Abstract
Single-atom nanozymes (SANs) possess unique features of maximum atomic utilization and present highly assembled enzyme-like structure and remarkable enzyme-like activity. By introducing SANs into immunoassay, limitations of ELISA such as low stability of horseradish peroxidase (HRP) can be well addressed, thereby improving the performance of the immunoassays. In this work, we have developed novel Fe-N-C single-atom nanozymes (Fe-Nx SANs) derived from Fe-doped polypyrrole (PPy) nanotube and substituted the enzymes in ELISA kit for enhancing the detection sensitivity of amyloid beta 1-40. Results indicate that the Fe-Nx SANs contain high density of single-atom active sites and comparable enzyme-like properties as HRP, owing to the maximized utilization of Fe atoms and their abundant active sites, which could mimic natural metalloproteases structures. Further designed SAN-linked immunosorbent assay (SAN-LISA) demonstrates the ultralow limit of detection (LOD) of 0.88 pg/mL, much more sensitive than that of commercial ELISA (9.98 pg/mL). The results confirm that the Fe-Nx SANs can serve as a satisfactory replacement of enzyme labels, which show great potential as an ultrasensitive colorimetric immunoassay.
1. Introduction
Single-atom catalysts (SACs), which contain exclusively isolated metal active sites, have attracted vast attention due to the precise design of nanomaterials at atomic levels [1–3]. Separated metal atoms enable SACs with remarkable catalytic activity and gratifying stability due to their much higher surface energy, maximum metallic atom utilization, homogeneity of active sites, and particular geometric structure [4–6]. Thereinto, SACs with enzyme-like characteristics are called single-atom nanozymes (SANs) and are regarded as ideal candidates to mimic the structure and catalytic activity of natural enzymes [7–10]. Nowadays, the SANs have found their extensive application as substitutions for natural enzymes in immunoassays [11, 12], environmental treatment [13], biodetection, and biosensing [8, 14, 15] owing to their excellent performance, high stability, ease of large-scale production, and economical price.
Commercial enzyme-linked immunosorbent assay (ELISA) has been a widely recognized standard in food safety, clinical diagnosis, and environmental evaluation due to its relatively high specificity and accuracy [16–19]. Its mechanism is to convert the interactions between antigen and antibody into visible color change so one can easily obtain the results from observation. However, there are still some limitations in the accurate detection of diseases utilizing a commercial ELISA, because the concentration of biomarkers is usually ultralow in the early stages of diseases and the performance of HRP used in ELISA is highly dependent on pH and temperature [20–22]. As such, it is urgent to seek a suitable substitution for natural peroxidase HRP and place continuous efforts on enhancing the sensitivity of ELISA. As we mentioned above, various kinds of SANs possess high enzyme-like characteristics and integrating them into traditional ELISA may enhance the detection performance of immunosorbent assay [15, 23].
Amyloid beta 1-40 (Aβ 1-40), as one of the most plentiful substances in humans, will easily form insoluble toxic Aβ 1-40 aggregation, which is a vital neuropathological hallmark to identify Alzheimer's disease commonly; the clinically relevant range of Aβ 1-40 is several ten to several hundred pg/mL [24, 25]. It is well known that AD begins in the human body years before symptoms present, so detecting Aβ 1-40 at low concentration is of great importance and can be used to estimate the risk or show the presence of AD at early stage [26–28]. Hence, we designed novel high-density Fe-Nx single-atom peroxidase-like nanozymes (Fe-Nx SANs) from pyrolyzed polypyrrole (PPy) nanotube via a nanoconfined strategy. A series of analyses revealed their ultrahigh surface area and superior peroxidase-like activity. The peroxidase-like catalytic activity of the Fe-Nx SANs was optimized and compared with natural HRP, which showed better thermal and pH stable catalytic properties. Then, the streptavidin- (SA-) functionalized Fe-Nx SANs were used to replace HRP in ELISA and detect Aβ 1-40. The detection performance of the proposed Fe-Nx SAN-linked immunosorbent assay (SAN-LISA) was examined and compared with commercial ELISA. The results show that the SAN-LISA exhibited higher sensitivity, making Fe-Nx SANs qualify as an enzyme replacement and providing satisfactory feasibility in clinical diagnosis.
2. Results and Discussions
The synthesis route of SA labeled Fe-Nx SANs is shown in Scheme 1. Methyl Orange (MO) micelle soft template was first created in the water phase, and pyrrole monomers and FeCl3 were added into the above solutions to form PPy nanotubes. Then, KMnO4 was added to form a layer of MnO2 coated on the surface of PPy nanotubes in order to produce more single-atom active sites due to nanoconfinement effect. Specifically, MnO2 coating can confine atoms into precursors, thus achieving high atomic distribution of Fe iron, reducing aggregation during pyrolysis, and greatly enhancing the number of Fe-Nx active sites. Moreover, free migration of iron species could be restricted, further improving its catalytic performance [29, 30]. Subsequently, the Fe-Nx SANs were acquired through a typical pyrolysis step under a N2 environment followed by an acid leaching process, in which the aggregated Fe atoms and MnO2 coating could be removed. Herein, the obtained Fe-Nx SANs can catalyze H2O2 to generate hydroxyl radicals via Fenton reaction, which can be recognized as a superior peroxidase-like activity [31]. Next, the synthesized Fe-Nx SANs were treated with N-(3-dimethylamino propyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), then modified with SA to bind biotinylated Aβ 1-40 antibody, which is proved by the Fourier transform infrared spectra successfully (Figure S1) [32]. Thereinto, the biotin can react with SA-conjugated labels, forming the strongest known noncovalent interaction between a protein and a ligand [33]. Notably, the interaction is rapid and maintains being robust in extreme conditions of pH and temperature. Through these steps, the obtained SA-modified Fe-Nx SANs can substitute HRP-streptavidin in the commercial ELISA. Therefore, we utilized these peroxidase-like Fe-Nx SANs to develop a new SAN-LISA kit to enhance the detection performance of Aβ 1-40.
Scheme 1.
