Abstract
EFSA was requested to estimate the cattle bovine spongiform encephalopathy (BSE) risk (C‐, L‐ and H‐BSE) posed by ruminant collagen and gelatine produced from raw material fit for human consumption, or from material classified as Category 3 animal by‐products (ABP), to be used in feed intended for non‐ruminant animals, including aquaculture animals. Three risk pathways (RP) were identified by which cattle could be exposed to ruminant feed cross‐contaminated with ruminant collagen or gelatine: 1) recycled former foodstuffs produced in accordance with Regulation (EC) No 853/2004 (RP1), 2) technological or nutritional additives or 3) compound feed, produced either in accordance with Regulation (EC) No 853/2004 (RP2a) or Regulation (EU) No 142/2011 (RP2b). A probabilistic model was developed to estimate the BSE infectivity load measured in cattle oral ID 50 (CoID 50)/kg, in the gelatine produced from the bones and hide of one infected animal older than 30 months with clinical BSE (worst‐case scenario). The amount of BSE infectivity (50th percentile estimate) in a member state (MS) with negligible risk status was 7.6 × 10–2 CoID 50/kg, and 3.1 × 10–4 CoID 50/kg in a MS with controlled risk status. The assessment considered the potential contamination pathways and the model results (including uncertainties) regarding the current epidemiological situation in the EU and current statutory controls. Given the estimated amount of BSE infectivity to which cattle would be exposed in a single year, and even if all the estimated undetected BSE cases in the EU were used for the production of collagen or gelatine (either using raw materials fit for human consumption or Category 3 ABP raw materials), it was concluded that the probability that no new case of BSE in the cattle population would be generated through any of the three RP is 99–100% (almost certain).
Keywords: BSE, collagen, feed, gelatine, risk, ruminants
Summary
In May 2019, the European Food Safety Authority (EFSA) was asked by the European Commission to deliver a scientific opinion on two Terms of Reference (ToRs). ToR1 required an estimate of the cattle bovine spongiform encephalopathy (BSE) risk (C‐, L‐ and H‐BSE) posed by the use of ruminant collagen/gelatine (C&G) produced in accordance with Section XIV and XV of Annex III to Regulation (EC) No 853/2004 in feed intended for non‐ruminant animals including aquaculture animals. ToR2 required an estimate of the cattle BSE risk (C‐, L‐ and H‐BSE) posed by the use of ruminant C&G classified as Category 3 as referred to in Article 10 of Regulation (EC) No 1069/2009 and produced in accordance with Regulation (EU) No 142/2011 for feed intended for non‐ruminant animals including aquaculture animals.
Four assessment questions (AQ) were agreed to address the ToRs. AQ1 – What are the risk pathways for cattle from the use of ruminant C&G in feed for non‐ruminant animals? AQ2 – What is the amount of infectivity in C&G produced from an infected animal at clinical stage? AQ3 – How many infected animals have to be processed and how many kg of C&G have to be produced from infected animals to accumulate 1 cattle oral infectious dose 50 (CoID50)? AQ4 – What is the residual BSE infectivity in the feed at the end of the risk pathways?
A probabilistic model was developed to answer the ToRs. The model estimates the BSE infectivity load, measured in CoID50, contained in the gelatine produced with the bones and hide of one adult animal (i.e. older than 30 months of age), infected with any of the three BSE strains (C, H and L), and at the clinical stage of the disease. The model represents the initial steps in the production chain of gelatine from the point at which an infected animal is slaughtered to the production of gelatine from its bones and hide. Some of the assumptions of the model correspond to worst‐case scenarios and, when data were available, the boundaries of probability distributions in the parameters describing uncertainty were selected to approximate to worst‐case scenarios. As a result, the probability that the infectivity is overestimated by the model is 99–100% (almost certain). The availability of more accurate data on the production of gelatine in terms of yield, infectivity reduction factors and processing methods led to the decision to only develop the model for the production of gelatine. However, some additional scenarios were run for comparison with the production of collagen, applying some assumptions of what is known about collagen production in the European Union (EU).
There are three potential risk pathways associated with the use of ruminant C&G, either produced from animals fit for human consumption or from ABP materials, in feed intended for non‐ruminant animals, including aquaculture animals. Risk pathway 1: C&G produced under Regulation (EC) No 853/2004 and used in former foodstuffs which are then recycled to produce a ‘bread meal’ for animal feed (relevant to ToR1). Risk pathway 2a: C&G produced under Regulation (EC) No 853/2004 and used in feed as technological additive (to encapsulate vitamins) or nutritional additive (supplement for dogs and horses), or as a component of compound feed (relevant to ToR1). Risk pathway 2b: C&G produced under Regulation (EU) No 142/2011 and used in feed as a technological additive (to encapsulate vitamins) or nutritional additive (supplement for dogs and horses), or as a component of compound feed (relevant to ToR2).
According to the results of the model, the 50th percentile of the amount of BSE infectivity per kg of gelatine extracted from the bones and hide of one adult animal with clinical BSE (C, H or L), slaughtered in a MS with negligible risk status, is 7.6 × 10−2 CoID50 (5th–95th percentile: 8 × 10−3–0.8 CoID50), and 3.1 × 10−4 CoID50 (5th–95th percentile: 2.9 × 10−5–4.1 × 10−3 CoID50), in a MS with controlled risk status. For collagen, the estimated amount of infectivity per kg was much lower than for gelatine, i.e. 1.3 × 10−6 (5th–95th percentile: 1.1 × 10−7–1.6 × 10−5 CoID50/kg) in both negligible and controlled risk countries. As a worst‐case scenario, if there was no reduction of BSE infectivity during collagen production, the final amount of infectivity per kg of collagen would be 5 × 10−3 (5th–95th percentile: 3.5 × 10−3–1.8 × 10−2 CoID50/kg), which is still more than 15 times lower than the estimate obtained for gelatine using bones and hides and with a reduction of infectivity during processing in a MS with negligible risk.
The 50th percentile of the number of adult animals with clinical BSE (C, H or L) slaughtered in a MS with negligible risk status that would be required to produce contaminated gelatine containing 1 CoID50, is 1.7 (5th–95th percentile: 0.1–16 infected animals), and 449.8 (5th–95th percentile: 33.8–4,745 infected animals) in a MS with controlled risk status. The 50th percentile of the amount of contaminated gelatine extracted from adult animals with clinical BSE (C, H or L) at clinical stage slaughtered in a MS with negligible risk status that would contain 1 CoID50 is 13.1 kg (5th–95th percentile: 1.2–125.3 kg), and 3,257 (5th–95th percentile: 244.9–34,360 kg) in a MS with controlled risk status. Considering all possible undetected BSE cases in the EU in one single year, estimated by the Cattle TSE Monitoring Model (C‐TSEMM), and the 50th percentile of the total amount of BSE infectivity estimated by the model, the cattle population of the EU would be exposed on average to up to 1.5 × 10−7 CoID50 per animal and per year if all infected animals were slaughtered in MS with negligible risk status and to 6.1 × 10−10 CoID50 if all the infected animals were slaughtered in MS with controlled risk status.
The uncertainties and data gaps in the three risk pathways precluded the execution of a full quantitative assessment of the risk posed through each of the risk pathways. The characterisation of the risk concluded that the probabilities that material from more than one positive animal could be included in the manufacture of any batch of collagen or gelatine and the contamination of ruminant feed with non‐ruminant feed containing C&G on‐farm are both 1–5% (extremely unlikely). Moreover, the final BSE risk to cattle in the three risk pathways is further reduced by additional factors such as the temporal (due to lack of clustering of cases, no multiple cases are slaughtered at the same time) and geographical (the small number of BSE cases that have recently been reported in the EU have been distributed across several countries) distribution of the exposure to any amount of infected material, and the individual host response to exposure. Cattle could be exposed via ruminant feed cross‐contaminated with non‐ruminant feed containing ruminant C&G from a BSE‐infected batch, or by accidental error accessing the wrong feed on farm.
It was concluded that the probability that no new case of BSE in the cattle population would be generated through any of the three risk pathways is 99–100% (almost certain), given the estimated amount of BSE infectivity to which cattle would be exposed.
A number of recommendations have been proposed: a) to maintain the EU‐wide current surveillance system, to periodically evaluate the new BSE data using epidemiological transmission models and to use the C‐TSEMM model on an annual basis with updated data in order to monitor the ability of the current surveillance system to detect BSE at both MS and EU level; b) to evaluate the impact of the specific industrial processes for the production of collagen and gelatine on the infectivity of naturally occurring BSE agents; c) to undertake research activities aimed at the production of new data regarding the susceptibility of cattle to infection with H‐BSE or L‐BSE via the oral route, and the quantitative distribution of infectivity in tissues of cattle preclinically and clinically affected with H‐ and L‐BSE; d) to produce new data measuring the reduction factors for cattle BSE infectivity provided by a variety of standard processing methods.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The Communication from the Commission to the European Parliament and the Council known as the Transmissible Spongiform Encephalopathies (TSE) Road Map 2, a strategy paper on TSE for 2010–2015 was adopted on 16 July 2010. It outlines areas where future possible changes to Union legislation on TSEs could be made. It also emphasises that any review of the TSE rules should be primarily driven by scientific advice and technical issues related to the control and enforcement of the new measures.
That Communication, inter alia, addresses the revision of the current feed ban rules laid down in Union legislation.
Based on the contents of two scientific opinions issued by the Panel on Biological Hazards (BIOHAZ) of the European Food Safety Authority (EFSA) on 24 January 2007 and on 17 November 2007 respectively, the Communication acknowledged that no TSE have been identified as occurring in non‐ruminant farmed animals under natural conditions. On 9 December 2010, the BIOHAZ Panel of EFSA adopted a scientific opinion on the revision of the quantitative risk assessment (QRA) of the bovine spongiform encephalopathy (BSE) risk posed by processed animal proteins (PAPs).
The QRA was updated on 7 June 2018. The updated model estimated a total BSE infectivity four times lower than that estimated in 2011, with less than one new case of BSE expected to arise each year.
European Parliament Resolutions of 8 March 2011 and of 6 July 2011 on EU legislation on TSEs, feed and food controls and the EU protein deficit, indicated that a solution for a long‐standing problem can be authorising the use of PAP declared fit for human consumption, for the production of feed for monogastric animals (pigs and poultry), provided that the ban on intra‐species recycling and forced cannibalism is fully implemented and controlled.
Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific rules for food of animal origin, establishes in Section XIV and XV of Annex III the specific requirements for the production of gelatine and collagen for food. The use of bones, other than specified risk materials as defined in Article 3(1)(g) of Regulation (EC) No 999/2001 of the European Parliament and of the Council, as raw material for the production of gelatine and collagen is permitted.
The ultimate purpose of the feed ban was to prevent the recycling of the BSE agent in cattle in the feed chain, and the subsequent risk of transmission to humans causing Variant Creutzfeldt‐Jacob Disease (vCJD). Thus, the control system laid down in Annex IV to Regulation (EC) No 999/2001 was also created to minimise presence of prions in feedstuffs and ultimately food.
In addition, Regulation (EC) No 1069/2009 and its Implementing Regulation (EU) No 142/2011 lay down the conditions for the production of gelatine and collagen for its use in feed.
1.1.2. Terms of Reference
EFSA is requested:
to estimate the cattle BSE risk (C‐, L‐ and H‐BSE) posed by the use of ruminant collagen/gelatine produced in accordance with Section XIV and XV of Annex III to Regulation (EC) No 853/2004 in feed intended for non‐ruminant animals including aquaculture animals.
to estimate the cattle BSE risk (C‐, L‐ and H‐BSE) posed by the use of ruminant collagen/gelatine classified as Category 3 as referred to in Article 10 of Regulation (EC) No 1069/2009 and produced in accordance with Regulation (EU) No 142/2011 for feed intended for non‐ruminant animals including aquaculture animals.
1.2. Interpretation of the Terms of Reference
During the discussion about the ToR, it was agreed to seek clarification from the requestor on two specific points:
if, only and specifically, the hazard to be considered is the BSE agent and not any other TSE agents;
if, only and specifically, the risk should be estimated for cattle and not for any other ruminants or species of farmed animals in general (target population in the risk characterisation).
It was highlighted to the requestor that the importance of clarifying the scope of the hazard and the target species is because not only has the BSE agent been identified in species other than cattle (goats), but there is also an increasing body of scientific evidence suggesting a change in the risk associated with TSE agents triggered by interspecies passage.
The European Commission clarified that EFSA is expected to consider only BSE (classical and atypical), and not other TSE agents, and to assess the risk in cattle only, and not in any other ruminant species.
After contact with industry stakeholders it became apparent that collagen and gelatine (C&G) are currently not directly used as a protein source in animal feed, and that only C&G produced from food‐grade material (i.e. derived from animals which are found fit for human consumption and produced in accordance with Section XIV and XV of Annex III to Regulation (EC) No 853/2004) is produced in the EU. The only feed applications of such food‐grade C&G are the micro encapsulation of vitamins and nutrients, and the use of some hydrolysed gelatine for pets and horses as a supplement to improve joint health. Industry stakeholders also highlighted that this scenario would not significantly change if the feed ban was lifted and that the main benefit to producers would result from the recycling, into feed intended for non‐ruminant animals, of former foodstuffs containing ruminant C&G. Taking into account all this background information, it was decided to address both ToRs together, by quantitatively estimating the cattle BSE risk posed by the use of ruminant gelatine produced from one adult animal older than 30 months of age infected with any of the three BSE strains (C, H and L) and at the clinical stage of the disease. Differences between ToR1 and ToR2 will be addressed qualitatively, highlighting the main factors that may increase or decrease the risk posed by the use of ruminant collagen/gelatine for each of the identified risk pathways (RP).
2. Data and methodologies
2.1. Data
2.1.1. Data related to production practices: contacts with the industry
To gain an insight into the current production practices for C&G production and their use in feed in the European Union, various producer and industry associations were consulted. General overviews (as described in Appendix A) on topics such as the source and type of raw materials used for C&G manufacture, the various processing methods or the use of C&G in the feed industry were included in the communications. A structured survey was not conducted, and differing levels of engagement were achieved with different stakeholders.
The information and data provided were reviewed and used to describe in a generic way the current practices for production and use of C&G in the EU and what changes could possibly occur in the C&G industry should the feed ban be lifted as described in the background of the mandate. All the stakeholders declared that the information provided to EFSA must be treated as confidential, therefore no specific references to any company or association have been included in the opinion.
2.1.2. Model data
Once the parameters of the model were identified and defined, data to populate the model were searched using the following data sources:
Data on tissue weights, tissue infectivity and contamination levels of bones and hides/skins were obtained primarily from previous EFSA opinions (EFSA, 2005a,b, 2006a) in which QRAs of the residual BSE risk in the production of C&G had been conducted. Information on these areas was also obtained from a number of scientific papers.
Data on the reduction of TSE infectivity by the application of alkaline and acidic methods as used in the production of C&G were sourced by reviewing previous Scientific Steering Committee (SSC) and EFSA Opinions (SSC, 2000a,b, 2001a,b, 2002, 2003; EFSA, 2005a,b) and key scientific publications.
The parameters specifically addressing industrial processes were defined using information sourced directly via personal communication with industry stakeholders. References have been added as personal communications.
For a small number of parameters, no data were available. In these cases, the parameter estimates were agreed based on the expert knowledge of the working group (WG) members.
The papers considered in this assessment were selected by experts of the working group based on the topic and their relevance. A systematic literature search was not performed.
When there were multiple data sources with different estimates, the WG discussed and agreed on the one to be included in the model based on the robustness of the data sources and their degree of similarity to the natural phenomena and processes that they represent.
2.2. Methodologies
2.2.1. Approach to address the ToRs
To address both ToRs, the following AQs were formulated:
AQ1 – What are the RP for cattle from the use of ruminant C&G in feed for non‐ruminant animals?
AQ2 – What is the amount of infectivity in C&G produced from an infected animal at clinical stage?
AQ3 – How many infected animals have to be processed and how many kg of C&G have to be produced from infected animals to accumulate 1 cattle oral infectious dose 50 (CoID50)?
AQ4 – What is the residual BSE infectivity in the feed at the end of the RPs?
Three separate RPs were identified (AQ1) that accounted for the main steps that may increase or decrease the cattle BSE risk posed by the use of ruminant C&G in non‐ruminant feed. The pathways describe the events from the outputs of the probabilistic model (C&G produced from bones and hide of an infected animal), which are addressed in AQ2 and AQ3, to the exposure of cattle to feed contaminated with C&G containing BSE infectivity. Based on the information made available through literature and stakeholders, the RPs assumed the possible cross‐contamination of ruminant feed with non‐ruminant feed that contained either: (i) former foodstuffs containing ruminant C&G; or (ii) ruminant C&G added directly as a protein source or as a component of a feed additive manufactured from raw materials fit for human consumption; or (iii) ruminant C&G added directly as a protein source or as a component of a feed additive manufactured from animal by‐products (ABP) as raw materials. In AQ4, a qualitative approach has been applied to conclude on the risk at the end of the pathways and to account for the differences between ToR1 and ToR2, respectively.
Due to the lack of data that exists for H‐ and L‐BSE strains, the risks associated with the three BSE strains (C, H and L) have been addressed together, highlighting any differences among them when enough information was available.
The final wording of the probabilities expressing uncertainty were agreed within the working group and followed the approximate probability scale, as in Table 2 of the EFSA's Guidance on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018a).
Table 2.
Summary of all input and output variables of the model, including model notation, the distributions used, the reference(s) from which estimates were calculated and any assumptions that were made. Scenarios have been tested based on: (a) the type of EU MS with regards to the BSE risk status: negligible (i = 1) and controlled (i = 2); (b) the possible cross‐contamination of infectivity from spinal cord/dorsal root ganglia (DRG)/brain to bones/hides within the same animal (CC: cross‐contamination vs NCC: no cross‐contamination)
| Code | Description | Unit (per individual unless stated otherwise) | Value | Assumptions | References |
|---|---|---|---|---|---|
| P1 | Live weight of one slaughtered animal | kg | 550 | All slaughtered animals are adults of approx. 550 kg live weight | EFSA (2005b) |
| P2 i | Weight of bones fit for human consumption | kg | P21 = P1 × 0.09 P22 = P1 × 0.067 | Bones for gelatine, di‐calcium phosphate and fats: i = 1 (negligible risk countries), if only the skull is removed, but the vertebral column is used: 9% of total live weight i = 2 (controlled risk countries), if both the skull and the vertebral column are removed: 6.7% of total live weight | EFSA (2005b) |
| P3 | Weight of the hide | kg | P3 = 0.07 × P1 | 7% of live weight | Devine and Dikeman (2014) |
| P4 | Weight of the brain | g | 475 | The mean weight of 475 g for adult cattle This is similar to other estimates available (e.g. 500 g, EFSA, 2005b) | Cooper and Bird (2002) |
| P5 | Weight of the spinal cord | g | 306.9 | Mean weight of 306.9 g for adult cattle | Vařechová (2015) |
| P6 | Weight of the DRG | g | 30 | The mean weight of 30 g for adult cattle | EFSA (2005b) |
| P7 i | Amount of contaminating spinal cord and DRG into bones | g | No cross‐contamination (NCC) PNCC71 = P6 PNCC72 = 0 Cross‐contamination (CC) PCC 71 = (0.025 × P5) + P6 PCC 72 = 0.025 × (P5 + P6) | In the no cross‐contamination route (NCC), for negligible risk countries, the DRG are in the carcass so any infectivity is carried forward entirely. In the controlled countries, it is removed In the cross‐contamination route (CC): For countries with negligible risk of BSE, since the vertebral column is left with the carcass, all the infectivity of the DRG is included plus 2.5% of the infectivity in the spinal cord For countries with controlled risk of BSE, since the vertebral column is removed from the carcass and disposed of as SRM, a 2.5% of the infectivity in both the spinal cord and DRG is included The 2.5% of infectivity cross‐contaminating bones was obtained from data reported in a previous EFSA QRA (2005b): ‘In 1% of the slaughters with removal of the spinal cord, approx. 2.5% of the spinal cord may remain attached to vertebrae when it is removed. If no vertebrae are removed (vertebrae are not an SRM), the above spinal infectivity would enter the production process, plus the entire weight of all the DRG (30 grams)’ | EFSA (2005b) |
| P8 | % of whole brain that spills out during slaughter | % | Uniform (0.005, 0.05) | The amount of brain that can spill out during slaughter was estimated as a % of the total weight. ‘Experts judgements with the SSC's working group yielded spillage estimates ranging from [0.1%–1%] to [0.5–5%] of the brain material. The lower value of the range would correspond with conditions where brain remains in the skull, SRMs are removed as a dedicated action and knives are cleaned and heat treated in hot water (c. 85°C) after this use (Knives are not sterilised in regard to TSE agents)’. (EFSA, 2005b). The WG decided to apply the upper boundary of the upper range (5%). Uncertainty around this value was applied using a uniform distribution | EFSA (2005b) |
| P9 | % of spilled brain contaminating the hide (excluding the part of the head) | % | Pert (0.001, 0.01, 0.1) | There are no data about the % of the spilled brain that could land on the surface of the hides. This parameter reflects the assumption that the majority of the spilled brain will remain on the hide of the head. Since the hide of the head is not used in the EU for the manufacture of gelatine, uncertainty was described by using a Pert distribution between 0.01 and 10% of the total weight of the spilled brain, with the most likely value being 1% | Expert agreement in the working group |
| P10 | Amount of contaminating brain into the hide (excluding the head) | G | P10 = P8*P9*P4 | ||
| P11 | BSE infectivity in spinal cord and DRG, per g | CoID50/g | Lognormal (2.5% = 1.25, 50% = 6.66, 97.5% = 33.3) | ‘The additional data resulted in a revised estimate of one cattle oral ID50 being equivalent to 102.7 mouse i.c./i.p. ID50/g (with a 95% confidence interval of 102.0–103.4). This ID50 estimate is equivalent to 0.15 g of the brain homogenate used in the experiment (previous estimate: 0.20 g), with a 95% confidence interval of 0.03–0.79 g. Therefore it is assumed that a median in 1g of brain = 1/0.15 = 6.66 CoID50 (2.5 percentile=1.25, 97.5 percentile=33.3)’. It is assumed that the infectivity in brain, spinal cord and DRG is the same | Konold et al. (2012) |
| P12 | BSE infectivity in brain, per g | CoID50/g | Lognormal (2.5% = 1.25, 50% = 6.66, 97.5% = 33.3) | Assumed to be the same as for spinal cord and DRG | Konold et al. (2012) |
| P13 | % of the weight of bones that corresponds to bone marrow | % | 32.5 | ‘25‐40% of the bone weight is marrow’. The central value of this range is applied | Ockerman and Hansen (1999) |
| P14 i | Amount of intrinsic BSE infectivity in bone marrow | CoID50 | P141 = 10−6 × P21 × P13 × 1,000 P142 = 10−6 × P22 × P13 × 1,000 | ‘In view of the small group size of four animals in the present titration study, this estimate must be regarded as an upper limit that equates to 10−6 cattle oral ID50/g (Wells et al., 2007)’ | Sohn et al. (2009) |
| P15 | Amount of intrinsic BSE infectivity in hide/split | CoID50/g | P15 = 10–6 × P3 × 1,000 | ‘In view of the small group size of four animals in the present titration study, this estimate must be regarded as an upper limit that equates to 10–6 cattle oral ID50/g (Wells et al., 2007)’. The WG agreed to use the same intrinsic infectivity for both hide and bones | Sohn et al. (2009) |
| P16 i | Amount of BSE infectivity in cross‐contaminated bones | CoID50 | No cross‐contamination (NCC) P16NCC1 = P7NCC1 × P11 P16NCC2 = P7NCC2 × P11 Cross‐contamination (CC) P16CC1 = P7CC1 × P11 P16CC2 = P7CC2 × P11 | ||
| P17 | Amount of BSE infectivity in cross‐contaminated hide | CoID50 | P17 = (P10 × P12) | ||
| P18 i | Amount of BSE infectivity in raw bone chips | Log10 CoID50 | No cross‐contamination (NCC) P18NCC1 = log10(P141 + P16NCC1) P18NCC2 = log10(P142 + P16NCC2) Cross‐contamination (CC) P18CC1 = log10(P141 + P16CC1) P18CC2 = log10(P142 + P16CC2) | As the possible reduction in infectivity due to processing (see P20) has been estimated in log, here the total infectivity is expressed in log units | |
| P19 | Amount of BSE infectivity in raw hide | Log10 CoID50 | No cross‐contamination (NCC) P19NCC = log10(P15) Cross‐contamination (CC) P19CC = log10(P15 + P17)) | As the possible reduction in infectivity due to processing (see P21) has been estimated in log, here the total infectivity is expressed in log units | |
| P20 | Reduction of infectivity due to the processing of bones | Log10 CoID50 | Pert (1, 2.6, 3.7) | Data on prion inactivation by gelatine manufacturing steps (excluding the UHT final sterilisation step), obtained using rodent adapted prions, indicate that strong acidic treatments used on bones provide RF ranging from 1.17 log10 to 3.7 log10, whereas strong alkaline treatments used on hides provide RF ranging from 2.1 log10 to 5.25 log10. Strong alkaline and/or strong acidic treatments were formally demonstrated to provide a 2.6 to 3.7 log10 reduction of the 301 V prion infectivity. Maximum value of RF by acidic method 3.7 (see Table 1) | Grobben et al. (2005), Expert agreement in the working group |
| P21 | Reduction of infectivity due to the processing of hides | Log10 CoID50 | Pert (2, 3.7, 5.25) | Data on prion inactivation by gelatine manufacturing steps (excluding the UHT final sterilisation step), obtained using rodent adapted prions, indicate that strong acidic treatments used on bones provide RF ranging from 1.17 log10 to 3.7 log10, whereas strong alkaline treatments used on hides provide RF ranging from 2.1 log10 to 5.25 log10. Strong alkaline and/or strong acidic treatment were formally demonstrated to provide a 2.6 to 3.7 log10 reduction of the 301 V prion infectivity. Maximum value of RF by alkaline method 5.25 (see Table 1) | Grobben et al. (2005), Expert agreement in the working group |
| P22 i | Amount of BSE infectivity in gelatine from bones | CoID50 | No cross‐contamination (NCC) P22NCC1 = 10^(P18NCC1‐P20) P22NCC2 = 10^(P18NCC2‐P20) Cross‐contamination (CC) P22CC1 = 10^(P18CC1‐P20) P22CC2 = 10^(P18CC2‐P20) | ||
| P23 | Amount of BSE infectivity in gelatine from hides | CoID50 | No cross‐contamination (NCC) P23NCC = 10^(P19NCC‐P21) Cross‐contamination (CC) P23CC= 10^(P19CC‐P21) | ||
| P24 | Yield of gelatine from bones | % | 4.5 | Industry data. 4–5%. The central value of this range is applied | Vermeulen (GME), by email on 18 March 2020 |
| P25 | Yield of gelatine from hides | % | 14.5 | Industry data. 12.5–16.6%. The central value of this range is applied | Vermeulen (GME), by email on 18 March 2020 |
| P26 i | Total amount of gelatine from bones | kg | P261 = P24 × P21 P262 = P24 × P22 | ||
| P27 | Total amount of gelatine from hide | kg | P27 = P25 × P3 | ||
| P28 i | Total amount of BSE infectivity/kg of gelatine | CoID50/kg | No cross‐contamination (NCC) P28NCC1 = ((P22NCC1 + P23NCC)/(P261 + P27)) P28NCC2 = (P22NCC2 + P23NCC)/(P262 + P27)) Cross‐contamination (CC) P28CC1 = ((P22CC1 + P23CC)/(P261 + P27)) P28CC2 = (P22CC2 + P23CC)/(P262 + P27)) | ||
| P29 | Frequency of cross‐contamination during slaughter between tissues within the same animal | % | Pert (0.001, 0.01, 0.05) | Cross‐contamination within the animal occurs at a certain frequency during slaughter. EFSA's QRA (2005b): ‘In approx. 1% of the slaughters with removal of the spinal cord, approx. 2.5% of the spinal cord may remain attached to vertebrae when it is removed’. Uncertainty around this value was applied using a Pert distribution. The WG decided to apply an upper boundary of the Pert distribution allowing for higher levels of cross‐contamination up to 5% | EFSA Working group (2020), EFSA (2005b) |
| P30w i | Total amount of BSE infectivity/kg of gelatine (WEIGHTED) | CoID50/kg | P30w1 = P28NCC1 × (1‐P29) + P28CC1 × P29 P30w2 = P28NCC2 × (1‐P29) + P28CC2 × P29 | The weighted amount of infectivity considers the frequency of cross‐contamination during slaughter, according to P29 | |
| P31 i | Number of BSE‐infected bovine animals needed to produce gelatine containing 1 CoID50 | Number | P311 = 1/P30w1 × (P261 + P27) P312 = 1/P30w2 × (P262 + P27) | ||
| P32 i | Amount of gelatine needed to contain 1 CoID50 | kg | P321 = 1/P30w1 P322 = 1/P30w2 |
2.2.2. Probabilistic model
A new probabilistic model was developed (as described in Section 3.7) to contribute to the answers to the ToRs. The model estimates the BSE infectivity load, measured in CoID50/kg, contained in the gelatine produced with the bones and hide of one adult animal (i.e. older than 30 months of age), infected with any of the three BSE strains (C, H and L) (subsequently collectively referred to as ‘BSE’), and at the clinical stage of the disease. The model represents the initial steps in the production chain of gelatine from the point at which an infected animal is slaughtered to the production of gelatine from its bones and hide. The structure, assumptions and parameters of the model are described in Sections 3.7.1 and 3.7.2, and the limitations are included in the section dealing with the uncertainty analysis (Section 3.7.4).
