial comparing the rhythm-control effectiveness of 2 ablation strategies: catheter ablation alone or combined with vein of Marshall ethanol infusion in de novo ablation of AF. The trial design has been previously described,14 and the full protocol and statistical analysis plan are available in Supplement 1.
The trial was approved by the institutional review boards of each center and was overseen by the US Food and Drug Administration under Investigational New Drug application No. 115,060. All patients provided written informed consent. A data and safety monitoring board, constituted by the National Heart, Lung, and Blood Institute, reviewed safety events, monitored study conduct, and reviewed and approved protocol modifications, safety, and efficacy interim analyses. A steering committee was responsible for study design and conduct. A data coordinating center was constituted at the Dan L. Duncan Institute for Clinical & Translational Research of Baylor College of Medicine. Independent study monitoring of data integrity was conducted at all sites. Adverse events and electrocardiographic monitoring data were monitored by independent committees, not involved in the procedure or patient follow-up, and blinded to the randomization outcome.
Study Population
Patients were recruited from 12 referral centers in the United States. Proceduralists received training in vein of Marshall cannulation and the ethanol infusion procedure. Patients were eligible if they were between 18 and 85 years of age and had symptomatic persistent AF (sustained AF lasting >7 days) refractory to at least 1 antiarrhythmic agent. Exclusion criteria included previous AF ablation attempts and left atrial diameter or volume exceeding 65 mm or 200 mL, respectively. Race and ethnicity—by patients’ self-identification—were collected as mandated by the National Institutes of Health. Detailed inclusion and exclusion criteria are provided in the eMethods in Supplement 2.
Trial Procedures
Patients were randomly assigned in a 1:1.15 ratio to either catheter ablation alone or vein of Marshall–catheter ablation (Figure 1). Randomization was performed using the method of permuted block randomization. The block sizes were randomly chosen as 4 and 6. Initial randomization was stratified by left atrial volume and AF duration. Stratification was eliminated in version 7 of the protocol to eliminate the need for computed tomography–based or magnetic resonance–based left atrial volume measurements and because patient characteristics and left atrial volume were balanced by randomization. The excess 15% patients in the vein of Marshall–catheter ablation group was designed to accommodate an expected 15% failure rate at cannulating the vein of Marshall in order to allow a prespecified secondary analysis of an equal number of successfully treated vein of Marshall–catheter ablation patients with the catheter ablation group (as-treated analysis) in addition to as-randomized analysis.14 The randomization algorithm was programmed into the data coordinating center and was activated when the research nurse at each site entered the patient enrollment in the database. The randomization outcome was communicated to the operator. Patients were blinded to randomization outcome, as were the committees evaluating adverse events and electrocardiographic data. All investigators were blinded to interim analyses.
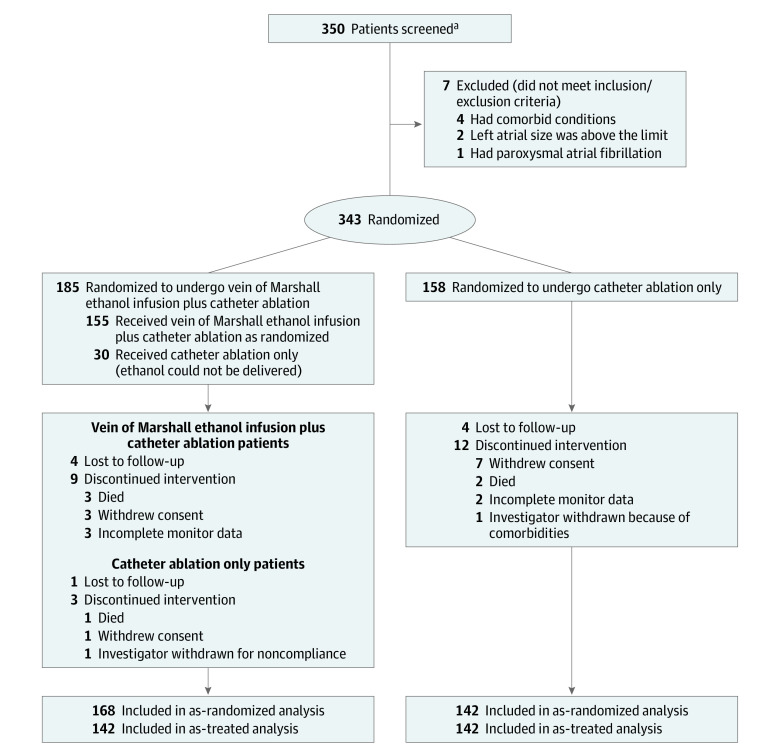
Figure 1. Clinical Trial Conduct.
aSites were not required to provide screening logs during the recruitment phase. Thus, the number of patients assessed for eligibility is not available.
The procedural steps are described in the eMethods in Supplement 2. Patients randomized to vein of Marshall–catheter ablation underwent the vein of Marshall procedure before catheter ablation. The vein of Marshall was identified by coronary sinus venography. If present, the vein of Marshall was cannulated with an angioplasty wire and balloon as previously described (eFigure 1 in Supplement 2).10,15 Following ethanol injection, a repeat voltage map was performed to quantify ethanol-induced scar. Catheter ablation followed in the same procedure.
