Abstract
The maintenance of neural stem cell function is vital to ensure neurogenesis throughout adulthood. During aging, there is a significant reduction in adult neurogenesis that correlates with a decline in cognitive function. Although recent studies have revealed novel extrinsic and intrinsic mechanisms that regulate the adult neural stem cell (NSC) pool and lineage progression, the precise molecular mechanisms that drive dysregulation of adult neurogenesis in the context of aging are only beginning to emerge. Recent studies have shed light on mechanisms that regulate the earliest step of adult neurogenesis, the activation of quiescent NSCs. Interestingly, the ability of NSCs to enter the cell cycle in the aged brain significantly declines suggesting a deepend state of quiescence. Given the likely contribution of adult neurogenesis to supporting cognitive function in humans, enhancing neurogenesis may be a strategy to combat age-related cognitive decline. This review highlights the mechanisms that regulate the NSC pool throughout adulthood and discusses how dysregulation of these processes may contribute to the decline in neurogenesis and cognitive function throughout aging.
Keywords: Adult neurogenesis, aging, cognitive decline, neural stem cell, NSC, quiescence
1. Introduction
Adult neurogenesis continues throughout life in the adult mammalian brain and supports brain plasticity, cognition, and sensory functions. Reservoirs of somatic neural stem cells (NSCs) that retain neurogenic potential are the source new neurons and glia in the adult. NSCs reside in two neurogenic niches in the adult rodent brain: the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus. The majority of NSCs reside in a reversible, cell cycle arrested state termed quiescence. Quiescent NSCs become activated in response to intrinsic or extrinsic cues and enter the cell cycle, which is the first functional step in neurogenesis. Activated NSCs in the SVZ generate newborn neurons in the olfactory bulb that function as inhibitory interneurons. NSCs in the SGZ of the hippocampus generate new excitatory granule neurons in the dentate gyrus that support learning and memory functions as well as mood regulation. In humans, evidence indicates that neurogenesis persists in the dentate gyrus in healthy adults (Boldrini et al., 2018; Eriksson et al., 1998; Moreno-Jimenez et al., 2019; Palmer et al., 2001; Spalding et al., 2013), though there has been some controversy over the extent to which neurogenesis declines postnatally in humans (Dennis et al., 2016; Kempermann et al., 2018; Snyder, 2019; Sorrells et al., 2018). Nonetheless, many studies, including recent work using improved methodologies to detect immature neurons, suggest that adult neurogenesis is present and sharply declines with age and in neurodegenerative disease in humans (Ernst et al., 2014; Knoth et al., 2010; Marxreiter et al., 2013; Mathews et al., 2017; Moreno-Jimenez et al., 2019; Spalding et al., 2013; Tobin et al., 2019).
Cognitive function can be improved by behavioral interventions, including physical activity, environmental enrichment, and dietary changes. Manipulations such as environmental enrichment and voluntary running paradigms increase neurogenesis in the dentate gyrus and improve learning in rodents (Brown et al., 2003; Leiter et al., 2019; van Praag et al., 1999a; van Praag et al., 1999b). Similarly, olfactory enrichment increases the number of newborn neurons in the olfactory bulb and improves odorant memory (Rochefort et al., 2002). Enrichment can also enhance neurogenesis in aged animals. For example, voluntary running paradigms are sufficient to ameliorate the loss of neurogenesis and learning deficits in old rodents (van Praag et al., 2005). Thus, newly born granule cells can functionally integrate into the aged dentate gyrus. Consistent with this notion, new granule cells in the aged brain express immediate early genes such as Arc in response to spatial processing (Marrone et al., 2012).
Evidence for an age-associated decline in NSC proliferation and neurogenesis was apparent even in early studies performed in rodents (Altman and Das, 1965; Kempermann et al., 1998; Kuhn et al., 1996; Seki and Arai, 1995). For example, starting after the first month of life, hippocampal neurogenesis decreases by about 40% each month for the first nine months in the mouse brain (Ben Abdallah et al., 2010). These and other data suggest that neurogenesis begins to decline by middle age (Bondolfi et al., 2004; Corenblum et al., 2016; Jinno, 2011). In aged animals, the loss of newly born neurons correlates with a severe loss of proliferation by 20–24 months of age in both NSC niches (Bondolfi et al., 2004; Enwere et al., 2004; Maslov et al., 2004; Tropepe et al., 1997). This loss seems to be specific to neurogenic populations as other niche cells such as SVZ astrocytes and ependymal cells remain intact in aged animals (Luo et al., 2006). Thus, the loss of the functional neural stem cell pool may drive the overall loss of neurogenesis and contribute to cognitive decline in aged animals.
Accumulating evidence suggests that defects in NSC activation are in part responsible for the age-associated decline in neurogenesis (Figure 1). In the aged brain, there is evidence for a strong reduction in the pool of cycling NSCs, but less of a reduction in total quiescent NSCs (Artegiani et al., 2017; Kalamakis et al., 2019; Lugert et al., 2010). This is consistent with studies showing the fraction of NSCs in the quiescent state increases throughout aging. These data, which are also supported by mathematical modeling of NSC states, suggest a “deepened quiescence” of aged NSCs (Ziebell et al., 2018). Once activated, aged and young NSCs may exhibit similar proliferation and differentiation potential (Kalamakis et al., 2019). Moreover, as discussed below, a number of interventions can mobilize NSCs out of the quiescent state. However, other studies suggest that NSCs can only undergo a limited number of divisions once activated, followed by differentiation into terminally differentiated astrocytes (Encinas et al., 2011). Since quiescent NSCs are a subtype of astrocytes, it remains unclear whether the terminally differentiated astrocytes observed in this study are NSCs in a form of deep quiescence that could be reversible under the appropriate conditions. Nevertheless, manipulations that rescue quiescent NSC function and/or activation remain promising targets to increase neurogenesis and cognitive function in aged animals. Here, we discuss the evidence implicating intrinsic and extrinsic changes that contribute to a deepening of quiescence with age, resulting in a decline in neurogenesis (summarized in Figure 2).