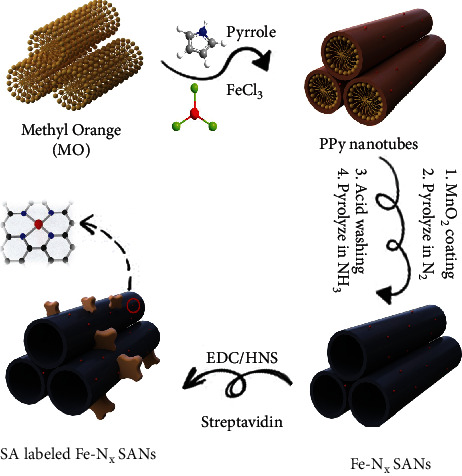
Schematic diagram of preparing SA-labeled Fe-Nx SANs.
The morphologies of PPy nanotube and MnO2 coating PPy nanotube were confirmed by Transmission electron microscopy (TEM), shown in Figure S2. In Scheme 1, the well-defined Fe-Nx SANs had a typical nanotube structure with a diameter of around 50 nm. Moreover, distorted graphite layers were found in Fe-Nx SANs (Figure 1(a)) by high-resolution TEM (HRTEM). This graphite structure could provide enriched defects and nanopores, which would anchor abundant atomic Fe-Nx moieties. A N2 adsorption/desorption test was carried out to evaluate the detailed textural structure (Figure S3). The Brunauer–Emmett–Teller (BET) surface area of Fe-Nx SANs was 648.16 m2/g. The large surface area enabled the synthesized Fe-Nx SANs to host more Fe–Nx–C moieties, thus achieving high peroxidase-like activity. Auxiliary energy-dispersive X-ray spectroscopy (EDS) elemental analysis demonstrated that the Fe-Nx SANs were comprised of C, N, and Fe (Figure 1(b)). Here, the absence of Mn signal meant that the MnO2 coating was removed successfully. The Si, Cu, and Au signals were introduced by TEM technology (such as EDS probes or TEM grid), which was also proved by the X-ray photoelectron spectroscopy (XPS) spectrum (Figure S4). Moreover, the EDS mapping of C, N, and Fe was conducted, as shown in Figure 1(c). All elements were distributed uniformly in the Fe-Nx SANs, indicating that the Fe-Nx could be incorporated into the PPy matrix. Besides, no Fe clusters were observed, which was because the aggregated Fe species were washed out during acid treatment and the remaining Fe existed as isolated atoms.
Figure 1.
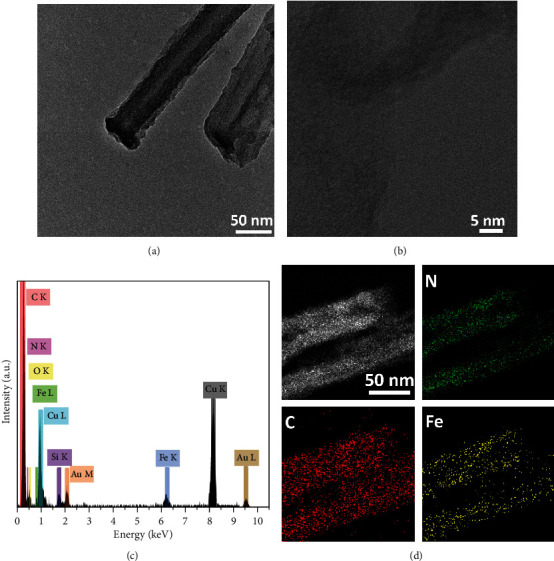
(a) TEM image of Fe-Nx SANs. (b) HRTEM image of the Fe-Nx SANs. (c) EDS elemental analysis of Fe-Nx SANs. (d) STEM image of Fe-Nx SANs and EDS elemental mapping results of C, N, and Fe.
In order to measure the chemical composition of Fe-Nx SANs, high-resolution XPS spectra with curve fitting of N 1 s and Fe 2p were adopted, and the results are shown in Figures 2(a) and 2(b). For N 1 s, the spectrum of Fe-Nx SANs could be fitted into four peaks at 397.7 eV, 399.7 eV, 400.7 eV, and 402.1 eV, corresponding to Fe-Nx or pyridinic N, pyrrolic N, graphitic N, and oxidized N, respectively [34]. Here, we fitted the pyridinic N and Fe-Nx in one peak because of the small difference in binding energy between Fe-Nx and pyridinic N [35]. For Fe 2p, four peaks of 707.9 eV, 712.1 eV, 718.9 eV, and 723.5 eV and 725.9 eV were assigned to Fe2+2p2/3, Fe3+2p2/3, Fe2+2p1/2, and Fe3+2p1/2 on the basis of binding energies, respectively [36]. The deconvolution method using Gaussian-Lorentz curve fittings was adopted to conduct the semiquantitative analysis of all the elements [37, 38]. Figure 2(c) shows that the N and Fe contents were 5.02 at.% and 0.41 at.%, respectively, which correspond to previously published works of single-atom Fe-N-C materials [34, 39]. The percentage of defective N configurations (pyridinic and pyrrolic N), regarded as coordination sites for single Fe atoms, was high. Moreover, compared to traditional PPy nanotube-based Fe-N-C materials (0.35 at.% [34]), the nanoconfinement strategy enhanced Fe loading significantly. The Fe K-edge X-ray absorption near-edge structure (XANES) spectra (Figure 2(d)) of Fe-Nx SANs and reference samples of iron (II) phthalocyanine FePc, Fe foil, FeO, and Fe2O3 were obtained. Obviously, the near-edge absorption energy of Fe-Nx SANs was located between standard bi- (FeO) and trivalent (Fe2O3) iron, illustrating that +2 and+3 coexisted in Fe-Nx SANs, consistent with XPS results (Figure 2(b)). The Fourier-transform EXAFS curve of Fe-Nx SANs in Figure 2(e) showed the Fe-N peak at 1.4 Å and no Fe-Fe peak at 2.1 Å was observed. Moreover, from the K-edge EXAFS oscillations, the spectrum of Fe-Nx SANs was distinct from those of Fe foil and Fe oxides, but almost the same as that of Fe single-atom reference FePc (Figure S5b), which could further demonstrate that Fe was atomically dispersed in Fe-Nx SANs. Such a structure is also similar to natural HRP (Figure S5a), thereby possessing intrinsic peroxidase activity.