When possible, the uncertainty on the parameter values has been described by probability distributions. Depending on the situation and the data available, either the Pert, log‐normal or the uniform distributions have been chosen. In a Monte Carlo analysis, these distributions have been used to assess the uncertainty in the model outputs. A group of parameters was identified as predominantly describing variability, and not uncertainty. As some preliminary analyses showed that this variability had little impact on the outputs, central values of the original distributions were assigned to those parameters as fixed values.
Worst‐case scenarios were not simulated in the model by assigning fixed values to parameters of the model. Nevertheless, some of the assumptions of the model corresponded to current worst‐case scenarios, and, when data were available to make a choice, the boundaries of probability distributions describing uncertainty in the parameters were selected to approximate worst‐case scenarios. As a result, the model overestimates the BSE infectivity in the final product. A sensitivity analysis was conducted to assess the contribution of the different model parameters to the total BSE infectivity estimation, and the association of sources of uncertainty in the input with the uncertainty in the output by looking at the parameters that are correlated the most with the outputs, measured by the Spearman correlation coefficient. All uncertainties that were not quantified in the model were listed and considered in the assessment of the overall uncertainty in the answer to the ToRs.
Given the uncertainties about collagen production, the model was also applied for a particular scenario: the production of collagen, only from hides, with and without reduction of infectivity during processing, the latter being considered as a worst‐case for the estimation of BSE infectivity if the infected carcass was devoted to collagen production.
2.2.3. Uncertainty assessment
The assessment of uncertainty was undertaken following the EFSA ‘Guidance on Uncertainty Analysis in Scientific Assessments’ (EFSA Scientific Committee, 2018a), the EFSA scientific opinion on ‘The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessments’ (EFSA Scientific Committee, 2018b) and the checklist for applying EFSA′s uncertainty guidance in a case‐specific assessment. Special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate, (ii) the identification of sources of uncertainty, which were listed, together with their expected impact on the outcome of the assessment, and (iii) the uncertainties in the answer to the ToRs. Following the recommendation of the guidance, probabilities expressing uncertainties were described using the numeric expression followed by the words (e.g., extremely unlikely, almost certain, etc.).
3. Assessment
3.1. Structure and biochemical properties of C&G
Collagen is a major fibrous protein in connective tissues, especially in skin, tendons and bones. It is a very large and complex proteinaceous structure consisting of a trimeric molecule based on three polypeptide chains (each about 1,000 amino acids long), forming a characteristic helical structure (Figure 2). Further intertwining of these triple helices due to the abundance of three amino acids (glycine, proline and hydroxyproline) results in the formation of fibrils and subsequently in more complex cross‐linked networks. Different collagen compositions allow a classification of 27 different types (Gómez‐Guillén et al., 2011), with collagen type I (the type present in skin, tendons and bones) being by far the most abundant form in connective tissue. Up to 80–90% of the collagen in the body consists of types I, II and III, while types I and III are the most common types in the skin (VKM, 2016).
Figure 2.
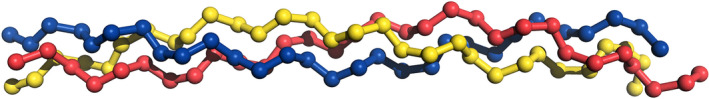
Typical structure of tropocollagen consisting of three intertwined peptide chains forming a characteristic helix. © Alexander V Grishin/Shutterstock.com
Collagen lacks tryptophan and has been considered as an ‘incomplete protein source’ (Paul et al., 2019). However, the presence of 19 different amino acids and the capacity to bind water have made collagen appealing for multiple applications. Due to its properties, it is commonly used as a coating, binding, gelling and glazing ingredient in food and feed, as a dietary supplement, in pet food and in the biomedical, cosmetic and pharmaceutical industries, with increasing demand in recent years.
Gelatine is obtained by the irreversible hydrolysation of the typical collagen triple helical structure into small peptides in the form of unordered single strands (denaturation) (Figure 3).
Figure 3.
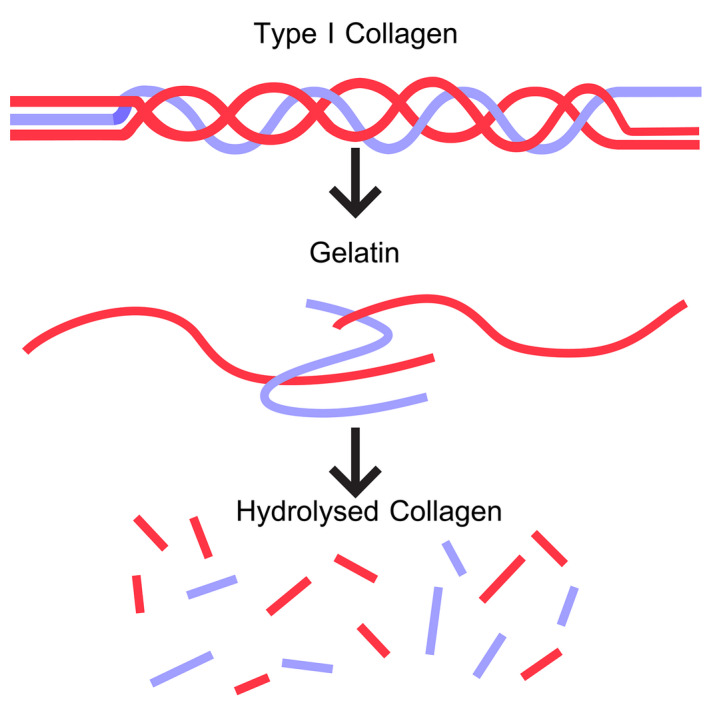
- Hydrolysed collagen is also referred to as collagen peptide.
Pure gelatine is white, odourless and tasteless and is 98–99% protein by dry matter (Francis, 2000). Due to its hydrophilic properties (gelling, thickening) and the high content of basic amino acids (as it is derived from collagen), gelatine is mostly used in the food and feed industries, but also has applications in the pharmaceutical, photographic and manufacturing industries.
3.2. Legal framework for the production of C&G
3.2.1. Collagen
Commission Regulation (EC) No 142/2011 for ABP and Commission Regulation (EC) No 853/2004 for foodstuff both define collagen as a protein‐based product derived from hides, skins, bones and tendons of animals.
Specifications for the production of collagen intended to be used in food are laid down in Point 1, Chapter I, Section XV, Annex III, Commission Regulation (EC) No 853/2004. For the production of collagen intended for use in food, the following raw materials may be used:
bones, other than specified risk materials as defined in Article 3(1)(g) of Regulation (EC) No 999/2001;
hides and skins of farmed ruminant animals;
pig skins;
poultry skin;
tendons and sinews;
wild game hides and skins; and
fish skin and bones.
Authorised raw materials must be derived from animals which have been slaughtered in a slaughterhouse and whose carcasses have been found fit for human consumption following ante‐ and post‐mortem inspection or, in the case of hides and skins from wild game, found fit for human consumption.
According to Chapter III, Section XV, Annex III of the Commission Regulation (EC) 853/2004, the production process for collagen must ensure that all ruminant bone material from animals born, reared or slaughtered in countries or regions with a controlled or undetermined BSE risk, as determined in accordance with Article 5 of Regulation (EC) No 999/2001, is subjected to a process which ensures that it is:
Finely crushed and degreased with hot water.
Treated with diluted hydrochloric acid (at a minimum concentration of 4% and pH < 1.5) over a period of at least 2 days.
pH adjusted using acid or alkali.
Rinsed either one or more times and subjected to at least one of the following processes: filtration, milling or extrusion, or any approved equivalent process.
All other raw materials must be subjected to:
treatment involving washing,
pH adjustment using acid or alkali,
either one or more rinses,
and at least one of the following processes: filtration, milling or extrusion, or any approved equivalent process.
The collagen yielded may undergo a drying process.
An overview of the collagen manufacturing process as determined by the legislation can be found in Figure 4.
Figure 4.
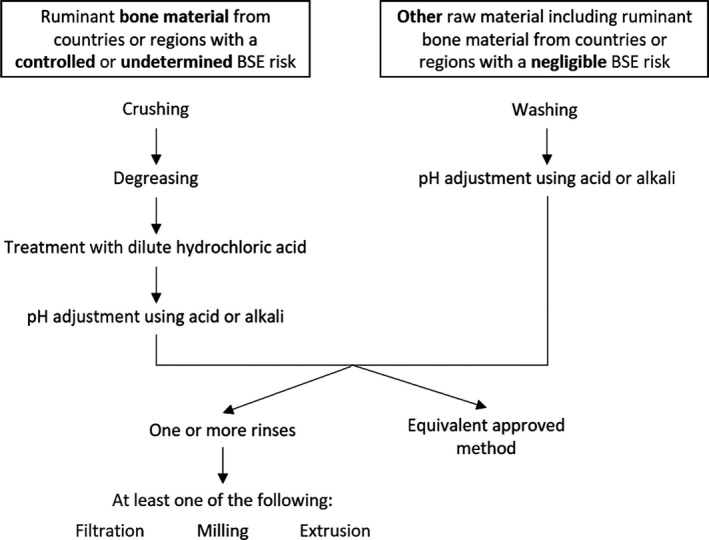
Collagen production process as specified by Regulation (EC) No 853/2004
In addition, collagen production from ABP is approved subject to certain conditions and requirements. In EU MS, ABP are regulated by EU Council Regulation No 1069/2009 and its Implementing Regulation (EU Regulation No 142/2011) (referred to as the ABP Regulations). The objectives of these Regulations are to promote the sustainable use of animal materials and a high level of protection of public and animal health in the European Union. As specified in Clause 1 of Commission Regulation No 142/2011:
‘Regulation (EC) No 1069/2009 lays down animal and public health rules for animal by‐products and products derived thereof. That Regulation determines the circumstances under which animal by‐products are to be disposed of, in order to prevent the spreading of risks for public and animal health. In addition, that Regulation specifies under which conditions animal by‐products may be used for applications in animal feed and for various purposes, such as in cosmetics, medicinal products and technical applications. It also lays down obligations for operators to handle animal by‐products within establishments and plants which are subject to official controls’.
Commission Regulation (EU) No 142/2011 lays down implementing rules for Regulation (EC) No 1069/2009, including definitions of ABPs, processing standards, hygiene conditions and the format for documentary evidence that has to accompany consignments of ABPs and derived products for the purposes of traceability.
With a view to preventing and minimising risks to the public and animal health arising from ABPs and products derived from them, Regulation (EC) No 1069/2009 assigns those products to specific categories that reflect the level of such risks and includes requirements on their safe use and disposal, as follows:
Category 1 ABPs are defined in Article 8 of Regulation (EC) No 1069/2009. This material is associated with the highest risk and consists principally of material that is considered a TSE risk, i.e. Specified Risk Material.
Category 2 ABPs are defined in Article 9 of Regulation (EC) No 1069/2009. This material is associated with medium risk. It includes fallen stock, manure and gastrointestinal tract contents. Category 2 is also the default status of any animal by‐product not defined in the ABP Regulation as either Category 1 or Category 3 material and includes such material as slaughterhouse drain‐trap waste.
Category 3 ABPs are defined in Article 10 of Regulation (EC) No 1069/2009. It is the lowest risk category of animal by‐product. It includes parts of animals that have been considered fit for human consumption in a slaughterhouse, but that are not intended for consumption for commercial or other reasons.
Specifications for the production of collagen that is intended to be used in feed and derived from ABPs are laid down in Section 8, Chapter II, Annex X of Commission Regulation (EC) No 142/2011. According to this Regulation, only ABPs that are Category 3 material or products that are derived from such ABPs can be used for the production of collagen intended for feed. All those Category 3 materials listed in Article 10 of Regulation (EC) No 1069/2009 may be used, except the following ones:
-
m
animals and parts thereof of the zoological orders of Rodentia and Lagomorpha;
-
n
hides and skins, hooves, feathers, wool, horns, hair and fur originating from dead animals that did not show any signs of disease communicable through that product to humans or animals other than those referred to in Point (b) of this Article 10 of Regulation (EC) No 1069/2009;
-
o
adipose tissue from animals which did not show any signs of disease communicable through that material to humans or animals, which were slaughtered in a slaughterhouse and which were considered fit for slaughter for human consumption following an ante‐mortem inspection in accordance with Community legislation;
-
p
catering waste other than as referred to in Article 8(f) of Regulation (EC) No 1069/2009.
According to Section 8, Chapter II, Annex X of Commission Regulation (EC) 142/2011, the production process for collagen must ensure that:
unless the collagen has been produced in accordance with the requirements for collagen set out in Section XV of Annex III to Regulation (EC) No 853/2004, it must be produced by a process ensuring that unprocessed Category 3 material is subjected to a treatment involving washing, pH adjustment using acid or alkali followed by one or more rinses, filtration and extrusion;
after that treatment, collagen may undergo a drying process.
In addition, collagen must be wrapped, packaged, stored and transported under satisfactory hygiene conditions. In particular:
-
a
a room or a dedicated place must be provided for storing materials for wrapping and packaging;
-
b
wrapping and packaging must take place in a room or in a place intended for that purpose.
3.2.2. Gelatine
Commission Regulation (EC) No 142/2011 and Regulation (EC) No 853/2004 define gelatine as a natural, soluble protein, gelling or non‐gelling, obtained by the partial hydrolysis of collagen produced from bones, hides and skins, tendons and sinews of animals.
Specifications for the production of gelatine intended to be used in food are laid down in Point 1, Chapter I, Section XV, Annex III, Commission Regulation (EC) No 853/2004. For the production of gelatine intended for use in food, the following raw materials may be used:
bones, other than specified risk materials as defined in Article 3(1)(g) of Regulation (EC) No 999/2001;
hides and skins of farmed ruminant animals;
pig skins;
poultry skin;
tendons and sinews;
wild game hides and skins; and
fish skin and bones.
Authorised raw materials must derive from animals which have been slaughtered in a slaughterhouse and whose carcases have been found fit for human consumption following ante‐ and post‐mortem inspection or, in the case of hides and skins from wild game, found fit for human consumption.
According to Chapter III, Section XIV, Annex III of the Commission Regulation (EC) No 853/2004, the production process for gelatine must ensure that all ruminant bone material from animals born, reared or slaughtered in countries or regions with a controlled or undetermined BSE risk, as determined in accordance with Article 5 of Regulation (EC) No 999/2001, is subjected to a process that ensures that it is:
finely crushed and degreased with hot water;
treated with diluted hydrochloric acid (at a minimum concentration of 4% and pH < 1.5) over at least 2 days;
-
treated by:
-
–
an alkaline treatment of saturated lime solution (pH > 12.5) for a period of at least 20 days with a heat treatment step of 138°C minimum for at least 4‐s; or
-
–
an acid treatment (pH < 3.5) for a minimum of 10 h, with a heat treatment step of 138 °C minimum for at least 4‐s; or
-
–
a heat‐and‐pressure process for at least 20 min with saturated steam at 133°C and more than 3 bar;
-
–
or any approved equivalent process.
-
–
All other raw material must be subjected to:
treatment with acid or alkali
one or more rinses
subsequent pH adjustment using acid or alkali
extraction by heating one or more times in succession
purification by filtration and heat treatment.
An overview of the gelatine manufacturing process as determined by the legislation is shown in Figure 5.
Figure 5.
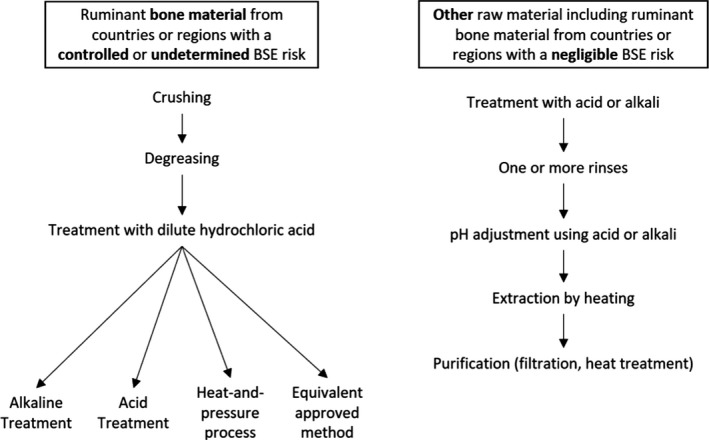
Gelatine production process as specified by Regulation (EC) No 853/2004
Specifications for the production of gelatine intended to be used in feed and derived from ABPs are laid down in Section 5, Chapter II, Annex X of Commission Regulation (EC) No 142/2011. According to this Regulation, the same raw materials can be used as for collagen production (see Section 3.2.1).
According to Section 5, the production process for gelatine must be carried out as follows:
Unless the gelatine has been produced in accordance with Section XIV of Annex III to Regulation (EC) No 853/2004, it must be produced by a process that ensures that Category 3 material is subjected to a treatment with acid or alkali, followed by one or more rinses.
The pH must be adjusted subsequently. Gelatine must be extracted by heating one or several times in succession, followed by purification by means of filtration and sterilisation.
After having been subjected to the processes referred to in Point 1, gelatine may undergo a drying process and, where appropriate, a process of pulverisation (ground to a powder) or lamination.
The use of preservatives, other than sulfur dioxide and hydrogen peroxide, shall be prohibited.
In addition, gelatine must be wrapped, packaged, stored and transported under satisfactory hygiene conditions. In particular:
-
a
A room or a dedicated place must be provided for storing materials for wrapping and packaging;
-
b
Wrapping and packaging must take place in a room or in a place intended for that purpose.
3.3. Industrial processes to produce C&G
3.3.1. Collagen production
There are various manufacturing processes for collagen extraction, and which one is used depends on the raw material and the desired function of the final collagen product. According to the Collagen Casings Trade Association (CCTA) only hides and skins from carcasses that have been found fit for human consumption, complying with Regulation (EC) No 853/2004 for the production of collagen for food, are used in Europe. All hides and skins have their sources clearly identified and are separated by species with bovine hide being the material that is predominantly used (CCTA, 2011). They can be obtained from slaughterhouses, tanneries, collection centres or intermediate processing plants. Approximately 1,835 tonnes of collagen produced from mammalian material were imported into the EU in 2019 (Juschus, 2020, TRACES assistant, by email on 16 January 2020).
In general, manufacturing methods for the production of collagen include the following steps.
Pretreatment
Ruminant hides are usually washed, dehaired, ‘split’1 and cut before further processing. To break down the collagenous cross‐links and separate collagen from unwanted material, a treatment with acid (pH < 5 to the core for a minimum of 1 h) or alkali (pH > 12 for at least 8 h), depending on the origin of the material, is conducted. Acidic solutions seem to be better for more fragile materials with softer cross‐links (e.g. pork skin and calf hides), whereas alkaline treatment with sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)2) seems to be commonly used for thicker and more mature materials like ruminant hides (Schmidt et al., 2016). The time for which hides are held in alkali can last from days to weeks (CCTA, 2011). The pretreatment leads to swelling of the starting material as preparation for further extraction. To ‘de‐fat’, an additional acetone or alcohol treatment may be applied (Li et al., 2009; Ran and Wang, 2014; Noorzai et al., 2019).
According to the Opinion and Report on Safety with Respect to TSE Risks of Collagen Produced from Ruminant Hide of the Scientific Steering Committee (SSC, 2001b), the production of collagen (from ruminant hides) generally involves an alkali processing step at pH 11.5–13 for 24–48 h followed by pH 13 for 12–13 h (with lime or a sodium sulfide or diluted sodium hydroxide solution).