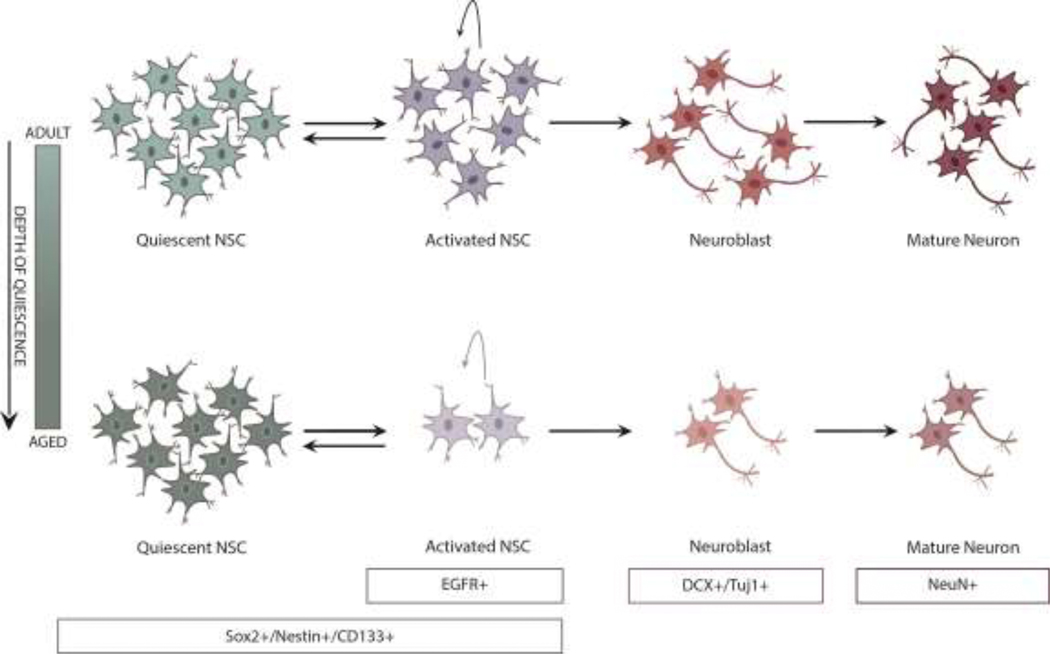
Figure 1. Neurogenesis declines in the aged mammalian brain.
Adult neurogenesis begins when quiescent NSCs enter the cell cycle, known as NSC activation. Following 2–3 rounds of proliferation, activated NSCs return to quiescence or commit to differentiation and functionally integrate into the circuitry. NSC in the aged brain drift deeper into quiescence and have a reduced capacity to become activated. As a result, there is a significant decline in the amount of activated NSCs, neuroblasts, and newly born mature neurons in the aged brain. Markers that are expressed along the neurogenic lineage include: quiescent NSCs (Sox2+/Nestin+/CD133+/EGFR-), activated NSCs (Sox2+/Nestin+/CD133+/EGFR+), doublecortin-expressing neuroblasts (DCX+/Tuj1+), and mature neurons (NeuN).
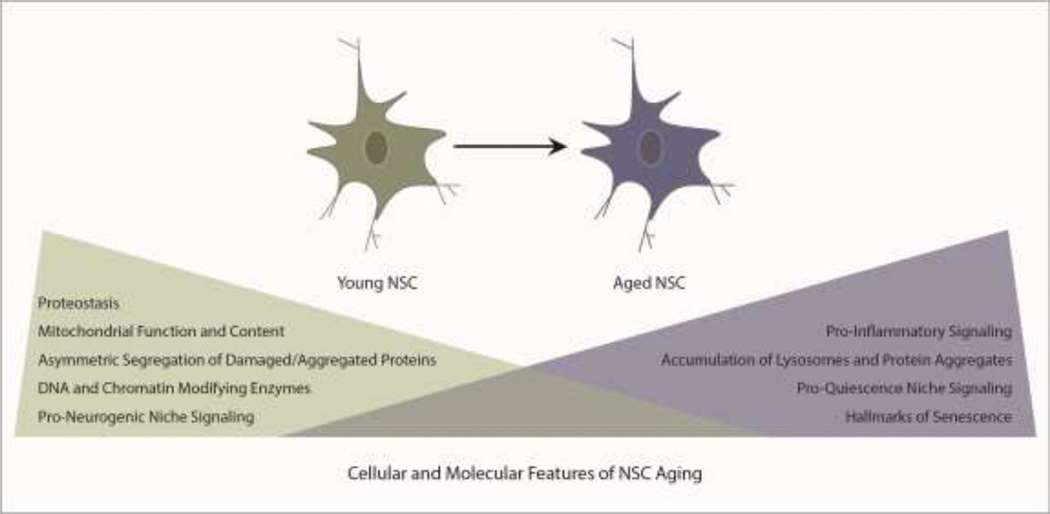
Figure 2. The molecular and cellular signatures of NSC aging.
The critical mechanisms that ensure homeostasis of the adult NSC pool become dysregulated during aging.
2. Intrinsic changes with age: altered metabolism, proteostasis, senescence, and epigenetic states
Metabolism
Specific metabolic mechanisms support NSC quiescence, proliferation, and the dynamic transition between the two states. The energetic needs of quiescent and activated NSCs differ substantially, with proliferating cells requiring significantly greater energy production. Quiescent NSCs are enriched for a glycolytic transcriptional program (Beckervordersandforth et al., 2017; Llorens-Bobadilla et al., 2015; Shin et al., 2015) and switch to predominantly utilizing mitochondrial oxidative phosphorylation (oxPhos) for energy production upon activation (Beckervordersandforth et al., 2017; Shin et al., 2015). Evidence suggets that this metabolic switch is critical to meet the energetic demands of proliferating NSCs, as pharmacological or genetic interruption of electron transport chain (ETC) and oxPhos activity significantly reduces proliferation and causes cell death (Beckervordersandforth et al., 2017).