Figure 2.
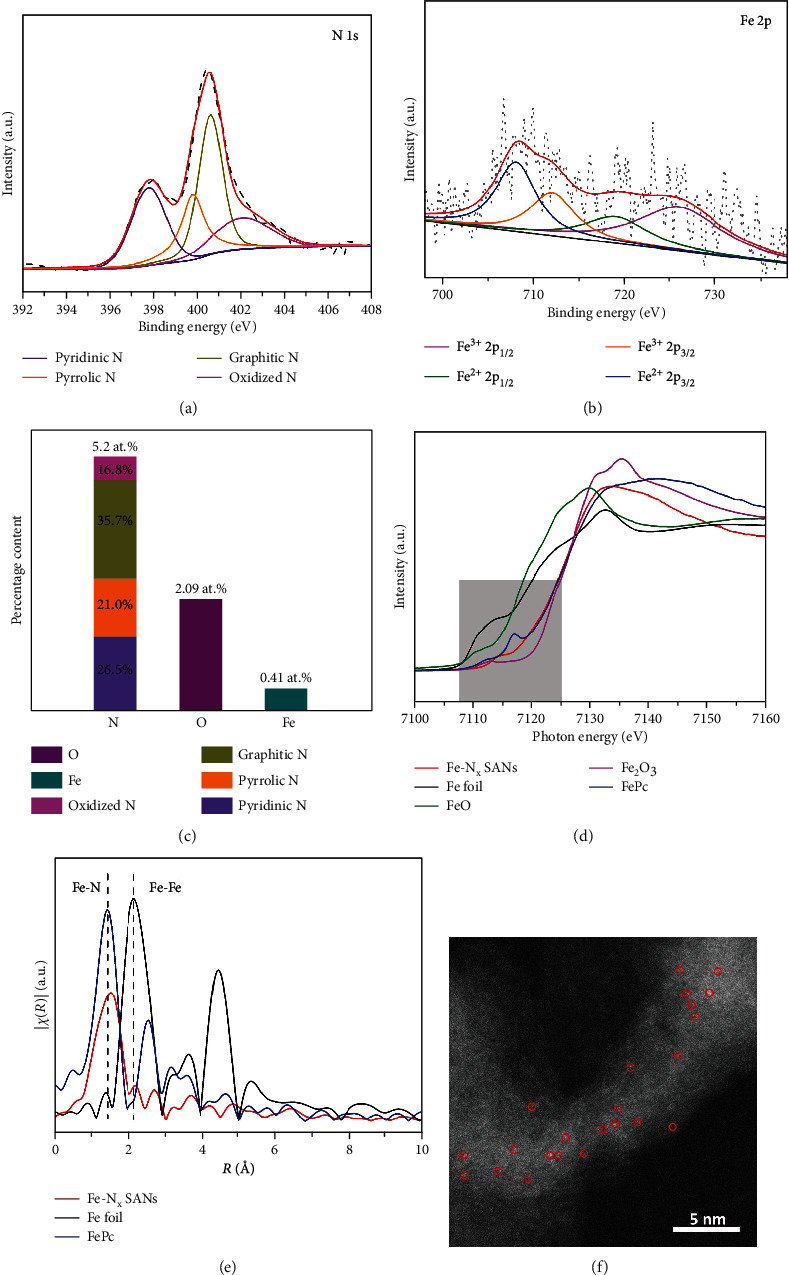
(a, b) High-resolution N 1 s and Fe 2p spectra of Fe-Nx SANs, respectively. (c) N, O, and Fe contents in Fe-Nx SANs. (d) Fe K-edge XANES spectrum of Fe-Nx SANs and reference samples of FePc, Fe foil, FeO, and Fe2O3. (e) FT k3-weighted EXAFS spectrum of Fe-Nx SANs, FePc, and Fe foil. (f) HAADF-STEM image of Fe-Nx SAN sample.
Subsequently, in order to confirm the distribution of Fe species in Fe-Nx SANs at atomic levels, aberration-corrected scanning TEM (STEM) characterizations were carried out (Figure 2(f)). It clearly showed that the Fe species were uniformly dispersed into the PPy matrix and formed single-atom Fe sites, which were the bright dots circled with red marks. In addition, no nanoparticles were observed at the atomic level, which again proved that no aggregated Fe species existed in Fe-Nx SANs. All the results illustrated that enriched atomic Fe-Nx moieties had been doped in the PPy matrix effectively.
To elucidate the possible peroxidase-like catalytic property of Fe-Nx SANs, we performed density functional theory (DFT) calculations to investigate the reaction process of the generation of hydroxyl radicals through catalyzing H2O2 with the Fe-N4 SAN model. As shown in Figure 3(a), the H2O2 molecule is firstly adsorbed on the Fe active site in the Fe-N4 SAN with an adsorption energy of -0.48 eV. Then, the H2O2 molecule easily dissociates and then a hydroxyl group desorbs from the adsorbed site, resulting in the generation of an active hydroxyl radical and adsorption of a hydroxyl group at the Fe-N4 active site. The energy diagram also matched previous reports [40, 41], and the calculated reaction energy from the initial step to the final step was 0.27 eV. Such low reaction energy confirmed the potential peroxidase-like catalytic property of Fe-Nx SANs. Further specific peroxidase-like activities and steady-state kinetics properties of Fe-Nx SANs were assessed in acetate buffer (pH = 3.6). Thereinto, 3,3′,5,5′-tetramethylbenzidine (TMB) was employed as the allochroic substrates. First, the TMB chromogenic reaction curve of absorbance to time was obtained, and the sample without adding H2O2 was served as a reference. The result is shown in Figure 3(b). It is clear that the absorbance at 652 nm increased with reaction time and the absorbance to reaction time was linear in the first minute with R2 coefficient close to 1 in linear regression analysis. The catalytic activity of Fe-Nx SANs expressed in units (U) was further assessed. Specifically, different amounts of Fe-Nx SANs were used to trigger chromogenic reaction of TMB. The first 60s was chosen as an initial time, and the results are in Figure 3(c). The peroxidase-mimic activity of the Fe-Nx SANs was calculated to be 64.79 U mg−1, which is higher than that of the reported Fe-Nx/SAN and conventional nanozymes (Table S1, Supporting information), further proving that the synthesized SANs possessed unprecedented peroxidase-like properties. This is due to the reason that those active sites of Fe-Nx have similar effective structures to natural enzymes. What is more, owing to single-atom Fe, the atom utilization could become 100% theoretically. In other words, every single atom can work as an active site to catalyze H2O2.