Catheter ablation was performed with the use of radiofrequency energy delivered by a catheter with an open, irrigated tip guided by 3-dimensional mapping system, and followed a sequential approach. All patients underwent pulmonary vein isolation. At the discretion of the operator, additional lesions could include isolation of the posterior wall, mitral isthmus ablation, and ablation of complex and fractionated electrograms. All ablation sites beyond pulmonary vein isolation were recorded in the database (eFigure 2 in Supplement 2). A final voltage map was obtained in all patients in both groups (eFigure 3 in Supplement 2). The total extent of ablated tissue (bipolar voltage <0.1 mV) was quantified.
Clinical assessments and 12-lead electrocardiograms were obtained at baseline and at 1, 3, 6, 9, and 12 months after the initial ablation. Patients underwent continuous 1-month monitoring (MediLynx) at 6 and 12 months after ablation. When present, data from implanted rhythm-monitoring devices (at least 30 days of data from pacemakers, defibrillators, or implanted loop recorders) replaced external monitor data. All rhythm data were read independently by a core laboratory and clinicians blinded to the randomization assignment.
During the first 3 months after the randomization procedure (blanking period), recurrent AF or atrial tachycardia was treated with antiarrhythmic drugs or cardioversion as needed, and not considered treatment failure.1 After the blanking period, recurrent AF or atrial tachycardia was considered treatment failure and treated with antiarrhythmic therapy, cardioversion, or repeat catheter ablation—repeat pulmonary vein isolation or ablation as directed by atrial tachycardia mapping—at the physicians’ discretion. Crossovers from the catheter ablation group to the vein of Marshall–catheter ablation group were allowed only after 2 failed catheter ablation procedures.
Outcomes
The primary outcome was freedom from AF or atrial tachycardia lasting longer than 30 seconds after the performance of a single procedure (vein of Marshall–catheter ablation or catheter ablation alone), without the use of antiarrhythmic medications and occurring after the blanking period, including monitoring at 6 and 12 months. A primary safety outcome of acute procedural complications and total mortality was initially included. An initial design included freedom from AF or atrial tachycardia after multiple procedures. Per recommendations from the data and safety monitoring board, in version 7 of the protocol (April 2015) (Supplement 1), the primary outcome was changed to single-procedure success because pilot data were based on single-procedure outcomes, and death from any cause was also considered to constitute treatment failure for the purpose of primary outcome analyses. Death from any cause as a treatment failure addresses potential bias from competing risks.
Secondary outcomes included freedom from AF or atrial tachycardia after more than 1 procedure or with antiarrhythmic drug treatment, AF burden (% time) on continuous monitoring, procedural parameters (total procedure, fluoroscopy, total extent of tissue ablated, achievement of perimitral block), recurrence as AF or atrial tachycardia, procedural complications, cardiovascular hospitalizations, left atrial function, and quality of life using the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire. Detailed definitions of the 12 secondary outcomes are provided in the eMethods in Supplement 2.
Statistical Analysis
Sample-size calculations were based on a pilot study (eMethods in Supplement 2). The expected single-procedure success was 38% in patients undergoing catheter ablation and 56% for those undergoing vein of Marshall–catheter ablation. A sample size of N1 = 180 (vein of Marshall–catheter ablation) and N2 = 156 (catheter ablation) at the final analysis achieved 91% power to detect a difference of 0.18 between a treatment group success proportion of 0.56 and a control group success proportion of 0.38 with an α of .05 and a 2-tailed Z test (unpooled). It was reasoned that an increase in single-procedure success from 38% to 56% would be clinically meaningful and consistent with testing of other adjunctive interventional approaches.1 A 3-stage group sequential randomized trial design was used, for which power and sample size determination were determined for an equality test of 2 success proportions using PASS version 12 (NCSS). Interim analyses at one- and two-thirds of enrollment were overseen by the data and safety monitoring board with prespecified O’Brien-Fleming futility boundaries.
Primary Analysis for Efficacy
The primary analysis for efficacy was based on a 2-tailed hypothesis test for equality of 2 independent success proportions. Patients randomized to vein of Marshall–catheter ablation in whom the vein of Marshall ethanol procedure was not completed underwent conventional catheter ablation procedure and remained part of the vein of Marshall–catheter ablation group. Primary outcome analyses were performed as randomized. A secondary, prespecified analysis excluding patients with failed vein of Marshall ethanol procedures (as treated) was also performed. Patients with missing primary outcomes were assumed to be failures for the primary analysis. Missing data analyses included multiple imputation via Markov chain Monte Carlo methods and by random replacement of missing outcome with failure or success (eMethods in Supplement 2). Crude odds ratios for primary outcome failures were determined using Woolf-based 95% CIs. The significance threshold was α of .05 and with 2-sided testing.
Baseline Demographics and Secondary Outcomes
Data are reported as mean (SD). Two-tailed t tests were used for equality of means hypothesis testing. The Bartlett test was used to test for heteroscedasticity among both treatment groups. If significant, a Welch t test was performed for which the degrees of freedom were modified. Categorical data were compared using χ2 contingency table analysis. AF burden and number of procedures per patient were compared with the Mann-Whitney U test. A type I error of α = .05 was assumed. Subgroup analyses were performed, including male vs female, longstanding persistent AF, left atrial volume, and left atrial scar tertiles; logistic regression was used to assess interactions between subgroup membership and treatment group. Interaction models used binarized primary outcome failure as the dependent variable, binarized treatment group and binarized subgroup membership as main effects, and the interaction between treatment group and subgroup membership. Stata (version 15, StataCorp) was used for all analyses. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory.