Extraction
The extraction of collagen can either be achieved by chemical hydrolysis (e.g. with 0.5M acetic acid for 24–72 h at 4°C with constant stirring) or by means of enzymatic hydrolysis (0.5M acetic acid containing selected enzymes (alcalase, collagenase, thermolysin, among other) for 48 h, at 4°C or room temperature, with constant stirring). A combination of enzymatic hydrolysis and ultrasound can shorten the extraction process (Li et al., 2009; Ran and Wang, 2014; Schmidt et al., 2016; Noorzai et al., 2019).
According to the SSC opinion and report, the extraction of collagen from bovine skins uses HCl (at pH between 0.8 and 3.3 for 6–48 h at room temperature) for gel formation (SSC, 2001b).
Purification
Following extraction, the collagen and residues from the manufacturing process are separated by filtration, and the collagen is subsequently precipitated with NaCl. The solid precipitate is collected by centrifugation and redissolved in acetic acid before a collagen gel is finally obtained by dialysis against water for 2 days at 4°C (Li et al., 2009; Ran and Wang, 2014; Schmidt et al., 2016; Noorzai et al., 2019). This gel can be freeze dried. The extrusion of collagen after filtering and hot air drying is possible as well (CCTA, 2011). In between the different processing steps, pH adjustments and washing/rinsing steps are carried out according to the legislation (CCTA, 2011; Noorzai et al., 2019).
In comparison with the gelatine manufacturing process, there seems to be more variability in processing parameters when collagen is produced (SSC, 2001a; Bierwagen (CCTA), by email on 27 January 2020).
3.3.2. Gelatine production
Depending on the manufacturing process there are different types of gelatine (type A, manufactured through acid processing; type B, manufactured through alkaline processing). Annually, there are 120,000 tonnes of gelatine and collagen peptides (obtained through further hydrolysation of gelatine) produced in Europe (Vermeulen (GME), by email on 19 December 2019). The worldwide demand for gelatine was about 620,000 tonnes in 2019.2 Approximately 22,030 tonnes of gelatine produced from mammalian material was imported into the EU in 2019 (Juschus (TRACES assistant), by email on 16 January 2020).
In Europe, most of the gelatine is obtained from pig skins, but pig bones, cattle hides, cattle bones as well as a small amount of fish skin are also used. In total, about 25% of the gelatine is estimated to be produced from bovine raw material (Vermeulen (GME), by email on 19 December 2019). Globally, more ruminant material is used to produce gelatine than in Europe, with approximately 29.4% being produced from bovine split and 23.1% from bovine bones (Karim and Bhat, 2009).
The final properties of the gelatine produced depend on the starting material and the extraction process. Raw materials are obtained from abattoirs and tanneries for skin and hides, and abattoirs, meat cutting plants and special degreasing units for bones. According to the Gelatine Manufacturers of Europe (GME), only hides, skins and bones from carcasses that have been found fit for human consumption, complying with the Regulation (EC) No 853/2004 for the production of gelatine for food, are used. Pig skin and bovine hides, as well as pig and bovine bones, are processed separately. To ensure full traceability, gelatine is generally produced in batches. Depending on the final application and the desired properties, gelatine derived from porcine material can be mixed with gelatine of bovine origin after production (Vermeulen (GME), by email 19 December 2019).
The production of gelatine using bones and hides requires mechanical preparation before the pretreatment is applied. The main steps in the standard production process are:
Preparation
Bones: bones are usually crushed to small pieces (1–1.5 cm) and degreased by immersion and stirring in a hot water bath (75–90°C) for 15–40 min, followed by drying in a stream of hot air (with possible temperatures of up to 400°C; SSC, 2002; Grobben et al., 2004; EFSA, 2006a). The purpose of this process is to remove any soft tissues (i.e. bone marrow, and any residual spinal cord and dorsal root ganglia (DRG) as further described in Section 3.6) that were adhering to the bones. Hot water or several solvents may be used to reduce the fat content, which should not exceed 1% following degreasing, and before the main extraction steps.3 Bones are then demineralised with hydrochloric acid (4–6% HCl, 2 days) in a cascade process (Grobben et al., 2004; EFSA, 2006a), and the remaining ossein (extracellular matrix of bone made of collagen) is washed to remove acidic residues (EFSA, 2006a).
Hides and skins: ruminant hides are usually washed and cut before further processing. Removal of hair from hides and degreasing are also necessary to prepare the hides and skins for the pretreatment.
Pretreatment
One of the following processes is applied:
Acid process: Treatment in an acidic solution (sulfuric or phosphoric acid, pH 2–3.5, 12–24 h) with subsequent washing steps to remove the acid (Grobben et al., 2004; EFSA, 2006a). The ossein obtained from this demineralisation process can additionally be immersed for 2 h in a solution of 0.3 M sodium hydroxide (NaOH) at pH 13 at room temperature (SSC, 2002). Within the gelatine industry, the gelatine obtained from acid‐treated raw material is called type A gelatine.
Alkaline process: This treatment commonly uses a saturated lime solution (pH > 12, 20–60 days) (Grobben et al., 2004; EFSA, 2006a) or, alternatively, NaOH (pH 13.5, 5–7 days) (SSC, 2002). The solution is replaced regularly and, from time to time, air is pumped into the mixture to prevent the growth of anaerobic microorganisms. At the end, the solution is first treated with sulfuric acid (H2SO4) to remove the lime and further washed with water to remove the acid (Grobben et al., 2004; EFSA, 2006a). Within the gelatine industry, the gelatine obtained from alkali‐treated raw material is called type B gelatine.
Heat/Pressure/Time process: The starting material is preheated in saturated steam to approximately 115°C before being autoclaved at 133°C with 3 bar for 20 min. The autoclaving process can be repeated up to eight times, but usually with lower temperatures (SSC, 2002; EFSA, 2006a). In Europe, no gelatine is currently being produced with the heat‐and‐pressure pretreatment process (Vermeulen, GME by email on 18 March 2020).
Extraction
To extract the gelatine, the pretreated material is mixed with hot water in a multi‐stage process. The temperature of the water rises with each extraction step until the residue is boiled to obtain the remaining gelatine. The final extract contains about 5% gelatine (Grobben et al., 2004; EFSA, 2006a).
Purification and concentration
Coarse particles are removed from the gelatine solution via filtration through diatomaceous earth or special filters. Salts are removed by deionisation (ion exchange with a cation and anion exchanger) (SSC, 2000a; Grobben et al., 2005; EFSA, 2006a). To concentrate the gelatine, water is extracted by means of vacuum evaporation. The gelatine extract is sterilised at ultra‐high temperature (138–140°C) for 4 s, and quickly cooled to obtain a gel that is finally dried in a stream of warm air. This step may be omitted when gelatine is manufactured by the heat‐and‐pressure process (Grobben et al., 2004; EFSA, 2006a).
3.3.3. Import of raw materials and C&G from outside the EU
There is limited availability of raw materials in the EU for the production of C&G and, currently, not all C&G sold in the EU is produced with raw materials from animals slaughtered in the EU. Gelatine producers in Europe use dried bone chips as raw material, produced by intermediate degreasing units that are not available in every MS. However, these plants are present in other countries, and dry bone chips can be imported. For bovine hides, the raw material or split is produced in tanneries and sorting establishments from which collagen or gelatine producers get their supplies.
Both the starting material and the final collagen or gelatine can be imported into the EU. Companies in non‐EU countries have to comply with the corresponding EU Regulations and need to be registered with the EU. The requirements for the importation of C&G, including the raw materials from which they can be manufactured, are set out in Commission Implementing Regulation (EU) 2019/626 and Commission Implementing Regulation (EU) 2019/628.
3.3.4. Concluding remarks to Sections 3.1, 3.2 and 3.3
Collagen is a protein‐based product derived from the hides, skins, bones, tendons and sinews of animals. Gelatine is a natural, soluble protein, obtained by the partial hydrolysis of collagen produced from the bones, hides and skins, tendons and sinews of animals.
Approximately 25% of the gelatine produced in the EU is made from bovine raw materials (the rest being mostly of porcine origin, or from fish).
-
Commission Regulation (EC) No 142/2011 for ABPs and Regulation (EC) No 853/2004 for foodstuff define C&G and specify the authorised material from which C&G are produced and the methods to produce them. Both Commission Regulation (EC) No 142/2011 and Regulation (EC) No 853/2004 allow flexibility in relation to the processes used for the production of C&G, resulting in a variety of industrial processes being used. This fact presents challenges in determining the effect of these processes in reducing any BSE infectivity that may be present in the raw materials, as for example:
-
–
There are various manufacturing processes for extracting collagen from hides, depending on the raw material used and the desired function of the final collagen product.
-
–
For collagen production, pretreatments may involve harsh acidic or alkaline treatments, and solvents may also be used before extraction of the collagen by chemical or enzymatic hydrolysis.
-
–
Gelatine manufacturing, which uses both hides and bones, is less variable and raw materials are crushed or chopped, washed and then treated with acid or alkali before the gelatine is extracted using water, then filtered and sterilised.
-
–
3.4. TSE infectivity: tissue distribution and calculation of infectious titre
The known bovine TSE agents replicate and accumulate in the central nervous system (CNS) and can disseminate along the nerves of the peripheral (autonomic and motor) nervous system. However, in a given host, the agent distribution and the level of infectivity in other tissues (relative to the brain) can vary substantially depending on the TSE strain and the stage of the incubation period (IP) (Arnold et al., 2007). When prion protein (PrP) distribution within tissues has been visualised by immunochemical methods, it is apparent that the visible accumulation of disease‐associated PrP is usually localised in those structures associated with the nervous or lymphatic systems (e.g. muscle spindles within muscle, and the autonomic ganglia and gut‐associated lymphoid tissue (GALT) in the digestive tract).
The limited data on tissue positivity are generally based on the presence/absence of detectable PrP rather than the direct demonstration of infectivity, and even when infectivity has been directly measured this too tends to be a single assay to establish the presence/absence of infectivity rather than an end‐point titration, although an estimation of titre can be calculated from such data (for full discussion of such methods, see EFSA, 2014). Where infectivity titres have been established/estimated, these titres are not absolute, because they are specific for the model used (e.g. conventional mice, cattle or bovinised transgenic mice), but some parallel titration studies have enabled conversion factors to be proposed (see Appendix B and EFSA, 2014). Failure to detect infectivity in a bioassay means that the tissue being tested is either negative, or that infectivity is below the limit of detection of the model (a threshold that will vary depending on the model used).
3.4.1. Classical BSE
The specified risk materials (SRM) listed in Regulation (EC) No 999/2001 are tissues that contain the highest infectious loads in cattle with classical BSE. Not all tissues with the potential to contain infectivity are included in this list. The current SRM definition is as follows:
The skull excluding the mandible and including the brain and eyes, and the spinal cord of animals aged over 12 months.
The vertebral column excluding the vertebrae of the tail, the spinous and transverse processes of the cervical, thoracic and lumbar vertebrae and the median sacral crest and wings of the sacrum, but including the DRG, of animals aged over 30 months, from animals whose origin is in a MS or non‐EU country or one of their regions with a controlled or undetermined BSE risk.
The tonsils, the last 4 m of the small intestine, the caecum and the mesentery from animals of all ages whose origin is in a MS or non‐EU country or one of their regions with a controlled or undetermined BSE risk.
3.4.2. Atypical BSE
In both H‐BSE and L‐BSE, disease‐related PrP accumulation has been reported consistently in CNS tissues, peripheral ganglia and nerves, muscles (predominantly the muscle spindles), adrenal glands and retina. All these tissues are also positive in C‐BSE. By contrast with C‐BSE, no lymphoid tissues or gastrointestinal tissues from H‐BSE‐ or L‐BSE‐affected animals have tested positive for the presence of disease‐specific PrP (by immunohistochemistry or western blot) or infectivity (bioassay).
The current lack of information on the possible presence or distribution of infectivity in tissues of atypical BSE‐infected cattle does not allow judgement of whether the current list of bovine SRM (see above) is fit for purpose for atypical BSE carcasses. Where data exist from both field cases and experimental animals (i.e. for L‐BSE only), there is good agreement of the data on abnormal PrP distribution (see EFSA, 2014, for review), but there are no quantitative data for infectivity. There are no data for field cases of H‐BSE.
3.4.3. BSE infectivity in hides, skins and bones
The number of studies providing data on potential tissue infectivity in bovine hides, skin and bones is very small and much of this seminal work, all of which relates to C‐BSE specifically, was undertaken before the development of transgenic mouse models. Most data come from a small number of natural cases and pathogenesis studies in the UK (Wells et al., 1998, 1999).
In the UK study, cattle were orally challenged with 100 g of C‐BSE‐infected brain tissues and tissues were harvested at various time points post challenge for bioassay in conventional mice (Wells et al., 1998, 1999; Arnold et al., 2009). Each inoculum was made by pooling the tissue samples from each of the animals killed at the same time point (maximum n = 4). Infectivity was reported in sternal bone marrow from animals near the clinical end‐point. No bioassays were performed on skin or compact bone.
Subsequent bioassays in cattle of tissue from these same experimentally challenged animals did not identify infectivity in bone marrow (Sohn et al., 2009). Bones were not tested. These negative results were interpreted as representing maximum hypothetical levels of infectivity of less than 0.1 cattle intracerebral (i.c.) ID50/g, which is beyond the end point of detection of the assay. Due to the small group size of four animals in that titration study, this estimate must be regarded as an upper limit that equates to 10–6 cattle oral ID50 per gram (CoID50/g) (Sohn et al., 2009).
A similar figure was obtained for skin in the UK pathogenesis study, in which cattle bioassays of skin were also negative.4 The analysis of these survival periods using a Poisson model for limiting dilution and applying the cattle i.c. to cattle oral conversion factor (see Appendix B) showed that skin could contain up to 10−6.1 CoID50/g (upper limit of the 95% confidence interval).
In the context of this risk assessment, the amount of infectivity in both bone marrow and hide included in the probabilistic model has been fixed at 10–6 CoID50/g. However, in natural disease, no infectivity has been detected in bone marrow or skin (SSC, 2002), so these calculated maximum infectivity levels represent a hypothetical worst‐case scenario. Compact bone has never been tested.
In a German pathogenesis study (Hoffmann et al., 2007), the bioassay was not used to test for infectivity, but immunohistochemistry and western blotting were applied to look for PrP accumulation as a marker of disease. Bone marrow was negative. Skin and bones were not tested.
Although various more recent studies have used the tissue distribution of PrP as a marker for possible infectivity in atypical BSE (see EFSA, 2014, for an exhaustive list), there are no data available on infectivity in bones, skins or hides for either H‐ or L‐BSE. Again, there is no evidence of infectivity in bones, skins or hides.
However, some positive tissues from within the same animal have the potential to cross‐contaminate these raw materials during their collection at slaughtering (see Section 3.6.1).
3.5. Inactivation of prions during the processing of raw materials for the production of C&G
At the peak of the C‐BSE crisis, several studies were conducted to estimate the prion infectivity reduction factors (RFs) attained by specific treatments used in the industrial production of gelatine and collagen. Together, the results of these studies (summarised below) support the general contention that the global industrial processes used to produce collagen/gelatine have the capacity to reduce TSE infectivity that could be present in the raw materials.
The data available on the effects on TSE titre of acidic or alkaline treatments, and other methods that may be used in the production of C&G (such as degreasing, and heat treatment), are presented in Table 1.
Table 1.
TSE inactivation during treatment processes for the production of gelatine
| Acidic treatments | |||||
|---|---|---|---|---|---|
| Process | TSE strain | Matrix | Bioassay model | log10 reduction | Source |
| Acid treatment | ME7 | Brain | Not reported | 1.17 | SSC, 2000a [Inveresk Research International 1998a, a] |
| Acid treatment | 301V | Bone and spinal cord | Not reported | 3.7 | SSC, 2001a [Grobben, 2001, a] |
| Acid process after extraction (including degreasing and demineralisation) | 301V | Bone | Not reported | 2.6 | Grobben et al. (2004) |
| Acid process (including filtration, ion‐exchange and UHT sterilisation) | 301V | Bone | Not reported | ≥ 4.8c | Grobben et al. (2004) |
| Alkaline treatments | |||||
| Process | TSE strain | Matrix | Bioassay model | log 10 reduction | Source |
| Lime treatment (20 days) | ME7 | Brain | Not reported | 2.33 | SSC, 2000a [Inveresk Research International, 1998a, a] |
| Lime treatment (45 days) | ME7 | Brain | Not reported | 2.23 | SSC, 2000a [Inveresk Research International, 1998a, a] |
| Lime treatment (60 days) | ME7 | Brain | Not reported | 2.1 | SSC, 2000a [Inveresk Research International, 1998a, a] |
| Soda alkali treatment (0.25M, 5 days, 15°C) | ME7 | Brain | Not reported | 4.82 | SSC, 2000a, 2001a [Shepherd, 1999, a] |
| Soda alkali treatment (0.3M, 7 days, 15°C) | ME7 | Brain | Not reported | 5.25 | SSC, 2000a, 2001a [Shepherd, 1999, a] |
| Alkaline treatment | 301V | Bone and spinal cord | Not reported | 4.2 | SSC, 2001a [Grobben, 2001, a] |
| Alkaline process after extraction (including degreasing and demineralisation) | 301V | Bone | Not reported | 3.7 | Grobben et al. (2004) |
| Alkaline process (including filtration, ion‐exchange and UHT sterilisation) | 301V | Bone | Not reported | ≥ 4.9 | Grobben et al. (2004) |
| Acidic and alkaline combinations | |||||
| Process | TSE strain | Matrix | Bioassay model | log 10 reduction | Source |
| Combined acid and lime treatment | ME7 | Brain | Not reported | 2.84 | SSC, 2000a [Inveresk Research International 1998b, a] |
| Short NaOH treatment after demineralisation in the acid process (0.3M, 2 h, pH 13) | 301V | Bone | Not reported | ≥ 5.4 | SSC, 2002 [Grobben, 2002, a] |
| Heat/pressure treatment | |||||
| Process | TSE strain | Matrix | Bioassay model | log 10 reduction | Source |
| 133°C/20’/3 bar | 301V | Bone | Not reported | ≥ 6.6 | SSC 2002 [Grobben, 2002, a] |
| Heat and pressure process and degreasing | 301V | Bone and spinal cord | Not reported | ≥ 6.5 | Grobben et al. (2005) |
| Autoclaving (133°C/20 min’/3 bar) and degreasing | 301V | Bone | Not reported | 4 | Grobben et al. (2006) |
| Degreasing, then heat and pressure | 301V | Bone | VM | 6.5 | Grobben et al. (2005) |
| Single steps | |||||
| Process | TSE strain | Matrix | Bioassay model | log 10 reduction | Source |
| Degreasing | NAb | NA | NA | 98–99% removal of infectivity – Indirect assumption based on removal of protein | EFSA (2005a) [Manzke et al., 1997, a] |
| Degreasing | NA | NA | NA | 1.5–2 (extrapolated from assumption above) | Grobben et al. (2004) |
| Degreasing | NA | NA | NA | 1.7–2 (extrapolated from assumption above) | Grobben et al. (2006) |
| Filtering | NA | NA | NA | 0.5–1 | EFSA (2005a) [Grobben, 2004 and unpublished resultsa] |
| Filtration, ion‐exchange (individual step in gelatine production) | 263K | Gelatine extracts (spiked at different points in the process) | Hamster | 1.5 | SSC (2002, 2003) [Rohwer, 2001, a] |
| UHT sterilisation (138–140°C, 4 s) (individual step in gelatine production) | 263K | Gelatine extracts (spiked after acid/alkali steps) | Hamster | 4 | SSC (2002, 2003) [Rohwer, 2001, a] |
References in squared brackets that have been used for the assessments undertaken in the respective opinions were not available for review because they were either provided to the SSC WG confidentially and not meant for public release, or never published.
NA: not applicable.
≥ in this column indicates the limits of detection for the assay that were reported.
These studies all used laboratory‐adapted rodent prion strains, such as ME7 or 263K (derived from naturally occurring scrapie) or 301V (derived from classical BSE) (Brown et al., 1990; Taylor, 1991, 1999), rather than material derived directly from animals with naturally occurring disease. They involved the comparative titration of infectivity, by bioassay, in samples before and after the application of a bench‐scale, downsized, protocol of the industrial step being assessed.
Several of these studies were sponsored by the industry and have never been published in peer‐reviewed journals (including some studies used by SSC and EFSA in previous risk assessments). Thus, for some of these studies the descriptions of the methodologies (including the matrix, the TSE agent, and the mouse models used for bioassay) are not available for contemporary scrutiny or comparison with newer data, which limits the usefulness of the RF estimates that were derived from these experiments.
Although the principles highlighted below are expected to be valid for bovine C‐BSE, the actual effect that the acid/alkaline/autoclaving conditions may have on the reduction of the infectious titre of H‐ and L‐BSE, as compared with C‐BSE, could differ.
The gelatine production process steps that were identified as being able to provide a potential reduction in prion infectivity in the final product were:
Washing with detergents, degreasing and demineralisation of raw material applied to bones, and washing and degreasing applied to hides.
Strong alkaline/acidic treatment used as a pretreatment.
Extraction by mixing with hot water in a multi‐stage process.
Purification and concentration via filtration of raw gelatine using diatomaceous earth, or other special filters (e.g. perlite), and salt removal by anion/cation exchange resins.
Ultra‐high temperature (UHT) sterilisation of the final product.
It cannot be assumed that the log reductions achieved by each step individually can be directly added to estimate a total RF. Changes to the physicochemical composition of the infectious agent might occur during individual steps, and could impact positively or negatively on the effectiveness of subsequent processes.
3.5.1. Degreasing
The degreasing process that is applied to crushed bones consists of immersion and stirring in a water bath at 75–90°C, followed by drying in a stream of hot air (Grobben et al., 2004). The purpose of this process is to remove soft tissues (i.e. bone marrow, and any residual spinal cord and DRG) that could be adhered to bones. The capacity of this step to remove the prion infectivity that might be associated with raw crushed bones has never been directly assessed. However, degreasing reduced the amount of several brain‐specific proteins by 98–99% (Manzke et al., 1997), and the final dried bone chips are required to have a fat content of no more than 1%, which is equivalent to a 2 log10 reduction. Therefore, degreasing is likely to reduce the level of infectivity that could be associated with CNS or DRG contamination of raw bone material by up to 2 log10.
3.5.2. Alkaline/acidic treatment
As strong alkaline or acidic solutions have the effect of reducing the infectious titre of TSE (Taylor, 2000). Mould et al. (1965) reported that at pH 2.1 infectivity was reduced by 0.9 log10 compared with pH 7.0. Similarly, at pH 10.5 it was reduced by 1 log10 compared to neutral pH.
Various acids/alkalis can be used to achieve the required conditions and it is believed that it is the strength of the acid/alkali solution and the duration of its application that determines the negative effect on the TSE titre (Brown et al., 1986). As a general rule, the harsher the inactivation conditions are the greater the associated reduction in the infectious titre (Taylor, 1994; Giles et al., 2008). Moreover, there is some evidence, although it is not well documented, that the chemical nature of the individual acids/alkalis used may also increase or decrease the extent to which inactivation of TSEs is achieved (Taylor, 1994; SSC, 2000a).
Strong alkaline, strong acidic and the combination of acidic/alkaline treatments used to extract gelatine have all been reported to reduce TSE infectivity in brain homogenate or bone material spiked with brain homogenate (see below and Table 1).
Strong alkaline, strong acidic and the combination of acidic/alkaline treatments used to extract gelatine have all been reported to reduce TSE infectivity in brain homogenate or bone material spiked with brain homogenate (see below and Table 1).
Studies that remain unpublished and inaccessible claimed an RF of 1.7–5.4 log10, with model systems using a variety of prion strains, matrices and treatments:
1.17 log10 (ME7 in brain matrix; SSC, 2000a) to 3.7 log10 (301V in brain/bone matrix; SSC, 2001a) for strong acid treatments;
2.1 log10 to 5.25 log10 (ME7 in brain; SSC, 2000a, 2001a) for strong alkaline treatments, with the ‘soda alkali’ treatments resulting in a higher RF than ‘lime’, and the RF achieved with lime not affected by treatment duration;
2.84 log10 (ME7 in brain matrix; SSC, 2000a) and > 5.4 log10 (301V in spiked bone; SSC, 2002) for a combined alkaline + acidic treatment.
Without access to the raw data, or information on specific aspects of the experimental design for these studies, no conclusions can be drawn about the robustness or comparability of these reported RF.
Data available in peer‐reviewed scientific literature indicate that acid or alkaline processes applied to bones, following the degreasing and demineralisation steps required for gelatine production, provide a prion (301V) RF of 2.6 log10 (acid process including degreasing and demineralisation) and 3.7 log10 (‘lime’ (CaO) alkali process including degreasing and demineralisation), respectively (Grobben et al., 2004). The RF observed by Grobben et al. (2004) using 301V prion strain and a bone and spinal cord matrix are considered to constitute the most reliable basis for the estimation of the impact of alkaline and acidic treatment on the cattle BSE agents that may enter the manufacturing process of C&G.