During aging, the metabolic balance between glycolysis and oxPhos shifts to favor a more quiescent state. Glycolytic enzymes show an age-associated upregulation whereas nuclear-encoded ETC proteins become downregulated (Stoll et al., 2011). Consistent with a shift toward glycolytic metabolism, aged NSCs also exhibit a significant loss of mitochondrial content, membrane potential, ATP production, and oxygen consumption that correlate with reduced proliferation (Beckervordersandforth et al., 2017; Stoll et al., 2011). Evidence suggests that aged NSCs can be “rejuvenated” through acitvation of oxPhos. For example, treatment of aged (18 month old) animals with a nootropic compound that enhances cognition increases the expression of mitochondrial complexes I-V in the dentate gyrus and stimulates neurogenesis (Beckervordersandforth et al., 2017). Similarly, elevated expression of the regulator of mitochondiral biogenesis, Pgc1a (proliferator-activated receptor gamma coactivator 1 alpha), enhances proliferation of aged NSCs in culture and neurogenesis in the aged brain (Stoll et al., 2015). Conversely, genetic disruption of mitochondrial oxPhos activity and the ETC in young adult NSCs reproduces many of the metabolic and neurogenic defects associated with aging. Thus, oxPhos levels are likely to directly impact the balance between NSC quiescence and activation, and modulation of mitochondrial content and oxPhos is sufficient to coerce old NSCs out of a dormant state.
A major byproduct of ATP production through oxPhos is the generation of reactive oxygen species (ROS). ROS are well known to cause cellular damage to DNA, proteins, and lipids that contribute to aging, but also function as second messengers in cell signaling. As a result of high oxPhos activity, actively proliferating NSCs have elevated ROS levels (Le Belle et al., 2011). Interestingly, treatment with exogenous ROS is sufficient to stimulate NSCs to proliferate and self-renew through activation of PI3K/AKT (Le Belle et al., 2011). In contrast, high levels of ROS are mitigated by the quiescence-promoting FOXO and HIF-1a transcription factors, which regulate ROS detoxifying genes and activities (Li et al., 2014; Mazumdar et al., 2010; Paik et al., 2009; Renault et al., 2009). Tight control of redox homeostasis is further reinforced by the redox-sensitive transcription factor NRF2, and the chromatin regulator PRDM16, which support the proliferative potential and self-renewal of NSCs (Chuikov et al., 2010; Corenblum et al., 2016). Moreover, mitochondrial DNA mutagenesis, which causes progeroid phenotypes, causes ROS/redox changes, reduces NSC self-renewal and the overall number of progenitors (Ahlqvist et al., 2012). Alterations in all of these factors may contribute to NSC dysfunction during aging. As a result, as oxidative metabolism decreases during aging, ROS levels are reduced, likely tipping the balance toward a deeper state of quiescence.
Exit from quiescence also coincides with altered lipid metabolism. Activated NSCs rely on fatty acid synthase-dependent lipogenesis for proliferative capacity, whereas quiescent NSCs require high levels of lipid breakdown through fatty acid oxidation (Knobloch et al., 2013; Knobloch et al., 2017). The role of lipid metabolism in stem cell behavior is only beginning to emerge and will undoubtedly be highly complex, as lipids function as energy sources, membrane components, and signaling molecules. Moreover, lipids can also be taken up from the circulation through the activity of fatty acid binding proteins. Environmental changes such as altered diet, systemic metabolism, and aging are likely to dramatically affect lipid-associated functions in aged NSCs, but the mechanisms have yet to be defined.
Together, these data suggest that metabolic programs play a key instructive role in balancing NSC quiescence and activation in the adult brain. Proteomics approaches revealed that metabolic factors make up one-third of all proteins with significant age-related alterations in NSCs (Stoll et al., 2011). Thus, NSCs undergo considerable metabolic reprogramming with age, yet we are only beginning to understand the mechanisms responsible.
Proteostasis
Proteostasis, or protein homeostasis, involves the integration of multiple mechanisms that ensure the proper synthesis, trafficking, folding, and degradation of proteins in the cell. Protein synthesis occurs at low rates in quiescent NSCs and is strongly upregulated during activation (Baser et al., 2017), and distinct mechanisms are in place in the two different cellular states to maintain healthy proteostasis. It is widely accepted that proteostasis is lost during aging across tissues and, similar to other cell types, NSCs accumulate ubiquitinated proteins with age in vivo (Moore et al., 2015). Precisely how proteostatic mechanisms support NSCs and contribute to their decline during aging is just beginning to be understood. There are three main branches of the protein homeostasis network: chaperone-mediated proteolysis, the autophagy-lysosomal system, and ubiquitin-mediated proteolysis. Each branch performs a specific function in either protein folding (chaperones) or degradation (autophagy and the proteasome). A number of studies provide evidence that these pathways are critical for adult neurogenesis (Audesse et al., 2019; Morrow et al., 2020; Vonk et al., 2020; Wang et al., 2013; Xi et al., 2016). In this section, we discuss recent evidence implicating these processes in NSC dormancy, activation, and aging.
Recent work revealed that quiescent and activated NSCs rely on different mechanisms to maintain healthy proteostasis. Quiescent NSCs highly express autophagy-lysosomal genes whereas activated NSCs upregulate proteasome and ubiquitin-mediated proteolysis networks. Quiescent NSCs accumulate enlarged lysosomes that contain protein aggregates, a property that is exacerbated with age. Interestingly, rapid clearance of lysosomal contents through autophagic induction is sufficient to increase NSC activation in the aged brain, implicating autophagic dysfunction as a primary driver of the decline in quiescent NSC function with age. The precise nature of the cargo loaded into the autophagy-lysosomal pathway remains to be defined, but aggregate-prone proteins have been detected as contents in NSCs (Moore et al., 2015; Morrow et al., 2020). In addition, a recent study shows the pro-mitogenic EGF receptors are rapidly degraded by quiescent NSC lysosomes (Kobayashi et al., 2019). As EGF is well known to drive NSC activation and proliferation, it remains unclear how reduced lysosomal function throughout aging, which would increase EGFR levels, may contribute to the decline in neurogenesis. Nevertheless, the autophagy-lysosomal pathway is vital for the maintenance of quiescence through a combination of protein quality control and signaling mechanisms.