Kaplan-Meier Analysis
A Kaplan-Meier secondary analysis was performed for time-to-event analysis of the primary outcome. The log-rank test was used to determine significance between the vein of Marshall–catheter ablation and catheter ablation treatment groups. Log-rank χ2 values greater than 3.84 (1 df) were assumed to be significant at the α = .05 level for all Kaplan-Meier tests. Cox proportional hazards regression analysis was used to calculate crude hazard ratios. The proportional hazards assumption based on Schoenfeld residuals was not significant for the as-randomized patients and as-treated patients (eMethods in Supplement 2).
Results
Patients
A total of 350 patients were screened between October 2013 and June 2018. Seven patients were found to meet exclusion criteria and were therefore excluded as screen failures. Of the remaining 343, 158 were randomly assigned to catheter ablation and 185 patients to vein of Marshall–catheter ablation (Figure 1). Baseline characteristics are shown in Table 1.
Table 1. Patient Demographic Characteristics.
| No. (%) | ||
|---|---|---|
| Vein of Marshall–catheter ablation (n = 185) | Catheter ablation (n = 158) | |
| Demographics | ||
| Age, mean (SD), y | 66.6 (9.6) | 66.4 (9.9) |
| Sex, No. (%) | ||
| Male | 137 (74) | 124 (78) |
| Female | 48 (26) | 34 (22) |
| Race and ethnicity | ||
| White | 169 (91) | 150 (95) |
| Black | 5 (3) | 2 (1) |
| Hispanic | 3 (2) | 1 (1) |
| Asian | 1 (1) | 2 (1) |
| Not stated | 7 (4) | 3 (2) |
| Medical history and risk factors | ||
| Hypertension | 144 (77) | 104 (66) |
| Diabetes | 52 (28) | 31 (20) |
| Coronary disease | 52 (28) | 41 (26) |
| Stroke-TIA | 19 (10) | 19 (12) |
| Heart failure | 48 (26) | 42 (27) |
| Body mass indexa | 31.2 (6.6) | 31.9 (6.5) |
| CHA2DS2-VASC scoreb | 2.9 (1.6) | 2.6 (1.6) |
| Cardiac parameters | ||
| Ejection fraction, % | 52.1 (10.1) | 53.4 (9.4) |
| Left atrial diameter, mm | 44.8.1 (7.9) | 47.0 (7.5) |
| Left atrial volume, mL | 110.9 (46.8) | 113.9 (46.3) |
| Time from first AF diagnosis | ||
| <6 mo | 15 (8) | 10 (6) |
| 6 mo to 2 y | 76 (41) | 65 (41) |
| >2 y | 94 (51) | 83 (52) |
| Longstanding persistent AF, No. (%)c | 99 (54) | 82 (52) |
Abbreviations: AF, atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/TIA/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65 to 75 years, sex category (female); TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared.
CHA2DS2-VASc score is a clinical estimation of the risk of stroke in patients with AF. Scores range from 0 to 9, and higher scores indicate a greater risk.
Longstanding persistent AF: continuous AF lasting for 1 year or more.
In 30 patients randomized to vein of Marshall–catheter ablation, the vein of Marshall ethanol injection procedure was not completed due to failure to cannulate the vein of Marshall (success in 83.7%). Adherence to the 30-day event monitor at 6 and 12 months was 85.1% and 83.3%, respectively. The rhythm follow-up data were obtained from implanted device recordings (pacemaker, defibrillators, or implantable loop recorders) in 43.8% and 44.8% of patients at 6 and 12 months, respectively, without significant monitoring differences between groups (eTable 1 in Supplement 2). Thirteen patients in the vein of Marshall–catheter ablation group and 14 in the catheter ablation group had missing primary outcome data due to incomplete monitoring follow-up (Figure 1).
In 10 patients (5 in each group), early recurrences were treated with repeat procedures within the blanking period—a protocol deviation. For the purpose of the primary outcome, they were all considered treatment failures. An alternative analysis using the patients’ outcomes after 90 days is described in the eMethods in Supplement 2.
Procedures
Vein of Marshall Ethanol Procedure
The total mean (SD) vein of Marshall procedure time was 42.5 (32.8) minutes and required 11.7 (11) minutes of fluoroscopy. Ethanol led to a low-voltage area measuring a mean (SD) of 4.9 (3.2) cm2. In 33 of 155 patients, ethanol caused left inferior pulmonary vein isolation. There were no complications directly attributed to the vein of Marshall ethanol procedure.
Ablation Procedure
eTable 2 in Supplement 2 shows the procedural parameters. The catheter ablation group had overall shorter total procedure, fluoroscopy, and left atrial instrumentation times and longer total radiofrequency application. The total ablated area had large variability but was overall similar for both groups. Successful pulmonary vein isolation was achieved in all patients in both groups. Additional ablation lesions beyond those required to achieve pulmonary vein isolation were delivered in 151 of 158 patients (95.6%) in the catheter ablation group and in 177 of 185 patients (95.7%) in the vein of Marshall–catheter ablation group. Isolation of the posterior wall and complex potential ablation were commonly performed in both groups. The vein of Marshall–catheter ablation group included more patients who underwent mitral isthmus ablation, and the catheter ablation group had more patients treated with right atrial or superior vena cava ablation.