Based on the general principles discussed above, it can be assumed that both the strong acidic and strong alkaline treatments used for the industrial manufacturing of gelatine and collagen will provide a reduction of C‐BSE, H‐BSE and L‐BSE infectivity. However, the RF achievable for C‐BSE, H‐BSE and L‐BSE could differ from those observed in rodent‐adapted strains, from each other, and according to the exact nature of the industrial process(es) applied (as shown by studies using rodent‐adapted TSE strains (see Table 1)).
Indeed, the strains involved in naturally occurring scrapie or BSE can display very different resistance/sensitivity to decontaminating treatments (e.g. autoclaving or chemical inactivation) (Giles et al., 2008; Spiropoulos et al., 2019; Chapman et al., 2020). Consequently, the resistance/sensitivity of C‐BSE, H‐BSE and L‐BSE prion strains in cattle to the inactivation treatments used for C&G production may vary substantially from that observed using rodent adapted strains.
Studies directly comparing the sensitivity of BSE agents and rodent‐adapted prion strains to alkaline or acidic treatments are extremely limited. However, a direct comparison of the efficacy of NaOH treatments (1M and 2M – 30 min, 60 min and 120 min) on cattle C‐BSE, ME7 and 263K hamster scrapie strains was reported by Taylor et al. (1994). Although non‐quantitative, this study supports the view that the resistance of cattle C‐BSE to alkaline treatment was similar to that observed for the rodent‐adapted prion strains assessed (Taylor et al., 1994).
3.5.3. Raw gelatine filtration
The reduction of TSE agent infectivity by filtration is a relatively well documented area (Cardone et al., 2012), but there are no publicly available aspects related to the impact of diatomaceous earth filtration on BSE agents(s) infectivity.
Ultrafiltration and nanofiltration were shown to provide significant (> 2–3 log10) reduction of TSE infectivity and are commonly used to increase the safety of biologically derived substances.
In contrast, the filtration of brain homogenates using devices with pores exceeding 520 nm was reported to have no or little effect on TSE infectivity (Stamp et al., 1959; Gibbs et al., 1965).
Diatomaceous earth filtration is a common procedure used in the agrifood industry to reduce the density of particles present in a liquid fraction. It has the potential to dramatically reduce the number of large size particles (> 1–2 μm) but has limited impact on particles with sizes that are lower than 0.5 μm (Reed and Picksley, 1987). Thus, filtering raw gelatine using diatomaceous earth has the potential to remove large solid particles, and therefore any TSE infectivity associated with them. However, it has little potential to eliminate TSE infectious particles present in a tissue homogenate.
Therefore, under a worst‐case scenario, it is considered unlikely that gelatine filtration using this process will have any impact on BSE infectivity.
3.5.4. Salts removal on anionic/cationic Amberlite resins
Both anion and cation Sepharose exchange columns were demonstrated to remove > 3 log10 of prions (based on both infectivity and PrPSc content) from spiked plasma products or saline solutions. However, prion removal by these chromatographic columns was shown to be the consequence of the direct binding of prions on the Sepharose matrix rather than from their interaction with the ion‐exchange groups (Thyer et al., 2006).
Examination of the limited data available about prion/Amberlite interactions suggests that in contrast with Toyopearl, Fractogel or Sepharose resins, the normal prion protein shows no detectable binding on Amberlite (either cationic or anionic). In consequence, it is unlikely that the desalting of gelatine by Amberlite treatment will provide any reduction of BSE infectivity.
3.5.5. UHT gelatine sterilisation
UHT treatment applied for the sterilisation of the gelatine extract was estimated to provide a higher than 2.2 log10 RF in experiments carried out at 137°C for 3.3 s using the 301V strain after an acidic treatment and a higher than 1.2 log10 RF after an alkaline treatment, respectively (Grobben et al., 2004). Extrapolation from data obtained using the 263K scrapie hamster model also suggested that UHT treatment could provide a 4 log10 reduction of infectivity for this particular prion strain (Rohwer, 1984).
Various studies in the literature suggest that autoclaving may be very effective at reducing the 301V strain. For instance, Grobben et al. (2005) reported RF exceeding 6 log10 at a temperature between 132.3 and 134.8°C for, e.g. 20 min, but, as is the case for most of the experimental protocols in this limited field, this parameter was not assessed in isolation. However, a study on prion infectivity carried out by Giles et al. (2008) clearly indicated that the heat inactivation profile, obtained in an acidic environment, strongly differed between C‐BSE and 301V/Sc237 hamster scrapie strains. Autoclaving at 134°C for 15 min resulted in a 6.2 log10 and 7.1 log10 reduction of infectivity for 301V (mouse/BSE strain) and Sc237 (hamster strain), respectively. In contrast, this treatment only provided a 2.9 log10 reduction of C‐BSE infectivity. These results suggest that UHT treatments (138–140°C for at least 4 s), applied as a final sterilisation step to gelatine, may have only a limited impact on any C‐BSE infectivity that could be present in this matrix.
3.6. The BSE agent in the production chain of ruminant C&G
The chains of events that constitute the scenarios proposed in the ToRs start with the extraction of the raw materials from cattle slaughtered at the abattoir and the declaration of parts of the processed animals as fit for human consumption and/or as ABP of different categories, of which Category 3 is relevant for ToR2.
In order to decide whether the raw material declared fit for human consumption merits different consideration to the Category 3 ABP material in terms of the risk of harbouring infectivity, if the animal is infected with BSE, it is necessary to consider the situation from the point of slaughter until C&G are produced.
3.6.1. Cross‐contamination of raw materials during slaughter
Cattle are generally stunned with a penetrating captive bolt before bleeding. Opening the cranial cavity can lead to spillage of blood, brain or other nervous tissues such as trigeminal ganglia (SSC 2001a, 2002) with an increased risk of spillage when stunning has to be repeated (FSANZ, 2002). Drops and aerosols will most likely contaminate the hide surrounding the opening of the head but can also fall on other parts of the carcase (Prendergast et al., 2003; EFSA, 2006b). Stunning‐related dissemination of CNS tissue via entry into the bloodstream has also been described (Anil et al., 2002; Coore et al., 2004) suggesting that contamination of hide vessels could be possible. Captive bolts that are not regularly cleaned can contaminate other animals (FAO, 2007).
According to a previous EFSA Opinion, the hide of the head is not used in the EU for the manufacture of gelatine. However, most head hide is exported to non‐EU countries and can be used there for this purpose, so gelatine or products containing it could be imported back into the EU (EFSA 2006b). Before further processing, the hides are thoroughly washed and usually salted (SSC 2001a).
The hides are removed before carcass opening, either before or after head removal. Head removal exposes central nervous tissue and should, therefore, be carried out with a clean knife or, ideally, with two knives (preferably colour coded with one being used for cutting the hide and the other for removing the central nervous tissue) (SSC, 2002; FAO, 2007).
The highest risk for the cross‐contamination of bones with tissue containing BSE infectivity is the splitting of the carcass (FAO, 2007). The spinal cord should stay intact during the halving of the carcass so that it can be removed completely. However, if the cut is not made near the midline of the spinal column, the spinal cord may not be fully exposed, and some could remain in the spinal column after the removal process (FSANZ, 2002). If the vertebral column is removed as part of the carcass dressing process, then the potential for its contamination by residual DRG is considered proportional to the risk from accidentally retained spinal cord. It was estimated that in approximately 1% of carcasses approximately 2.5% of the spinal cord remains attached to the vertebrae (EFSA, 2006a). If the vertebral column is retained with the carcass (i.e. in countries with ‘negligible’ BSE risk) the DRG will be retained in the raw edible material (EFSA, 2006a).
Water is used during sawing and can potentially splash and contaminate the vertebral column of the carcass with nervous tissues (FAO, 2007); however, the risk of cross‐contamination of bones in this way can be considered negligible because most bones other than the vertebral column are covered by other tissues and, therefore, will not be exposed to aerosols or drops (EFSA, 2006a).
Brain and spinal cord present the greatest risk for the cross‐contamination of other parts of the carcass. However, the contamination of hides seems likely to have little impact on the risk associated with gelatine and collagen production because most such cross‐contamination would be on the hide of the head, which is not used in Europe, as mentioned above. Furthermore, hides are thoroughly washed before processing, potentially removing any residual contaminating tissue, and the splitting of hides to remove the epidermis and associated hair would further reduce any remaining superficial contamination. In contrast, bones (especially vertebral column), which are currently used in Europe only for gelatine and not for collagen production, are not immediately washed after harvesting and may be collected by intermediate processors for drying and chipping.
Appropriate slaughtering techniques (especially stunning and carcass splitting) can reduce these cross‐contamination risks. Containers for different materials must be cleaned and disinfected after use. Cross‐contamination of adjacent carcasses with infected tissues may also be possible, but it is less likely than the cross‐contamination that can occur between tissues in the same carcass.
All the events described above could lead to the redistribution of some BSE infectivity to tissues usually not harbouring it in a single animal/carcass, but not to an overall increase in the total amount of infectivity in the tissues of an infected animal.
3.6.2. Cross‐contamination of raw materials fit for human consumption in the production of C&G
Currently, in the EU, only raw materials derived from animals fit for human consumption (complying with the Regulation (EC) No 853/2004) are used for the production of C&G. Pig and bovine materials are both used in the production of food gelatine. In Europe, collagen for casings is made almost exclusively from bovine hides. There is no production of food‐grade collagen or gelatine from small ruminants due to the need to harvest large numbers of skins to make the production process cost‐effective.
Bones from carcasses fit for human consumption are usually harvested in cutting and processing plants. The risk of cross‐contamination with non‐food‐grade material during extraction, storage and transport to the collagen/gelatine factory or to an intermediate plant (bone chipping factories) is likely to be low. However, there is a potential for inadvertent placement of ABP from different risk categories in the raw materials to be used for the production of C&G. There is also the potential for some of the other non‐compliances, including incorrect labelling and cross‐contamination during transport, identified in EU audit reports in relation to ABP (see Section 3.6.3), to occur with raw materials being used for the production of C&G under EU Regulation No 853/2004.
Overall, despite the potential opportunities for cross‐contamination, given the controls in place, the probability that contamination of raw materials fit for human consumption with ABP material occurring at the slaughterhouse is considered to be 1–5% (extremely unlikely).
Hides may go to tanneries or intermediate plants. Those used for the production of collagen or gelatine under EU Regulation No 853/2004 must be segregated from hides not in conformity with the requirements set out in this Regulation throughout the period of receipt, storage, processing and dispatch, therefore reducing the possibility of cross‐contamination (see Section 3.6.3).
Once the raw material arrives to the collagen or gelatine factory, there is no possibility of cross‐contamination with non‐food‐grade material as ABP raw material is currently not used in the EU. For imported raw material or C&G, the standards required for export to the EU are the same as those for production within the EU. However, as previously mentioned, although the hide from the head is not used for the production of C&G in EU plants, it is sometimes used in non‐EU manufacturing plants.
3.6.3. Cross‐contamination of ABP raw materials in the production of C&G
As seen in Figure 1, C&G derived from non‐ruminants can be currently used as a feed for ruminants, non‐ruminants, fish and fur animals and as pet food, whereas C&G derived from ruminants can only be used as food for pets and as feed for fur animals.
Figure 1.
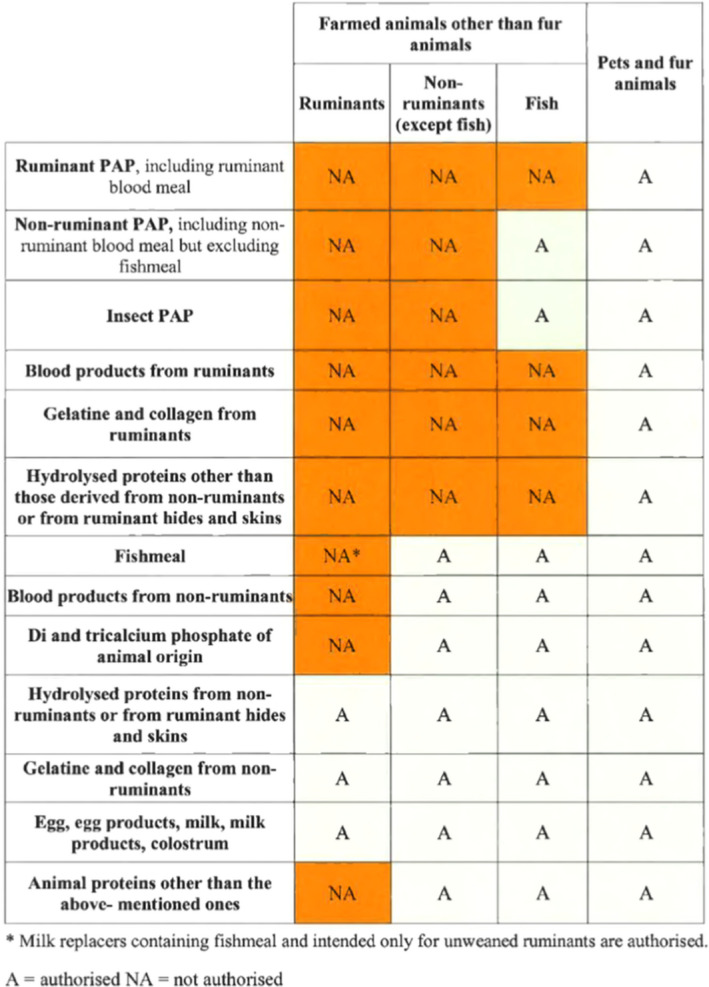
Summary of feed ban rules as laid down in the TSE Regulation (updated in May 2019)
As mentioned in Section 3.2, under the ABP legislation, additional raw materials can be used for the production of C&G compared with the raw materials used for the production of food‐grade C&G. The specific raw materials that are most likely to be used are those listed under points 10(a), 10(b)(i), 10(b)(iii), 10(e) and 10(f) of Article 10 of Regulation (EU) No 1069/2009.
Detailed rules are set out in EU legislation to ensure that ABP of different categories are segregated at the different stages of the ABP chain. According to the ABP Regulations, Category 1 material should be segregated, labelled and transported separately for rendering into meat and bone meal (MBM) and animal fat, marked indelibly to enable its identification if there are suspicions of re‐use, and subsequently destroyed by incineration, conversion into biofuel or otherwise disposed of or used in accordance with Article 12 of Regulation (EU) No 142/2011. Given the nature of the tissues being segregated into these categories (e.g. heads, spinal columns), visual inspection should be sufficient to distinguish the Category 1 material, but additional visual marking may be undertaken to aid identification. For example, under Commission Regulation No 2016/1396, there is a requirement if removal of vertebral column as a specified risk material is required, the carcass shall be identified by a clearly visible red stripe.
If different categories of ABPs are mixed, then the material assumes the highest risk category. For example, in some MS Category 2 material is mixed with Category 1 at slaughterhouses or rendering plants for commercial reasons, and all this material is then classified as Category 1.
Detailed rules are set out in Annex IV of EU Regulation No 999/2001 for appropriate segregation of materials from ruminants and non‐ruminants in establishments such as slaughterhouses, rendering plants and feed mills that are involved in the production of animal feed. Slaughterhouses vary regarding the number of animal species they process. Larger slaughterhouses are more likely to be specialised on single species, whereas others with lower throughput might slaughter multiple species in separate lines or in series on the same line.
Generally, Category 3 ABP intended to be used for the production of PAPs or other animal feeds must come from slaughterhouses that are registered by the competent authority as not slaughtering ruminants. However, by way of derogation from that specific condition, the competent authority may authorise the production of ABPs, intended to be used for feed, in slaughterhouses handling ruminants if measures to prevent cross‐contamination of ruminant and non‐ruminant by‐products are in place. Annex IV of EU Regulation No 999/2001 requires that MS keep up‐to‐date and publicly available lists of slaughterhouses, processing plants and compound feed establishments involved in the production of animal feed. This includes slaughterhouses and other plants that would be producing raw materials for the production of collagen or gelatine for animal feed.
In a slaughterhouse or a cutting plant, Category 1 material could potentially be placed inadvertently in Category 3 bins, therefore increasing the BSE risk, should the Category 1 material be sourced from BSE‐infected animals. For example, Category 1 vertebral column might be put into bins for Category 3 materials, if deboned at the abattoir, and subsequently be used for the production of C&G. In fact, as described in a previous EFSA Opinion (EFSA BIOHAZ Panel, 2018), some audit reports documented isolated instances of the mixing of SRM with Category 2 and/or Category 3 ABP due to an incorrect identification of bins for the different categories, and/or their misuse. As a result, ABP leaving a slaughterhouse could either contain material from a different category or could be wrongly identified. The audit reports analysed in EFSA BIOHAZ Panel (2018) also documented:
deficiencies in the recording and documentation of the category of the material, in particular absent or incorrect labelling at the unloading bays for raw ABP or storage of SRM in unidentified containers, not distinguishable from those of Category 3 material, which in some instances resulted in SRM being sent to a Category 3 processing plant or to mixing of Category 1 material containing full bovine bodies with Category 3 material before processing as Category 3;
some lack of enforcement of the requirements laid down by Regulation on the marking of Category 1 material with glyceroltriheptanoate (GTH);
communications from inspectors reporting that official controls pay little attention to Category 3 material at retailers or to the risk of cross‐contamination during transport.
Procedures for the prevention of cross‐contamination of Category 3 materials with Category 1 materials during transport are set out in the ABP Regulations. Cross‐contamination of Category 3 ABP with Category 1 ABP could occur if the same containers or vehicles were used to subsequently store and transport materials from a different category, since material of ruminant origin could remain in small amounts following routine cleaning and disinfection of containers or vehicles, given the resistance of the BSE agent to most conventional disinfection procedures (Department of Health and Social Care, UK, 2015).
Overall, despite the potential opportunities for cross‐contamination of ABP, given the controls in place, the probability that contamination of Category 3 material with Category 1 material occurs at the slaughterhouse is considered to be 1–5% (extremely unlikely).
The path that hides follow from the abattoir requires separate consideration. Under the ABP Regulations, Category 3 hides can be kept in stores approved for that purpose. Hides from dead (fallen stock) animals are also classified as Category 3. While they cannot be used for the production of C&G, they can be kept in the same hide store as Category 3 hides being used for C&G production. Thus, there is a possibility of cross contamination of hides or inadvertent mixing of hides from fallen stock being used for collagen or gelatine production.
There is a greater possibility that hides from fallen stock could be contaminated with brain material due to the less hygienic conditions present in premises dealing with this type of ABP compared with food premises and the higher prevalence of TSE in this surveillance category. In particular, contamination of hides could occur during the sampling of brainstem for TSE testing. This could provide an additional risk for C&G produced under the ABP Regulations compared to that produced under Regulation (EC) No 853/2004. However, given that only hides from TSE‐negative animals are classified as Category 3, any additional risk is likely to be minor.
3.6.4. BSE infectivity in raw materials for the production of C&G
Raw materials from any BSE‐infected animal used for the production of food‐grade C&G would be diluted through the mixing of raw materials from that animal with raw materials derived from other animals. The tonnage of material used to make a batch of gelatine can vary from 1 to 120 tonnes of hide (i.e. hides from approximately 26 to 3,116 animals) or 15 to 160 tonnes of bones (i.e. bones from between 303 and 3,232 animals) (Vermeulen (GME), by email on 18 March 2020). The same applies to the Category 3 raw materials from a BSE‐infected animal that could be used for the production of C&G.
Although, as explained in Section 3.6.3, more theoretical opportunities for the cross‐contamination of raw materials with BSE‐infected tissues may arise if Category 3 ABP are used for the production of C&G, the probability that ABP raw materials included in a batch for the production of C&G would contain higher levels of BSE infectivity than raw materials being used in a batch for the production of food grade collagen/gelatine is considered to be 1–5% (extremely unlikely). Therefore, it can be assumed that the level of BSE infectivity, in terms of the CoID50 units, in the raw materials that would be used in the production of collagen/gelatine under the ABP Regulations would be of a similar order of magnitude to that in the materials that would be used for the production of collagen/gelatine under the Regulation (EC) No 853/2004.
The very low number of BSE cases annually occurring in the EU, combined with the temporal and geographical spread of these cases, makes the probability that material from more than one positive animal could be included in the manufacture of a single batch of collagen or gelatine is considered to be 1–5% (extremely unlikely). Therefore, despite the extra potential residual risk in the Category 3 raw materials compared with the raw materials from animals fit for human consumption and based also on the ‘lack of data’ uncertainty associated with some of the events involved in the production and usage of C&G, it was decided to assess the potential risk at the initial stage of the production chain by applying the worst‐case scenario in which all raw materials (bones and hide) from one infected animal (either fit or unfit for human consumption) are devoted to the production of C&G, and then quantifying the amount of BSE infectivity being carried forward by such material. The results of this approach will apply equally to the two end products, i.e. food grade C&G to be used as an ingredient in foodstuff or feedstuff and ABP C&G hypothetically used as an ingredient in feedstuff. The estimation of the BSE infectivity in C&G has been performed by applying an ad hoc probabilistic model, described in Section 3.7.
3.6.5. Concluding remarks to Sections 3.4, 3.5 and 3.6
No infectivity or disease‐related PrP accumulation has ever been recorded in cattle skin or bones – the tissues used as raw material for the production of C&G. However, under the worst‐case scenario (based on the sensitivity limits of infectivity assays that gave negative results), the infectivity both in bone marrow and in hide included in the probabilistic model has been fixed at 10−6 CoID50/gram.
All quantitative data on BSE infectivity in tissues are derived from studies on C‐BSE. No equivalent data exist for H‐BSE or L‐BSE.
There is no comprehensive experimental study regarding the inactivation or reduction in infectivity of cattle C‐BSE, H‐BSE or L‐BSE prions associated with industrial gelatine or collagen manufacturing steps or processes. Most published data on the inactivation of prions by processing methods representative of those prevailing during C&G production have been derived from mouse‐adapted TSE strains, not naturally occurring BSE. When necessary, data derived from inactivation studies on rodent‐adapted TSE strains have been considered by the WG to parameterise the probabilistic model (Section 3.7).
Available data support the view that the industrial processes used to produce C&G have the capacity to reduce the TSE infectivity that could be present in the raw materials.
It cannot be assumed that log reductions achieved by each step individually will necessarily add up to provide a realistic total RF estimate, given the changes to the physicochemical composition of the infectious agent that might occur during such processes.
Data on prion inactivation by gelatine manufacturing steps (excluding the UHT final sterilisation step), obtained using rodent‐adapted prions, indicate that strong acidic treatments, used on bones, provide RF ranging from 1.17 log10 to 3.7 log10, whereas strong alkaline treatments, used on hides, provide RF ranging from 2.1 log10 to 5.25 log10.
C‐BSE, H‐BSE and L‐BSE infectivity RF achieved by gelatine manufacturing steps could differ from those observed for rodent‐adapted strains, between each other and according to the exact nature of the applied industrial processes.
Cross‐contamination of bones and hides with brain, spinal cord and DRG can occur during slaughter and carcass dressing. Further cross‐contamination of bones and hides can also occur during transport, storage and processing in intermediate plants.
Although there is a greater potential possibility of cross‐contamination in Category 3 raw materials than in those deemed fit for human consumption, the probability that the level of infectivity, in terms of the CoID50 units, in the raw materials that would be used in the production of collagen/gelatine under the ABP Regulations would be different to that in the materials that would be used for the production of collagen/gelatine under the Regulation (EC) No 853/2004 is considered to be 1–5% (extremely unlikely).
The process for C&G production under the ABP Regulations is essentially the same as that under Regulation (EC) No 853/2004. Therefore, it can be assumed that the reduction of the BSE infectivity measured in CoID50 per kg caused by the processing of raw materials to produce collagen/gelatine under the ABP Regulations would be of a similar level as that produced under the Regulation (EC) No 853/2004.
3.7. QRA of the residual BSE infectivity in gelatine
3.7.1. Model structure
A probabilistic model was developed to estimate the BSE infectivity load, measured in CoID50, contained in the gelatine produced with the bones and hide of one adult animal older than 30 months of age infected with any of the three BSE strains (C, H and L) (subsequently referred to as ‘BSE’) and at the clinical stage of the disease.
The objective of the model was to assess a worst‐case scenario in which one BSE‐infected animal at clinical end‐stage escapes on‐farm and slaughterhouse detection by the surveillance system (clinical suspect or fallen stock) and is consequently slaughtered and its bones and hide used to produce gelatine. In this worst‐case scenario, all the raw materials from the infected animal, i.e. bones (excluding the head) and the hide (excluding that of the head), are used to produce gelatine in the same batch. The availability of more accurate data on the production of gelatine in terms of yield, infectivity RF and processing methods led to the decision to only develop the model for the production of gelatine. However, some additional scenarios have been run on the production of collagen for comparison.