Figure 3.
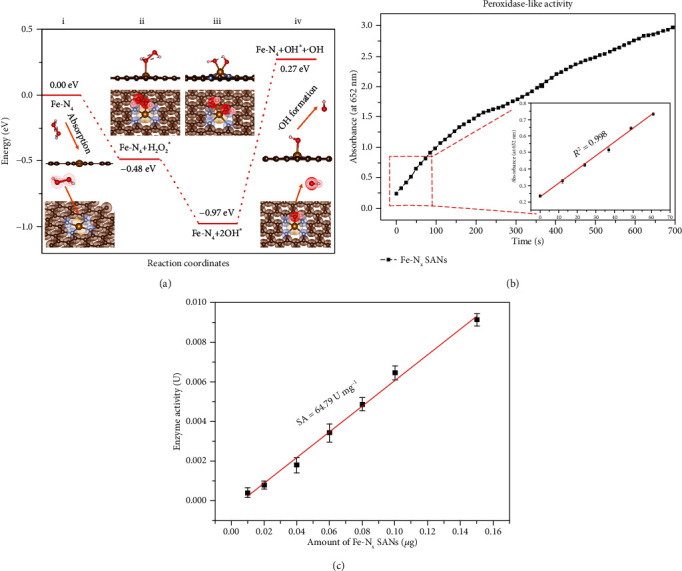
(a) The free energy diagrams of the Fe-N4 model in catalytic process. (b) Absorbance-time curve of TMB chromogenic reaction catalyzed by Fe-Nx SANs and the corresponding magnified initial linear portion. (c) Specific activities of Fe-Nx SANs.
Then, the kinetics of peroxidase-mimicking catalysis of Fe-Nx SANs was analyzed, as shown in Figures 4(a) and 4(b). The steady-state kinetics curves of Fe-Nx SANs towards TMB substrates and H2O2 were obtained. Moreover, Michaelis constants (Km) of the steady-state kinetics were obtained by fitting in the Michaelis-Menten model and compared with that of HRP. The Km of Fe-Nx SANs with TMB and H2O2 as the substrate is slightly lower than that of HRP, demonstrating that the synthesized SAN has a comparable affinity of HRP. Besides, the steady-state kinetics curves of HRP toward H2O2 and TMB are shown in Figure S6; Kcat and Kcat/Km of Fe-Nx SANs and HRP were calculated and listed in Table S2, again indicating the excellent catalytic performance of Fe-Nx SANs. Also, the stability of Fe-Nx SANs in harsh environments was evaluated, as shown in Figures 4(c) and 4(d). The curve demonstrated that the SANs maintained excellent stability with pH and temperature variation, while HRP gradually lost its activities when pH was higher than four or the temperature was not close to 40°C. These results indicate that the Fe-Nx SANs can retain much better robustness in harsh environments.
Figure 4.
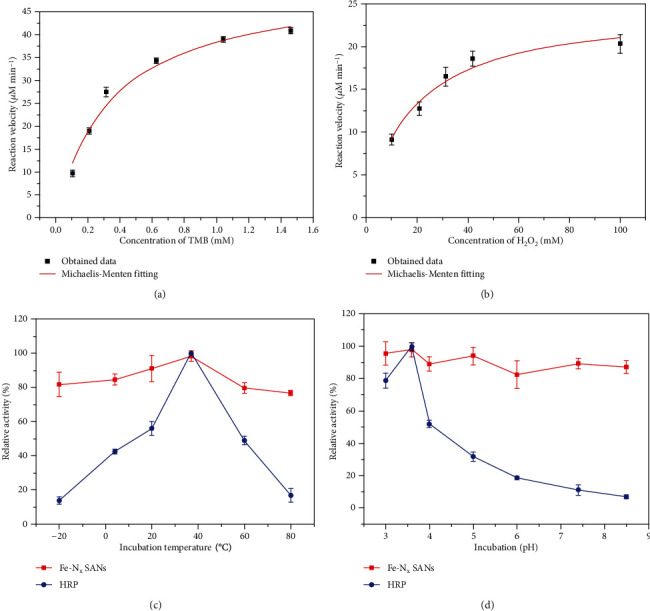
(a, b) Steady-state kinetics curves of Fe-Nx SANs toward TMB and H2O2, respectively; (c, d) Robustness of Fe-Nx SANs against the harsh environment of temperature and pH, respectively.
