Key Points
Question
For patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI), is ticagrelor, compared with clopidogrel, associated with better outcomes in routine clinical practice?
Findings
In a retrospective cohort study that included 62 580 propensity score–matched patients, ticagrelor use, compared with clopidogrel, was not associated with statistically significant difference in the risk of net clinical adverse events (defined as recurrent myocardial infarction, revascularization, ischemic stroke, hemorrhagic stroke, and gastrointestinal bleeding) at 12 months (hazard ratio, 1.05).
Meaning
Ticagrelor, compared with clopidogrel, was not associated with better outcomes for patients with ACS treated with PCI in clinical practice.
Abstract
Importance
Current guidelines recommend ticagrelor as the preferred P2Y12 platelet inhibitor for patients with acute coronary syndrome (ACS), primarily based on a single large randomized clinical trial. The benefits and risks associated with ticagrelor vs clopidogrel in routine practice merits attention.
Objective
To determine the association of ticagrelor vs clopidogrel with ischemic and hemorrhagic events in patients undergoing percutaneous coronary intervention (PCI) for ACS in clinical practice.
Design, Setting, and Participants
A retrospective cohort study of patients with ACS who underwent PCI and received ticagrelor or clopidogrel was conducted using 2 United States electronic health record–based databases and 1 nationwide South Korean database from November 2011 to March 2019. Patients were matched using a large-scale propensity score algorithm, and the date of final follow-up was March 2019.
Exposures
Ticagrelor vs clopidogrel.
Main Outcomes and Measures
The primary end point was net adverse clinical events (NACE) at 12 months, composed of ischemic events (recurrent myocardial infarction, revascularization, or ischemic stroke) and hemorrhagic events (hemorrhagic stroke or gastrointestinal bleeding). Secondary outcomes included NACE or mortality, all-cause mortality, ischemic events, hemorrhagic events, individual components of the primary outcome, and dyspnea at 12 months. The database-level hazard ratios (HRs) were pooled to calculate summary HRs by random-effects meta-analysis.
Results
After propensity score matching among 31 290 propensity-matched pairs (median age group, 60-64 years; 29.3% women), 95.5% of patients took aspirin together with ticagrelor or clopidogrel. The 1-year risk of NACE was not significantly different between ticagrelor and clopidogrel (15.1% [3484/23 116 person-years] vs 14.6% [3290/22 587 person-years]; summary HR, 1.05 [95% CI, 1.00-1.10]; P = .06). There was also no significant difference in the risk of all-cause mortality (2.0% for ticagrelor vs 2.1% for clopidogrel; summary HR, 0.97 [95% CI, 0.81-1.16]; P = .74) or ischemic events (13.5% for ticagrelor vs 13.4% for clopidogrel; summary HR, 1.03 [95% CI, 0.98-1.08]; P = .32). The risks of hemorrhagic events (2.1% for ticagrelor vs 1.6% for clopidogrel; summary HR, 1.35 [95% CI, 1.13-1.61]; P = .001) and dyspnea (27.3% for ticagrelor vs 22.6% for clopidogrel; summary HR, 1.21 [95% CI, 1.17-1.26]; P < .001) were significantly higher in the ticagrelor group.
Conclusions and Relevance
Among patients with ACS who underwent PCI in routine clinical practice, ticagrelor, compared with clopidogrel, was not associated with significant difference in the risk of NACE at 12 months. Because the possibility of unmeasured confounders cannot be excluded, further research is needed to determine whether ticagrelor is more effective than clopidogrel in this setting.
This cohort study compares net risk for ischemic vs hemorrhagic events at 12 months among patients treated with ticagrelor vs clopidogrel during and after percutaneous coronary intervention (PCI) management of acute coronary syndrome (ACS).
Introduction
Ticagrelor is an oral, reversible, direct-acting P2Y12 inhibitor that exhibits more profound platelet inhibition with more rapid onset on the basis of platelet function testing, compared with clopidogrel.1 Guidelines from the American College of Cardiology, American Heart Association, the European Society of Cardiology, and the European Association for Cardio-Thoracic Surgery recommend using ticagrelor with aspirin in preference to clopidogrel for patients with acute coronary syndrome (ACS).2,3 These recommendations are based on the Platelet Inhibition and Patient Outcomes (PLATO) trial, which demonstrated that ticagrelor was more effective than clopidogrel in patients with ACS.4 On the basis of the evidence from PLATO and the revised guidelines, the use of ticagrelor increased rapidly worldwide.5,6,7
Questions remain, however, about the greater efficacy of ticagrelor compared with clopidogrel. In the PLATO trial, the benefit was not evident in patients from North America and Asia.8 Other concerns about the safety of ticagrelor include the drug-induced dyspnea and higher hemorrhagic events associated with its use, which can lead to early discontinuation.9,10 Recent observational studies have raised questions about whether ticagrelor is associated with better outcomes compared with clopidogrel in clinical practice.11
This study aimed to evaluate the association of ticagrelor vs clopidogrel with clinical outcomes in patients undergoing percutaneous coronary intervention (PCI) due to ACS in the US and Korea using the Observational Health Data Sciences and Informatics (OHDSI) network.12
Methods
Study Design and Data
This retrospective observational study, which used 2 electronic health record (EHR)-based databases in the United States (US Optum EHR; US IQVIA Hospital) and 1 nationwide administrative claims database in South Korea (Health Insurance Review and Assessment service [HIRA]) from November 2011 to March 2019, was approved by the institutional review board of Ajou University (AJIRB-MED-MDB-17-289). Written informed consent was waived due to the deidentified nature of the databases. Analyses of deidentified data were performed in accordance with local laws and regulation with approvals from respective scientific and ethics committees. All 3 databases were standardized to the Observational Medical Outcomes Partnership common data model, version 5 (eMethod 1 in Supplement 1).13
We performed distributed network analyses.14 The statistical analytic protocol (Supplement 2) was prespecified before execution. According to this protocol, the study package for the entire process was built on the OHDSI Methods Library in R and released with a Docker image providing a computational reproducible environment.15 This package was executed locally inside a firewall for each database. Next, the predesignated statistical results (without patient-level information) were shared for interpretation and database-level meta-analysis.
Study Population and Exposure
We identified adult patients (aged ≥20 years) who underwent PCI for the first time (according to their medical history) following a diagnosis of ACS. The index date was defined as the date of PCI. To avoid left censoring, we excluded patients who had been enrolled in the database for less than 1 year before the index date. The other exclusion criteria were a history of ischemic stroke, hemorrhagic stroke, or gastrointestinal bleeding any time before the index date; and a prescription of prasugrel, clopidogrel (for the ticagrelor group), or ticagrelor (for the clopidogrel group) during the 30 days preceding the index date. Further details of the cohort definition are presented in eMethod 2 in Supplement 1. Ticagrelor or clopidogrel use was ascertained from prescription records within the 7 days before the index date.
Outcomes
The primary outcome was net adverse clinical events (NACE), which included ischemic events (recurrent acute myocardial infarction [AMI], revascularization, or ischemic stroke) and hemorrhagic events (hemorrhagic stroke or gastrointestinal bleeding) from day 1 to 365 days after PCI. The secondary outcomes consisted of an extended definition of NACE that included mortality (NACE or mortality), all-cause mortality, composite ischemic events (recurrent AMI, revascularization, or ischemic stroke), composite hemorrhagic events (hemorrhagic stroke or gastrointestinal bleeding), and individual components of the primary outcome within 1 year. The 1-year risk of dyspnea, a well-known adverse event of ticagrelor, was also evaluated.