Primary Outcome
Freedom from any clinical AF or atrial tachycardia, more than 30 seconds of AF or atrial tachycardia on monitoring at 6 and 12 months, or repeat procedures and without the use of antiarrhythmic drugs was reached in 38% of patients (60/158) randomized to catheter ablation and in 49.2% of patients (91/185) randomized to vein of Marshall–catheter ablation (P = .04), with an absolute difference of 11.2% (95% CI, 0.8%-21.7%) and an odds ratio of 0.63 (95% CI, 0.41-0.97). Excluding patients in whom vein of Marshall ethanol infusion was not performed (as-treated analysis), the primary outcome was reached in 80 of the 155 patients in the vein of Marshall–catheter ablation group (51.6%, P = .02), with an absolute difference of 13.6% (95% CI, 2.7%-24.6%) and an odds ratio of 0.57 (95% CI, 0.37-0.90) (Table 2).
Table 2. Trial Outcomes.
| As-randomized analysis | As-treated analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Absolute percentage difference (95% CI) | Odds ratio (95% CI) | P value | Vein of Marshall–catheter ablation as treated (n = 155), No. (%) | Absolute percentage difference (95% CI) | Odds ratio (95% CI) | P value | ||
| Vein of Marshall–catheter ablation as randomized (n = 185) | Catheter ablation (n = 158) | ||||||||
| Primary outcomea | |||||||||
| Freedom from AF or tachycardia >30 s after 90 d | 91 (49.2) | 60 (38) | 11.2 (0.8 to 21.7) | 0.63 (0.41 to 0.97) | .04 | 80 (51.6) | 13.6 (2.7 to 24.6) | 0.57 (0.37 to 0.90) | .02 |
| Primary outcome components | |||||||||
| Atrial tachycardia or fibrillation recurrence (excluding deaths and missing data) | 77/168 (45.8) | 82/142 (57.7) | –11.9 (–23 to –0.8) | 0.62 (0.39 to 0.97) | .04 | 62/142 (43.7) | –14 (–2.5 to –25.5) | 0.57 (0.36 to 0.91) | .02 |
| Symptomatic recurrence of AF or tachycardia | 58 (31.3) | 59 (37.3) | –6 (–16.1 to 4.1) | .24 | 45 (29) | –8.3 (–18.7 to –2.1) | .12 | ||
| Recurrence only on monitoring: AF or tachycardia >30 s | 19 (10.3) | 23 (14.6) | –4.3 (–11.3 to 2.7) | .23 | 17 (11) | –3.6 (–11 to 3.8) | .34 | ||
| Secondary outcomes | |||||||||
| Freedom from AF or tachycardia >30 s after 90 d and repeat procedures | 115 (62.2) | 84 (53.8) | 8.4 (–2.1 to 18.9) | .12 | 101 (65.2) | 11.4 (0.6–22.2) | .04 | ||
| Recurrence as AF | 47 (25.4) | 50 (31.6) | –6.2 (–15.8 to 3.4) | .20 | 35 (22.6) | –9 (–18.8 to 0.8) | .07 | ||
| Recurrence as atrial tachycardia | 30 (16.2) | 32 (20.3) | –4.1 (–12.3 to 4.1) | .33 | 27 (17.4) | –2.9 (–11.6 to 5.8) | .51 | ||
| AF burden on 6- and 12-mo monitoringb | .03 | .01 | |||||||
| 0% burden | 232/306 (75.8) | 184/271 (67.9) | 7.9 (0.6 to 15.2) | 206/263 (78.3) | 10.4 (2.9 to 17.9) | ||||
| >0-<5% burden | 37/306 (12.1) | 55/271 (20.3) | –8.2 (–14.2 to –2.2) | 30/263 (11.4) | –8.9 (–15 to –2.8) | ||||
| >5% burden | 37/306 (12.1) | 32/271 (11.8) | 0.3 (–5 to 5.6) | 27/263 (10.3) | –1.5 (–6.8 to 3.8) | ||||
| Bidirectional perimitral block obtainedc | 137 (74.0) | 81 (51.3) | 22.7 (12.7 to 32.7) | 2.7 (1.7 to 4.2) | <.001 | 125 (80.6) | 29.3 (19.3 to 39.3) | 3.9 (2.4 to 6.5) | <.001 |
| Mitral isthmus ablation time, min | 8.1 (9.1) | 12.7 (15.5) | –4.6 (–7.2 to –1.9) | <.001 | 7.6 (9.1) | –5.1 (–7.9 to –2.3) | <.001 | ||
| Repeat ablation(s) performedd | 32 (17.3) | 40 (25.3) | .04 | 27 (17.4) | .05 | ||||
Abbreviation: AF, atrial fibrillation.
The primary outcome is reported as randomized and as treated. Missing data (13 patients in the vein of Marshall–catheter ablation group and 14 in the catheter ablation group) are counted as failures, as are deaths and all repeat procedures. Imputations for missing data are available in eMethods in Supplement 2.