The model produces two different sets of outputs, one for MS with a negligible BSE risk status and the other for MS with a controlled or undetermined BSE risk status. The reason for this is the different list of tissues removed as SRM in countries with negligible or controlled risk status, respectively (see below). Based on risk assessment and epidemiological data, each EU MS has been assigned a risk status according to OIE provisions (Chapter 11.4. of the OIE Terrestrial Code) and EU legislation. As a result of strict enforcement of risk management measures at EU level, most of the EU MS (24 out of 27) are currently recognised as having a negligible risk with the other 3 (France, Greece, and Ireland) having a controlled risk. The United Kingdom (except for Northern Ireland) also has a controlled risk status.
Depending on the risk status of a country, the list of SRM, i.e. tissues that may harbour BSE infectivity and cannot enter the food and feed chains, differs (see Section 3.4.1). In countries classified as having a negligible BSE risk, the vertebral column is not considered SRM. Thus, the vertebral column, and any tissue attached to it that might contain BSE infectivity (i.e. DRG) remains attached to the carcass. In addition, cross‐contamination may occur directly from the spinal cord. As a result, the bones can carry infectivity along the gelatine production chain. In countries with controlled or undetermined BSE risk status, the vertebral column and the attached DRG will be removed, leaving only the possibility of cross‐contamination of the bones from a small section of spinal cord (and associated DRG).
The model does not estimate the cattle BSE risk posed by the use of ruminant C&G, produced with food‐grade material or Category 3 ABP, in feed intended for non‐ruminant animals including aquaculture animals. Instead, it provides three different estimates based on the residual infectivity at the end point of gelatine production:
Infectivity (parameter P30wi): the amount of infectivity, measured in CoID50, per kg of gelatine produced with the bones and hide of one infected animal, older than 30 months of age, at the clinical stage of BSE.
Infected animals (parameter P31i): the number of BSE‐infected animals older than 30 months of age, and at the clinical stage of disease, that would be required to produce gelatine containing 1 CoID50.
Gelatine (parameter P32i): the amount of gelatine (kg), produced exclusively with bones and hides from BSE‐infected bovine animals older than 30 months of age, and at the clinical stage of disease, that would be required to accumulate 1 CoID50.
The conceptual framework of the model can be visualised in Figure 6.
Figure 6.
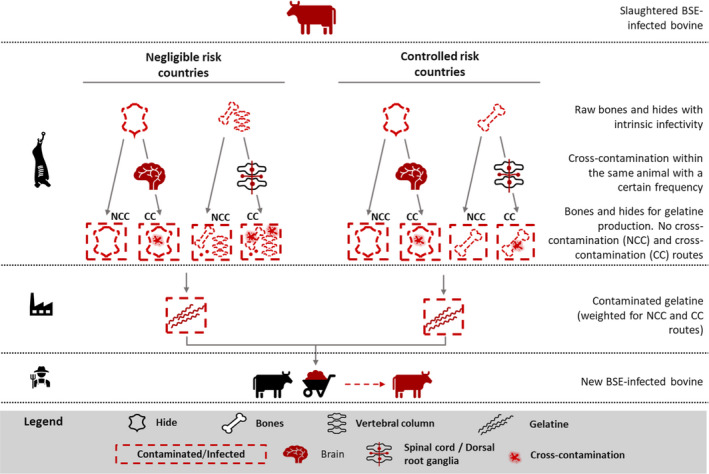
Conceptual framework of the probabilistic model
3.7.2. Model assumptions and parameters
The description of the parameters in the model, including model notation, the distributions used, the references from which the estimates were calculated, and explanations on the sources of data and equations, are given in Table 2. The model uses the following assumptions:
The starting point of the model is one adult animal (> 30 months of age) infected with any of the three BSE strains (C, H and L) and at clinical stage, slaughtered in a slaughterhouse in an EU MS. The model assumes that all the bones (excluding SRM) and the hide (excluding the head hide) of such animal are devoted to the production of gelatine.
The very limited data available suggest that the infectivity load and tissue distribution in H‐BSE and L‐BSE may not be as widespread as in C‐BSE. In the absence of any data on tissue infectivity in atypical BSE, the worst‐case scenario applied is that infectivity in the tissues would be the same as in classical BSE and with the same tissue distribution, even though there is no evidence of lymphoid involvement in these disease variants. Thus, the identity of the potentially cross‐contaminating tissues, and the amount of infectivity passing to the raw material, are assumed to be the same for the three BSE strains.
Two scenarios have been tested based on the type of EU MS for BSE risk status: negligible and controlled. The exclusion of the vertebral column in countries with controlled risk has been considered when calculating the weight of the bones and the amount of spinal cord and DRG that could contaminate them. The rest of the parameters are the same for infected animals slaughtered in a country with negligible or controlled risk.
The infectivity contained in raw bones and hide can come from two sources: the intrinsic infectivity contained in the target tissues, and that coming from cross‐contamination by other tissues, from the same animal, during slaughter and processing.
Due to the very limited presence of nervous or lymphatic tissues in compact bone it has been assumed that, for the purposes of the model, any intrinsic infectivity related to skeletal material would be in the bone marrow.
Similarly, any infectivity related to skin would not be in the hair or epidermis and would be retained in the ‘split’ within the dermis and subdermal structures during pre‐processing of a contaminated hide to produce the split. It is assumed that this does not cause any reduction in the infectivity, all of which would be carried forward in the production chain. This is a worst‐case scenario because some reduction of infectivity during washing and splitting would be expected.
Despite the lack of infectivity detected in bone marrow or hides, a worst‐case scenario (see Section 3.4) was used in the model, assuming that the bone marrow and the split contain a maximum level of homogeneously distributed BSE infectivity corresponding to the limits of detection of the bioassays at the time these tissues were investigated.
It is assumed that the only tissues containing BSE infectivity that can cross‐contaminate the bones are the spinal cord and the DRG.
It is assumed that the only tissue containing BSE infectivity that can cross‐contaminate the hide is the brain.
The amount of brain displaced via the stun hole during killing, and/or the process of disarticulating the head, is assumed to remain mostly on the surface of the head with only a fraction of it reaching the rest of the hide. The hide used for the production of gelatine is assumed not to contain the head skin.
It is assumed that all the BSE infectivity in contaminating tissues, i.e. in the brain, the spinal cord and the DRG, is homogeneously distributed. So whatever volume of tissue cross‐contaminates, it will contain a uniform level of infectivity.
Two chains of the model have been developed: one representing the scenario where, during slaughter, there is cross‐contamination of infectivity from brain, spinal cord or DRG to the hides and bones within the same animal. The second where this cross‐contamination does not take place.
For an animal slaughtered in a negligible risk country, the infectivity in the chain with no cross‐contamination of tissues will consist of all the infectivity in the DRG (as the vertebral column remains attached) and the intrinsic infectivity of the bones and hide, whereas the chain with cross‐contamination will include all the infectivity from the DRG (as the vertebral column remains attached), the intrinsic infectivity of the bones and hide plus any cross‐contaminated BSE infectivity from the spinal cord and brain.
For an animal slaughtered in a controlled risk country, the infectivity in the route without cross‐contamination will consist only of the intrinsic infectivity of the bones and hide, whereas the infectivity in the cross‐contamination route will consist of the intrinsic infectivity of the bones and hide plus some infectivity from spinal cord, DRG and brain material that have cross‐contaminated the bones and hide, respectively.
The frequency with which cross‐contamination occurs is based on previous data on detection of cross‐contamination during inspection at abattoirs. The final output of the model P30wi has been calculated weighting of the estimates for the cross‐contamination and non‐cross‐contamination routes according to their frequency, expressed as a probability distribution, as described in Table 2, accounting for the uncertainty of such an event.
The processing of bones or hides to produce gelatine follows an acidic or an alkaline pretreatment, respectively, and achieves an overall level of reduction of the BSE infectivity through the implementation of the multiple steps as described in Section 3.5. Hides are usually processed with the alkaline method, whereas bones can be processed both with acidic and alkaline treatments. Because acidic treatments seem to be more common and result in a lower reduction factor, the model considers only the acidic treatment for the bones as a worst‐case scenario.
Other processes in the production of C&G, such as washing, degreasing, demineralisation, purification and concentration via filtration, salt removal or UHT sterilisation, may not be applied consistently and the specific effect of each of them on prion infectivity may be minimal or peer‐reviewed data are not available. Therefore, the individual effects of these processes have not been included in the model.
It is assumed that all the residual BSE infectivity in gelatine produced from the bones and hide of an infected animal is homogeneously distributed throughout the end product.
3.7.3. Results of the model
The outputs of the model reflect the uncertainty of the parameters. They are presented as the 50th percentile of the probability distributions of the different outputs and the 5th and 95th percentiles (presented in brackets), which indicate the range within which 90% of the results lie.
According to the model, the mass of gelatine produced from the bones of an adult animal, slaughtered in a country with negligible or controlled risk status, is 2.2 and 1.7 kg, respectively. The mass of gelatine produced from the hide of an adult animal slaughtered in any country, irrespective of its risk status, is 5.6 kg.
For controlled risk countries, the removal of the vertebral column implies the removal of the DRG as well, and therefore of a substantial amount of infectivity that would otherwise be liable to cross‐contaminate the raw materials for gelatine production. This results in a lower estimated amount of infectivity in the end product from animals slaughtered in controlled risk countries, and a larger calculated number of infected animals and amount of gelatine needed to reach 1 CoID50.
It should be noted that the model outputs do not take into account the prevalence of BSE in negligible risk or controlled risk countries as both scenarios start from a single infected bovine animal regardless of the risk status of the country. The outputs of the model for both negligible and controlled risk countries are (see Table 3):
The 50th percentile of the amount of BSE infectivity per kg of gelatine extracted from the bones and hide of one adult animal with clinical BSE (C, H or L), slaughtered in a MS with negligible risk status, is 7.6 × 10−2 CoID50 (5th–95th percentile: 8 × 10−3–0.8 CoID50).
The 50th percentile of the number of adult animals with clinical BSE (C, H or L) slaughtered in a MS with negligible risk status that would be required to produce contaminated gelatine containing 1 CoID50 is 1.7 (5th–95th percentile: 0.1–16 infected animals).
The 50 th percentile of the amount of contaminated gelatine extracted from adult animals with clinical BSE (C, H or L) at clinical stage slaughtered in a MS with negligible risk status that would contain 1 CoID50 is 13.1 kg (5th–95th percentile: 1.2–125.3 kg).
Table 3.
Model outputs taking the uncertainty of (some) input parameters into consideration
| Risk status of country of origin/outputs | Percentiles of the output distribution | ||
|---|---|---|---|
| Negligible | 5th | 50th | 95th |
| Infectivity contained in the gelatine produced from 1 BSE‐infected animal (CoID 50 /kg) | 8.0 × 10−3 | 7.6 × 10−2 | 0.8 |
| No. of BSE‐infected animals required to produce gelatine containing 1 CoID 50 of BSE infectivity (number) | 0.1 | 1.7 | 16 |
| Amount of gelatine from infected animals required to contain 1 CoID 50 of BSE infectivity (kg) | 1.2 | 13.1 | 125.3 |
| Controlled | 5th | 50th | 95th |
| Infectivity contained in the gelatine produced from 1 BSE‐infected animal (CoID 50 /kg) | 2.9 × 10−5 | 3.1 × 10−4 | 4.1 × 10−3 |
| No. of BSE‐infected animals required to produce gelatine containing 1 CoID 50 of BSE infectivity (number) | 33.8 | 449.8 | 4,745 |
| Amount of gelatine from infected animals required to contain 1 CoID 50 of BSE infectivity (kg) | 244.9 | 3,257 | 34,360 |
The corresponding figures from a MS with controlled risk status are reported in Table 3.
The model can be reproduced in the Monte Carlo tool (risk assessment using Monte Carlo) of the EFSA's Shiny proxy open source platform, at the following link (it requires registration):
The six files required to reproduce the three outputs of the model for both negligible and controlled risk countries can be accessed in the following link:
3.7.4. Uncertainty analysis of the model
The results of a sensitivity analysis show that the uncertainty about the parameters P11 (BSE infectivity in spinal cord and DRG per gram) and P20 (reduction of infectivity due to the processing of bones) most affected the uncertainty in the outputs P30w, P31 and P32 of the model. For the uncertainty in the outputs in controlled risk countries, the third most relevant parameter was P29 (frequency of cross‐contamination). Tornado diagrams for the six outputs showing the results of the sensitivity analysis are displayed in Figure 7.
Figure 7.
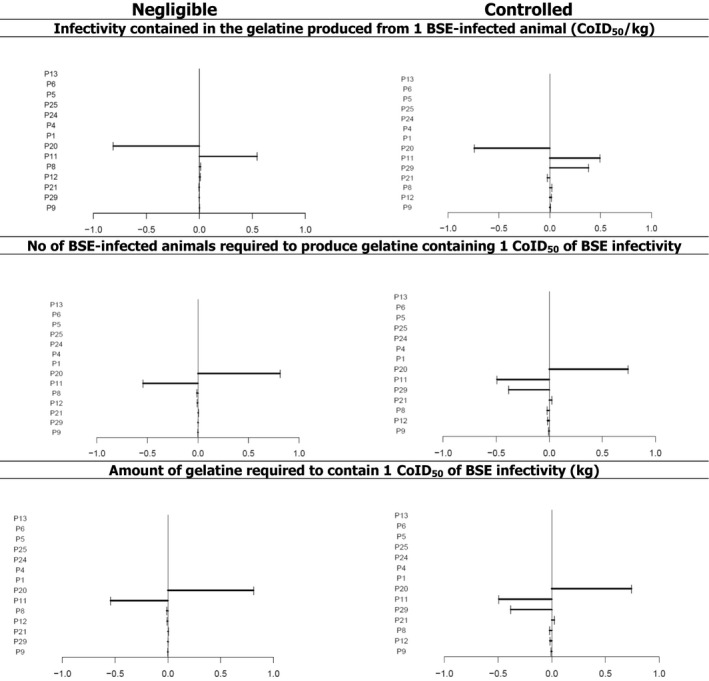
Results of the sensitivity analysis for the six outputs of the model
The model represents the initial common pathway from the point at which an infected animal is slaughtered until the gelatine from the bones and hide of this animal is produced. In general, the model simulates the uncertainty around the true amount of BSE infectivity in the final product. Where possible, uncertainty has been described by appropriate probability distributions. The uncertainties accounted for include the amount of brain that can spill over the hide (P8), the percentage of spilled brain contaminating the hide (P9), the amount of infectivity in spinal cord, DRG and brain (P11 and P12, respectively), the reduction of infectivity due to the processing of bones and hides for the production of gelatine (P20 and P21, respectively) and the frequency of cross‐contamination during slaughter between tissues within the same animal (P29).
Worst‐case scenarios were not simulated in the model by assigning fixed values to parameters of the model. When data were available to make a choice, the boundaries of probability distributions in the parameters describing uncertainty were selected to approximate to worst‐case scenarios. This approach was used for the percentage of whole brain that spills out during slaughter (P8) and the frequency of cross‐contamination during slaughter between tissues within the animal (P29), the latter being one of the most relevant parameters of the model.
In addition, some of the assumptions of the model correspond to worst‐case scenarios: all the bones and the hide of one infected animal are devoted to the production of gelatine; bones are processed with an acidic treatment (which causes lower reduction of BSE infectivity than the alkaline treatment), which corresponds to the parameter P20 of the model; the amount of infectivity in peripheral tissues in L‐BSE and H‐BSE is similar to that in C‐BSE; there is no reduction of infectivity during splitting; the infectivity is homogeneously distributed in the bones and hide of an infected animal and in the end product; the bone marrow and hide contain a certain amount of intrinsic infectivity even though this has never been detected, corresponding in the model to parameters P14 and P15, respectively.
Therefore, the estimated output of the model overestimates the actual amount of infectivity per kg of gelatine produced from an infected animal with a probability of 99‐100% (almost certain). The main uncertainties associated with the model are shown in Table 4.
Table 4.
Sources of uncertainty associated with the model and their possible impact on the estimates obtained
| Source of uncertainty | Cause of the uncertainty | Impact of the uncertainty on the conclusions |
|---|---|---|
| TSE epidemiology |
|
|
| Infectivity in bones and hides |
|
|
| BSE infectivity cross‐contaminating bones and hides |
|
|
| Homogeneous distribution of BSE infectivity |
|
|
| Inactivation of BSE during gelatine production |
|
|
| Different behaviour of C‐, H‐ and L‐BSE agents |
|
|
| Differences between C&G production processes in reducing infectivity |
|
|
C&G can be produced through different processing standards (acidic, alkaline or heat/pressure processing), which may impact differently on the level of reduction achieved. There is insufficient information to compare specific industrial process parameters with the decontaminating effects of these processes on any prion strain (see Section 3.5), or any data to indicate whether the effects of individual steps can be directly additive or might potentiate or detract from the effects of subsequent steps.
The model only estimates the BSE infectivity contained in the gelatine produced from one infected animal, older than 30 months of age, at the clinical stage of the disease. The residual BSE infectivity remaining in collagen is expected to be lower than that in gelatine. In a scenario for collagen production, where the model only considers infectivity in the hide, cross‐contamination from the brain to the hide, and a higher yield of collagen from hides (29%5), the final amount of infectivity per kg of collagen is much lower: 1.3 × 10−6 (5th–95th percentile: 1.1 × 10−7–1.6 × 10−5 CoID50/kg) in both negligible and controlled risk countries, as most of the infectivity is harboured in the cross‐contaminated bones, instead of in the hide. If it is assumed that collagen is produced only with hides, the collagen obtained would harbour a much lower amount of infectivity than if bones were incorporated into the production.
Collagen is usually made of cattle hides, which are hard to hydrolyse. Thus, the preferred method would be the alkaline one, resulting in a very high pH and a long retention time. According to the model, the alkaline method achieves a greater reduction factor as compared with the acidic method that is more commonly applied to the bones.
For comparison with the results of the model assuming gelatine production from both bones and hides, if the model uses only hides and considers, as a worst‐case scenario, no reduction of infectivity during processing and a higher yield of collagen from hides (29%), the final amount of infectivity per kg of collagen would be 5 × 10−3 (5th–95th percentile: 3.5 × 10−3–1.8 × 10−2 CoID50/kg), which is still more than 15 times lower than the estimate obtained for gelatine using bones and hides and with reduction of infectivity during processing in a MS with negligible risk.
The four files required to reproduce the outputs of the model for collagen can be accessed in the following link:
3.8. The interpretation of the CoID50 in the BSE risk assessment at population level
3.8.1. Situation of BSE in the EU
Despite a potential global underestimation of the epidemiological situation due to the heterogeneity in the sensitivities of the surveillance systems (e.g. outside Europe, passive surveillance is the main approach to detect the disease), the progressive fading out of BSE globally is clear.
Currently, the number of cases of BSE detected through surveillance activities is extremely low. Since 2015 only 27 cases have been reported worldwide, mostly (23) from the EU, whereas in the previous 5‐year period there were about five times more (140) (OIE, online; ProMed, online; EFSA, 2016, 2019). Most of the temporal decline can be explained by the reduction of C‐BSE cases (Figure 8) and, as a consequence, the atypical forms (i.e. H‐ and L‐BSE) are becoming more common than C‐BSE (since 2015 the H‐, L‐ and C‐BSE cases have been, respectively, 13, 9 and 5).
Figure 8.
- Three additional atypical cases (1 from Poland in 2012 and 2 from Switzerland in 2011) were not included as discrimination was not achieved.
In 2017, for the first time since BSE was reported, no cases of classical BSE were reported worldwide, although one case has been reported since. In 2019, four new cases were reported globally, all of them atypical.
Focusing on the European situation, the declining trend of C‐BSE is shown by prevalence data from active surveillance (i.e. the proportion of cases among the cattle subjected to rapid testing): an annual decrease of 39% (p < 0.0001) has been observed over the 2009–2018 period, whereas the proportion of cases per number of tested animals of the two atypical BSE forms remained stable (EFSA, 2019). Currently, the overall prevalence of the different forms of BSE looks to be similar, but the real disease detection capability is unknown. Over time, amendments to the EU surveillance programme, such as the exclusion from rapid testing of healthy slaughter animals, may have led to a certain degree of under‐detection, particularly for H‐BSE and L‐BSE.
Moreover, in Europe the decreasing occurrence of the disease has been further confirmed by the output of modelling exercises. An ad hoc model, the Cattle TSE Monitoring Model (C‐TSEMM) (Adkin et al., 2012, 2016), was developed to evaluate the performance of different BSE monitoring regimes in cattle in the EU. The C‐TSEMM was applied in 2018 (EFSA BIOHAZ Panel, 2018) taking into account diagnostic sensitivity, different MS demographics, the 2017 surveillance testing requirements (no healthy slaughter testing) and testing data from four surveillance streams, i.e. animals clinically suspected of being infected by BSE, healthy slaughtered animals, fallen stock and emergency slaughtered animals (including animals with clinical signs at ante‐mortem inspection).
The C‐TSEMM model showed that, statistically, since 2009 the number of BSE cases in the total population have been declining at an exponential rate, lowering the total number of infected cases contributing to infectivity loads, even though the exponential decline of the C‐BSE cases could be observed from much earlier. Moreover, the output of an additional analysis (Arnold et al., 2017) restricted to BARB cases, i.e. the cases born after the date of entry into force of the EU total feed ban (BARB) (i.e. after 1 January 2001 for ‘old Member States’, and after 1 May 2004 for the central and eastern European countries that which joined the EU on that date), is consistent with their exponential decline in each birth cohort of the nine MS considered in the analysis, including those most affected.
3.8.2. Potential for infectivity in ruminant C&G
At population level, the worst‐case scenario of the estimation of the residual risk can be calculated applying certain assumptions. The infectivity calculated using the probabilistic model (Section 3.7) for the gelatine produced from one infected animal can be extrapolated to estimate the residual infectivity levels, expressed in CoID50, in gelatine produced from all BSE‐infected animals in the EU in a single year that might escape the surveillance system.
The total number of infected cattle that may escape the surveillance monitoring in a single year has been estimated to be equal to 11.4 (2.75th–97.5th percentiles: 3.6–19.8) (EFSA BIOHAZ Panel, 2018), across the whole of the EU. According to the model, the mass of gelatine produced from the bones of an adult animal, slaughtered in a MS with negligible or controlled risk status, is 2.2 and 1.7 kg, respectively. The mass of gelatine produced from the hide of an adult animal slaughtered in any country, according to the estimates used in the model (Section 3.7.2) irrespective of the risk status of the MS, is 5.6 kg. Thus, the total amount of gelatine produced by a single animal is 7.5 kg (7.3–7.8 kg, for countries with negligible or controlled risk status, respectively).
The maximum amount of gelatine that could be produced with the median number of infected animals escaping the surveillance monitoring in the EU in a single year (11) is approximately 82.5 kg. If they were all slaughtered in MS with negligible risk status, the total BSE infectivity contained in such product would be approximately 6.3 CoID50 (5th–95th percentile: 0.66–66.2 CoID50) in the entire EU in a single year, to be diluted within the entire amount of gelatine and collagen peptides produced in the EU annually.
If the median number of infected animals escaping the surveillance monitoring in the EU in a single year (11) were all slaughtered in MS with controlled risk status, the total BSE infectivity contained in such product would be approximately 2.6 × 10−2 CoID50 (5th–95th percentile: 2.4 × 10−3–0.34 CoID50) in the entire EU in a single year, to be diluted within the entire amount of gelatine and collagen produced in the EU annually.
It has to be noted that the estimation of cases by the C‐TSEMM model included animals at different stage of the IP, including non‐clinical. As the level of infectivity in tissues in non‐clinical cases might be lower than in clinical cases, the overall infectivity would result lower than 6.3 CoID50 in MS with negligible risk status and 2.6 × 10−2 CoID50 in MS with controlled risk status.
As the results of the estimations performed show infectivity levels that are very small, the question has been raised of how such low levels of infectivity can be interpreted.
3.8.3. The cattle oral ID50 as a measure of BSE infectivity
In a previous ‘QRA of the animal BSE risk posed by MBM with respect to the residual BSE risk’ (EFSA, 2005c), the interpretation of the CoID50 in the context of risk assessments is thoroughly described. In this opinion, the 2000 SSC opinion on ‘Oral exposure of humans to the BSE agent: infective dose and species barrier’ (SSC, 2000b), in which it was ‘assumed for the time being a linear dose–response curve down to the low dose range’ as a ‘conservative assumption’, was quoted. However, the assumption of a linear dose–response has been questioned by, for example, Gale (1998, 2001). The reason argued by these authors was that it ‘is a worst‐case in assuming that there is no threshold dose for prions’, i.e. that up to a single PrPres molecule could result in infection. It is unknown whether there is a need to have: i) just an individual PrP molecule to initiate the conversion of PrPsen to PrPres or ii) the co‐operative effect of prions at the molecular level, making the quantification of a minimum infective dose or threshold dose unfeasible.