Amyloid beta 1-40 is a typical biomarker of detecting Alzheimer's disease (AD). However, the concentration of Aβ 1-40 is in pg/mL to ng/mL level in human serum which requires sensitive and accurate detection in the early diagnosis of AD. Herein, a typical sandwich-type SAN-linked immunosorbent assay (SAN-LISA) was built to detect Aβ 1-40, shown in Figure 5(a). The curve of SANs detecting Aβ 1-40 was obtained and is shown in Figure 5(b). The linear range was 1 pg/mL to 2000 pg/mL. The low concentration range between 0 and 15 pg/mL is shown in the inserted figure in Figure 5(b). By applying to the equation 3S/K, where S and K referred to the standard deviation of blank sample and slope of the standard curve, respectively, the limit of detection (LOD) was calculated to be 0.88 pg/mL, which is low enough to meet the detection requirement of human serum. Absorbance spectra of various concentrations of Aβ 1-40 detected by SAN-LISA and their corresponding colorimetric signal are shown in Figure 5(c). It was shown clearly that the signal intensities increased with elevated concentrations of Aβ 1-40. As comparison, the calibration curve of commercial ELISA for the Aβ 1-40 detection was analyzed and is shown in Figure S7. By applying the previous equation, the LOD of the traditional ELISA was calculated to be 9.98 pg/mL, which was more than ten times higher than that of SAN-LISA. The enhanced sensitivity was due to the ultrahigh surface area which could hold more active sites. Furthermore, we evaluated the sensitivity of SAN-LISA by comparing the signal between traditional ELISA and proposed SAN-LISA (Figure 5(d)). The results proved that SAN-LISA has better sensitivity, with much higher absorbance on much lower concertation of Aβ 1-40. What is more, we further studied the detection performance of the two methods with the same Aβ 1-40 concentrations to further prove the satisfactory detection sensitivity of SAN-LISA, shown in Figure S8. As shown in Table 1, compared with previously reported detection results of Aβ 1-40 using different methods, the proposed SAN-LISA method exhibits superior detection performance. Lastly, the specificity of SAN-LISA was analyzed, as displayed in Figure 5(e). Aβ 1-40 exhibited a distinct signal, while the other competing proteins had negligible signals, indicating the satisfactory specificity of SAN-LISA.
Figure 5.
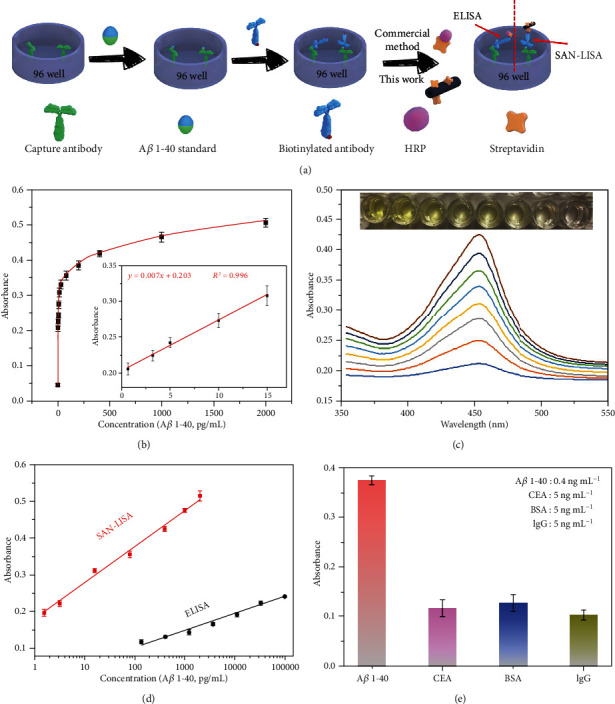
(a) Schematic illustration of SAN-LISA for the detection of Aβ 1-40. (b) The curve of SAN-LISA for the detection of Aβ 1-40 ranging from 1 pg/mL to 2000 pg/mL. (c) Absorbance spectra of various concentrations of Aβ 1-40 detected by SAN-LISA. (d) Standard curves of SAN-LISA (Aβ 1-40 ranging from 1 to 2000 pg/mL) and ELISA (Aβ 1-40 ranging from 100 pg/mL to 100 ng/mL). (e) Specificity of SAN-LISA (Aβ 1-40 of 400 pg/mL; CEA, BSA and IgG of 5 ng/mL, respectively).
Table 1.
Reviews of the detection of Aβ 1-40 with different methods.
| Techniques | LOD (pg mL−1) | Linear range (pg mL−1) | Reference |
|---|---|---|---|
| SAN-LISA | 0.88 | 1-2000 | This work |
| Electrochemical immunoassay | 19 | 20-12500 | [42] |
| SWV∗ at GCE∗ | 7 × 105## | Nonlinear | [43] |
| Microfluidic droplet | 2165# | NP | [44] |
| EIS∗ | 2468# | 43.3-4.33 × 105# | [45] |
| SPR∗ | 86.6### | 86.6-865.9# | [46] |
| SWV∗ | 8.6 × 105# | 1.772 × 106-8.66 × 106# | [47] |
SWV: Square Wave Voltammetry; GCE: Glassy Carbon Electrode; SPR: Surface Plasmon Resonance; ECL: Electrochemiluminescence (ECL) immunosensor; EIS: Electrochemical Impedance Spectroscopy. #Value was expressed in nM and converted to pg mL−1. ##Value was expressed in μg mL−1 and converted to pg mL−1. ###Value was expressed in pM and converted to pg mL−1.
3. Conclusion
In summary, we have successfully synthesize a Fe-Nx single-atom catalyst with outstanding peroxidase-mimicking activity, which is mainly attributed to the ultra-large surface area of carbon support that forms more active sites and enables 100% Fe atom utilization. It also shows excellent robustness in harsh environments. Most importantly, novel Fe-Nx SAN-LISA has been developed to enhance the detection performance of Aβ 1-40, exhibiting a sensitivity with LOD of 0.88 pg/mL. This result is much lower than that of the commercial ELISA kit (9.98 pg/mL), which meets the requirement of effective detection of Aβ 1-40. Based on the high activity of Fe-Nx SANs and improved ELISA performance, the peroxidase-like SANs show great potential and pave a new way to design ELISA kits with improved sensitivity for detecting various target biomarkers.
4. Experimental Section
4.1. Preparation of Fe-Nx SANs
500 mg of MO was dissolved in DI water; then, 5 g of FeCl3 and 1.5 mL pyrrole were added under vigorous stirring to form a Fe3+-doped PPy nanotube. MnO2-coated PPy nanotubes were prepared by dispersing a certain amount of KMnO4 into the aforementioned solution. The product was pyrolyzed at 900°C under the nitrogen atmosphere, and the MnO2 coating could be removed by leaching for 8 h with 5% H2SO4 (v/v) [48]. Finally, the Fe-Nx SANs were obtained after the second heat treatment at 900°C under ammonia.