Proportions indicate number of patient monitors at 6 and 12 months with a range of AF or atrial tachycardia burden (percentage of time in AF or tachycardia over total monitored time) divided by total number of monitored patients.
Bidirectional block refers to elimination of electrical propagation across the mitral isthmus in both directions.
Repeat procedures compared by Mann-Whitney U test.
Differences in the primary outcome were driven by a reduced recurrence of AF or atrial tachycardia in the vein of Marshall–catheter ablation group (Table 2). Missing data imputation analysis was consistent with the results of the primary analysis (eTables 3-5 and eFigures 4-5 in Supplement 2).
Time-to-event analyses are shown in Figure 2. Kaplan-Meier plots showed significant reduction in AF or atrial tachycardia recurrence in the vein of Marshall–catheter ablation group, both in the as-randomized analysis (hazard ratio, 0.73 [95% CI, 0.53-1.00]; P = .05) and the as-treated analysis (hazard ratio, 0.67 [95% CI, 0.47-0.93]; P = .02) (eTables 6-9 in Supplement 2).
Figure 2. Time to Recurrence of Atrial Fibrillation or Tachycardia and Atrial Fibrillation Burden.
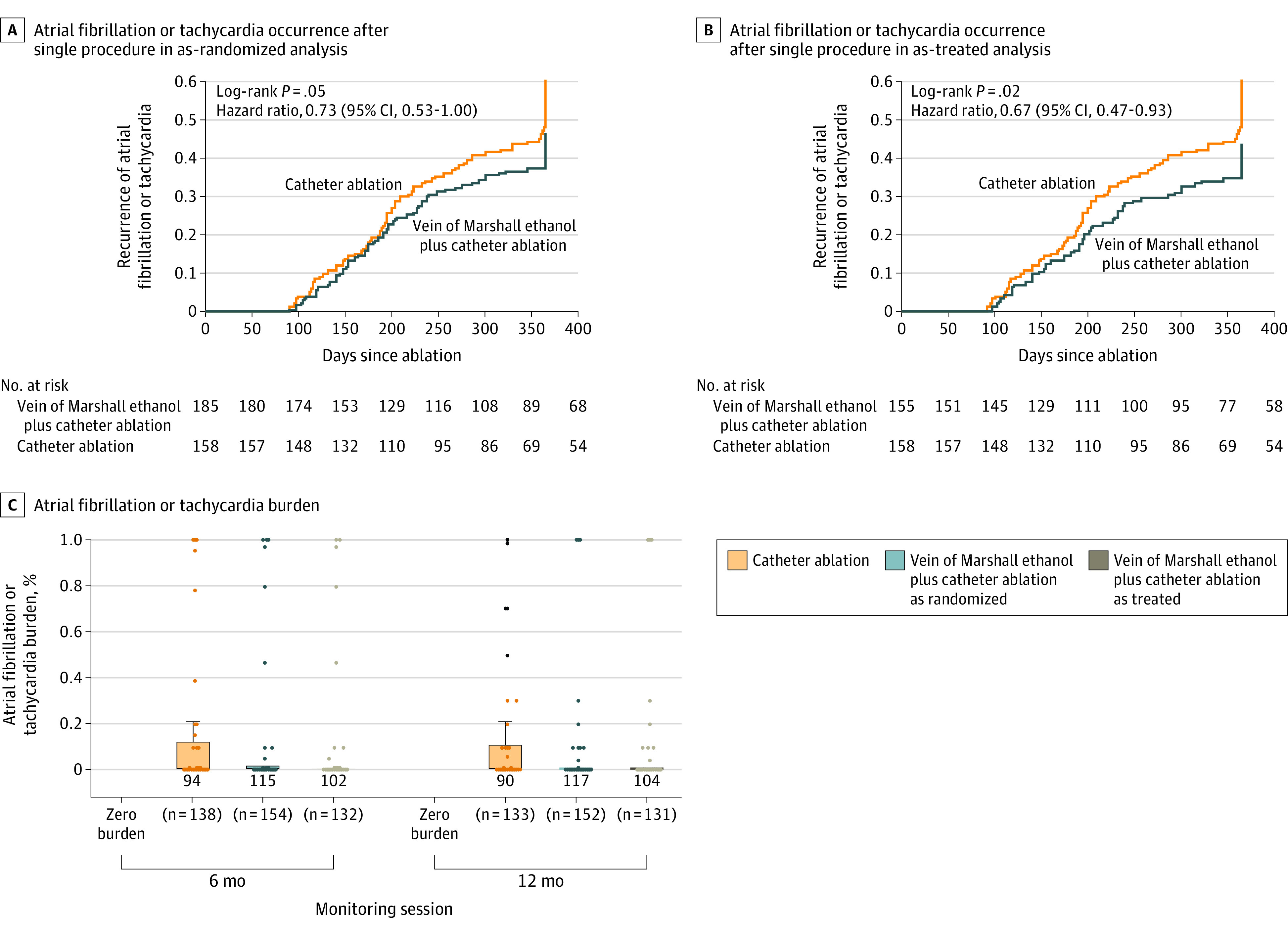
The figure shows time to recurrence of atrial fibrillation or tachycardia in the as-randomized (A) and as-treated (B) analyses and atrial fibrillation burden (C), quantified as percentage of atrial fibrillation or tachycardia over 1-month monitoring at 6 and 12 months of follow-up. Boxes represent interquartile ranges (zero for vein of Marshall ethanol plus catheter ablation groups). Whiskers represent maximum values (third quartile plus 1.5 times interquartile range − zero for vein of Marshall ethanol plus catheter ablation groups). Dots represent outliers.