Asymmetric segregation of protein aggregates during NSC division provides an additional mechanism to preserve the neurogenic lineage. During cell division, NSCs utilize the ER membrane as a diffusion barrier to asymmetrically distribute damaged and aggregated proteins to non-stem daughters, thereby lowering the aggregate load of the cells that return to quiescence. The ER diffusion barrier weakens with age, resulting in more symmetric distribution of damaged and aggregated proteins during NSC proliferation (Moore et al., 2015). The intermediate filament vimentin was recently identified as a critical factor in ensuring asymmetric inheritance of damage. Vimentin is asymmetrically inherited along with polyubiquitinated proteins and functions to recruit proteasomes to clear damaged proteins under proteotoxic stress. Accordingly, vimentin-ablated NSCs fail to proliferate or fully exit quiescence under high aggregate load. As loss of vimentin accelerates the age-dependent decline of NSC function and neurogenesis, age-associated loss of vimentin that causes increased symmetric inheritance of aggregates could be a primary mechanism that drives the enhanced quiescence of aged NSCs (Morrow et al., 2020).
Although it is clear that protein quality control mechanisms are essential across the neurogenic lineage, a number of questions remain unanswered. For example, whether a loss of lysosomal number or function drives the age-associated autophagic defects and accumulation of protein aggregates in quiescent NSCs remains unknown. A pulse of the transcription factor TFEB, a master regulator of lysosomal biogenesis, is sufficient to rescue the age-associated loss of proliferation in NSCs. The pro-longevity transcription factor FOXO3, which has been implicated in the maintenance of quiescence and neuronal maturation, directly binds and functionally regulates an autophagy gene network in NSCs (Paik et al., 2009; Renault et al., 2009; Schaffner et al., 2018; Webb et al., 2013). In the absence of FOXOs, NSCs accumulate protein aggregates (Audesse et al., 2019). Whether loss or alteration of these transcriptional networks throughout aging drives the age-associated decline in autophagy and NSC function remains to be explored. Despite the unknowns, it is clear that induction of proteostasis pathways can be altered to rescue the age-associated decline in NSC activation and neurogenesis.
Senescence
Cellular senescence is an irreversible state of cell cycle arrest accompanied by a number of phenotypic changes, including morphological alterations, metabolic reprogramming, and epigenetic changes. Senescent cells accumulate during aging and clearance of these cells delays the hallmarks of aging and improves age-associated diseases (Kirkland and Tchkonia, 2017; Xu et al., 2018). Senescent cells are identified by markers such as p16/Cdkn2a, p21/Cdkn1a, senescence associated β-galactosidase activity, as well as a specific secretory signature known as the SASP (senescence associated secretory phenotype). Our understanding of when these markers are elevated in the NSC lineage is limited to a small number of studies, but data indicate that the hallmarks of senescence emerge in NSCs similar to other cells. For example, aged (22–26 month) NSCs in culture contain about 30% SA-β-gal positive cells, which is about a 2-fold increase compared to 2–5 month old adult NSCs (Ahlenius et al., 2009). p16/Cdkn2a is detectable in vivo in the mouse SVZ niche between 12–18 months of age (Molofsky et al., 2006). Moreover, p16/Cdkn2a ablation is sufficient to increase neurogenesis in these mice indicating that senescence is detrimental to the niche.
Data on the impact of clearing senescent cells in the brain are only starting to emerge, but initial studies suggest improvements in neurodegenerative disease models (Bussian et al., 2018; Chinta et al., 2018; Musi et al., 2018). These studies specifically demonstrated a role for senescent glia in neurodegeneration, and this role likely holds true for NSCs as well due to their glial identity (Doetsch et al., 1999). Indeed, in the context of high fat diet-induced senescence, new neuron formation is impaired and clearance of senescent cells reversed neurogenesis in the dentate gyrus and anxiety-associated behaviors (Ogrodnik et al., 2019). Thus, initial studies on the emergence of senescent NSCs and niche cells implicate increased senescence as a mechanism responsible for the loss of neurogenesis in the aged brain. Since senescent cells are still a minority of cells in any tissue with age, the impact of senescence on neurogenesis is likely to be largely driven by the SASP or other cell non-autonomous mechanisms, which may contribute to deepening of quiescence. This is an important area for future studies.
Epigenetics
Young, healthy NSCs rely on stable epigenetic programs to support neurogenesis. For example, early studies investigating the polycomb transcriptional repressor BMI1 in SVZ NSCs revealed a critical role for chromatin modifiers in self-renewal (Fasano et al., 2007; Molofsky et al., 2003). The accumulation of epigenetic changes is a major feature of aging, often referred to as epigenetic drift. Age-associated epigenetic changes include the loss or accumulation of covalent histone modifications, DNA modifications, or global alterations to chromatin conformation. Indeed, recent work demonstrated that covalent histone modifications change with age in primary NSCs, including the mark associated with transcription start sites, H3K4me3, as well as features found at “super-enhancers” (Benayoun et al., 2019).