As a post hoc analysis, we compared the occurrence of cardiovascular-related mortality and major adverse cardiovascular events (MACE, including cardiovascular mortality, recurrent AMI, and stroke) in the Optum EHR and HIRA databases (this information was not available in the IQVIA database). Cardiovascular-related mortality was identified by a death record with at least 1 cardiovascular-related diagnosis (AMI, stroke, sudden cardiac death, or hospitalization for heart failure) in the 30 days before death as described previously.14 Further details of the outcome definitions, including International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; International Classification of Diseases, Ninth Revision, Clinical Modification; and Current Procedural Terminology (CPT)-4 codes, and the results of validation are provided in eMethod 3 in Supplement 1.
Statistical Analysis
We used regularized logistic regression16,17 to estimate the propensity scores, which used more than 10 000 baseline patient characteristics between the ticagrelor and clopidogrel groups, including all the available demographic characteristics, as well as the medical, medication, procedure, and device exposure history in each database. For the Optum EHR, we also included race and baseline laboratory values in the logistic regression model because they were available and can be associated with the selection of the drug and clinical outcomes. Categories of race were determined according to self-reported responses in the EHR: African American, Asian, White, and Other/Unknown. However, due to statistical deidentification rules for race based on geography, if there was a small number of one group, such as Asians in a particular region, they were categorized as Other/Unknown.
All variables except laboratory values were binary (yes/no) and all missing binary variables were considered as not present and coded as no. The missingness in laboratory values were matched between the ticagrelor and clopidogrel groups and missing values were not imputed. Next, the study populations were matched using one-to-one greedy matching of the propensity score.18 We used Cox proportional hazard regression models to estimate the association of exposures with outcomes. The Cox proportionality assumption was tested based on the Schoenfeld residuals, and no relevant violations were found for the primary outcome. Patients were censored when they were no longer observed in the database, while they remained in the primary analysis if they discontinued the allocated drug or switched the drug within the first year. We then performed random-effects meta-analysis to calculate summary hazard ratio (HR) pooling effect estimates across the databases.19
Sensitivity Analyses
To assess the robustness of the findings, a large set of sensitivity analyses were conducted using differing definitions of the at-risk time window, the statistical approaches, the study population, and the outcomes. First, in addition to one-to-one propensity score matching, 2 additional propensity score adjustments were performed: (1) variable-ratio propensity score matching with a maximum ratio of 10; and (2) propensity score stratification using deciles of the propensity score distribution. Second, 2 more time-at-risk windows were applied—a 5-year period and an on-treatment period. The on-treatment period was defined as the time from 1 day after the index date until the end of persistent exposure to the drug, allowing a 7-day gap or the end of a patient’s record. Third, in every analysis, we added sensitivity analyses with a “blanking period” rule that excluded the occurrence of the outcome within the initial 28 days after PCI because such early outcomes may reflect duplicated diagnoses, either due to transfer between hospitals or clinical coding practices.20
Fourth, since a diagnosis code–based outcome definition may include false-positive cases, we applied a restricted definition of outcome that only considered primary diagnoses. Fifth, we conducted an additional analysis to apply an identical study period to all databases, namely March 2013 and December 2016, because ticagrelor was covered by national insurance in South Korea from March 2013. Sixth, as a post hoc analysis and since the individual events in NACE are not equivalent, we calculated the weighted incidence of NACE, weighted by the HR of individual events, for 1-year mortality after an event relative to death after ischemic stroke (eMethod 4 in Supplement 1).
In addition, we employed a total of 96 falsification end points using a data-rich algorithm to quantify systematic error (eMethod 5 in Supplement 1).21,22 These outcomes are not known to be related to either ticagrelor or clopidogrel (eg, chalazion and pneumothorax). We performed empirical calibration of the CIs by fitting an empirical null distribution to point estimates of these falsification end points.23 Overall, 144 different analyses (3 statistical approaches × 3 time windows × 2 blanking period rules × 2 outcome definitions × 2 study periods × 2 empirical calibrations) were performed for each outcome.
To assess the association of ticagrelor vs clopidogrel with clinical outcomes in the United States, we also performed a post hoc meta-analysis that was restricted to the 2 US databases. To measure adherence, the medication possession ratio was calculated by summing days supplied of the allocated drug divided by days in the time-at-risk period (ie, a value of 1 indicates 100% adherence) using the HIRA database, which provides the complete longitudinal drug usage history after the index date.24 We compared the incidences of individual ischemic or hemorrhagic outcomes in the ticagrelor group from our study with those from the recent head-to-head randomized trial (TICA-KOREA).25 A prespecified 2-sided P value of less than .05 was considered to indicate statistical significance. Because of the potential for type 1 error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory. All analyses were performed using R programming language version 3.5.1. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Results
Cohort Characteristics
A total of 183 579 patients across the 3 databases were included in the analysis (43 578 in the ticagrelor group [31 197 person-years of follow-up]; and 140 001 in the clopidogrel group [110 939 person-years of follow-up]) (Figure 1). The proportion of patients in the ticagrelor group in the entire study population consistently increased in all 3 databases over time, such that it reached 32.0% in South Korea by 2016 and 43.0% in the 2 US databases by 2018 (eFigure 1 in Supplement 1).
Figure 1. Study Flowchart of Patients With Acute Coronary Syndrome Who Underwent Percutaneous Coronary Intervention and Received Ticagrelor or Clopidogrel.