Prespecified Secondary Outcomes
Eliminating AF or atrial tachycardia of greater than 30 seconds’ duration after multiple procedures was successful in 53.8% of patients (85/158) treated with catheter ablation alone and in 65.2% of patients (101/155) treated with vein of Marshall–catheter ablation (absolute difference, 11.4% [95% CI, 0.6%-22.2%]; P = .04).
AF burden was extremely variable in both groups and followed a skewed distribution (Table 2 and Figure 2). At 6 and 12 months, freedom from any AF or atrial tachycardia on 1-month monitoring (zero burden), after repeat procedures, or with the use of antiarrhythmic drugs was achieved in 67.8% of monitoring sessions (184/271) in the catheter ablation group, in 75.8% (232/306) in the vein of Marshall–catheter ablation group (absolute difference, 8% [95% CI, 0.6%-15.2%]; P = .03), and in 78.3% of those undergoing successful vein of Marshall ethanol infusion procedures (206/263) (as treated: absolute difference, 10.5% [95% CI, 2.9%-17.9%]; P = .008) (eTables 10-12 in Supplement 2).
Recurrence as AF vs atrial tachycardia was not significantly different between groups. In the subgroup analyses, female sex, longstanding persistent AF, and left atrial volume greater than 75 mL/m2 were associated with a greater effect in the vein of Marshall–catheter ablation group. However, testing for interaction did not reach significance for any subgroup (eFigure 6 and eTable 13 in Supplement 2).
Overall quality of life (AFEQT score) was not statistically different between groups (eFigure 7 in Supplement 2).
Post Hoc Analyses
A post hoc analysis of the rate of repeat ablations showed that these were performed in 40 of 158 patients in the group receiving catheter ablation alone (25.3%, or a mean [SD] of 1.3 [0.6] procedures per patient) and in 32 of 185 patients in the group randomized to vein of Marshall–catheter ablation (17.3%, or a mean [SD] of 1.2 [0.4] procedures per patient) (P = .04; eFigure 8 and eTables 14-15 in Supplement 2).
Adverse Events
The most common intraprocedural adverse events were vascular access complications (hematoma or pseudoaneurysm, 11 events) and intraprocedural pericardial effusion (3 events). After the procedure, subacute pericardial effusion requiring pericardiocentesis occurred in 4 patients. There were 7 patients with cerebrovascular events and 7 with pneumonia after the procedure. Symptomatic inflammatory pericarditis not requiring drainage occurred in 11 patients in the vein of Marshall–catheter ablation and in 6 in the catheter ablation group (Table 3). Fluid overload requiring diuretic treatment occurred in 10 patients in the vein of Marshall–catheter ablation group and in 2 patients in the catheter ablation group. There were 6 deaths in the study, none related to the ablation or vein of Marshall procedures.
Table 3. Adverse Events.
| Vein of Marshall–catheter ablation as randomized (n = 185) | Catheter ablation (n = 158) | Vein of Marshall–catheter ablation as treated (n = 155) | |
|---|---|---|---|
| Intraprocedural pericardial effusion | 2 | 1 | 1 |
| Subacute pericardial effusion requiring drainage | 2 | 2 | 2 |
| Subacute pericardial effusion/pericarditis not requiring drainage | 11 | 6 | 10 |
| Vascular access complications | |||
| Hematoma | 3 | 6 | 3 |
| Pseudoaneurysm | 0 | 2 | 0 |
| Stroke | 1 | 2 | 1 |
| Transient ischemic attack | 2 | 2 | 1 |
| Fluid overload | 10 | 2 | 7 |
| Pneumonia | 3 | 4 | 2 |
| Atrioesophageal fistula | 0 | 0 | 0 |
| Death | 4a | 2b | 3c |
Deaths due to pancreatic cancer, lung transplant failure, hypokalemic cardiac arrest, and unknown cause.
Deaths due to esophageal cancer and pneumonia.
Deaths due to pancreatic cancer, lung transplant failure, and hypokalemic cardiac arrest.
Discussion
In this randomized clinical trial, among patients with persistent AF, addition of vein of Marshall ethanol infusion to catheter ablation, compared with catheter ablation alone, increased the likelihood of remaining free of AF or atrial tachycardia at 6 and 12 months.
Despite the outcome differences between groups, the overall ablation success, as measured by the primary outcome, was low. These outcomes are still suboptimal and worse than previously reported in other studies.3,4 More than half of the patients enrolled had longstanding persistent AF, which is known to respond poorly to ablation.16 Electrocardiographic monitoring in this trial, which was continuous for 1 month in 2 sessions, was more prolonged than in previous studies (with 24- to 72-hour Holter or event monitors),3,4,17 which could have increased the probability of detecting short or sporadic AF episodes.