More recent studies, such as the stochastic‐birth death model for scrapie (Fryer and McLean, 2011), have confirmed that only at low dose there is a linear relationship between dose and probability of infection (< ID50), and without any evidence that a threshold for infection exists. Above the ID50, the use of a linear relation for doses will lead to an overestimation of the risk.
There are also host‐specific factors that might influence the susceptibility of an exposed individual, and as a result, the consequences of exposure of an animal to different amounts of infectivity are difficult to predict. This is a significant caveat with respect to the interpretation of the animal BSE risk with respect to the residual BSE risk posed by collagen and gelatine.
The EFSA QRA (EFSA, 2005c) on the BSE risk posed by MBM estimated that on average one case of BSE in the EU, per period of time (and considering an animal population of about 45 million cattle), will occur when the residual risk is around 10−7 CoID50/animal per period of time. Following this reasoning, it can be considered that at EU level any residual risk equal to, or higher than, 10−7 CoID50/animal/period of time represents a risk of disease in cattle.
3.8.4. Contextual interpretation of the model outputs
From a practical point of view, we can estimate the ‘individual risk’ by calculating the ‘total risk/number of individuals exposed’, i.e. by dividing the total BSE infectivity contained in the gelatine produced in the entire EU in a single year by the total cattle population (approximately 42 million individuals6).
According to the outputs of the current model, considering all possible undetected BSE cases in the EU in one single year and the 50th percentile of the total amount of BSE infectivity (6.3 CoID50 for cattle slaughtered in MS with negligible risk and 2.6 × 10−2 CoID50 in MS with controlled risk, respectively, Section 3.8.2), the cattle population of the EU could be exposed on average to up to 1.5 × 10−7 CoID50 per animal and year if all infected animals were slaughtered in MS with negligible risk status and to 6.2 × 10−10 CoID50 if all the infected animals were slaughtered in MS with controlled risk status. These calculations assume a homogeneous distribution of infectivity within a batch and the full utilisation of all raw materials of all undetected BSE‐infected cattle in the EU for the production of gelatine.
Considering all these factors, together with the production of C&G from hides and bones being separated into different batches, further dilutions of any BSE infectivity contained in food and feed (see Section 3.10), as well as the feed ban on ruminant material being incorporated into ruminant feed, that a new case of BSE could not be generated in the cattle population of the EU by this route is considered to be 99‐100% (almost certain).
3.9. The BSE RP arising from the use of ruminant C&G
Currently, bones and hides from non‐ruminant animals fit for human consumption or ABP Category 3 materials derived from non‐ruminants may be used as a raw material for the production of C&G intended to be included in feed for livestock. Thus, collagen or gelatine being used as a protein source in feed could be produced in accordance with Section XIV and XV of Annex III to Regulation (EC) No 853/2004 or in accordance with Regulation (EU) No 142/2011. In line with this mandate, consideration is now being given to the use of ruminant‐derived C&G as a feed for non‐ruminants.
The production of C&G is always separated by species of origin (pig gelatine, bovine collagen, etc.), to furnish the requirements of halal or kosher food markets, and mandatory food traceability in the food industry. So, it would be feasible to identify non‐ruminant C&G for their use as a protein source in feed, but due to considerations, such as the high cost of production (Vermeulen (GME), by email on 18 March 2020) and the uncertain nutritional value (Khalaji et al., 2016; Asadi Kermani et al., 2017), they are currently not used in compound feed for any livestock.
C&G in their pure state, and intended for human use, may occasionally be recategorised as ABP‐produced C&G for commercial reasons for their use in compound feed for livestock. In addition, former foodstuffs containing collagen or gelatine of non‐ruminant origin are sometimes recycled for their use as an animal feed, but, according to the industry associations, there are major constraints on this use. Gelatine (generally of porcine origin) is used in feed for the micro‐encapsulation of vitamins, and collagen peptides are used as a supplement for dogs and horses to improve joint health, nutrition and digestion, among other benefits described in marketed products. According to the manufacturers, some collagen production surplus is sold to pet food producers and it is possible to find pet food advertised with the message ‘containing collagen’ in the market.7
No collagen or gelatine is currently produced from Category 3 animal by‐products in Europe (Vermeulen (GME), by email 19 December 2019; Bierwagen (CCTA), by email on 27 January 2020; CCTA, 2011). So, products used for feed, pet food or technical purposes all meet the standards of food‐grade C&G.
If the legislation is changed to allow ruminant‐derived C&G to be used in feed for non‐ruminant animals, it is possible that the use of this material could increase and that C&G could be produced under the ABP legislation as well as the food legislation.
Taking this into account, there are three potential RP for cattle of the use of ruminant collagen/gelatine in feed intended for non‐ruminant animals, including aquaculture animals, once the C&G have been produced from animals fit for human consumption or ABP:
Risk pathway 1: C&G produced under Regulation (EC) No 853/2004 and used in foodstuffs that are then recycled to produce a bread meal for animal feed (relevant to ToR1).
Risk pathway 2a: C&G produced under Regulation (EC) No 853/2004 and used in feed as a technological additive (to encapsulate vitamins) or nutritional additive (supplement for dogs and horses), or as a component of compound feed (relevant to ToR1).
Risk pathway 2b: C&G produced under Regulation (EU) No 142/2011 and used in feed as a technological additive (to encapsulate vitamins) or nutritional additive (supplement for dogs and horses), or as a component of compound feed (relevant to ToR2).
3.9.1. Risk pathway 1
Collagen or gelatine for human consumption must be produced in accordance with Section XIV and XV of Annex III to Regulation (EC) No 853/2004. So, this risk pathway is related to ToR1 of this mandate.
The extent to which former foodstuffs containing non‐ruminant collagen or gelatine are currently used in livestock feed is not clear. According to the European Former Foodstuff Processors Association (EFFPA), such foodstuffs are often not incorporated into livestock feed because of the logistical difficulties of separating former foodstuffs containing collagen or gelatine of non‐ruminant origin from former foodstuffs containing collagen or gelatine of ruminant origin. The possibility of being allowed to recycle former foodstuffs containing ruminant C&G and mixing them with former foodstuffs containing non‐ruminant C&G may be an incentive for Food Business Operators (FBO) to consider using more ruminant‐derived C&G in food.
According to the feedback received from stakeholders, food processors currently pay for the disposal of waste foodstuffs containing ruminant C&G, generally through biogas plants. The food industry claims that 100,000 tonnes8 of former foodstuff containing ruminant collagen and/or gelatine must be disposed of annually even though, on average, the gelatine content in any final food does not exceed ± 2% on average, with the exception of certain types of confectionery (up to 8% gelatine) (van den Brink (EFFPA), by email on 21 February 2020). It is not envisaged that such small amounts of C&G would be retrieved for use as a protein source, if the feed ban was lifted, not least because of the cost of achieving this, and the unpredictability of its availability. However, some former foodstuffs (e.g. biscuits, bread, breakfast cereals, chocolate bars, pasta, savoury snacks and sweets) are typically used in feed because of their high energy content in the form of sugars, oils and starch (EFFPA, 2019) rather than the proteins (including C&G) these meals can contain. The transformation of these former foodstuffs containing C&G would result in a ‘bread meal’ or ‘biscuit meal’ that could be added as an ingredient to compound feed or fed directly to non‐ruminant livestock.
Section 10, Chapter II Annex X of Commission Regulation (EC) No 142/2011 states that ‘Category 3 material comprising of foodstuffs containing products of animal origin originating from MS that are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise’, referred to in Article 10(f) of Regulation (EC) No 1069/2009, may be placed on the market for feeding to farmed animals, other than fur animals, without further treatment, if the material:
has undergone processing as defined in Article 2(1)(m) of Regulation (EC) No 852/2004 or in accordance with this Regulation;
is composed of or contain one or more of the following Category 3 materials referred to in Article 10(f) of Regulation (EC) No 1069/2009: milk, milk‐based products, milk‐derived products, eggs, egg products, honey, rendered fats, collagen, gelatine;
has not been in contact with any other Category 3 materials; and
all necessary precautions have been taken to prevent the contamination of the material’.
However, while allowed under the ABP Regulations, the use of this material is currently prohibited under EU Regulation No 999/2001 for feeding livestock if it contains collagen or gelatine derived from ruminants.
This risk pathway would encompass the cross‐contamination of ruminant feed with non‐ruminant feed that contains a bread meal extracted from former foodstuffs containing BSE‐infected food‐grade collagen or gelatine.
3.9.2. Risk pathway 2a
When either collagen or gelatine produced under Regulation (EC) No 853/2004 are being used as feed rather than food, they come under the ABP Regulations. However, since they are being produced under Regulation (EC) No 853/2004, this risk pathway is related to ToR1 of this mandate.
The extent to which C&G in its pure form are rejected for use in food and are instead diverted for use in compound feed is uncertain but based on information provided by the industry it is extremely unlikely.
Gelatine of non‐ruminant origin is currently used in feed as an additive. Collagen or gelatine of non‐ruminant origin may also occasionally be diverted for their use as a feed when they are rejected by a food business operator. While it is generally not economical to use C&G in compound feed, this could change in the future. If the legislation is changed, C&G of ruminant origin could be used.
Nevertheless, it is unclear to what extent, if any, ruminant derived C&G produced under Regulation (EC) No 853/2004 might be used for the production of compound feed if the legislation is changed and if commercial conditions change.
This risk pathway would encompass the cross‐contamination of ruminant feed with non‐ruminant feed that contains food‐grade collagen or gelatine (as a encapsulation agent for vitamins supplement or as a protein source) that may contain BSE‐infectivity.
3.9.3. Risk pathway 2b
If collagen or gelatine were to be produced for use as a feed ingredient under Regulation (EC) No 142/2011, it is not clear as to what types of feed the collagen and/or gelatine would be incorporated and in what proportion C&G would be included in such feedstuffs.
There are strict criteria set out in relation to the production and use of C&G in compound feed for non‐ruminants, which would apply to both C&G produced under Regulation (EC) No 853/2004 and under Regulation (EC) No 142/2011.
Collagen or gelatine that are being used as feed must also comply with the requirements for feed labelling as set out in Commission Regulation (EU) No 2015/1905 of 22 October 2015 amending Annex II to Regulation (EC) No 183/2005 of the European Parliament and of the Council and the requirements of Commission Regulation (EU) No 68/2013.
If the legislation is changed and C&G of ruminant origin could be added to compound feed for species other than ruminants, including aquaculture, this might serve as an incentive for using ABP as a starting material. Vitamins and collagen supplements could also be produced under Regulation (EC) No 142/2011.
This risk pathway would encompass the cross‐contamination of ruminant feed with non‐ruminant feed that contains ruminant collagen or gelatine that have been produced from ABPs containing BSE agent/s and are used as a vitamin supplement or as a protein source, and is therefore related to ToR2 of this mandate.
The conceptual framework of the three RP is shown in Figure 9.
Figure 9.
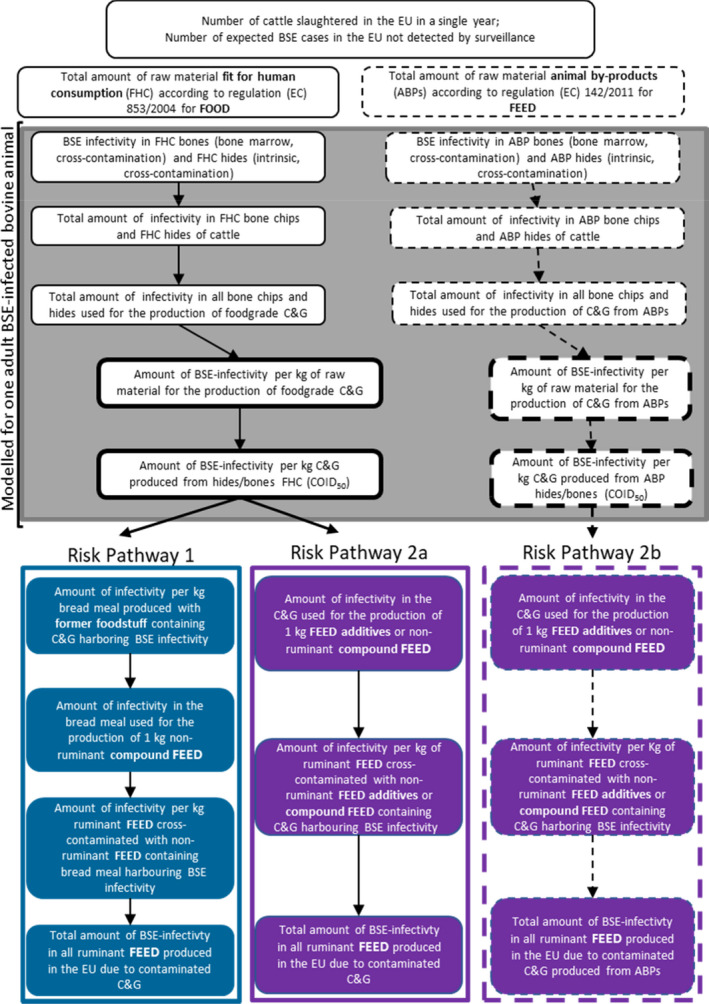
Diagram of the risk pathways identified in the assessment
3.10. Characterisation of the BSE cattle risk in the RP
3.10.1. The characterisation of the risk at the end of RP 1
For risk pathway 1, the only piece of data available is the estimated ‘100,000 tonnes of former foodstuffs containing ruminant gelatine that currently go for disposal and therefore are under‐utilised within the EU, as ruminant gelatine is prohibited in feed’, as described in the scoping paper entitled Reducing food waste by lifting the feed ban on ruminant gelatine written by the GME, EFFPA, FEFAC (European Feed Manufacturers’ Federation) and FoodDrinkEurope, and referred to in the background of the mandate. However, there is no reference on how this figure was calculated at the time of producing the scoping paper and how this figure could have varied in the last 10 years.
There are several data gaps and uncertainties identified in relation to some of the factors characterising RP 1, such as: which specific foodstuffs contain C&G; what is the average content of C&G in them; the total production of foodstuffs containing C&G in the EU; the proportion of former foodstuffs containing C&G that is currently processed as bread meal in the EU; the total amount of foodstuffs containing C&G that are currently disposed of and could be recycled as bread meal if the feed ban was lifted; and whether, and if so, to what extent, the bread meal production process reduces the BSE infectivity.
The uncertainties and data gaps mentioned above preclude the execution of a full quantitative assessment of the risk posed by the entire risk pathway. However, using the outputs of the model, some calculations on the amount of BSE infectivity along RP 1 have been made, applying certain assumptions on the dilution of infectivity in the batch production.
Using the data from the model, if we assume that the weight of a positive animal at slaughter is 550 kg (P1), the weight of bones (P2) available for collagen or gelatine production would be 9% of the live weight in animals from negligible and 6.7% in animals from controlled risk countries, and the weight of hides (P3) 7% of live weight regardless of the risk status of the country, equating to 49.5 kg or 36.8 kg of bones for negligible and controlled risk countries, respectively, and 38.5 kg of unsplit hide.
As detailed in Section 3.6.4, the tonnage of material used to make a batch of gelatine can vary from 1–120 tonnes of hide (i.e. hides from approximately 26 to 3,116 animals) or 15‐160 tonnes of bones (i.e. bones from approximately 303 to 3,232 animals).
In the case that raw materials obtained from one infected animal entered production in a standard batch of hides (Figure 10), according to the assumptions made in the model, this would mean that the infectivity per kilogram of final commercial gelatine in the batch would be 9.6 × 10−8 CoID50/kg for the minimum batch size, and 8 × 10−10 CoID50/kg for the maximum batch size. For gelatine produced with bones only, the infectivity would be between 8.8 × 10−4 and 8.3 × 10−5 CoID50/kg in negligible risk countries and 3.2 × 10−6 and 3 × 10−7 CoID50/kg gelatine in controlled risk countries, respectively (Table 5).
Figure 10.
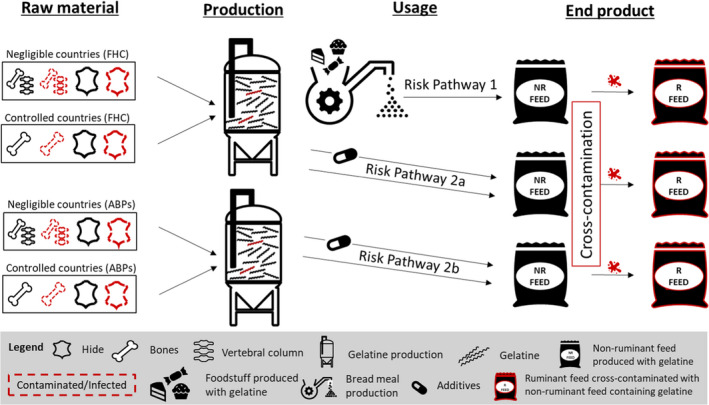
- FHC = fit for human consumption, ABPs = animal by‐products.
Table 5.
Amount of CoID50 (50th percentile) in batches of gelatine produced with raw materials from one infected cow as described in the probabilistic model and in former foodstuffs produced with the gelatine, respectively
| Hide | Bones (negligible) | Bones (controlled) | |
|---|---|---|---|
| Weight of raw materials (550 kg bovine animal) [kg] | 38.5 | 49.5 | 36.85 |
| Yield of gelatine [%] | 14.5 | 4.5 | 4.5 |
| Amount of gelatine produced with the raw materials from one bovine animal (550 kg) [kg] | 5.6 | 2.2 | 1.7 |
| Infectivity per kg gelatine produced with raw materials from one infected animal [CoID 50 ] | 2.5 × 10−6 | 2.7 × 10−1 | 1.3 × 10−3 |
| Infectivity in all gelatine produced with raw materials from one infected animal [CoID 50 ] | 1.4 × 10−5 | 6 × 10−1 | 2.2 × 10−3 |
| Batch size [kg] | 1,000–120,000 | 15,000–160,000 | 15,000–160,000 |
| Infectivity per kg gelatine in a batch produced with raw materials from one infected animal [CoID 50 /kg gelatine] | 9.6 × 10−8 to 8 × 10−10 | 8.8 × 10−4 to 8.3 × 10−5 | 3.2 × 10−6 to 3 × 10−7 |
| Infectivity per kg of former foodstuff with 2% gelatine from a batch produced as above [CoID 50 /kg former foodstuffs] | 1.9 × 10−9 to 1.6 × 10−11 | 1.8 × 10−5 to 1.7 × 10−6 | 6.4 × 10−8 to 6 × 10−9 |
Given the epidemiological presentation of BSE in the EU, the probability that more than one infected animal will end up in the same batch for gelatine production, and that the final amount of BSE infectivity per batch will be higher than the estimates above and in Table 5 is considered < 1% (almost impossible). Nevertheless, the worst‐case scenario that includes the use of all BSE‐infected animals in a single year in the EU for the production of C&G has been expanded on in Section 3.8.1.
Currently, food‐grade C&G are added to many foods, food supplements and feed supplements. The presence of these ingredients of ruminant origin in former foodstuff limits the recycling of surplus stocks into non‐ruminant feed. Such foodstuffs rendered into ‘bread meal’ to be used as a carbohydrate ingredient (see Section 3.9.1) are currently only allowed to contain non‐ruminant C&G.
If the average gelatine content of foodstuff is 2% (20 g/kg) and the gelatine included in the foodstuff was sourced from the batch of gelatine produced with hides (1,000–120,000 kg) including one infected animal, as described above, the amount of infectivity contained in 1 kg of that foodstuff would be 1.9 × 10−9 CoID50, if sourced from the minimum‐sized batch, or 1.6 × 10−1 CoID50, if sourced from the maximum‐sized batch.
If the average gelatine content of foodstuff is 2% (20 g/kg) and the gelatine included in foodstuff was sourced from the batch of gelatine produced with bones (15,000–160,000 kg) including one infected animal, as described above, the amount of infectivity would range between 1.8 × 10−5 and 1.7 × 10−6 CoID50, if sourced from the minimum and maximum‐sized batch, respectively, in animals from MS with negligible risk status, and 6.4 × 10−8 and 6 × 10−9 CoID50, if sourced from the minimum and maximum‐sized batch, respectively, in animals from controlled risk countries (see Table 5).
Such foodstuffs, rendered into ‘bread meal’, would undergo a further dilution of the infectivity via the processing of the former foodstuffs (temperature and drying) and inclusion into a batch of bread meal made from multiple sources, types and quantities of former foodstuffs, most of them not containing any infectivity. It is uncertain if the process for producing biscuits and other foodstuffs using collagen or gelatine would reduce the level of BSE infectivity if the collagen or gelatine is derived from a BSE‐infected animal and is used as an ingredient. Likewise, it is not known if the foodstuff production process would have any effect on the level of BSE infectivity contained in the raw collagen or gelatine added as an ingredient.
Thus, in RP 1, if ruminant feed is cross‐contaminated with non‐ruminant feed containing this bread meal produced from former foodstuffs made with gelatine from an infected batch, even with high levels of cross‐contamination, the amount of infectivity will be significantly diluted.
3.10.2. The characterisation of the risk at the end of RP 2a and 2b
There are several data gaps and uncertainties identified in relation to feed additives in RP 2a and 2b, such as: which specific feedstuffs contain encapsulated/protected vitamins or other additives produced using gelatine or supplements containing collagen; what is the average content of C&G in those vitamins or supplements; the total production of feedstuffs containing vitamins or supplements in the EU; the proportion of all vitamins or supplements for feed used in the EU that contain C&G; whether and, if so, to what extent the production process of the vitamins or supplements reduces the BSE infectivity; and if the usage of ruminant gelatine in vitamin capsules or collagen in supplements would increase after a lifting of the feed ban.
There are also several data gaps and uncertainties identified in relation to collagen or gelatine added to feed as a protein source in RP 2a and 2b, such as: the changes in the production of C&G out of ABPs in the EU as a result of a possible lifting of the feed ban; the future total production of feedstuffs containing C&G out of ABPs in the EU; the proportion of C&G out of ABPs included in feedstuff, and whether and, if so, to what extent the production process of the compound feed reduces the BSE infectivity.
It is not clear what the future total production of feed containing C&G in the EU under Regulation (EC) No 142/2011 would be, either as capsules for vitamins, protein supplements or for their incorporation into compound feed if the ban on the use of ruminant raw materials was lifted. According to the industry, it is very unlikely that collagen or gelatine would be used as a protein source in feed for livestock, even if it was authorised for this purpose. If collagen and/or gelatine were to be produced for use as a feed under Regulation (EC) No 142/2011, it is not clear to what extent the process for producing C&G under the Regulation would reduce the BSE infectivity. As legal requirements for producing C&G under the ABP Regulations are very similar to those set out in Regulation (EC) 853/2004, it is likely that the level of reduction would be very similar.
Currently, gelatine is used to provide feed technological additives. Collagen supplements derived from non‐ruminants are used for dogs and horses for the treatment or prevention of joint, and other, problems, as nutritional additives. Given these very specific uses, it is likely that any collagen or gelatine contaminated with the BSE agent would be greatly diluted during the manufacturing process.
According to industry information, it is extremely unlikely that collagen or gelatine will be used as a protein source in feed for livestock due to the low profitability and questionable nutritional value, even if it is authorised for this purpose. Therefore, the cross‐contamination of ruminant feed with BSE‐infected collagen or gelatine through this route represents an extremely unlikely risk pathway for cattle, even if the feed ban, as postulated in the ToRs, was lifted.
3.10.3. Cross‐contamination of ruminant feed with non‐ruminant feed
As set out in Section B of Annex IV of EU Regulation No 999/2001, compound feed intended for non‐ruminant farmed animals shall be produced in establishments that do not produce compound feed for ruminants. By way of a derogation from this, the production of compound feed for non‐ruminants in feed mills that also produce compound feed for ruminants may be permitted by the competent authority if the manufacturing, storage, transport and packaging of the ruminant and non‐ruminant feed is completely separate. The manufacturers are obliged to keep detailed records of the purchase of the ABP used in these premises for at least 5 years. They are also obliged to carry out regular testing of the compound feed intended for ruminants to verify the absence of unauthorised constituents of animal origin.
In addition to EU Regulation No 999/2001, there are several other Regulations in force to monitor the feed chain for compliance with the feed ban (Commission Regulation No 51/2013 and Commission Regulation (EC) No 691/2013 amending Regulation (EC) No 152/2009). The correct application of such Regulations would in principle allow the detection of cross‐contamination events.