Other chromatin modifying enzymes have important roles in supporting adult neurogenesis, and likely affect the depth of NSC quiescence during aging. For example, the Sirtuin family of nicotine adenine dinucleotide (NAD+) dependent deacetylases, which have been linked to brain aging (Satoh et al., 2017), are also regulators of adult neurogenesis (Ma et al., 2014; Okun et al., 2017). SIRT1, which functions as a histone deacetylase, has been shown to suppress proliferation of neural progenitors in the adult dentate gyrus (Ma et al., 2014). Interestingly, metabolic stress induced by 2-deoxyglucose increases SIRT1 activity, thereby suppressing proliferation of the NSC pool. Thus, during aging, increased stress may deepen quiescence through enhanced SIRT1 activity in the NSC lineage. However, NSC-specific ablation of the rate-limiting enzyme for NAD+ biosynthesis, NAMPT, impairs cell cycle progression (Stein and Imai, 2014). NAMPT activity is reduced with age in the hippocampus and elevating NAD+ levels in the aged hippocampus restores the functional NSC pool. It remains unclear why loss of SIRT1 and NAMPT ablation in NSCs have apparently opposite phenotypes. However, since NAD+ functions as a redox cofactor for a number of enzymes, other metabolic functions are likely disrupted in the context of NAMPT ablation. Nonetheless, these data support an important function for histone acetylation/deacetylation in the balance between NSC quiescence and activation with age.
DNA modifications have also been linked to NSC proliferation, aging and rejuvenation. Cytosine methylation (5mC) is an evolutionarily conserved modification that is widespread across the genome and functions in the regulation of gene expression. Both methylation and demethylation of cytosines is critical for the maintenance of neurogenesis in the postnatal and adult brain (Hutnick et al., 2009; Ma et al., 2009; Wu et al., 2010). More recently, hydroxylation of 5mC was identified as an additional epigenetic modulator catalyzed by Tet family dioxygenases (ten eleven translocation methylcytosine dioxygenase). Specifically, the TET1 and TET2 enzymes, which catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), were identified as regulators of adult neurogenesis. Tet1 knockout mice have hippocampal neurogenesis defects due to reduced NSC proliferation, and display impaired learning and memory (Zhang et al., 2013). Interestingly, the function of TET1 is to promote DNA demethylation in the dentate gyrus, thereby protecting genes from methylation and reversing silencing (Guo et al., 2011). TET2 and overall 5hmC levels decrease with age in the hippocampus, and elevating TET2 levels is sufficient to reverse age-associated loss of neurogenesis and enhances cognition in contextual fear conditioning and radial arm maze paradigms (Gontier et al., 2018).
Despite these advances, we are only beginning to understand how epigenetic drift affects NSC quiescence and activation in aged individuals. One challenge in understanding the epigenetic underpinnings of NSC aging is that many of the necessary approaches (e.g. biochemical assays and genomics technologies) require large numbers of cells. The recent establishment of single cell epigenomics technologies will shed new light on this area and open up new opportunities such as the heterogeneity of the NSC pool in vivo during aging.
3. Extrinsic mechanisms that impact NSC quiescence and activation during aging
Signals from the extracellular environment impact levels of neurogenesis in the adult brain through regulation of quiescence, proliferation, differentiation, and integration. Locally, adult neurogenesis is regulated by a number of secreted signaling molecules in the niche. The signals can be produced by different cell types, including astrocytes, microglia, neurons, endothelial cells, and NSCs themselves. A number of studies have reported changes in levels of these factors with age, resulting in a loss of NSC proliferative capacity. IGF1, IGF2, EGF, FGF2, VEGF and WNT3 are reduced with age in the NSC niche, and enhancing some of these signals can rejuvenate the niche in aged animals, at least to some extent (Enwere et al., 2004; Jin et al., 2003; Kang and Hebert, 2015; Licht et al., 2016; Lichtenwalner et al., 2001; Miranda et al., 2012; Okamoto et al., 2011; Shetty et al., 2005; Steinmetz et al., 2016). As a result of reduced mitogenic signaling cues with age, NSC transcriptional programs shift toward signatures less supportive of growth and neurogenesis, and more indicative of deep quiescence. For example, reduced insulin/IGF signaling causes increased activity of the FOXO family of transcription factors, which preserve a more quiescent state (Audesse et al., 2019; Paik et al., 2009; Renault et al., 2009; Webb et al., 2013). Reduction of IGF receptors has also been linked to reduced activation of NSCs with age in zebrafish, indicating evolutionary conservation of this mechanism (Obermann et al., 2019). In contrast to insulin/IGF, increased levels of bone morphogenetic protein (BMP) signaling in the aged brain impair NSC proliferation and push cells deeper into quiescence. For example, levels of BMP4 and BMP6 are increased in the NSC niche with age, resulting in enhanced quiescence and reduced neurogenesis (Diaz-Moreno et al., 2018; Mira et al., 2010; Yousef et al., 2015b). Similarly, TGFβ signaling, which restricts hippocampal neurogenesis, increases with age, and can be attenuated to restore more youthful neurogenesis (Buckwalter et al., 2006; Yousef et al., 2015a).
Interventions that promote healthy aging, such as caloric restriction and inhibition of mTOR signaling also affect NSC activation. Caloric restriction, the most highly conserved intervention to extend lifespan, robustly enhances NSC proliferation and progenitor survival in the rodent dentate gyrus and SVZ (Apple et al., 2019; Lee et al., 2000; Lee et al., 2002; Park et al., 2013). The mTOR (mammalian target of rapamycin) protein kinase is a key integrator of extracellular signals to sense nutrients and control stem cell proliferation, growth, and differentiation. mTORC1 activity is reduced with age in NSCs in the dentate gyrus, and enhancing TOR activity in these cells can restore proliferation in aged animals. Functionally, mTOR acts in parallel to PI3K signaling to promote the balance between NSC quiescence and activation, and as mTOR activity dampens with age, NSCs drift deeper into quiescence (Nieto-Gonzalez et al., 2019; Sato et al., 2010; Zhou et al., 2018). The mechanism underlying reduced TOR signaling with age remains to be determined, as mTOR is regulated by a number of upstream cues including EGF, IGF, and amino acids. Moreover, the full spectrum of cellular effects downstream of altered mTOR activity remains undefined, but recent work suggests action through the lysosome-autophagy system.