4.2. Fabrication of SA-Labeled Fe-Nx SANs
First of all, the tubed Fe-Nx SANs were shattered under vigorous sonication and dispersed in PBS (0.5 mg/ml), then adjusted by K2CO3 to reach pH = 6.0 and ultrasonicated for 1 h. Secondly, the solution was activated by N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC: 2 mg/mL) and N-hydroxysuccinimide (NHS: 4 mg/mL) under shaking for 30 minutes, and then, the mixture was centrifuged and washed three times to form the activated Fe-Nx SANs. SA (100 μg/ml in PBS) was incubated with activated Fe-Nx SANs at 37°C for 1 hour, and the mixture was centrifuged for three times to remove unbonded SA. Lastly, the products were passivated with 1% BSA for 30 minutes and dispersed in 1 mL of PBS. Herein, the SA-labeled Fe-Nx SANs were broken down to nanosize via an intense ultrasound treatment before further using in ELISA.
4.3. Detection of Amyloid Beta 1-40 by SAN-Linked Immunosorbent Assay (SAN-LISA)
Schematic illustration of the procedures to determine the level of amyloid beta 1-40 through the ultrasensitive ELISA method employing the Fe-Nx SANs is shown in Figure 5(a). Firstly, different amounts of amyloid beta 1-40 standard were added into a 96-well plate and incubated at 37°C for 2.5 h. Each well was washed three times, and then, 200 μL of PBST (PBS containing 0.5 wt % of TWEEN-20) containing 1 wt % BSA was added into it to block the unbonded primary antibody at 37°C for 1.5 h. Secondly, 100 μL of the prepared biotinylated amyloid beta 1-40 was added to each well and incubated for 1 h with gentle shaking, then the plate was washed with wash buffer for three times. Thirdly, 50 μL of SA labeled Fe-Nx SANs was added into each well and shaken for 45 min. Finally, a chromogenic reaction was conducted. Specifically, 100 μL TMB was added to each well and the mixture was incubated for 10 min at room temperature under gentle shaking. Then, 50 μL stop solution was added to stop the reaction and absorbance data were collected at 450 nm immediately upon color change.
Acknowledgments
This work was supported by a start-up fund from Washington State University. Y. Cheng would like to thank the supports from the Agency for Science, Technology and Research (A∗STAR) and the use of A∗STAR Computational Resource Centre, Singapore (ACRC), and National Supercomputing Centre, Singapore (NSCC). XAS measurements were done at beamline 12-BM of the Advanced Photon Source (APS), which is a User Facility operated for the U.S. Department of Energy Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
Contributor Information
Dan Du, Email: annie.du@wsu.edu.
Yuehe Lin, Email: yuehe.lin@wsu.edu.
Conflicts of Interest
The authors declare no competing interests.
Authors' Contributions
Zhaoyuan Lyu and Shichao Ding contributed equally to this work. Zhaoyuan Lyu and Shichao Ding carried out the experiment and wrote the original draft of the manuscript, which was led by Dan Du and Yuehe Lin. Maoyu Wang, Mingjie Xu, and Zhenxing Feng contributed to the characterization or synthesis of the materials. Nan Zhang, Yuan Cheng, and Chao Zhang did the computation part. All authors contributed to the writing, reviewing, and editing of the manuscript.
Supplementary Materials
Figure S1: the Fourier transform infrared spectra of the Fe-Nx SANs and SA-labeled Fe-Nx SANs. The strong peak at 1638 cm−1 which corresponds to the amide I shows that streptavidin is already successful labeled on Fe-Nx SANs. Figure S2: morphology of PPy nanotube and MnO2-coated PPy nanotube. Figure S3: N2 adsorption-desorption isotherm of Fe-Nx SANs. Figure S4: X-ray photoelectron spectroscopy (XPS) spectrum of Fe-Nx SANs. Figure S5: structure of natural (a) HRP and (b) iron (II) phthalocyanine (FePc). Figure S6: steady-state kinetics curves of HRP toward (a) H2O2 and (b) TMB. Figure S7: standard curve of commercial EISA for the detection of Aβ 1-40 (Aβ 1-40 ranging from 0.1 to 100 ng/mL) and its linear range. Figure S8: standard curve of commercial ELISA and SAN-LISA (Aβ 1-40 ranging from 100 pg/mL to 10 ng/mL). Table S1: comparison of peroxidase-like specific activity (U/mg) of Fe-Nx, other published nanozymes, and natural HRP. Table S2: comparison of steady-state kinetics parameters of Fe-Nx SANs and natural HRP.
References
- 1.Qiao B., Wang A., Yang X., et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nature chemistry. 2011;3(8):634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 2.Zhu C., Fu S., Shi Q., du D., Lin Y. Single-atom electrocatalysts. Angewandte Chemie International Edition. 2017;56(45):13944–13960. doi: 10.1002/anie.201703864. [DOI] [PubMed] [Google Scholar]
- 3.Zhu C., Shi Q., Feng S., Du D., Lin Y. Single-atom catalysts for electrochemical water splitting. ACS Energy Letters. 2018;3(7):1713–1721. doi: 10.1021/acsenergylett.8b00640. [DOI] [Google Scholar]
- 4.Wei H., Liu X., Wang A., et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nature Communications. 2014;5(1):p. 5634. doi: 10.1038/ncomms6634. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H., Liu G., Shi L., Ye J. Single-atom catalysts: emerging multifunctional materials in heterogeneous catalysis. Advanced Energy Materials. 2018;8(1):p. 1701343. doi: 10.1002/aenm.201701343. [DOI] [Google Scholar]
- 6.Zhu C., Shi Q., Xu B. Z., et al. Hierarchically porous M–N–C (M = Co and Fe) single-atom electrocatalysts with robust MNxActive moieties enable enhanced ORR performance. Advanced Energy Materials. 2018;8(29):p. 1801956. doi: 10.1002/aenm.201801956. [DOI] [Google Scholar]