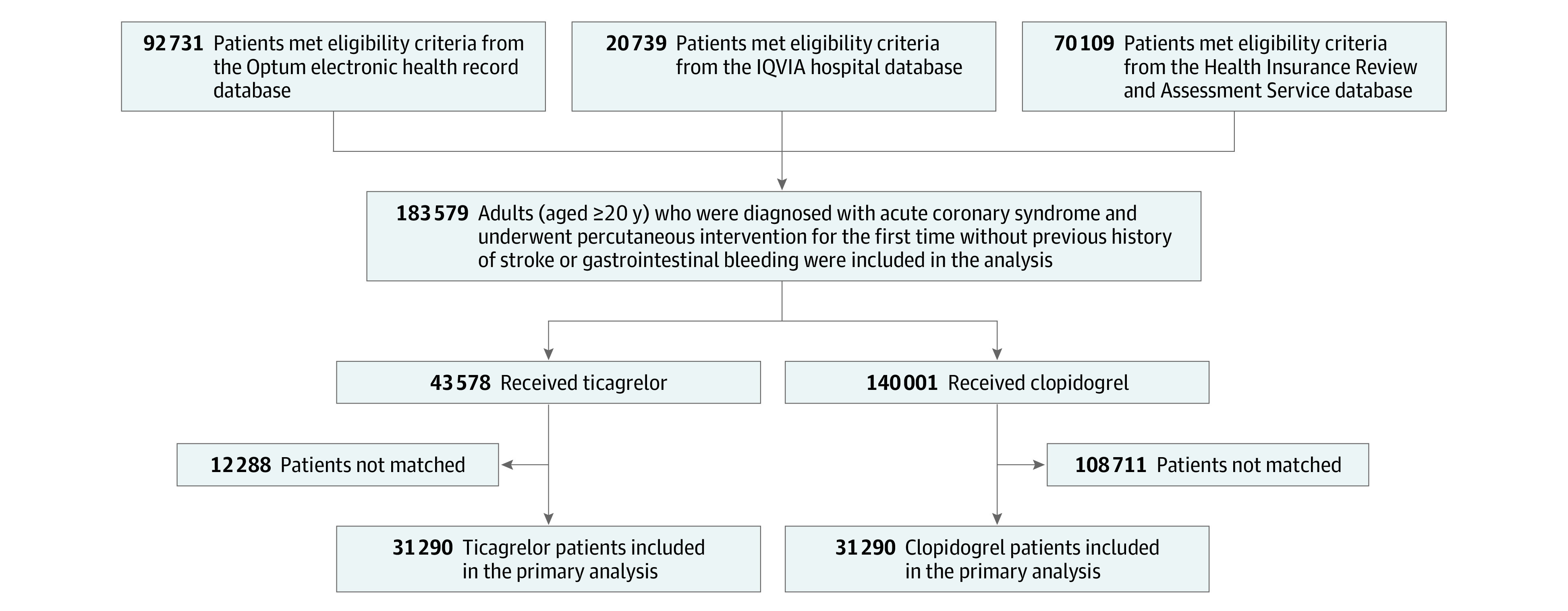
The baseline characteristics of the overall study population before and after propensity score matching are reported in the Table. The baseline characteristics before and after propensity score matching in each database are presented online (eTables 1-3 in Supplement 1). After propensity score matching, among 31 290 propensity-matched pairs, the absolute standardized differences for all the baseline patient characteristics between the ticagrelor and clopidogrel users were less than 0.1 within each data source (eFigure 2 in Supplement 1). The aggregated patient cohort size, follow-up duration, incidences of NACE, and minimum detectable relative risk before and after propensity score matching in the 3 databases are shown in eTable 4 in Supplement 1. Among matched patients, 95.5% of the study population used aspirin together with ticagrelor or clopidogrel. At 1 year after the index date, mean medication possession ratios were 0.59 (interquartile range [IQR], 0.13-1.00) for patients who received ticagrelor and 0.77 (IQR, 0.55-1.00) for patients who received clopidogrel in the HIRA database (eTable 5 in Supplement 1).
Table. Baseline Characteristics of Patients With Acute Coronary Syndrome Who Underwent Percutaneous Coronary Intervention and Received Ticagrelor or Clopidogrel.
| Before propensity score matchinga | After propensity score matchinga | |||||
|---|---|---|---|---|---|---|
| Ticagrelor (n = 43 578)b | Clopidogrel (n = 140 001)b | Standardized difference | Ticagrelor (n = 31 290)b | Clopidogrel (n = 31 290)b | Standardized difference | |
| Age group, %c | ||||||
| 30-34 | 0.5 | 0.3 | 0.03 | 0.5 | 0.5 | 0.01 |
| 35-39 | 1.7 | 1.0 | 0.05 | 1.5 | 1.6 | 0.01 |
| 40-44 | 4.1 | 2.6 | 0.08 | 3.6 | 3.6 | <0.01 |
| 45-49 | 7.6 | 5.2 | 0.10 | 6.9 | 7.0 | <0.01 |
| 50-54 | 12.0 | 9.1 | 0.09 | 11.0 | 11.1 | <0.01 |
| 55-59 | 15.7 | 12.5 | 0.09 | 14.9 | 14.6 | 0.01 |
| 60-64 | 15.8 | 13.9 | 0.05 | 15.3 | 15.7 | 0.01 |
| 65-69 | 13.7 | 14.3 | 0.02 | 14.1 | 14.1 | <0.01 |
| 70-74 | 11.7 | 14.3 | 0.08 | 12.6 | 12.4 | 0.01 |
| 75-79 | 9.0 | 12.3 | 0.11 | 9.8 | 9.9 | <0.01 |
| 80-84 | 5.8 | 10.5 | 0.17 | 6.9 | 6.6 | 0.01 |
| 85-89 | 2.2 | 3.5 | 0.08 | 2.6 | 2.7 | <0.01 |
| Sex, %d | ||||||
| Men | 72.2 | 66.6 | 0.12 | 70.6 | 70.8 | <0.01 |
| Women | 27.7 | 33.4 | 0.12 | 29.4 | 29.2 | <0.01 |
| Medical history, %e | ||||||
| Essential hypertension | 71.4 | 75.5 | 0.09 | 72.5 | 72.2 | 0.01 |
| Hyperlipidemia | 67.2 | 70.6 | 0.07 | 68.0 | 67.5 | 0.01 |
| Congestive heart failure | 10.9 | 15.3 | 0.13 | 11.8 | 11.7 | <0.01 |
| Type 2 diabetes | 9.6 | 8.8 | 0.03 | 9.9 | 9.8 | <0.01 |
| Type 1 diabetes | 0.6 | 0.6 | <0.01 | 0.7 | 0.6 | 0.01 |
| Peripheral arterial occlusive disease | 0.6 | 0.9 | 0.04 | 0.7 | 0.6 | 0.01 |
| Kidney failure syndrome | 0.5 | 1.0 | 0.05 | 0.6 | 0.7 | <0.01 |
| Persistent atrial fibrillation | 0.4 | 0.6 | 0.03 | 0.4 | 0.6 | 0.02 |
| Medication use, %f | ||||||
| Aspirin | 95.8 | 95.8 | <0.01 | 95.5 | 95.5 | <0.01 |
| Statins | 91.3 | 89.2 | 0.07 | 90.6 | 90.8 | 0.01 |
| β-Blockers | 80.5 | 79.4 | 0.03 | 80.5 | 80.8 | 0.01 |
| Calcium channel blockers | 51.8 | 52.0 | <0.01 | 51.6 | 51.8 | <0.01 |
| ACE inhibitors | 41.9 | 40.3 | 0.03 | 41.4 | 41.4 | <0.01 |
| Proton pump inhibitors | 36.5 | 37.0 | 0.01 | 36.7 | 36.9 | <0.01 |
| Diuretics | 30.5 | 36.5 | 0.13 | 31.3 | 30.9 | 0.01 |
| Angiotensin II antagonists | 23.1 | 26.4 | 0.08 | 24.0 | 23.8 | 0.01 |
| Insulins and analogues | 20.0 | 22.2 | 0.05 | 20.5 | 20.2 | 0.01 |
| Blood glucose–lowering drugs, except insulins | 18.2 | 21.4 | 0.08 | 19.0 | 18.4 | 0.02 |
| Abciximab | 7.4 | 4.4 | 0.13 | 5.6 | 6.1 | 0.02 |
| Warfarin | 1.5 | 3.8 | 0.15 | 1.9 | 1.9 | 0.01 |
| Apixaban | 0.7 | 0.8 | 0.01 | 0.8 | 0.9 | <0.01 |
| Dabigatran | 0.1 | 0.3 | 0.04 | 0.1 | 0.2 | 0.02 |
| Rivaroxaban | 0.0 | 0.0 | <0.01 | 0.0 | 0.0 | 0.01 |
Abbreviation: ACE, angiotensin-converting enzyme.
To account for baseline differences between patients with ticagrelor and clopidogrel, propensity score–based matching was used. Propensity scores were calculated in each database independently, based on available demographic characteristics, as well as the medical, medication, procedure, device exposure history, and baseline laboratory values of each database. Here, we reported the aggregated balance before and after matching only for limited covariates from 3 databases. The whole balance data before and after propensity score adjustment for more than 10 000 baseline covariates in each database are provided elsewhere.26
Values are presented as proportion of the patients (%) not otherwise specified.