The mechanisms of improved rhythm control by vein of Marshall ethanol may be related to enhanced atrial denervation,12 more reliable conduction block at the mitral isthmus,9 or elimination of AF triggers.11 Other studies that have evaluated ablative approaches beyond pulmonary vein isolation have failed to show a consistent benefit. Linear lesions or ablation of complex potentials failed to improve the outcome of pulmonary vein isolation in previous randomized trials.3,4,18,19 Isolation of the posterior wall has been reported as beneficial in some20 but not all studies.21,22 In this trial, most patients (>95%) in both groups underwent lesion sets aiming to ablate AF substrates beyond pulmonary vein isolation, but specific vein of Marshall arrhythmogenesis was only targeted in the ethanol group.
The benefits of vein of Marshall ethanol were observed in other outcomes of clinical relevance, such as AF burden rate of repeat procedures. The vein of Marshall ethanol infusion procedure did not increase procedural complications. Adverse events were consistent with the overall aggressive ablation approach used in both groups.
Limitations
This study has several limitations. First, patients in both groups underwent ablation beyond pulmonary vein isolation, including posterior wall and other locations. Given the single-blinded design, investigator bias to modify procedural aspects (potentially toward more aggressive ablation) in the catheter ablation group is possible. However, the only significant differences included right atrial ablation, which was more frequent in the catheter ablation–only group, and mitral isthmus ablation with successful bidirectional perimitral block, which was more frequent in the vein of Marshall–catheter ablation group. Right atrial ablation is unlikely to affect outcomes aside from prevention of peritricuspid flutter. A more robust mitral isthmus ablation could have reduced recurrence as perimitral atrial tachycardia.9 However, recurrences as atrial tachycardia were not statistically different between groups.
Second, the vein of Marshall ethanol infusion procedure was not completed in all patients randomized to undergo it. Both as-randomized and as-treated analyses support a treatment effect, but technical failures may limit its applicability.
Third, adherence to monitoring was incomplete, but consistent with4 or better than17 other clinical trials that used shorter monitoring sessions.
Fourth, there were 27 patients in whom the primary outcome could not be ascertained because of lacking monitoring data and 10 patients had repeat procedures performed during the blanking period. Sensitivity analyses of potential outcomes of missing patients and reanalysis of early repeat ablation patients did not alter the trial results.
Fifth, although the increase in ablation success with vein of Marshall ethanol infusion was less than in the pilot study, the results were within the range of detectable differences. Whether an as-treated absolute difference of 14% (43% reduction in recurrence) represents a clinically relevant improvement is subject to judgment, but is consistent with that of other adjunctive interventional approaches.1
Conclusions
Among patients with persistent AF, addition of vein of Marshall ethanol infusion to catheter ablation, compared with catheter ablation alone, increased the likelihood of remaining free of AF or atrial tachycardia at 6 and 12 months. Further research is needed to assess longer-term efficacy.
Trial Protocol
eMethods.
eFigure 1. Procedural Steps of the Vein of Marshall Ethanol Injection Procedure
eFigure 2. Procedural Steps of the Catheter Ablation Procedure
eFigure 3. Left Atrial Voltage Maps Obtained During the Procedure
eFigure 4. Z-score Values From B=500 Two-Tailed Tests of Proportions as a Function of Levels of Missingness, Based on Results From Algorithm 1
eFigure 5. Random Replacement of Missing Primary Outcome With Success or Failure
eFigure 6. Subgroup Analysis of the Primary Outcome
eFigure 7. Quality of Life (Overall AFEQT Score)
eFigure 8. Number of Procedures in Each Group
eTable 1. Monitoring Characteristics and Compliance
eTable 2. Procedural Characteristics
eTable 3. Primary Outcome Analyses, With and Without Early Repeat Procedures, as Randomized and as Treated Analyses
eTable 4. Multiple Imputation by Monte Carlo Markov Chain
eTable 5. Primary Outcome Simulations of Missing Data as a Function of Failure Missingness
eTable 6. Log-rank Test Results
eTable 7. Cox Regression Analysis for Time to Atrial Fibrillation/Atrial Tachycardia Recurrence by as Randomized Analysis
eTable 8. Cox Regression Analysis for Time to Atrial Fibrillation/Atrial Tachycardia Recurrence by as Treated Analysis
eTable 9. PH Assumption Test
eTable 10. Atrial Fibrillation (AF) Burden Comparisons
eTable 11. Atrial Fibrillation Burden Groups
eTable 12. Atrial Fibrillation Burden Comparisons
eTable 13. Subgroup Analysis for the Primary Outcome
eTable 14. Total Number of Procedures per Patient
eTable 15. Number of Procedures per Patient (Mann-Whitney test)
Data Sharing Statement
References
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659-666. doi: 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 3.Dixit S, Marchlinski FE, Lin D, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol. 2012;5(2):287-294. doi: 10.1161/CIRCEP.111.966226 [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Jiang CY, Betts TR, et al. ; STAR AF II Investigators . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812-1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Tai CT, Hsieh MH, et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol. 2005;46(6):1054-1059. doi: 10.1016/j.jacc.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 6.Ulphani JS, Arora R, Cain JH, et al. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293(3):H1629-H1635. doi: 10.1152/ajpheart.00139.2007 [DOI] [PubMed] [Google Scholar]