- 7.Huang L., Chen J., Gan L., Wang J., Dong S. Single-atom nanozymes. Science Advances. 2019;5(5):p. eaav5490. doi: 10.1126/sciadv.aav5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y., Jiao L., Luo X., et al. Oxidase-like Fe-N-C single-atom nanozymes for the detection of acetylcholinesterase activity. Small. 2019;15(43):p. 1903108. doi: 10.1002/smll.201903108. [DOI] [PubMed] [Google Scholar]
- 9.Jiao L., Xu W., Yan H., et al. Fe–N–C single-atom nanozymes for the intracellular hydrogen peroxide detection. Analytical Chemistry. 2019;91(18):11994–11999. doi: 10.1021/acs.analchem.9b02901. [DOI] [PubMed] [Google Scholar]
- 10.Jiao L., Yan H., Wu Y., et al. When nanozymes meet single-atom catalysis. Angewandte Chemie. 2020;132(7):2585–2596. doi: 10.1002/ange.201905645. [DOI] [PubMed] [Google Scholar]
- 11.Cheng N., Li J.‐. C., Liu D., Lin Y., Du D. Single-atom nanozyme based on nanoengineered Fe–N–C catalyst with superior peroxidase-like activity for ultrasensitive bioassays. Small. 2019;15(48):p. 1901485. doi: 10.1002/smll.201901485. [DOI] [PubMed] [Google Scholar]
- 12.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews. 2013;42(14):6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 13.Yan H., Cheng H., Yi H., et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1, 3-butadiene. Journal of the American Chemical Society. 2015;137(33):10484–10487. doi: 10.1021/jacs.5b06485. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y., Wu J., Jiao L., et al. Cascade reaction system integrating single-atom nanozymes with abundant Cu sites for enhanced biosensing. Analytical Chemistry. 2020;92(4):3373–3379. doi: 10.1021/acs.analchem.9b05437. [DOI] [PubMed] [Google Scholar]
- 15.Niu X., Cheng N., Ruan X., Du D., Lin Y. Nanozyme-based immunosensors and immunoassays: recent developments and future trends. Journal of The Electrochemical Society. 2020;167:p. 037508. [Google Scholar]
- 16.Jiao L., Yan H., Xu W., et al. Self-assembly of all-inclusive allochroic nanoparticles for the improved ELISA. Analytical Chemistry. 2019;91(13):8461–8465. doi: 10.1021/acs.analchem.9b01527. [DOI] [PubMed] [Google Scholar]
- 17.Sanjay S. T., Li M., Zhou W., Li X., Li X. J. A reusable PMMA/paper hybrid plug-and-play microfluidic device for an ultrasensitive immunoassay with a wide dynamic range. Microsystems & Nanoengineering. 2020;6(1):p. 1. doi: 10.1038/s41378-020-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjay S. T., Dou M., Sun J., Li X. J. A paper/polymer hybrid microfluidic microplate for rapid quantitative detection of multiple disease biomarkers. Scientific Reports. 2016;6(1):p. 30474. doi: 10.1038/srep30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu G., Sanjay S. T., Dou M., Li X. J. Nanoparticle-mediated photothermal effect enables a new method for quantitative biochemical analysis using a thermometer. Nanoscale. 2016;8(10):5422–5427. doi: 10.1039/C5NR09051B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao L., Zhang L., du W., Li H., Yang D., Zhu C. Hierarchical manganese dioxide nanoflowers enable accurate ratiometric fluorescence enzyme-linked immunosorbent assay. Nanoscale. 2018;10(46):21893–21897. doi: 10.1039/C8NR07096B. [DOI] [PubMed] [Google Scholar]
- 21.Ye R., Zhu C., Song Y., et al. Bioinspired synthesis of all-in-one organic–inorganic hybrid nanoflowers combined with a handheld pH meter for on-site detection of food pathogen. Small. 2016;12(23):3094–3100. doi: 10.1002/smll.201600273. [DOI] [PubMed] [Google Scholar]
- 22.Cheng N., Shi Q., Zhu C., Li S., Lin Y., du D. Pt–Ni (OH) 2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides. Biosensors and Bioelectronics. 2019;142:p. 111498. doi: 10.1016/j.bios.2019.111498. [DOI] [PubMed] [Google Scholar]
- 23.Mazzu-Nascimento T., Morbioli G. G., Milan L. A., et al. Improved assessment of accuracy and performance indicators in paper-based ELISA. Analytical Methods. 2017;9(18):2644–2653. doi: 10.1039/C7AY00505A. [DOI] [Google Scholar]
- 24.Zhao G., Wang Y., Li X., et al. Dual-quenching electrochemiluminescence strategy based on three-dimensional metal–organic frameworks for ultrasensitive detection of amyloid-β. Analytical Chemistry. 2019;91(3):1989–1996. doi: 10.1021/acs.analchem.8b04332. [DOI] [PubMed] [Google Scholar]
- 25.Yoo Y. K., Yoon D. S., Kim G., et al. An enhanced platform to analyse low-affinity amyloid β protein by integration of electrical detection and preconcentrator. Scientific reports. 2017;7(1):p. 14303. doi: 10.1038/s41598-017-14338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar G. M., Li S., Mehta T. H., et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Medicine. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roychaudhuri R., Yang M., Hoshi M. M., Teplow D. B. Amyloid β-protein assembly and Alzheimer disease. Journal of Biological Chemistry. 2009;284(8):4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein S. L., Dupuis N. F., Lazo N. D., et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nature Chemistry. 2009;1(4):326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shifa T. A., Vomiero A. Confined catalysis: progress and prospects in energy conversion (Adv. Energy Mater. 40/2019) Advanced Energy Materials. 2019;9(40):p. 1970158. doi: 10.1002/aenm.201970158. [DOI] [Google Scholar]
- 30.Guo J., Li Y., Cheng Y., Dai L., Xiang Z. Highly efficient oxygen reduction reaction electrocatalysts synthesized under nanospace confinement of metal–organic framework. ACS Nano. 2017;11(8):8379–8386. doi: 10.1021/acsnano.7b03807. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W., Wu Y., Dong H.-J., et al. Sparks fly between ascorbic acid and iron-based nanozymes: a study on Prussian blue nanoparticles. Colloids and Surfaces B: Biointerfaces. 2018;163:379–384. doi: 10.1016/j.colsurfb.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z., Jiang L., Galli F., et al. A graphene oxide streptavidin complex for biorecognition–towards affinity purification. Advanced Functional Materials. 2010;20(17):2857–2865. doi: 10.1002/adfm.201000761. [DOI] [Google Scholar]