Age groups younger than 30 years or older than 90 years were omitted.
Before matching, information for sex was not available for 0.1% of patients in the ticagrelor group.
Medical history was identified by coded medical diagnosis within 1 year prior to the catheterization.
Medication use was identified by medication records within 7 days of catheterization. Both anatomical therapeutic chemical class-level and ingredient-level drug uses were used to fit the propensity score model. We reported ingredient-level balances for antithrombotic agents, with class-level balances for other drugs before and after propensity score matching.
Primary Outcome Assessment
The survival curves for the 1-year NACE after propensity score matching are presented in Figure 2. The risk of NACE was not significantly different between the ticagrelor and clopidogrel groups in the IQVIA Hospital (HR, 1.06 [95% CI, 0.90-1.24]; P = .52) and HIRA databases (HR, 1.02 [95% CI, 0.96-1.09]; P = .50). In the Optum EHR, the risk of NACE was significantly higher in the ticagrelor group than the clopidogrel group (HR, 1.08 [95% CI, 1.00-1.17]; P = .05). Overall, 1-year incidence of NACE was 15.1% (3484/23 116 person-years) in the ticagrelor group and 14.6% (3290/22 587 person-years) in the clopidogrel group. The meta-analysis showed no significant difference in overall NACE between the ticagrelor and clopidogrel groups (summary HR, 1.05 [95% CI, 1.00-1.10]; P = .06; Figure 3).
Figure 2. Kaplan-Meier Plots for the Risks of the Primary Outcome (Net Adverse Clinical Events) Associated With Ticagrelor and Clopidogrel.
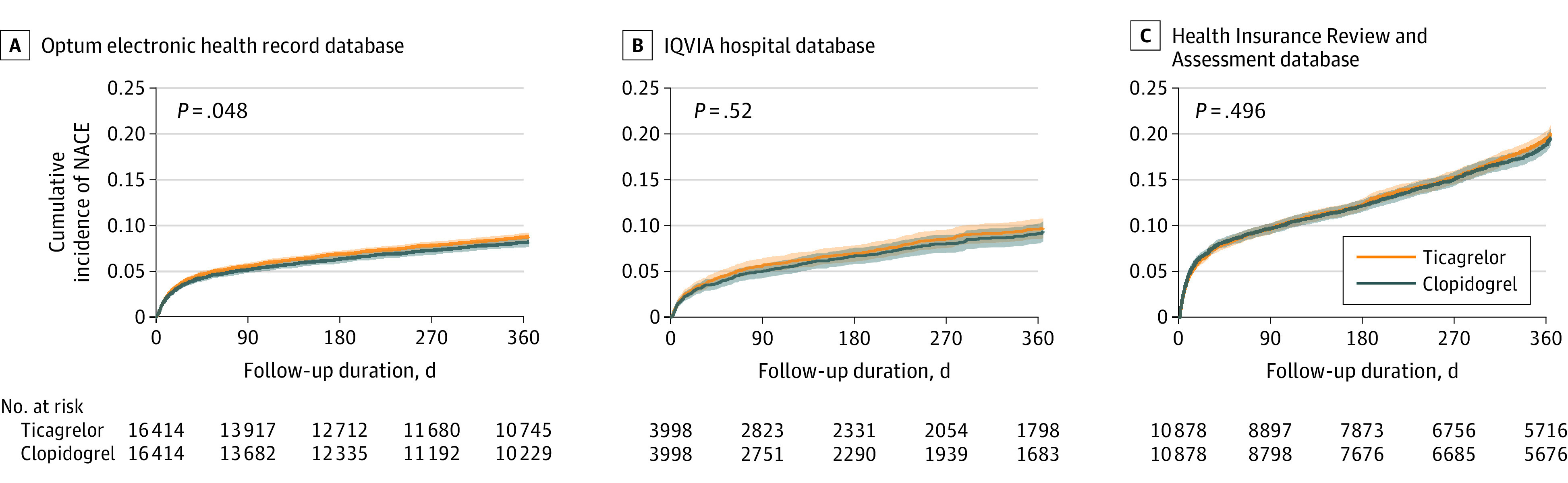
Net adverse clinical events comprise ischemic events (recurrent acute myocardial infarction, revascularization, or ischemic stroke) and hemorrhagic events (hemorrhagic stroke or gastrointestinal bleeding) in propensity score–matched cohorts from each data source. A, For the Optum electronic health record source, median follow-up was 365 days for ticagrelor patients (interquartile range [IQR], 213-365) and for clopidogrel patients, 365 days (IQR, 181-365). B, For the IQVIA hospital source, median follow-up was 285 days for ticagrelor patients (IQR, 63-365) and for clopidogrel patients, 253 days (IQR, 55-365). C, For the Health Insurance Review and Assessment Service source, median follow-up was 365 days for ticagrelor patients (IQR, 154-365) and for clopidogrel patients, 365 days (IQR, 138-365). P values in survival curves were estimated using Cox proportional hazard regression models. Shading in the survival curves indicates 95% CIs.
Figure 3. Risk of the Primary Outcome (NACE) at 1 Year.
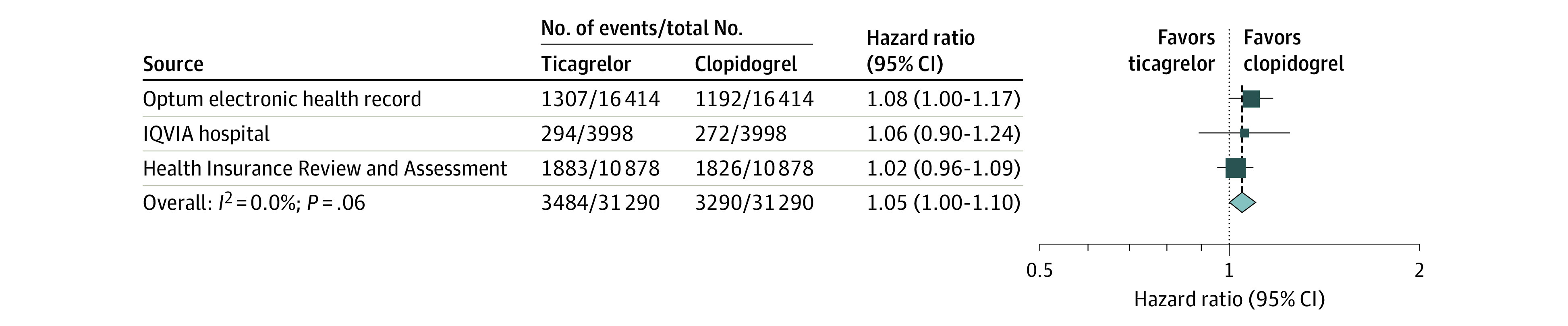
Net adverse clinical events comprise ischemic events (recurrent acute myocardial infarction, revascularization, or ischemic stroke) and hemorrhagic events (hemorrhagic stroke or gastrointestinal bleeding) in propensity score-matched cohorts from each data source. The summary hazard ratios were calculated using a random-effects model. A hazard ratio greater than 1 indicates higher risk in the ticagrelor group. The size of the data marker indicates the weight of the study. Error bars indicate 95% CIs.