Despite these regulatory requirements, cross‐contamination of ruminant feed with non‐ruminant feed could occur both at feed mill level and at farm level (AFSSA, 2001). Some mixed feed mills produce feed for both monogastric and ruminant species and are potentially vulnerable to cross‐contamination events, even when completely separate processing lines are used, as the EU Regulation requires. In addition, the specialisation of feed mills is not an economically feasible option in some countries or regions with low animal density (Ducrot et al., 2013).
Overall, the probability of contamination of ruminant feed with non‐ruminant feed containing collagen or gelatine at feed mills is considered to be 1–5% (extremely unlikely), and if it occurred, the amount of cross‐contaminated material would be extremely low, as there will be a considerable dilution of any BSE‐infected collagen or gelatine due to mixing with other feed materials in the production of animal feed.
3.10.4. Exposure of cattle to BSE infectivity and final risk
The feeding of cattle with ruminant feed cross‐contaminated during production by non‐ruminant feed containing collagen or gelatine (Figure 10) is not the only exposure route. As explained in the EFSA Opinion on the QRA of the BSE risk posed by PAP (EFSA BIOHAZ Panel, 2018), farms with mixed species may take delivery of compound feeds for different species (or mix their own). Contamination could possible occur through deliberate or accidental feeding of the wrong food by the farmer, inappropriate storage of feeds (e.g. poor segregation), or poor facilities, which could lead, for example, to animal breakouts, giving cattle access to the wrong feed.
Under Section B of Annex IV of EU Regulation No 999/2001, the use of compound feed containing ABPs, such as fishmeal, that is currently permitted in non‐ruminant feed, is only allowed on farms where only non‐ruminants are kept. Moreover, home compounders using such feed must be registered with the competent authority. Due to these requirements, the contamination of ruminant feed with non‐ruminant feed containing collagen or gelatine on farm is also considered to be 1–5% (extremely unlikely).
Cattle gaining accidental access to pet food containing collagen or gelatine could be another example of this route of exposure. However, while feed for different farmed animals (e.g. pigs and cattle) are likely to be stored close to each other on the same premises, pet food is likely to be stored and handled separately. Pets are more likely to share the same environment as the human residents, rather than that of the farmed animals, which are usually well confined.
If any batch of animal feed containing BSE infectivity, due to cross‐contamination or error, is consumed by cattle, it would be consumed by a number of animals rather than one, therefore reducing the possibility that an infectious dose would be consumed by a single animal.
As stated in Section 3.8.2, under the worst‐case scenario of all the BSE cases in the EU in 1 year being devoted to the production of collagen or gelatine, and cattle being exposed to all of the resulting infectivity, the threshold to generate one new case of BSE in the EU cattle population in a single year would not be exceeded.
The final BSE risk to cattle via any of the three RP detailed in the previous sections is affected by additional factors such as the temporal (due to lack of clustering of cases, no multiple cattle are slaughtered at the same time) and geographical (multiple countries report cases of BSE in the EU) distribution of the exposure to the entire amount of infected material, and the individual host response to exposure, that will also affect the outcome.
Finally, as described earlier, the dilution of collagen or gelatine containing BSE infectivity also plays a significant role, as only a fraction produced from one or all infected animal/s in the EU would be part of the same batch of feed for non‐ruminants and only a very small proportion of the feed for non‐ruminants could cross‐contaminate a batch of ruminant feed.
In summary, if ruminant feed was cross‐contaminated with non‐ruminant feed containing ruminant collagen or gelatine from a BSE‐infected batch, either as former foodstuffs recycled for animal feed produced under Regulation (EC) No 853/2004, or as additives or as a protein source, produced under Regulation (EC) No 853/2004 or under Regulation (EC) No 142/2011, the probability that no new case of BSE in the cattle population would be generated through any of the three risk pathways is 99‐100% (almost certain), given the estimated amount of BSE infectivity to which cattle would be exposed.
3.10.5. Concluding remarks for Sections 3.7, 3.8, 3.9 and 3.10
A probabilistic model was developed to assess a worst‐case scenario in which one adult BSE‐infected animal older than 30 months of age at clinical end‐stage escapes on‐farm and slaughterhouse detection by the surveillance system (clinical suspect or fallen stock) and is consequently slaughtered and its bones and hide used to produce gelatine.
In the model, it is assumed that the infectivity contained in raw bones and hide come from the intrinsic infectivity contained in the target tissues, and from cross‐contamination by other tissues, from the same animal, during slaughter and processing.
The model produces two different sets of outputs depending on the BSE risk status (negligible vs controlled) of the MS. A different list of SRM in the two situations may affect the residual amount of potential for cross‐contamination. As an example, for controlled risk countries, the removal of the vertebral column implies the removal of a substantial amount of infectivity.
Some of the assumptions of the model correspond to worst‐case scenarios, and when data were available, the boundaries of probability distributions in the parameters describing uncertainty were selected to approximate to worst‐case scenarios. As a result, the model overestimates the actual amount of infectivity per kg of gelatine produced from an infected animal.
Based on the model, in a negligible risk country the estimates for the three outputs were respectively: 7.6 × 10−2 CoID50/kg gelatine (5th–95th percentile: 8 × 10−3–0.8 CoID50/kg); 1.7 infected animals needed to reach 1 CoID50 (5th–95th percentile: 0.1–16 infected animals); and 13.1 kg of gelatine from infected animals needed to reach 1 CoID50 (5th–95th percentile: 1.2–125.3 kg).
The corresponding figures in a controlled risk country were respectively: 3.1 × 10−4 CoID50/kg (5th–95th percentile: 2.9 × 10−5–4.1 × 10−3 CoID50/kg); 449.8 infected animals (5th–95th percentile: 33.8–4,745 infected animals); and 3,257 kg (5th–95th percentile: 244.9–34,360 kg).
The sensitivity analysis showed that the parameters ‘BSE infectivity in spinal cord and DRG per g’ and ‘Reduction of infectivity due to the processing of bones’ were the parameters that most affected the outputs of the model.
The current occurrence of any form of BSE in the EU is extremely low and the number of infected cattle that may go undetected each year is estimated to be equal to 11.4 (2.75th–97.5th percentiles: 3.6–19.8). If all the undetected BSE cases in the EU in a single year contributed raw material to the production of gelatine, the 50th percentile of the total infectivity in the gelatine obtained would be 6.3 CoID50 and 2.6 × 10−2 CoID50, if the animals were slaughtered in negligible or controlled risk countries, respectively.
At individual animal level, the consequences of exposure to the maximum amount of BSE infectivity produced in the EU in a single year via C&G are difficult to predict. Applying a linear dose response at low dose levels the average amount of infectivity to which every individual in the 42 million cattle in the EU would be exposed to in one single year would be below a previously estimated threshold of 10−7 CoID50/animal per year, required to generate a new case of BSE.
Once the C&G have been produced, if ruminant C&G (produced from animals fit for human consumption or ABP) are authorised for inclusion in feed for non‐ruminants, including aquaculture animals, there are three potential RPs identified from their use: two apply to C&G produced under the Regulation (EC) No 853/2004 and used in foodstuffs (relevant to ToR1) or in feed (relevant to ToR1); the third one refers to C&G produced under the Regulation (EU) No 142/2011 and used in feed (relevant to ToR2).
There are data gaps and uncertainties in relation to several of the factors characterising the three RPs, mainly on how the C&G is or would be used in feed, which preclude the execution of a full quantitative assessment of the risk posed by the entire RP.
The dilution effect of one infected animal in a batch of gelatine was estimated within the outputs of the model, showing that the residual infectivity per kg of gelatine in the batch would be extremely low, either if produced from hides only or from bones only, or both.
The last steps in the RP make it evident that an additional dilution effect of any residual infectivity from C&G included into non‐ruminant feed will occur through the potential cross‐contamination of ruminant feed.
Finally, the BSE risk to cattle through exposure to any residual BSE‐infectivity in feed will be also affected by additional factors such as the geographical and temporal distribution of cattle exposure to the entire infected material, as well as the individual host response to exposure to BSE infectivity.
Overall, it was concluded that the probability that no new case of BSE in the cattle population would be generated through any of the three RPs is 99‐100% (almost certain), given the estimated amount of BSE infectivity to which cattle would be exposed.
3.11. Other sources of uncertainty
Apart from the sources of uncertainty (presented in Section 3.7.4) related to the assumptions and factors included in the model and that can affect the response to AQ2 and AQ3, other sources of uncertainty were identified, not related to the model outcomes, that can mainly affect the responses to AQ1 and AQ4 (Table 6).
Table 6.
Other sources of uncertainty not related to the model and their possible impact on the outcome of the assessment
| Source of uncertainty | Cause of the uncertainty | Impact of the uncertainty on the conclusions |
|---|---|---|
| Raw materials used for C&G production |
|
|
| Differences between the level of infectivity in the raw materials used to produce C&G under the ABP Regulation compared with the Regulation (EC) No 853/2004 |
|
|
| Total amount of former foodstuffs containing C&G and the content of C&G in specific former foodstuffs |
|
|
| Impact of the process for producing food and ‘bread meal’ on BSE infectivity |
|
|
| Impact of the process for producing supplements on BSE infectivity |
|
|
| Impact of the process for producing compound feed on BSE infectivity |
|
|
| Use of ruminant C&G and former foodstuffs containing ruminant C&G in animal feed for non‐ruminants if the ban is lifted |
|
|
| Cross‐contamination of ruminant feed with feed for non‐ruminants |
|
|
| Dose‐response relationship of BSE infectivity expressed via CoID50 |
|
|
4. Answers to the ToRs
AQ1 – What are the RP for cattle from the use of ruminant C&G in feed for non‐ruminant animals?
-
Taking into account the background information on processes for the industrial production of C&G and their possible addition to animal feed, there are three plausible RPs for cattle from the use of ruminant C&G for non‐ruminant feed:
-
–
Risk pathway 1: C&G produced under Regulation (EC) No 853/2004 and used in former foodstuffs that are then recycled to produce a ‘bread meal’ for animal feed (relevant to ToR1).
-
–
Risk pathway 2a: C&G produced under Regulation (EC) No 853/2004 and used in feed, either as technological or nutritional additives, or as a component of compound feed (relevant to ToR1).
-
–
Risk pathway 2b: C&G produced under Regulation (EC) No 142/2011 and used in feed, either as technological or nutritional additives, or as a component of compound feed (relevant to ToR2).
-
–
AQ2 – What is the amount of infectivity in C&G produced from an infected animal at clinical stage?
According to a probabilistic model that was developed to estimate the BSE infectivity load, the estimated 50th percentile of the amount of BSE infectivity per kg of gelatine extracted from the bones and hide of one adult animal with clinical BSE (C, H or L), slaughtered in a MS with negligible risk status, is 7.6 × 10−2 CoID50/kg gelatine (5th–95th percentile: 8 × 10−3–0.8 CoID50/kg), and 3.1 × 10−4 CoID50/kg (5th–95th percentile: 2.9 × 10−5–4.1 × 10−3 CoID50/kg) in a MS with controlled risk status. Given that worst‐case scenarios were considered in some assumptions of the model and that several input variables were approximated to worst‐case estimates, the probability that the infectivity is overestimated by the model is 99–100% (almost certain).
Currently, only hides are used for the production of collagen in the EU. If the model only considers infectivity in the hide and cross‐contamination from the brain to the hide within the same animal, assuming higher yield and the same reduction factor, the final amount of infectivity per kg of collagen is much lower than for gelatine (for which infectivity in the hide and the bones and cross‐contamination from the brain to the hide and from the spinal cord and DRG to the bones also have to be considered). The estimate of the amount of BSE infectivity per kg of collagen is 11.3 × 10−6 (5th–95th percentile: 1.1 × 10−7–1.6 × 10−5 CoID50/kg) in both negligible and controlled risk countries. For comparison as a worst‐case scenario, with no reduction of infectivity during processing, the final amount of infectivity per kg of collagen would be 5 × 10−3 (5th–95th percentile: 3.5 × 10−3–1.8 × 10−2 CoID50/kg), which is still more than 15 times lower than the estimate obtained for gelatine using bones and hides and with reduction of infectivity during processing in a MS with negligible risk.
AQ3 – How many infected animals have to be processed and how many kg of C&G have to be produced from infected animals to accumulate 1 CoID 50 ?
-
Using the probabilistic model estimates of BSE infectivity per kg, the following two outputs have been calculated for gelatine:
-
–
The 50th percentile estimate of the number of infected animals required to produce gelatine containing 1 CoID50 is 1.7 animals (5th–95th percentile: 0.1–16) in a MS with negligible risk status, and 449.8 animals (5th–95th percentile: 33.8–4,745) in a MS with controlled risk status.
-
–
The 50th percentile estimate of the number of kg of gelatine from infected animals required to accumulate 1 CoID50 is 13.1 kg (5th–95th percentile: 1.2–125.3 kg) in a MS with negligible risk status and 3,257 kg (5th–95th percentile: 244.9–34,360 kg) in a MS with controlled risk status.
-
–
Some of the assumptions of the model correspond to worst‐case scenarios and, when data were available, the boundaries of probability distributions in the parameters describing uncertainty were selected to approximate to worst‐case scenarios. As a result, the probability that the number of infected animals which have to be processed and the number of kg of gelatine which have to be produced from infected animals to accumulate 1 CoID50 are greater than the estimates, is considered to be 99–100% (almost certain).
-
–
For collagen, the number of infected animals that have to be processed, and the number of kg of collagen that have to be produced from infected animals, to accumulate 1 CoID50 would be much higher than those for gelatine, given the lower amount of BSE infectivity per kg in the raw materials.
AQ4: What is the residual BSE infectivity in the feed at the end of the RPs?
The procedures for separation, storage and prevention of cross‐contamination of Category 3 materials in a slaughterhouse and the production process for C&G under the ABP Regulations are similar to those for raw materials being used for the production of C&G under Regulation (EC) No 853/2004.
There is no evidence to support the contention that the BSE risk to cattle from ruminant C&G produced using Category 3 ABP raw materials (as referred to in Article 10 of Regulation (EC) No 1069/2009 and produced in accordance with Regulation (EU) No 142/2011) would be different than the BSE risk from ruminant C&G produced from raw materials fit for human consumption, and produced according to Regulation (EC) No 853/2004.
The probabilistic model developed within this mandate has attempted to quantify the amount of BSE infectivity associated with the manufacture of collagen or gelatine before they are used as food, or by the feed industry.
There are multiple events along the three RP identified for cattle that will result in the dilution of the maximum amount of infectivity contained in the collagen or gelatine produced from an infected animal. Moreover, the final BSE risk to cattle is also affected by additional factors such as the temporal and geographical distribution of the exposure to the entire amount of infected material, and the individual host response to exposure.
Taking these multiple events into account, together with the results of the model, the current epidemiological situation in the EU, the statutory controls in place, and accounting for uncertainties (regardless of whether they are included in the model or not), it was concluded that the probability that no new case of BSE in the cattle population would be generated through any of the three RPs is 99–100% (almost certain), given the estimated amount of BSE infectivity to which cattle would be exposed. This conclusion remains valid, even if all the estimated undetected BSE cases in the EU in a single year were used for the production of collagen or gelatine, either using raw materials fit for human consumption or Category 3 ABP raw materials.
5. Recommendations
-
The BSE risk posed by the use of ruminant C&G in feed directly depends on the prevalence of the BSE agents (C‐BSE, H‐BSE and L‐BSE) in the cattle population. It is recommended:
-
–
to maintain the current EU‐wide surveillance system in order to: (1) monitor the final stages of the BSE epidemic; (2) detect a potential re‐emergence of BSE; and (3) detect new BSE forms in cattle, should they appear.
-
–
to use the C‐TSEMM model on an annual basis with updated data in order to monitor the ability of the current surveillance system to detect BSE at both MS and EU level.
-
–
To evaluate the impact of the specific industrial processes employed by the industry for the production of C&G on the infectivity of naturally occurring BSE agents. In particular, to undertake research activities aimed at the production of new data measuring the sensitivity/resistance of cattle BSE agents (C‐, H‐ and L‐BSE independently, but in parallel) to a variety of standard processing methods (e.g. acid and alkaline treatments and heat), and, when possible, to specifically quantify the RF achievable.
To undertake research activities aimed at the production of new data regarding the susceptibility of cattle to infection with H‐BSE or L‐BSE via the oral route, and the quantitative distribution of infectivity in tissues of cattle preclinically and clinically affected with H‐ and L‐BSE. The current lack of such data poses a major limitation for the assessment of the probability of exposure to prion disease associated with the use of cattle‐derived materials in food and feed.
To periodically reassess the risks addressed in this opinion, should the current ban be lifted in the future, therefore changing the relative plausibility and likelihood of RP 1, 2a and 2b.
Abbreviations
- ABP
animal by‐products
- AQ
assessment question
- BARB
cases born after the date of entry into force of the EU total feed ban
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- C&G
collagen and gelatine
- CC
cross‐contamination
- CCTA
Collagen Casings Trade Association
- CNS
central nervous system
- C i.c. ID50
cattle intracerebral infectious dose
- CoID50
cattle oral infectious dose
- C‐TSEMM
Cattle TSE Monitoring Model
- DRG
dorsal root ganglia
- EFFPA
European Former Foodstuff Processors Association
- FBO
food business operator
- FEFAC
European Feed Manufacturers’ Federation
- GALT
autonomic ganglia and gut‐associated lymphoid tissue
- GME
Gelatine Manufacturers of Europe
- GTH
glyceroltriheptanoate
- IP
incubation period
- MBM
meat‐and‐bone meal
- MS
Member States
- NCC
no cross‐contamination
- PAP
processed animal proteins
- PrPC
normal cellular prion protein
- PrPres
Protease K resistant prion protein
- PrPSc
abnormal protease resistant isoform of prion protein
- PrPsen
Protease K sensitive prion protein
- QRA
quantitative risk assessment
- RF
reduction factors
- RP
risk pathway
- SRM
specified risk materials
- SSC
Scientific Steering Committee
- ToR
Terms of Reference
- TSE
transmissible spongiform encephalopathy
- UHT
ultra‐high temperature
- vCJD
variant Creutzfeldt‐Jacob disease
- WG
working group
Appendix A – Contacts with the industry
1.
To gain insight into the current practices associated with C&G production in the European Union, a number of informal written and/or verbal communications took place with different industry stakeholders.
The questions sent to the industry stakeholders were adapted for each type of production or area covered by the company or association. In general, for associations, they included:
Which of these two proteins is used/produced in the industry: collagen or gelatine?
What is the end use of the C&G in the … industry?
What was the volume (approx..) of C&G used in the … industry in 2019?
What was the volume of C&G (approx.) used in the … industry (as ingredient) in the last five years in the European Union?
What is the current use of food waste containing collagen or gelatine in the … industry?
If ruminant collagen/gelatine was authorised to feed non‐ruminants and fish, what would be the impact of this change on the collagen/gelatine industry in Europe?
Do you think the impact will be more likely to occur in the recycling of food waste containing ruminant C&G (if any) or in the production of C&G as ingredient of non‐ruminant feed?
Do you think the… industry will have an incentive to include ruminant C&G in compound feed or will it still be too expensive to be considered?
Do you envisage a decrease in the price of C&G in general as a consequence of this lift of the feed ban?
In general, for producers, they included:
Which raw materials are commonly used for the production of C&G?
What is the percentage of the C&G produced that originates from ruminants?
Are hides and skins clearly sourced and separated by species?
Are bones used for the production of C&G?
Who selects the raw material for a producer: abattoir, renderers, tanneries, intermediaries, food producers…?
Traceability of the raw materials?
What is the percentage of the starting material (yield) as final product? Is it different for each material? Yield of C&G extracted from cattle bones, yield of C&G extracted from cattle hides
Is the heat‐and‐pressure processing method to extract C&G commonly used as an alternative to the alkaline or acidic methods?
Which would be the most common temperature/pressure combination when applied? Is it applied to both bones and hides?
Is C&G always produced in batches (batch size, traceability for each batch possible)? What are the main processes applied by European companies to produce C&G?
Can you provide us with data on combinations of temperature, time, pH, pressure of the current methods applied by the industry in Europe? If there are many, is there much variability?
Are you aware of import from non‐EU countries of raw materials to produce C&G or end products into the EU? If Yes, do you have any idea about the origin of the imports, species and amount? Which countries would be importers?
Appendix B – Calculation of the infectious load of tissues infected with TSE agents
1.
The infectious load of the cattle by‐products varies with the type of tissue, the titre of infectivity, its weight and with the age of the animal, relative to the incubation period (IP). There are different methods to calculate the infectious load of tissues infected with TSE agents. The most accurate method for the determination of the concentration of infectivity of TSE agents in tissues is the bioassay, using end‐point titration.
A method was developed in the UK using the data from cattle challenged experimentally p.o. with a pool of C‐BSE brainstem homogenate: 100 g on 3 consecutive days or single doses of 100 g, 10 g or 1 g (first phase) and 1 g, 100 mg, 10 mg or 1 mg (second phase) with a known end‐point titre of the BSE brainstem homogenate, as determined by bioassay in RIII mice (Konold et al., 2012).
In order to estimate titres from mouse bioassays data, it is essential to know the dose response for a standard BSE inoculum in a given mouse line, in terms of both the probability of survival and the incubation period for a given dose (Arnold et al., 2009). By assuming a dose‐dependent attack rate that follows a logistic regression curve, the probability of infection for a given dose could be estimated by fitting the IP of all inoculated animals. Then, as the titre of the initial inoculum is known only in terms of mouse i.c./i.p. ID50 units, the dose is expressed in terms of mouse i.c./i.p. ID50. This ID50 estimate is equivalent to a certain amount of the brain homogenate used in the experiment, with 95% confidence intervals (Wells et al., 2007; Konold et al., 2012).
Hawkins et al. (2000) showed, by using parallel titration of the same inoculum in cattle and mice, that one mouse i.c./i.p. ID50 equals 102.7 cattle i.c., and from there it can be extrapolated that one cattle oral ID50 equals 105.4 cattle i.c. ID50. Applying this method, Wells et al. (2007) estimated that the 102.8 mouse i.c./i.p./g in the initial inoculum was equivalent to 0.2 g of the brain homogenate (95% CI: 0.04–1 g). A few years later and with full data of the second phase of the experimental challenge using lower doses, Konold et al. (2012) refined the previous estimates to 102.7 mouse i.c./i.p. ID50/g and the equivalence in brain to 0.15 g (95% CI: 0.03–0.79 g). On the basis of these data, the range of infectivity in 1 g of brain could be approximately 0.52 to 5 cattle oral ID50 (EFSA's Quantitative assessment of the residual BSE risk in bovine-derived products, 2005b). The report mentioned that with higher titres of BSE‐affected brain the range could extend to 300, as titres of 105 mouse i.c./i.p. ID50/g have been recorded. However, using the same reasoning, Wells et al. (2007) indicated that the range should be revised to 1.0–20, and it was agreed that, with higher titres of BSE‐affected brain as starting material, the range could extend upward.
Adkin et al. (2014) described in an EFSA external scientific report (2014) an algorithm to determine the titre from mouse bioassays data when end‐point titration data are not available, based on Arnold et al. (2009). Instead of using IP, as in a pathogenesis study, the attack rate (proportion of mice that become clinical) was used. The authors argued that this system provides a more accurate estimate of titre at lower doses than solely using the mean IP. In order to derive the dose/incubation relationship to be used to convert the incubation periods from the TgBov XV (transgenic mice overexpressing bovine PrP) and RIII (conventional inbred mouse) bioassays into infectivity titre estimates, data were analysed from titrations of several different pools of brain material (whole brain or brain stem) from clinical field cases of BSE in RIII mice. A full description of the mathematical background can be found in the report (Adkin et al., 2014).
The authors reported the estimated probability of BSE infection for RIII and TgBov XV mice versus the dose in terms of RIII mouse i.c. i.p. log10 ID50/g. Fitting the log10 dose (RIII mouse i.c. i.p. LD50) against the probability of BSE infection, the authors estimated that the equivalent TgBov XV ID/g dose would be 4.76 log10 higher than the RIII dose.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Bolton DJ, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman LM, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Andreoletti O, Griffin J, Spiropoulos J, Ortiz‐Pelaez A and Alvarez‐Ordóñez A, 2020. Scientific Opinion on the potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non‐ruminant farmed animals. EFSA Journal 2020;18(10):6267, 68 pp. 10.2903/j.efsa.2020.6267
Requestor: European Commission
Question number: EFSA‐Q‐2019–00436
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Kostas Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Waiver: In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to John Griffin, Working Group member. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the discussions and in the drafting of the scientific output but was not allowed to be or act as chair, vice‐chair or rapporteur of the Working Group. Any competing interests are recorded in the respective minutes of the meetings of the Working Group, available online: https://www.efsa.europa.eu/sites/default/files/wgs/biological-hazards/wg-ruminant-collagen-gelatine.pdf
Acknowledgements: The Panel wishes to thank Katrin Bote for her contributions to the drafting of this scientific output, and the industry stakeholders that provided information and data: management and members of the Gelatine Manufacturers of Europe (GME), Collagen Casings Trade Association (CCTA); European Feed Manufacturers’ Federation (FEFAC); EU Association of Specialty Feed Ingredients and their Mixtures (FEFANA) and European Former Foodstuff Processors Association (EFFPA).