- 33.Xu D., Wegner S. V. Multifunctional streptavidin–biotin conjugates with precise stoichiometries. Chemical Science. 2020;11(17):4422–4429. doi: 10.1039/d0sc01589j. [DOI] [Google Scholar]
- 34.Liu D., Li J.-C., Shi Q., et al. Atomically isolated iron atom anchored on carbon nanotubes for oxygen reduction reaction. ACS Applied Materials & Interfaces. 2019;11(43):39820–39826. doi: 10.1021/acsami.9b12054. [DOI] [PubMed] [Google Scholar]
- 35.Liang H.-W., Wei W., Wu Z.-S., Feng X., Müllen K. Mesoporous metal–nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. Journal of the American Chemical Society. 2013;135(43):16002–16005. doi: 10.1021/ja407552k. [DOI] [PubMed] [Google Scholar]
- 36.Li J.-C., Cheng M., Li T., et al. Carbon nanotube-linked hollow carbon nanospheres doped with iron and nitrogen as single-atom catalysts for the oxygen reduction reaction in acidic solutions. Journal of Materials Chemistry A. 2019;7(24):14478–14482. doi: 10.1039/C9TA00508K. [DOI] [Google Scholar]
- 37.Seah M. P., Brown M. T. Validation and accuracy of peak synthesis software for XPS. Applied Surface Science. 1999;144-145:183–187. doi: 10.1016/s0169-4332(98)00787-9. [DOI] [Google Scholar]
- 38.Ding S., Hu X., Guan P., et al. Preparation of surface-imprinted microspheres using ionic liquids as novel cross-linker for recognizing an immunostimulating peptide. Journal of Materials Science. 2017;52(13):8027–8040. doi: 10.1007/s10853-017-1005-x. [DOI] [Google Scholar]
- 39.Li J.-C., Yang Z.-Q., Tang D.-M., et al. N-doped carbon nanotubes containing a high concentration of single iron atoms for efficient oxygen reduction. NPG Asia Materials. 2018;10(1):e461–e461. doi: 10.1038/am.2017.212. [DOI] [Google Scholar]
- 40.Xu B., Wang H., Wang W., et al. A single-atom nanozyme for wound disinfection applications. Angewandte Chemie International Edition. 2019;58(15):4911–4916. doi: 10.1002/anie.201813994. [DOI] [PubMed] [Google Scholar]
- 41.Huo M., Wang L., Wang Y., Chen Y., Shi J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano. 2019;13(2):2643–2653. doi: 10.1021/acsnano.9b00457. [DOI] [PubMed] [Google Scholar]
- 42.de la Escosura-Muñiz A., Plichta Z., Horák D., Merkoçi A. Alzheimer′ s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosensors and Bioelectronics. 2015;67:162–169. doi: 10.1016/j.bios.2014.07.086. [DOI] [PubMed] [Google Scholar]
- 43.Vestergaard M.'d., Kerman K., Saito M., Nagatani N., Takamura Y., Tamiya E. A rapid label-free electrochemical detection and kinetic study of Alzheimer's amyloid beta aggregation. Journal of the American Chemical Society. 2005;127(34):11892–11893. doi: 10.1021/ja052522q. [DOI] [PubMed] [Google Scholar]
- 44.Mai T. D., Ferraro D., Aboud N., et al. Single-step immunoassays and microfluidic droplet operation: towards a versatile approach for detection of amyloid-β peptide-based biomarkers of Alzheimer’s disease. Sensors and Actuators B: Chemical. 2018;255:2126–2135. doi: 10.1016/j.snb.2017.09.003. [DOI] [Google Scholar]
- 45.Lien T. T. N., Takamura Y., Tamiya E., Vestergaard M.'d. C. Modified screen printed electrode for development of a highly sensitive label-free impedimetric immunosensor to detect amyloid beta peptides. Analytica Chimica Acta. 2015;892:69–76. doi: 10.1016/j.aca.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 46.Xia N., Liu L., Harrington M. G., Wang J., Zhou F. Regenerable and simultaneous surface plasmon resonance detection of aβ (1−40) and aβ (1−42) peptides in cerebrospinal fluids with signal amplification by streptavidin conjugated to an n-terminus-specific antibody. Analytical Chemistry. 2010;82(24):10151–10157. doi: 10.1021/ac102257m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhulkar S., Piatyszek R., Cirrito J. R., Wu Z. Z., Li C. Z. Microbiosensor for Alzheimer’s disease diagnostics: detection of amyloid beta biomarkers. Journal of Neurochemistry. 2012;122(2):374–381. doi: 10.1111/j.1471-4159.2012.07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hariprasad D., Dash B., Ghosh M. K., Anand S. Leaching of manganese ores using sawdust as a reductant. Minerals Engineering. 2007;20(14):1293–1295. doi: 10.1016/j.mineng.2007.07.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: the Fourier transform infrared spectra of the Fe-Nx SANs and SA-labeled Fe-Nx SANs. The strong peak at 1638 cm−1 which corresponds to the amide I shows that streptavidin is already successful labeled on Fe-Nx SANs. Figure S2: morphology of PPy nanotube and MnO2-coated PPy nanotube. Figure S3: N2 adsorption-desorption isotherm of Fe-Nx SANs. Figure S4: X-ray photoelectron spectroscopy (XPS) spectrum of Fe-Nx SANs. Figure S5: structure of natural (a) HRP and (b) iron (II) phthalocyanine (FePc). Figure S6: steady-state kinetics curves of HRP toward (a) H2O2 and (b) TMB. Figure S7: standard curve of commercial EISA for the detection of Aβ 1-40 (Aβ 1-40 ranging from 0.1 to 100 ng/mL) and its linear range. Figure S8: standard curve of commercial ELISA and SAN-LISA (Aβ 1-40 ranging from 100 pg/mL to 10 ng/mL). Table S1: comparison of peroxidase-like specific activity (U/mg) of Fe-Nx, other published nanozymes, and natural HRP. Table S2: comparison of steady-state kinetics parameters of Fe-Nx SANs and natural HRP.