Secondary Outcome Assessments
Figure 4 and Figure 5 show the meta-analyses for the secondary outcomes. There was no significant difference between ticagrelor and clopidogrel use in risk of NACE or mortality (incidence rate at 1 year, 16.8% in the ticagrelor group vs 16.6% in the clopidogrel group; summary HR, 1.03 [95% CI, 0.98-1.08]; P = .21), ischemic events (13.5% in the ticagrelor group vs 13.4% in the clopidogrel group; summary HR, 1.03 [95% CI, 0.98-1.08]; P = .32), ischemic stroke (0.7% in the ticagrelor group vs 0.8% in the clopidogrel group; summary HR, 0.93 [95% CI, 0.76-1.13]; P = .46), recurrent AMI (8.1% in the ticagrelor group vs 8.2% in the clopidogrel group; summary HR, 1.01 [95% CI, 0.94-1.08]; P = .81), revascularization (4.3% in the ticagrelor group vs 4.3% in the clopidogrel group; summary HR, 1.08 [95% CI, 0.85-1.38]; P = .51), or all-cause mortality (2.0% in the ticagrelor group vs 2.1% in the clopidogrel group; summary HR, 0.97 [95% CI, 0.81-1.16]; P = .74).
Figure 4. Risks of the Net Adverse Clinical Events or Mortality, Ischemic Event, Ischemic Stroke, Acute Myocardial Infarction, Revascularization, and Hemorrhagic Event at 1 Year.
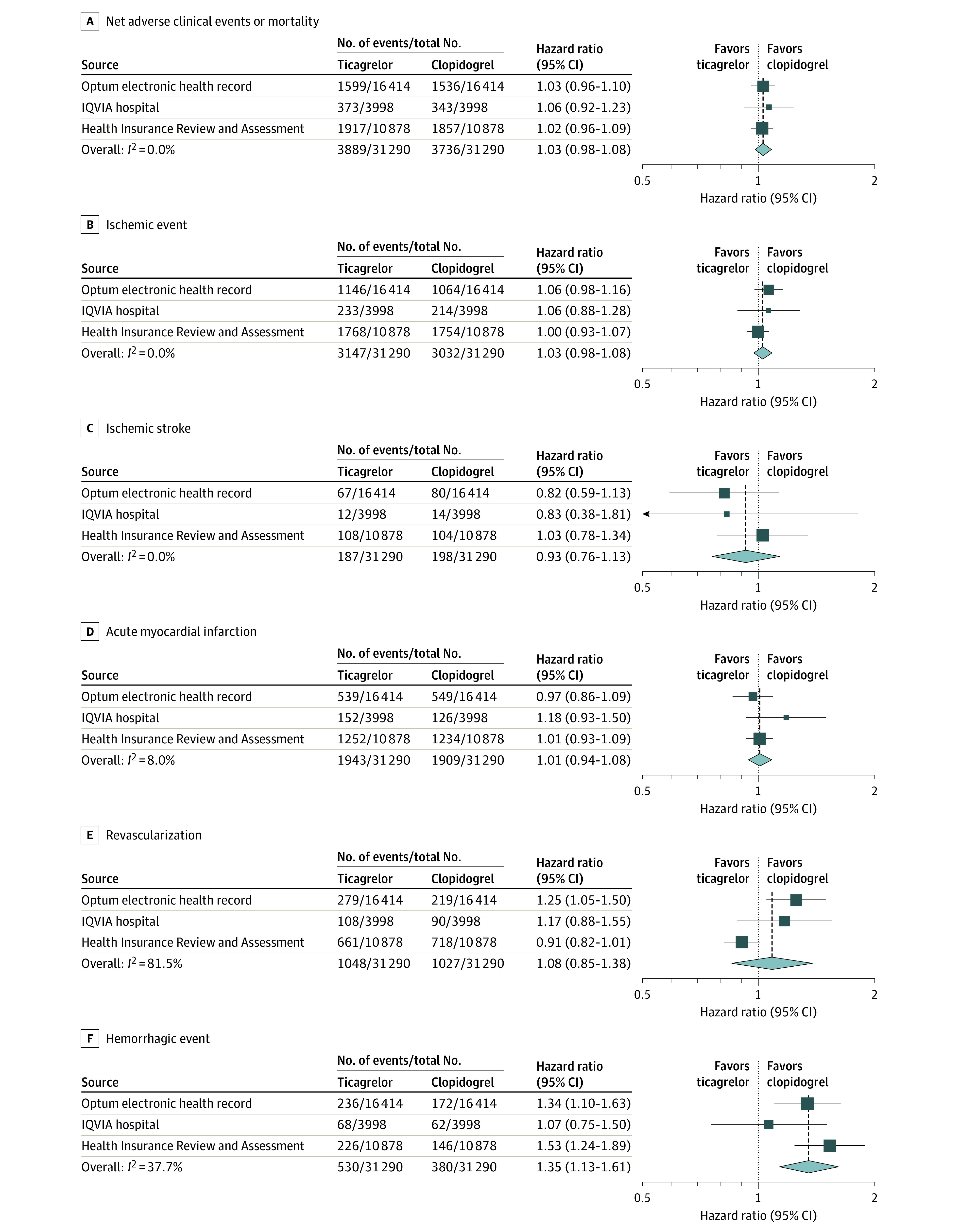
The summary hazard ratios were calculated using a random-effects model. A hazard ratio greater than 1 indicates higher risk in the ticagrelor group. The size of the data marker indicates the weight of the study. Error bars indicate 95% CIs.
Figure 5. Risks of Hemorrhagic Stroke, Gastrointestinal Bleeding, All-Cause Mortality, Cardiovascular-Related Mortality, Major Adverse Cardiovascular Events, and Dyspnea at 1 Year.
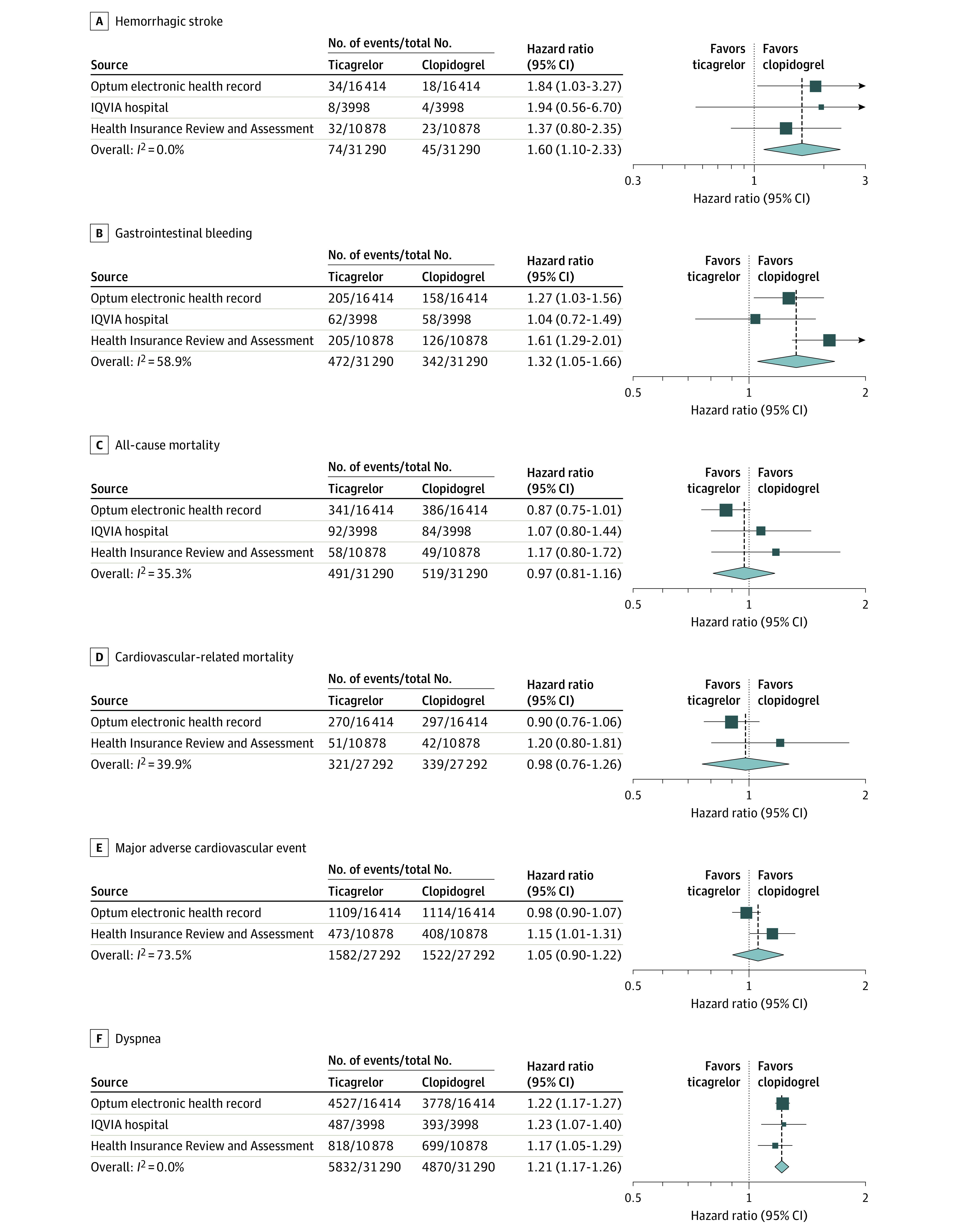
The summary hazard ratios were calculated using a random-effects model. A hazard ratio greater than 1 indicates higher risk in the ticagrelor group. The size of the data marker indicates the weight of the study. Error bars indicate 95% CIs.