- 7.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36(4):1324-1327. doi: 10.1016/S0735-1097(00)00819-6 [DOI] [PubMed] [Google Scholar]
- 8.Kamanu S, Tan AY, Peter CT, Hwang C, Chen PS. Vein of Marshall activity during sustained atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(8):839-846. doi: 10.1111/j.1540-8167.2006.00516.x [DOI] [PubMed] [Google Scholar]
- 9.Báez-Escudero JL, Morales PF, Dave AS, et al. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012;9(8):1207-1215. doi: 10.1016/j.hrthm.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valderrábano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol. 2009;2(1):50-56. doi: 10.1161/CIRCEP.108.818427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave AS, Báez-Escudero JL, Sasaridis C, Hong TE, Rami T, Valderrábano M. Role of the vein of Marshall in atrial fibrillation recurrences after catheter ablation: therapeutic effect of ethanol infusion. J Cardiovasc Electrophysiol. 2012;23(6):583-591. doi: 10.1111/j.1540-8167.2011.02268.x [DOI] [PubMed] [Google Scholar]
- 12.Báez-Escudero JL, Keida T, Dave AS, Okishige K, Valderrábano M. Ethanol infusion in the vein of Marshall leads to parasympathetic denervation of the human left atrium: implications for atrial fibrillation. J Am Coll Cardiol. 2014;63(18):1892-1901. doi: 10.1016/j.jacc.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pambrun T, Denis A, Duchateau J, et al. MARSHALL bundles elimination, pulmonary veins isolation and lines completion for anatomical ablation of persistent atrial fibrillation: MARSHALL-PLAN case series. J Cardiovasc Electrophysiol. 2019;30(1):7-15. doi: 10.1111/jce.13797 [DOI] [PubMed] [Google Scholar]
- 14.Valderrábano M, Peterson LE, Bunge R, et al. Vein of Marshall ethanol infusion for persistent atrial fibrillation: VENUS and MARS clinical trial design. Am Heart J. 2019;215:52-61. doi: 10.1016/j.ahj.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valderrábano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6(11):1552-1558. doi: 10.1016/j.hrthm.2009.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60(19):1921-1929. doi: 10.1016/j.jacc.2012.04.060 [DOI] [PubMed] [Google Scholar]
- 17.Gillinov AM, Gelijns AC, Parides MK, et al. ; CTSN Investigators . Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372(15):1399-1409. doi: 10.1056/NEJMoa1500528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogler J, Willems S, Sultan A, et al. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol. 2015;66(24):2743-2752. doi: 10.1016/j.jacc.2015.09.088 [DOI] [PubMed] [Google Scholar]
- 19.Fink T, Schlüter M, Heeger CH, et al. Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the randomized alster-lost-af trial (ablation at St G0 rg Hospital for long-standing persistent atrial fibrillation). Circ Arrhythm Electrophysiol. 2017;10(7):e005114. doi: 10.1161/CIRCEP.117.005114 [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Shin SY, Na JO, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? a prospective randomized clinical trial. Int J Cardiol. 2015;181:277-283. doi: 10.1016/j.ijcard.2014.12.035 [DOI] [PubMed] [Google Scholar]
- 21.Tamborero D, Mont L, Berruezo A, et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009;2(1):35-40. doi: 10.1161/CIRCEP.108.797944 [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Shim J, Park J, et al. ; POBI-AF Investigators . The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5(11):1253-1261. doi: 10.1016/j.jacep.2019.08.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eFigure 1. Procedural Steps of the Vein of Marshall Ethanol Injection Procedure
eFigure 2. Procedural Steps of the Catheter Ablation Procedure
eFigure 3. Left Atrial Voltage Maps Obtained During the Procedure
eFigure 4. Z-score Values From B=500 Two-Tailed Tests of Proportions as a Function of Levels of Missingness, Based on Results From Algorithm 1
eFigure 5. Random Replacement of Missing Primary Outcome With Success or Failure
eFigure 6. Subgroup Analysis of the Primary Outcome
eFigure 7. Quality of Life (Overall AFEQT Score)
eFigure 8. Number of Procedures in Each Group
eTable 1. Monitoring Characteristics and Compliance
eTable 2. Procedural Characteristics
eTable 3. Primary Outcome Analyses, With and Without Early Repeat Procedures, as Randomized and as Treated Analyses
eTable 4. Multiple Imputation by Monte Carlo Markov Chain
eTable 5. Primary Outcome Simulations of Missing Data as a Function of Failure Missingness
eTable 6. Log-rank Test Results
eTable 7. Cox Regression Analysis for Time to Atrial Fibrillation/Atrial Tachycardia Recurrence by as Randomized Analysis
eTable 8. Cox Regression Analysis for Time to Atrial Fibrillation/Atrial Tachycardia Recurrence by as Treated Analysis
eTable 9. PH Assumption Test
eTable 10. Atrial Fibrillation (AF) Burden Comparisons
eTable 11. Atrial Fibrillation Burden Groups
eTable 12. Atrial Fibrillation Burden Comparisons
eTable 13. Subgroup Analysis for the Primary Outcome
eTable 14. Total Number of Procedures per Patient
eTable 15. Number of Procedures per Patient (Mann-Whitney test)
Data Sharing Statement