Adopted: 22 September 2020
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 2: © Alexander V Grishin/Shutterstock.com; Figure 3: © 1168group/Shutterstock.com
Notes
The ‘split’ refers to the intermediate layer, or corium, of the hide which is used for the production of collagen or gelatine. The corium consists of a network of collagen fibres, very intimately woven and joined together.
Food Standards Agency (FSA) project 3006/3007 https://webarchive.nationalarchives.gov.uk/20141103170648/http://www.foodbase.org.uk/results.php?f_report_id=507
References
- Adkin A, Simons R and Arnold ME, 2012. Model for the evaluation of different options for the monitoring of transmissible spongiform encephalopathies in cattle in the European Union (C‐TSEMM). EFSA supporting publications 2012:EN‐349, 55 pp. 10.2903/sp.efsa.2012.en-349 [DOI] [Google Scholar]
- Adkin A, Simons R and Arnold M, 2014. TSE infectivity model (TSEi) in animal tissues: bovine intestines and mesenteries. EFSA supporting publication 2014:EN‐559, 74 pp. 10.2903/sp.efsa.2014.en-559 [DOI] [Google Scholar]
- Adkin A, Simons R and Arnold M, 2016. Assessing the sensitivity of European surveillance for detecting BSE in cattle according to international standards. Preventive Veterinary Medicine, 135, 113–122. [DOI] [PubMed] [Google Scholar]
- AFSSA , 2001. Les risques sanitaires liés aux différents usages des farines et graisses d'origine animale et aux conditions de leur traitement et de leur élimination, Avis de l'Afssa en date du 7 avril 2001. Available online: http://www.vie-publique.fr/documents-vp/avis_afssa_070401.pdf [Google Scholar]
- Anil MH, Love S, Helps CR and Harbour DA, 2002. Potential for carcass contamination with brain tissue following stunning and slaughter in cattle and sheep. Food Control, 13, 431–436. [Google Scholar]
- Arnold ME, Ryan JB, Konold T, Simmons MM, Spencer YI, Wear A, Chaplin M, Stack M, Czub S, Mueller R, Webb PR, Davis A, Spiropoulos J, Holdaway J, Hawkins SA, Austin AR and Wells GA, 2007. Estimating the temporal relationship between prpsc detection and incubation period in experimental bovine spongiform encephalopathy of cattle. Journal of General Virology, 88, 3198–3208. 10.1099/vir.0.82987-0 [DOI] [PubMed] [Google Scholar]
- Arnold ME, Hawkins SAC, Green R, Dexter I and Wells GAH, 2009. Pathogenesis of experimental bovine spongiform encephalopathy (BSE): estimation of tissue infectivity according to incubation period. Veterinary Research, 40, 8 10.1051/vetres:2008046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ME, Simons RRL, Hope J, Gibbens N and Adkin AL, 2017. Is there a decline in bovine spongiform encephalopathy cases born after reinforced feed bans? A modelling study in EU member states. Epidemiology and Infection, 145, 2280–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi Kermani Z, Shahir MH and Baradaran N, 2017. Effect of gelatin supplementation on growth performance and blood metabolites of broiler chickens fed diets varying in crude protein. Livestock Science, 201, 5–12. 10.1016/j.livsci.2017.04.008 [DOI] [Google Scholar]
- Bierwagen RM. 2020. RBI050 EFSA questions on collagen and gelatine [BB‐DMS.FID119579]. Message to Katrin Bote. 27 January 2020. Email.
- van den Brink A, 2020. Re: EFSA consultation EFFPA. Message to Angel Ortiz‐Pelaez. 21 February 2020. Email. Document EFFPA Vision – 10 Key Working Priorities for a Stable & Growing Former Foodstuff Processor Sector – December 2019.
- Brown P, Rohwer RG and Gajdusek DC, 1986. Newer data on the inactivation of scrapie virus or Creutzfeldt‐Jakob disease virus in brain tissue. Journal of Infectious Diseases, 153, 1145–1148. Available online: www.jstor.org/stable/30105224 [DOI] [PubMed] [Google Scholar]
- Brown P, Liberski PP, Wolff A and Gajdusek DC, 1990. Resistance of scrapie infectivity to steam autoclaving after formaldehyde fixation and limited survival after ashing at 360°C: practical and theoretical implications. Journal of Infectious Diseases, 161, 467–472. 10.1093/infdis/161.3.467 [DOI] [PubMed] [Google Scholar]
- Cardone F, Simoneau S, Arzel A, Puopolo M, Berardi VA, Abdel‐Haq H, Galeno R, De Pascalis A, Sbriccoli M, Graziano S, Valanzano A, Porte P, Diringer H, Brown P, Flan B and Pocchiari M, 2012. Comparison of nanofiltration efficacy in reducing infectivity of centrifuged versus ultracentrifuged 263K scrapie‐infected brain homogenates in spiked albumin solutions. Transfusion, 52, 953–962. 10.1111/j.1537-2995.2011.03425.x [DOI] [PubMed] [Google Scholar]
- CCTA (Collagen Casings Trade Association), 2011. CCTA Position Paper on the Safety of Edible Collagen Casings. 3 pp. Available online: http://collagencasings.Org/Upload/Documents/Ccta44positionpaperforwebsite.pdf
- Chapman GE, Lockey R, Beck KE, Vickery C, Arnold M, Thorne L, Thorne JK, Walker SR, Keulen L, Casalone C, Griffiths PC, Simmons MM, Terry LA and Spiropoulos J, 2020. Inactivation of H‐type and L‐Type bovine spongiform encephalopathy following recommended autoclave decontamination procedures. Transboundary and Emerging Diseases, 67, 1872–1878. 10.1111/tbed.13513 [DOI] [Google Scholar]
- Cooper JD and Bird SM, 2002. UK dietary exposure to BSE in head meat: by birth cohort and gender. Journal of Cancer Epidemiology and Prevention, 7, 71–83. [DOI] [PubMed] [Google Scholar]
- Coore RR, Love S, McKinstry JL, Weaver HR, Phillips A, Hillman T, Hiles MJ, Shand A, Helps CR and Anil MH, 2004. Dissemination of brain emboli following captive bolt stunning of sheep: capacity for entry into the systemic arterial circulation. Journal of Food Protection, 67, 1050–1052. [DOI] [PubMed] [Google Scholar]
- Department of Health and Social Care, UK , 2015. General principles of decontamination and waste disposal. ANNEX C. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/427855/Annex_C_v3.0.pdf
- Devine C and Dikeman M, 2014. Encyclopedia of Meat Sciences. Elsevier, ISBN0123847346, 9780123847348, 1712 pp.
- Ducrot C, Paul M and Calavas D, 2013. BSE risk and the use of meat and bone meal in the feed industry: perspectives in the context of relaxing control measures. Natures Sciences Societes, 21, 3–12. [Google Scholar]
- EFFPA (European Former Foodstuff Processors Association), online. 2019. Effpa Vision ‐ 10 Key Working Areas for a Stable and Growing Former Foodstuff Processing Sector. Form of Item. Available online: https://www.effpa.eu/what-are-former-foodstuffs/
- EFSA (European Food Safety Authority), 2005a. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on the safety of collagen and a processing method for the production of collagen. EFSA Journal2005;174, 9 pp. 10.2903/j.efsa.2005.174 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005b. EFSA QRA Report 2004 (BIOHAZ) on quantitative assessment of the residual BSE risk in Bovine‐derived products. EFSA Journal2005;3(12):307, 135 pp. 10.2903/j.efsa.2005.307 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005c. Opinion on the Quantitative risk assessment of the animal BSE risk posed by meat and bone meal with respect to the residual BSE risk. EFSA Journal2005;3(9):257, 30 pp. 10.2903/j.efsa.2005.257 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006a. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on the Quantitative assessment of the human BSE risk posed by gelatine with respect to residual BSE. EFSA Journal2006;4(1):312, 29 pp. 10.2903/j.efsa.2006.312 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006b. Opinion of the Scientific Panel on Biological Hazards (BIOHAZ) on the BSE risk from cohort animals: bovine hides and skins for technical purposes. EFSA Journal2006;367, 25 pp. 10.2903/j.efsa.2006.367 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Protocol for further laboratory investigations into the distribution of infectivity of atypical BSE. EFSA Journal 2014;12(7):3798, 55 pp. 10.2903/j.efsa.2014.3798 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. The European Union Summary Report on data of the surveillance of ruminants for the presence of transmissible spongiform encephalopathies (TSEs) in 2015. EFSA Journal 2016;14(12):4643, 62 pp. 10.2903/j.efsa.2016.4643 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. The European Union summary report on surveillance for the presence of transmissible spongiform encephalopathies (TSE) in 2018. EFSA Journal 2019;17(2):e05925, 61 pp. 10.2903/j.efsa.2019.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlstr€om H, Adkin A, Greiner M, Marchis D, Prado M, Da Silva Felicio T, Ortiz‐Pelaez A and Simmons M, 2018. Scientific opinion on an updated quantitative risk assessment (QRA) of the BSE risk posed by processed animal protein (PAP). EFSA Journal 2018;16(7):5314, 111 pp. 10.2903/j.efsa.2018.5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018a. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Germini A, Martino L, Merten C, Mosbach‐Schulz O, Smith A and Hardy A, 2018b. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2007. Management of transmissible spongioform encephalopathies in meat production. 83 pp. Available online: http://www.fao.org/3/a-a1002e.pdf
- Francis FJ, 2000. Wiley Encyclopedia of Food Science and Technology. 4 vols, 2nd Edition John Wiley & Sons, New York: pp. 1183–1188. [Google Scholar]
- Fryer HR and McLean AR, 2011. There is no safe dose of prions. PLoS ONE, 6, e23664 10.1371/journal.pone.0023664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2002. Final Assessment Report, Proposal P238, BSE risk assessment and risk management strategy. Available online: https://www.foodstandards.gov.au/code/proposals/documents/P238%20FAR%20-%20BSE.pdf
- Gale P, 1998. Quantitative BSE risk assessment: relating exposures to risk. Letters in Applied Microbiology, 27, 239–242. 10.1046/j.1472-765x.1998.00449.x [DOI] [PubMed] [Google Scholar]
- Gale P, 2001. Developments in microbiological risk assessment for drinking water. Journal of Applied Microbiology, 91, 191–205. 10.1046/j.1365-2672.2001.01421.x [DOI] [PubMed] [Google Scholar]
- Gibbs CJ Jr, Gajdusek DC and Morris JA, 1965. Viral characteristic of the scrapie agent in mice. in slow, latent, and temperate virus infections In: Gibbs CJ, Jr, Gajdusek DC. and Alpers M. (eds.). NINDB2 monograph. Vol no. 2 US Government Printing Office, Washington, DC: pp. 195–202. [Google Scholar]
- Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ and Prusiner SB, 2008. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathogen, 4, e1000206 10.1371/journal.ppat.1000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Guillén MC, Giménez B, López‐Caballero ME and Montero MP, 2011. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids, 25, 1813–1827. [Google Scholar]
- Grobben AH, 2001. Enhancing the safety of gelatine with regard to the bovine spongiform encephalopathy agent. In: Scientific Steering Committee (SSC). 2001. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides from Cattle, Sheep or Goats. Adopted by the Scientific Steering Committee at its Meeting of 28–29 June 2001.
- Grobben AH, 2002, Report on the inactivation/removal of TSE infectivity by the manufacturing processes of gelatine. Status report on 13 June 2002 provided to the secretariat of the Scientific Steering Committee not for public release. 13 pp. In: Scientific Steering Committee (SSC). 2002. Updated Opinion on the Safety with Regard To TSE Risks of Gelatine Derived from Ruminant Bones or Hides. Adopted by the Scientific Steering Committee at its Meeting of 5–6 December 2002.
- Grobben AH, Steele PJ, Somerville RA and Taylor DM, 2004. Inactivation of the bovine‐spongiform‐encephalopathy (BSE) agent by the acid and alkaline processes used in the manufacture of bone gelatine. Biotechnology and Applied Biochemistry, 39, 329–338. [DOI] [PubMed] [Google Scholar]
- Grobben AH, Steele PJ, Somerville RA, Taylor DM and Schreuder BE, 2005. Inactivation of the BSE agent by the heat‐and‐pressure process for manufacturing gelatine. Veterinary Record, 157, 277–281. [DOI] [PubMed] [Google Scholar]
- Grobben AH, Steele PJ, Somerville RA and Taylor DM, 2006. Inactivation of BSE infectivity on chips of bone by autoclaving during the manufacture of gelatine. Veterinary Record, 158, 94–96. [DOI] [PubMed] [Google Scholar]
- Hawkins S, Wells G, Austin A, Ryder S, Dawson M, Blamire I and Simmons M, 2000. Comparative Efficiencies of the Bioassay of BSE Infectivity in Cattle and Mice. Alexandria, VA, USA. [Google Scholar]
- Hoffmann C, Ziegler U, Buschmann A, Weber A, Kupfer L, Oelschlegel A, Hammerschmidt B and Groschup MH, 2007. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. Journal of General Virology, 88, 1048–1055. 10.1099/vir.0.82186-0 [DOI] [PubMed] [Google Scholar]
- Inveresk Research International , 1998a. Validation of the clearance of scrapie from the manufacturing process of gelatine. Final report. Inverest Project No. 855028. Inveresk Report No. 14682. Tranent (Scotland), 41 pp. In: Scientific Steering Committee (SSC), 2000. Scientific Report and Opinion on the Safety of Gelatine. Updated by the Scientific Steering Committee at its Meeting of 20–21 January 2000.
- Inveresk Research International , 1998b. Validation of the clearance of scrapie from the manufacturing process of gelatine: additional stage. Final report. Inverest Project No. 855080. Inveresk Report No. 14683. Tranent (Scotland), 28 pp. In: Scientific Steering Committee (SSC), 2000. Scientific Report and Opinion on the Safety of Gelatine. Updated by the Scientific Steering Committee at its Meeting of 20–21 January 2000.
- Juschus F, 2020. RE: [IM0018552452] EFSA mandate on Collagen and Gelatine. Message to Angel Ortiz‐Pelaez. 16 January 2020. Email.
- Karim AA and Bhat R, 2009. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids, 23, 563–576. [Google Scholar]
- Khalaji S, Manafi M, Olfati Z, Hedyati M, Latifi M and Veysi A, 2016. Replacing soybean meal with gelatin extracted from cow skin and corn protein concentrate as a protein source in broiler diets. Poultry Science, 95, 287–297. 10.3382/ps/pev330 [DOI] [PubMed] [Google Scholar]
- Konold T, Arnold ME, Austin AR, Cawthraw S, Hawkins SA, Stack MJ, Simmons MM, Sayers AR, Dawson M, Wilesmith JW and Wells GA, 2012. Bovine spongiform encephalopathy: the effect of oral exposure dose on attack rate and incubation period in cattle – an update. BMC Research Notes, 5, 674 10.1186/1756-0500-5-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mu C, Cai S and Lin W, 2009. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrasonic Sonochemistry, 16, 605–609. [DOI] [PubMed] [Google Scholar]
- Manzke U, Schlaf G, Poethke R, Felgenhauer K and Maeder M, 1997. On the removal of nervous proteins from materials used for gelatin manufacturing during processing. Drugs made in Germany 40, 32–36. In: EFSA (European Food Safety Authority). 2005. Opinion of the Scientific Panel on Biological Hazards (Biohaz) on the safety of collagen and a processing method for the production of collagen. EFSA Journal 2005:3(3):174, 9 pp. 10.2903/j.efsa.2005.174 [DOI] [Google Scholar]
- Mould DL, Dawson AM and Smith W, 1965. Scrapie in mice. the stability of the agent to various suspending media, pH and solvent extraction. Research in Veterinary Science, 6, 151–154. [PubMed] [Google Scholar]
- Noorzai S, Verbeek CJR, Lay MC and Swan J, 2019. Collagen extraction from various waste bovine hide sources 10.1007/s12649-019-00843-2 [DOI] [Google Scholar]
- Ockerman HW and Hansen CL, 1999. Animal by‐Product Processing & Utilization: CRC Press. Boca Raton, Florida, USA: 522 pp. [Google Scholar]
- OIE , online. Available online: https://www.oie.int/animal-health-in-the-world/bse-situation-in-the-world-and-annual-incidence-rate/annual-incidence-rate/ (until 2016) and https://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Countryreports (after 2016)
- Paul C, Leser S and Oesser S, 2019. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nutrients, 11, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast DM, Sheridan JJ, Daly DJ, McDowell DA and Blair IS, 2003. Dissemination of central nervous system tissue from the brain and spinal cord of cattle after captive bolt stunning and carcass splitting. Meat Science, 65, 1201–1209. [DOI] [PubMed] [Google Scholar]
- ProMED , online. Available online: https://promedmail.org/promed-posts/
- Ran XG and Wang LY, 2014. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by‐products. Journal of the Science of Food and Agriculture, 94, 585–590. [DOI] [PubMed] [Google Scholar]
- Reed RJR and Picksley MA, 1987. The Determination of the True Filtration Characteristics of Diatomaceous Earth. Journal of the American Society of Brewing Chemists, 45, 48–53. 10.1094/asbcj-45-0048 [DOI] [Google Scholar]
- Rohwer RG, 1984. Virus like sensitivity of the scrapie agent to heat inactivation. Science, 223, 600–602. 10.1126/science.6420887 [DOI] [PubMed] [Google Scholar]
- Rohwer RG, 2001, Intermediate data on the removal and inactivation of TSE agents by the individual process steps of the finishing unit operations of the gelatine manufacturing process (provided in confidence). In: Scientific Steering Committee (SSC). 2003. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides. Adopted by the Scientific Steering Committee at its Meeting of 6–7 March 2003.
- Schmidt MM, Dornelles RCP, Mell RO, Kubota EH, Mazutti MA, Kempka AP and Demiate IM, 2016. Collagen extraction process. International Food Research Journal, 23, 913–922. [Google Scholar]
- Shepherd A, 1999. Validation of the manufacturing process for gelatine to show Reduction of the Scrapie Agent. Inveresk Report No. 16032 sponsored by Leiner Davis Gelatine (International) (Botany, Australia). Tranent (Scotland), 29 pp. (Confidential). In: Scientific Steering Committee (SSC). 2001. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides from Cattle, Sheep or Goats. Adopted by the Scientific Steering Committee at its Meeting of 28–29 June 2001.
- Sohn HJ, Lee YH, Green RB, Spencer YI, Hawkins SA, Stack MJ, Konold T, Wells GA, Matthews D, Cho IS and Joo YS, 2009. Bone marrow infectivity in cattle exposed to the bovine spongiform encephalopathy agent. Veterinary Record, 164, 272–273. 10.1136/vr.164.9.272 [DOI] [PubMed] [Google Scholar]
- Spiropoulos J, Lockey R, Beck KE, Vickery C, Holder TM, Thorne L, Arnold M, Andreoletti O, Simmons MM and Terry LA, 2019. Incomplete inactivation of atypical scrapie following recommended autoclave decontamination procedures. Transboundary and Emerging Disease, 66, 1993–2001. 10.1111/tbed.13247 [DOI] [PubMed] [Google Scholar]
- SSC (Scientific Steering Committee), 2000a. Scientific Report and Opinion on the Safety of Gelatine. Updated by the Scientific Steering Committee at its Meeting of 20–21 January 2000.
- SSC (Scientific Steering Committee), 2000b. Oral Exposure of Humans to the BSE Agent: Infective Dose and Species Barrier. Adopted by the Scientific Steering Committee at its Meeting of 2–3 March 2000.
- SSC (Scientific Steering Committee), 2001a. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides from Cattle, Sheep or Goats. Adopted by the Scientific Steering Committee at its Meeting of 28–29 June 2001.
- SSC (Scientific Steering Committee), 2001b. Opinion and Report on the Safety with Respect to TSE Risks of Collagen Produced from Ruminant Bones or Hides from Cattle, Sheep or Goats. Adopted by the Scientific Steering Committee at its Meeting of 10–11 May 2001.
- SSC (Scientific Steering Committee), 2002. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides. Adopted by the Scientific Steering Committee at its Meeting of 5–6 December 2002.
- SSC (Scientific Steering Committee), 2003. Updated Opinion on the Safety with Regard to TSE Risks of Gelatine Derived from Ruminant Bones or Hides. Adopted by the Scientific Steering Committee at its Meeting of 6–7 March 2003.
- Stamp JT, Brotherston JG, Zlotnik I, Mackay JMK and Smith W, 1959. Further Studies on scrapie. Journal of Comparative Pathology and Therapeutics, 69, 268–280. 10.1016/s0368-1742(59)80026-6 [DOI] [PubMed] [Google Scholar]
- Taylor DM, 1991. Inactivation of BSE agent. Developments in Biological Standardization, 75, 97–102. Available online: http://europepmc.org/abstract/MED/1794635 [PubMed] [Google Scholar]
- Taylor KC, 1994. Suspected vertical transmission of BSE. Veterinary Record, 134, 175 10.1136/vr.134.7.175-a [DOI] [PubMed] [Google Scholar]
- Taylor DM, Fraser H, McConnell I, Brown DA, Brown KL, Lamza KA and Smith GR, 1994. Decontamination studies with the agents of bovine spongiform encephalopathy and scrapie. Arch Virol., 139, 313–326. 10.1007/BF01310794. PMID: 7832638. [DOI] [PubMed] [Google Scholar]
- Taylor DM, 1999. Inactivation of prions by physical and chemical means. Journal of Hospital Infection, 43, S69–S76. 10.1016/s0195-6701(99)90067-1 [DOI] [PubMed] [Google Scholar]
- Taylor DM, 2000. Inactivation of transmissible degenerative encephalopathy agents: a review. Veterinary Journal, 159, 10–17. 10.1053/tvjl.1999.0406 [DOI] [PubMed] [Google Scholar]
- Thyer J, Unal A, Thomas P, Eaton B, Bhashyam R, Ortenburg J, Uren E, Middleton D, Selleck P and Maher D, 2006. Prion‐removal capacity of chromatographic and ethanol precipitation steps used in the production of albumin and immunoglobulins. Vox Sanguinis, 91, 292–300. 10.1111/j.1423-0410.2006.00829.x [DOI] [PubMed] [Google Scholar]
- Vařechová E, 2015. Specified risk material and its amounts removed from cattle carcasses. Maso International – Journal of Food Science and Technology, 02 Available online: http://www.maso-international.cz/download/115_124_022015.pdf. http://www.maso-international.cz/download/115_124_022015.pdf [Google Scholar]
- Vermeulen M, 2019. RE: EFSA mandate on Collagen and Gelatine. Message to Angel Ortiz‐Pelaez. 19 December 2019. Email.
- Vermeulen M, 2020. RE: EFSA mandate on Collagen and Gelatine. Message to Angel Ortiz‐Pelaez, 18, March 2020. Email. [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety), 2016. Risk assessment of other substances – collagen from fish skin. Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics of the Norwegian Scientific Committee for Food Safety. ISBN: 978‐82‐8259‐255‐0. [Google Scholar]
- Wells GA, Hawkins SA, Green RB, Austin AR, Dexter I, Spencer YI, Chaplin MJ, Stack MJ and Dawson M, 1998. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Veterinary Record, 142, 103–106. 10.1136/vr.142.5.103 [DOI] [PubMed] [Google Scholar]
- Wells GA, Hawkins SA, Green RB, Spencer YI, Dexter I and Dawson M, 1999. Limited detection of sternal bone marrow infectivity in the clinical phase of experimental bovine spongiform encephalopathy (BSE). Veterinary Record, 144, 292–294. 10.1136/vr.144.11.292 [DOI] [PubMed] [Google Scholar]
- Wells GA, Konold T, Arnold ME, Austin AR, Hawkins SA, Stack M, Simmons MM, Lee YH, Gavier‐Widen D, Dawson M and Wilesmith JW, 2007. Bovine spongiform encephalopathy: the effect of oral exposure dose on attack rate and incubation period in cattle. Journal of General Virology, 88, 1363–1373. 10.1099/vir.0.82421-0 [DOI] [PubMed] [Google Scholar]