Ticagrelor use, compared with clopidogrel, was associated with significantly higher risks of dyspnea (27.3% vs 22.6%; summary HR, 1.21 [95% CI, 1.17-1.26]; P < .001), hemorrhagic events (2.1% in the ticagrelor group vs 1.6% in the clopidogrel group; summary HR, 1.35 [95% CI, 1.13-1.61]; P = .001), hemorrhagic stroke (0.3% in the ticagrelor group vs 0.2% in the clopidogrel group; summary HR, 1.60 [95% CI, 1.10-2.33]; P = .01), and gastrointestinal bleeding (1.9% in the ticagrelor group vs 1.4% in the clopidogrel group; summary HR, 1.32 [95% CI, 1.05-1.66]; P = .02). The risk of cardiovascular-related mortality (1.4% for ticagrelor vs 1.5% for clopidogrel; summary HR, 0.98 [95% CI, 0.76-1.26]; P = .86) and MACE (7.3% for ticagrelor vs 7.2% for clopidogrel; summary HR, 1.05 [95% CI, 0.90-1.22]; P = .51) did not significantly differ between groups. The incidence rate differences between the ticagrelor and clopidogrel groups using random-effects meta-analysis are described for the primary and secondary outcomes in eTable 6 in Supplement 1. The incidences of individual ischemic and hemorrhage events in the ticagrelor group are comparable with those from the TICA-KOREA trial (eTable 7 in Supplement 1).
Falsification End Point Analyses and Sensitivity Analyses
In the analyses of falsification end points, 95.2% (80/84) of the nominal 95% CIs covered 1 (eFigure 3 in Supplement 1). In on-treatment analysis, the risk of NACE was not significantly different between the ticagrelor and clopidogrel groups (summary HR, 1.00 [95% CI, 0.93-1.08]; P = .90). Across various statistical approaches, different risk windows, and clinical outcome definitions or the blanking period rules, ticagrelor use was not associated with significantly different risk of NACE compared with clopidogrel (eFigure 4 in Supplement 1). This finding was robust after empirical calibration of CIs (eFigure 5 in Supplement 1). The results, using limited cohort study year (eFigure 6 in Supplement 1), were similar. The overall distribution of the point estimates for the primary outcome from the 144 different analyses is shown in eFigure 7 in Supplement 1. In the meta-analysis conducted using only the US databases, the risk of NACE was significantly higher in the ticagrelor group than the clopidogrel group (summary HR, 1.08 [95% CI, 1.00-1.16]; P = .04], eFigure 8 in Supplement 1). The weighted incidences of NACE were 461.8 per 1000 person-years in the ticagrelor group and 374.0 per 1000 person-years in the clopidogrel group (eMethod 4 in Supplement 1).
Ticagrelor use was not associated with a different risk of ischemic events compared with clopidogrel in sensitivity analyses. However, many sensitivity analyses showed a higher risk of hemorrhagic events in the ticagrelor group (eFigures 9-17 in Supplement 1).
Discussion
In this retrospective cohort study, the use of ticagrelor, compared with clopidogrel, was not associated with a statistically significantly different risk of NACE in patients with ACS who underwent PCI. Ticagrelor was associated with higher risks for hemorrhagic events and dyspnea than clopidogrel. There was no significant difference in risks for ischemic events or MACE between the ticagrelor and clopidogrel groups. These results were consistent across the health databases and in numerous analyses using different statistical approaches and time windows.
The findings are consistent with those from 2 relatively small clinical trials of patients in East Asia25,27 and recent clinical trials of older patients in Europe28 but different from the overall results of PLATO, the single large pivotal trial comparing ticagrelor vs clopidogrel. However, the finding is consistent with the result of North America PLATO, as there was a significant interaction by region of enrollment.8 Also, findings from the TICA-KOREA25 and PHILO27 trials indicated an increased hemorrhagic risk with ticagrelor compared with clopidogrel, without a protective effect to reduce ischemic events in South Korea, Japan, and Taiwan. In the recent open-label Clopidogrel vs Ticagrelor or Prasugrel in Patients Aged 70 Years or Older with Non-ST-Elevation Acute Coronary Syndrome (POPular-AGE) trial, ticagrelor led to more bleeding events without superior net clinical benefit than clopidogrel in elderly Dutch patients.28 Observational studies have reported lower risk of cardiovascular outcomes in the ticagrelor group compared with the clopidogrel group20 or no significant difference.11,29,30 Nonetheless, each of these observational studies was confined to a single country and produced without assessment of residual bias by using falsification end points. To our knowledge, the association between use of ticagrelor compared with clopidogrel and ischemic and hemorrhagic outcomes has not been evaluated for more than 50 000 patients across 2 continents under comprehensive analyses with identical study protocols.
There are several possible explanations for the lack of a significant difference in risk of NACE for patients treated with ticagrelor compared with clopidogrel. Owing to the observational nature of the study, residual confounding might have produced this finding. However, this study performed empirical calibration using falsification end points to measure and reduce the degree of systematic error, which demonstrated the low degree of residual bias. The drug adherence in the ticagrelor group was lower than in the clopidogrel group. Nonadherence with the use of antiplatelet agents is a well-known factor associated with higher incidences of all-cause mortality, cardiovascular mortality, AMI, unplanned revascularization,31 and even hemorrhagic events.7 Low adherence with ticagrelor, compared with other agents, has been consistently reported in recent randomized clinical trials, mostly because of nonserious adverse events such as dyspnea or nonmajor bleeding.9,25,28,32,33 In routine clinical practice, twice-daily dosing and costs also may be important factors for nonadherence.7 In the Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction (PRAGUE)-18 trial, approximately 45% of patients assigned to ticagrelor switched to clopidogrel for economic reasons, mostly within 8 days.34 Dayoub et al7 argued that increased prescription of newer, more expensive P2Y12 inhibitors in clinical practice has reduced the overall adherence of P2Y12 inhibitors after PCI. Furthermore, it has exacerbated socioeconomic health disparities because adherence disproportionately affects the most economically disadvantaged patients, even among the insured population in the United States.7
The overall improvement in the clinical outcomes of patients with coronary diseases35 may be another possible explanation for the diminished benefit of ticagrelor in the modern era; in particular, this may be driven by progress in the use of drug-eluting stents36 and poststenting care. More than 60% of the study population in PLATO, who were treated using PCI, received bare metal stents, and most of the remaining patients received first-generation drug-eluting stents.4 The recent clinical trials, which reported comparable thrombotic event rates between the ticagrelor and clopidogrel groups, mostly used second-generation drug-eluting stents,28,32 which might have reduced the need for a stronger P2Y12 inhibitor. In addition, it may be that despite the finding from a single trial, ticagrelor is not more effective than clopidogrel.
Limitations
This study has several limitations. First, detailed information for dosage of aspirin, angiographic findings, or PCI procedure were not included. The unmeasured baseline characteristics may lead to residual confounding such as confounding by indication in the observational study. However, this study applied the large-scale propensity score model, which has low sensitivity to balancing on unmeasured baseline characteristics.14 Consistency in the results was also identified from the large set of sensitivity analyses and low degree of systematic error by using falsification end points as described previously. Second, since this study only included 2 bleeding events in the definition of hemorrhagic event, without assessment of clinical significance, the burden of overall bleeding events can be underestimated or overestimated. The incidence of each of the ischemic or hemorrhagic events in this study was comparable with the results from the TICA-KOREA trial. Furthermore, consistent findings were demonstrated across various sensitivity analyses with blanking period or stricter outcome definition. Third, it is not uncommon in routine clinical practice for patients to change their antiplatelet drug after PCI.37,38 This study did not quantify the proportion of patients who switched drugs nor the effects of this. However, a sensitivity analysis was conducted using the on-treatment risk window, the results of which were consistent with those of the primary analysis. Furthermore, a previous observational study showed that switching from ticagrelor to clopidogrel was not associated with a higher risk of ischemic events.38 Fourth, this study did not assess the effect of pretreatment with an antiplatelet agent. However, it has been reported that pretreatment with an antiplatelet agent does not significantly affect outcome.39 Fifth, the mortality rate might be underestimated, since only hospital-reported mortality was recorded. Sixth, the data to calculate 1-year adherence to use of the medication were available only from the Korean HIRA database. Seventh, this study did not include patients who used prasugrel for dual antiplatelet therapy. Since a recent large clinical trial (ISAR-REACT 5) reported that ticagrelor was less effective than prasugrel,40 the comparative effectiveness of prasugrel vs ticagrelor or clopidogrel should be investigated in future research.
Conclusions
Among patients with ACS who underwent PCI in routine clinical practice, ticagrelor, compared with clopidogrel, was not associated with significant difference in the risk of NACE at 12 months. Because the possibility of unmeasured confounders cannot be excluded, further research is needed to determine whether ticagrelor is more effective than clopidogrel in this setting.
eMethod 1. Data Source
eMethod 2. Cohort Definitions
eMethod 3. Individual Outcome Definitions
eMethod 4. Weighted Incidence of Net Adverse Clinical Event
eMethod 5. Falsification Endpoints
eTable 1. Baseline Characteristics of the Optum EHR Database
eTable 2. Baseline Characteristics of the IQVIA Hospital Database
eTable 3. Baseline Characteristics of the HIRA Database
eTable 4. Patient Cohort Sizes, Primary Endpoint Events, Incidence Rates, and Minimum Detectable Relative Risk
eTable 5. Drug Adherence After the Index Date in the HIRA Database
eTable 6. Incidence Rate Difference Using Random-Effects Meta-Analysis
eTable 7. Incidence Rates of Secondary Endpoint Events at One Year in the Tacagrelor Groups From This Study and TICA-KOREA
eFigure 1. Proportion of Ticagrelor Group Among the Whole Study Population, 2011-2019
eFigure 2. Covariate Balance Plot Before and After Propensity Score Matching
eFigure 3. Systematic Error Control of Effect Estimation in the Meta-Analysis Comparing the Risk of Net Adverse Clinical Event Between the Ticagrelor and Clopidogrel Group Under One-Year, 1-to-1 Propensity Score Matching Design
eFigure 4. Sensitivity Analyses for Risks of the Primary Outcome (NACE) Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 5. Risks of NACE Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings After Empirical Calibration
eFigure 6. Risks of NACE Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings From 2013 to 2015
eFigure 7. Distribution of Risk Estimates for NACE From 144 Analyses Before and After Empirical Calibration
eFigure 8. Meta-Analysis Results Using Only US Databases
eFigure 9. Sensitivity Analyses for Risks of Ischemic Event (Recurrent Acute Myocardial Infarction, Revascularization, or Ischemic Stroke) and Hemorrhagic Event (Hemorrhagic Stroke or Gastrointestinal Bleeding) Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 10. Risks of Ischemic Stroke Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 11. Risks of Recurrent Acute Myocardial Infarction Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 12. Risks of Revascularization Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 13. Risks of Hemorrhagic Stroke Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 14. Risks of GI Bleeding Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 15. Risks of All-Cause Mortality Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 16. Risks of Cardiovascular-Related Mortality Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 17. Risks of Major Adverse Cardiovascular Event Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eReferences.
Trial Protocol
References
- 1.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577-2585. doi: 10.1161/CIRCULATIONAHA.109.912550 [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. doi: 10.1016/j.jacc.2016.03.513 [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 5.Yudi MB, Clark DJ, Farouque O, et al. ; Melbourne Interventional Group . Clopidogrel, prasugrel or ticagrelor in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Intern Med J. 2016;46(5):559-565. doi: 10.1111/imj.13041 [DOI] [PubMed] [Google Scholar]
- 6.Sahlén A, Varenhorst C, Lagerqvist B, et al. Contemporary use of ticagrelor in patients with acute coronary syndrome: insights from Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Eur Heart J Cardiovasc Pharmacother. 2016;2(1):5-12. doi: 10.1093/ehjcvp/pvv034 [DOI] [PubMed] [Google Scholar]
- 7.Dayoub EJ, Seigerman M, Tuteja S, et al. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008-2016. JAMA Intern Med. 2018;178(7):943-950. doi: 10.1001/jamainternmed.2018.0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahaffey KW, Wojdyla DM, Carroll K, et al. ; PLATO Investigators . Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124(5):544-554. doi: 10.1161/CIRCULATIONAHA.111.047498 [DOI] [PubMed] [Google Scholar]
- 9.Bonaca MP, Bhatt DL, Oude Ophuis T, et al. Long-term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS-TIMI 54 trial. JAMA Cardiol. 2016;1(4):425-432. doi: 10.1001/jamacardio.2016.1017 [DOI] [PubMed] [Google Scholar]
- 10.Johnston N, Weinman J, Ashworth L, Smethurst P, El Khoury J, Moloney C. Systematic reviews: causes of non-adherence to P2Y12 inhibitors in acute coronary syndromes and response to intervention. Open Heart. 2016;3(2):e000479. doi: 10.1136/openhrt-2016-000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgeon RD, Koshman SL, Youngson E, et al. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern Med. 2020;180(3):420-428. doi: 10.1001/jamainternmed.2019.6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574-578. [PMC free article] [PubMed] [Google Scholar]
- 13.Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19(1):54-60. doi: 10.1136/amiajnl-2011-000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394(10211):1816-1826. doi: 10.1016/S0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You SC. GitHub website. OHDSI network study: net adverse clinical event between ticagrelor and clopidogrel in patients with acute coronary syndrome. Accessed October 6, 2020. https://github.com/ohdsi-studies/TicagrelorVsClopidogrel
- 16.Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23(1). doi: 10.1145/2414416.2414791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005-2014. doi: 10.1093/ije/dyy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.Sahlén A, Varenhorst C, Lagerqvist B, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37(44):3335-3342. doi: 10.1093/eurheartj/ehw284 [DOI] [PubMed] [Google Scholar]
- 21.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72-81. doi: 10.1016/j.jbi.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct P values. Stat Med. 2014;33(2):209-218. doi: 10.1002/sim.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 25.Park DW, Kwon O, Jang JS, et al. ; TICAKOREA Investigators . Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140(23):1865-1877. doi: 10.1161/CIRCULATIONAHA.119.041766 [DOI] [PubMed] [Google Scholar]
- 26.You SC. GitHub website. OHDSI network study: whole balance data before and after propensity score adjustment for more than 10 000 baseline covariates in each database. Accessed October 6, 2020. https://github.com/OHDSI/ShinyDeploy/tree/master/TicagrelorVsClopidogrel/data
- 27.Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome—randomized, double-blind, phase III PHILO study. Circ J. 2015;79(11):2452-2460. doi: 10.1253/circj.CJ-15-0112 [DOI] [PubMed] [Google Scholar]
- 28.Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395(10233):1374-1381. doi: 10.1016/S0140-6736(20)30325-1 [DOI] [PubMed] [Google Scholar]
- 29.Zocca P, Kok MM, van der Heijden LC, et al. High bleeding risk patients with acute coronary syndromes treated with contemporary drug-eluting stents and clopidogrel or ticagrelor: insights from change DAPT. Int J Cardiol. 2018;268:11-17. doi: 10.1016/j.ijcard.2018.03.116 [DOI] [PubMed] [Google Scholar]
- 30.Wang HY, Li Y, Xu XM, Li J, Han YL. Impact of baseline bleeding risk on efficacy and safety of ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin Med J (Engl). 2018;131(17):2017-2024. doi: 10.4103/0366-6999.239306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews R, Peterson ED, Honeycutt E, et al. Early medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study. Circ Cardiovasc Qual Outcomes. 2015;8(4):347-356. doi: 10.1161/CIRCOUTCOMES.114.001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078. doi: 10.1093/eurheartj/ehx175 [DOI] [PubMed] [Google Scholar]
- 33.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 34.Motovska Z, Hlinomaz O, Kala P, et al. ; PRAGUE-18 Study Group . 1-year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. J Am Coll Cardiol. 2018;71(4):371-381. doi: 10.1016/j.jacc.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 35.Simonsson M, Wallentin L, Alfredsson J, et al. Temporal trends in bleeding events in acute myocardial infarction: insights from the SWEDEHEART registry. Eur Heart J. 2020;41(7):833-843. doi: 10.1093/eurheartj/ehz593 [DOI] [PubMed] [Google Scholar]
- 36.Piccolo R, Bonaa KH, Efthimiou O, et al. ; Coronary Stent Trialists’ Collaboration . Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393(10190):2503-2510. doi: 10.1016/S0140-6736(19)30474-X [DOI] [PubMed] [Google Scholar]
- 37.Angiolillo DJ, Patti G, Chan KT, et al. De-escalation from ticagrelor to clopidogrel in acute coronary syndrome patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2019;48(1):1-10. doi: 10.1007/s11239-019-01860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biscaglia S, Campo G, Pavasini R, Tebaldi M, Tumscitz C, Ferrari R. Occurrence, causes, and outcome after switching from ticagrelor to clopidogrel in a real-life scenario: data from a prospective registry. Platelets. 2016;27(5):484-487. doi: 10.3109/09537104.2015.1119815 [DOI] [PubMed] [Google Scholar]
- 39.Effron MB, Wang TY, Fonarow GC, et al. The safety and effectiveness of adenosine diphosphate receptor inhibitor pretreatment among acute myocardial infarction patients treated with percutaneous coronary intervention in community practice: insights from the TRANSLATE-ACS study. Catheter Cardiovasc Interv. 2018;91(2):242-250. doi: 10.1002/ccd.27145 [DOI] [PubMed] [Google Scholar]
- 40.Schüpke S, Neumann F-J, Menichelli M, et al. ; ISAR-REACT 5 Trial Investigators . Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524-1534. doi: 10.1056/NEJMoa1908973 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethod 1. Data Source
eMethod 2. Cohort Definitions
eMethod 3. Individual Outcome Definitions
eMethod 4. Weighted Incidence of Net Adverse Clinical Event
eMethod 5. Falsification Endpoints
eTable 1. Baseline Characteristics of the Optum EHR Database
eTable 2. Baseline Characteristics of the IQVIA Hospital Database
eTable 3. Baseline Characteristics of the HIRA Database
eTable 4. Patient Cohort Sizes, Primary Endpoint Events, Incidence Rates, and Minimum Detectable Relative Risk
eTable 5. Drug Adherence After the Index Date in the HIRA Database
eTable 6. Incidence Rate Difference Using Random-Effects Meta-Analysis
eTable 7. Incidence Rates of Secondary Endpoint Events at One Year in the Tacagrelor Groups From This Study and TICA-KOREA
eFigure 1. Proportion of Ticagrelor Group Among the Whole Study Population, 2011-2019
eFigure 2. Covariate Balance Plot Before and After Propensity Score Matching
eFigure 3. Systematic Error Control of Effect Estimation in the Meta-Analysis Comparing the Risk of Net Adverse Clinical Event Between the Ticagrelor and Clopidogrel Group Under One-Year, 1-to-1 Propensity Score Matching Design
eFigure 4. Sensitivity Analyses for Risks of the Primary Outcome (NACE) Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 5. Risks of NACE Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings After Empirical Calibration
eFigure 6. Risks of NACE Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings From 2013 to 2015
eFigure 7. Distribution of Risk Estimates for NACE From 144 Analyses Before and After Empirical Calibration
eFigure 8. Meta-Analysis Results Using Only US Databases
eFigure 9. Sensitivity Analyses for Risks of Ischemic Event (Recurrent Acute Myocardial Infarction, Revascularization, or Ischemic Stroke) and Hemorrhagic Event (Hemorrhagic Stroke or Gastrointestinal Bleeding) Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 10. Risks of Ischemic Stroke Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 11. Risks of Recurrent Acute Myocardial Infarction Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 12. Risks of Revascularization Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 13. Risks of Hemorrhagic Stroke Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 14. Risks of GI Bleeding Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 15. Risks of All-Cause Mortality Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 16. Risks of Cardiovascular-Related Mortality Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eFigure 17. Risks of Major Adverse Cardiovascular Event Associated With Ticagrelor and Clopidogrel Use, Analyzed Using a Meta-Analysis and Various Time-at-Risk, Statistical, and Clinical Definition Settings
eReferences.
Trial Protocol