Recent studies implicate changes in the niche environment in the reduction of neurogenesis with age. Activation of niche microglia precedes the loss of neurogenesis in the SVZ and the pro-inflammatory milieu produced by activated microglia is sufficient to reduce proliferation and neurogenic potential in culture (Solano Fonseca et al., 2016). In vivo, interferon-γ producing T cells invade the old SVZ niche, likely enhancing the inflammatory effect caused by microglia (Dulken et al., 2019). Multiple studies using heterochronic parabiosis showed age-associated alterations in chemokines, corticosteroids and other systemic blood-borne factors (e.g. CCL11 and β2-microglobulin) alter neurogenesis in the hippocampus and SVZ (Katsimpardi et al., 2014; Smith et al., 2018; Villeda et al., 2011; Villeda et al., 2014). In some cases, exposure to factors in young blood is sufficient to revitalize the aged hippocampus and rescue cognitive performance (Castellano et al., 2015; Katsimpardi et al., 2014; Villeda et al., 2014). SVZ NSCs receive additional cues from the lateral ventricle choroid plexus that secretes factors vital to NSC function and proliferation (Silva-Vargas et al., 2016). Thus, both the systemic and local niche environment undergo significant age-associated changes. Collectively, these studies support the notion that the aged neurogenic niche remains permissive for new neuron formation, and that quiescent NSCs can be stimulated to form functional neurons in the aged environment given the right signaling cues.
4. Conclusions
In recent years, our understanding of the molecular mechanisms responsible for the loss of neurogenesis has greatly advanced. Accumulating evidence indicates that populations of NSCs are maintained in the aged mammalian brain but have retreated deeper into quiescence. This view is supported by studies manipulating intrinsic factors including metabolic states, proteostasis pathways, and epigenetic features. Additionally, extrinsic factors such as BMP signaling and inflammatory cytokines push cells deeper into quiescence with age, which can be reversed through modulation of systemic cues. It should be noted that interventions that activate NSCs in the aged brain have thus far not succeeded in restoring neurogenesis back to young adult levels, indicating that other factors depletion of the NSC pool likely contribute to the loss of neurogenesis. Nonetheless, coercing the cells out of quiescence and along a neurogenic path enhances cognition and sensory function in aged rodents. Therefore, understanding the mechanisms that drive NSCs into a deeper state of quiescence with age is critical to fully capture the potential of these cells.
While significant progress has been made in recent years in our understanding on NSC aging, a number of questions remain unanswered. For example, our understanding of the mechanisms of senescence in NSCs is rudimentary at this time. There is currently little evidence for double stranded breaks in aged NSCs (Kalamakis et al., 2019), and thus our understanding of how DNA damage may contribute to the decline in neurogenesis is limited. Moreover, although telomerase activity is important for maintaining adult NSC proliferation (Caporaso et al., 2003; Ferron et al., 2004; Lobanova et al., 2017), little is known about how telomere maintenance affects NSC aging. Telomere attrition has been linked to senescence, including in NSCs (Zhang et al., 2006), and restoring telomerase activity in mice with telomere dysfunction can increase NSC proliferation (Jaskelioff et al., 2011). Whether the depth of quiescence is affected in this context, as opposed to senescence or apoptosis, is not known. Finally, given the recent reports of abundant neurogenesis in the adult human dentate gyrus, and its steady decline with age and neurodegeneration, it will be critical to gain a deeper mechanistic understanding of why NSCs become defective in the aged human brain, and identify strategies to rejuvenate them.
HIGHLIGHTS.
During aging, there is a significant reduction in adult neurogenesis that correlates with a decline in cognitive function
In humans, there is strong evidence that neurogenesis persists in the dentate gyrus in healthy adults, and declines in aged individuals
Recent evidence suggests that the depth of NSC quiescence increases with age
We discuss cell intrinsic and extrinsic changes that impact NSC quiescence and activation during aging
Acknowledgements
We thank Kelsey Babcock for critically reading and providing feedback on the manuscript. Work in the Webb laboratory is funded by NIH grant R01 AG053288 to A.E.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z, 2009. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 4408–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, Suomalainen A, 2012. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell metabolism 15, 100–109. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD, 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124, 319–335. [DOI] [PubMed] [Google Scholar]
- Apple DM, Mahesula S, Fonseca RS, Zhu C, Kokovay E, 2019. Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging (Albany NY) 11, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, Lyubimova A, Muraro M, van Es JH, van Oudenaarden A, Clevers H, 2017. A Single-Cell RNA Sequencing Study Reveals Cellular and Molecular Dynamics of the Hippocampal Neurogenic Niche. Cell reports 21, 3271–3284. [DOI] [PubMed] [Google Scholar]
- Audesse AJ, Dhakal S, Hassell LA, Gardell Z, Nemtsova Y, Webb AE, 2019. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS genetics 15, e1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baser A, Skabkin M, Martin-Villalba A, 2017. Neural Stem Cell Activation and the Role of Protein Synthesis. Brain Plast 3, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Ebert B, Schaffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Holter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, Ming GL, Toni N, Jessberger S, Song H, Lie DC, 2017. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 93, 560–573 e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP, 2010. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of aging 31, 151–161. [DOI] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Singh PP, Mahmoudi S, Harel I, Casey KM, Dulken BW, Kundaje A, Brunet A, 2019. Remodeling of epigenome and transcriptome landscapes with aging in mice reveals widespread induction of inflammatory responses. Genome Res 29, 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ, 2018. Human Hippocampal Neurogenesis Persists throughout Aging. Cell stem cell 22, 589–599 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M, 2004. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiology of aging 25, 333–340. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG, 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17, 2042–2046. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T, 2006. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol 169, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ, 2018. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV, 2003. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci 23, 693–702. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kirby ED, Wyss-Coray T, 2015. Blood-Borne Revitalization of the Aged Brain. JAMA Neurol 72, 1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK, 2018. Cellular Senescence Is Induced by the Environmental Neurotoxin Paraquat and Contributes to Neuropathology Linked to Parkinson’s Disease. Cell reports 22, 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuikov S, Levi BP, Smith ML, Morrison SJ, 2010. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nature cell biology 12, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corenblum MJ, Ray S, Remley QW, Long M, Harder B, Zhang DD, Barnes CA, Madhavan L, 2016. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging cell 15, 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT, 2016. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol Appl Neurobiol 42, 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Moreno M, Armenteros T, Gradari S, Hortiguela R, Garcia-Corzo L, Fontan-Lozano A, Trejo JL, Mira H, 2018. Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proceedings of the National Academy of Sciences of the United States of America 115, 11625–11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A, 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716. [DOI] [PubMed] [Google Scholar]
- Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, George BM, Boutet SC, Hebestreit K, Pluvinage JV, Wyss-Coray T, Weissman IL, Vogel H, Davis MM, Brunet A, 2019. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G, 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell stem cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S, 2004. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 8354–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH, 1998. Neurogenesis in the adult human hippocampus. Nature medicine 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J, 2014. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S, 2007. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell stem cell 1, 87–99. [DOI] [PubMed] [Google Scholar]
- Ferron S, Mira H, Franco S, Cano-Jaimez M, Bellmunt E, Ramirez C, Farinas I, Blasco MA, 2004. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 131, 4059–4070. [DOI] [PubMed] [Google Scholar]
- Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA, 2018. Tet2 Rescues Age-Related Regenerative Decline and Enhances Cognitive Function in the Adult Mouse Brain. Cell reports 22, 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H, 2011. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G, 2009. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Human molecular genetics 18, 2875–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, Horner JW, Maratos-Flier E, Depinho RA, 2011. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA, 2003. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging cell 2, 175–183. [DOI] [PubMed] [Google Scholar]
- Jinno S, 2011. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol 519, 451–466. [DOI] [PubMed] [Google Scholar]
- Kalamakis G, Brune D, Ravichandran S, Bolz J, Fan W, Ziebell F, Stiehl T, Catala-Martinez F, Kupke J, Zhao S, Llorens-Bobadilla E, Bauer K, Limpert S, Berger B, Christen U, Schmezer P, Mallm JP, Berninger B, Anders S, Del Sol A, Marciniak-Czochra A, Martin-Villalba A, 2019. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 176, 1407–1419 e1414. [DOI] [PubMed] [Google Scholar]
- Kang W, Hebert JM, 2015. FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 10217–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisen J, 2018. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell stem cell 23, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1998. Experience-induced neurogenesis in the senescent dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, 2017. Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S, 2013. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Pilz GA, Ghesquiere B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S, 2017. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell reports 20, 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G, 2010. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PloS one 5, e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Piao W, Takamura T, Kori H, Miyachi H, Kitano S, Iwamoto Y, Yamada M, Imayoshi I, Shioda S, Ballabio A, Kageyama R, 2019. Enhanced lysosomal degradation maintains the quiescent state of neural stem cells. Nature communications 10, 5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH, 1996. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience 16, 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI, 2011. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell 8, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP, 2000. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci 15, 99–108. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP, 2002. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80, 539–547. [DOI] [PubMed] [Google Scholar]
- Leiter O, Seidemann S, Overall RW, Ramasz B, Rund N, Schallenberg S, Grinenko T, Wielockx B, Kempermann G, Walker TL, 2019. Exercise-Induced Activated Platelets Increase Adult Hippocampal Precursor Proliferation and Promote Neuronal Differentiation. Stem cell reports 12, 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Candelario KM, Thomas K, Wang R, Wright K, Messier A, Cunningham LA, 2014. Hypoxia inducible factor-1alpha (HIF-1alpha) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 16713–16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Rothe G, Kreisel T, Wolf B, Benny O, Rooney AG, Ffrench-Constant C, Enikolopov G, Keshet E, 2016. VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America 113, E7828-E7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR, 2001. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience 107, 603–613. [DOI] [PubMed] [Google Scholar]
- Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A, 2015. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell stem cell 17, 329–340. [DOI] [PubMed] [Google Scholar]
- Lobanova A, She R, Pieraut S, Clapp C, Maximov A, Denchi EL, 2017. Different requirements of functional telomeres in neural stem cells and terminally differentiated neurons. Genes & development 31, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C, 2010. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell stem cell 6, 445–456. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC, 2006. The aging neurogenic subventricular zone. Aging cell 5, 139–152. [DOI] [PubMed] [Google Scholar]
- Ma CY, Yao MJ, Zhai QW, Jiao JW, Yuan XB, Poo MM, 2014. SIRT1 suppresses self-renewal of adult hippocampal neural stem cells. Development 141, 4697–4709. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H, 2009. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone DF, Ramirez-Amaya V, Barnes CA, 2012. Neurons generated in senescence maintain capacity for functional integration. Hippocampus 22, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxreiter F, Regensburger M, Winkler J, 2013. Adult neurogenesis in Parkinson’s disease. Cell Mol Life Sci 70, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC, 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews KJ, Allen KM, Boerrigter D, Ball H, Shannon Weickert C, Double KL, 2017. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging cell 16, 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC, 2010. O2 regulates stem cells through Wnt/beta-catenin signalling. Nature cell biology 12, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, Colak D, Gotz M, Farinas I, Gage FH, 2010. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell stem cell 7, 78–89. [DOI] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK, 2012. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging cell 11, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ, 2003. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ, 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Pilz GA, Arauzo-Bravo MJ, Barral Y, Jessberger S, 2015. A mechanism for the segregation of age in mammalian neural stem cells. Science 349, 1334–1338. [DOI] [PubMed] [Google Scholar]
- Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M, 2019. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature medicine 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Morrow CS, Porter TJ, Xu N, Arndt ZP, Ako-Asare K, Heo HJ, Thompson EAN, Moore DL, 2020. Vimentin Coordinates Protein Turnover at the Aggresome during Neural Stem Cell Quiescence Exit. Cell stem cell 26, 558–568 e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME, 2018. Tau protein aggregation is associated with cellular senescence in the brain. Aging cell 17, e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Gonzalez JL, Gomez-Sanchez L, Mavillard F, Linares-Clemente P, Rivero MC, Valenzuela-Villatoro M, Munoz-Bravo JL, Pardal R, Fernandez-Chacon R, 2019. Loss of postnatal quiescence of neural stem cells through mTOR activation upon genetic removal of cysteine string protein-alpha. Proceedings of the National Academy of Sciences of the United States of America 116, 8000–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann J, Wagner F, Kociaj A, Zambusi A, Ninkovic J, Hauck SM, Chapouton P, 2019. The Surface Proteome of Adult Neural Stem Cells in Zebrafish Unveils Long-Range Cell-Cell Connections and Age-Related Changes in Responsiveness to IGF. Stem cell reports 12, 258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D, 2019. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell metabolism 29, 1061–1077 e1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T, 2011. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25, 3570–3582. [DOI] [PubMed] [Google Scholar]
- Okun E, Marton D, Cohen D, Griffioen K, Kanfi Y, Illouz T, Madar R, Cohen HY, 2017. Sirt6 alters adult hippocampal neurogenesis. PloS one 12, e0179681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA, 2009. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell stem cell 5, 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH, 2001. Cell culture. Progenitor cells from human brain after death. Nature 411, 42–43. [DOI] [PubMed] [Google Scholar]
- Park JH, Glass Z, Sayed K, Michurina TV, Lazutkin A, Mineyeva O, Velmeshev D, Ward WF, Richardson A, Enikolopov G, 2013. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci 37, 1987–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A, 2009. FoxO3 regulates neural stem cell homeostasis. Cell stem cell 5, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM, 2002. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 2679–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sunayama J, Matsuda K, Tachibana K, Sakurada K, Tomiyama A, Kayama T, Kitanaka C, 2010. Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett 470, 115–120. [DOI] [PubMed] [Google Scholar]
- Satoh A, Imai SI, Guarente L, 2017. The brain, sirtuins, and ageing. Nat Rev Neurosci 18, 362–374. [DOI] [PubMed] [Google Scholar]
- Schaffner I, Minakaki G, Khan MA, Balta EA, Schlotzer-Schrehardt U, Schwarz TJ, Beckervordersandforth R, Winner B, Webb AE, DePinho RA, Paik J, Wurst W, Klucken J, Lie DC, 2018. FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis. Neuron 99, 1188–1203 e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y, 1995. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport 6, 2479–2482. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA, 2005. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51, 173–186. [DOI] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, Song H, 2015. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell stem cell 17, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F, 2016. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell stem cell 19, 643–652. [DOI] [PubMed] [Google Scholar]
- Smith LK, White CW 3rd, Villeda SA, 2018. The systemic environment: at the interface of aging and adult neurogenesis. Cell Tissue Res 371, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, 2019. Recalibrating the Relevance of Adult Neurogenesis. Trends Neurosci 42, 164–178. [DOI] [PubMed] [Google Scholar]
- Solano Fonseca R, Mahesula S, Apple DM, Raghunathan R, Dugan A, Cardona A, O’Connor J, Kokovay E, 2016. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem cells and development 25, 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A, 2018. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J, 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai S, 2014. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO journal 33, 1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Johnson SA, Iannitelli DE, Pollonini G, Alberini CM, 2016. Insulin-like growth factor 2 rescues aging-related memory loss in rats. Neurobiology of aging 44, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, Horner PJ, 2011. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. The Journal of biological chemistry 286, 38592–38601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Makin R, Sweet IR, Trevelyan AJ, Miwa S, Horner PJ, Turnbull DM, 2015. Neural Stem Cells in the Adult Subventricular Zone Oxidize Fatty Acids to Produce Energy and Support Neurogenic Activity. Stem Cells 33, 2306–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O, 2019. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell stem cell 24, 974–982 e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D, 1997. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. The Journal of neuroscience : the official journal of the Society for Neuroscience 17, 7850–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience 2, 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH, 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, CouillardDespres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T, 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature medicine 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk WIM, Rainbolt TK, Dolan PT, Webb AE, Brunet A, Frydman J, 2020. Differentiation Drives Widespread Rewiring of the Neural Stem Cell Chaperone Network. Molecular cell 78, 329–345 e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liang CC, Bian ZC, Zhu Y, Guan JL, 2013. FIP200 is required for maintenance and differentiation of postnatal neural stem cells. Nature neuroscience 16, 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Pollina EA, Vierbuchen T, Urban N, Ucar D, Leeman DS, Martynoga B, Sewak M, Rando TA, Guillemot F, Wernig M, Brunet A, 2013. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell reports 4, 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE, 2010. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 329, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y, Dhaliwal JS, Ceizar M, Vaculik M, Kumar KL, Lagace DC, 2016. Knockout of Atg5 delays the maturation and reduces the survival of adult-generated neurons in the hippocampus. Cell Death Dis 7, e2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, 2018. Senolytics improve physical function and increase lifespan in old age. Nature medicine 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef H, Conboy MJ, Morgenthaler A, Schlesinger C, Bugaj L, Paliwal P, Greer C, Conboy IM, Schaffer D, 2015a. Systemic attenuation of the TGF-beta pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 6, 11959–11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef H, Morgenthaler A, Schlesinger C, Bugaj L, Conboy IM, Schaffer DV, 2015b. Age-Associated Increase in BMP Signaling Inhibits Hippocampal Neurogenesis. Stem Cells 33, 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Furukawa K, Opresko PL, Xu X, Bohr VA, Mattson MP, 2006. TRF2 dysfunction elicits DNA damage responses associated with senescence in proliferating neural cells and differentiation of neurons. J Neurochem 97, 567–581. [DOI] [PubMed] [Google Scholar]
- Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL, 2013. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell stem cell 13, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bond AM, Shade JE, Zhu Y, Davis CO, Wang X, Su Y, Yoon KJ, Phan AT, Chen WJ, Oh JH, Marsh-Armstrong N, Atabai K, Ming GL, Song H, 2018. Autocrine Mfge8 Signaling Prevents Developmental Exhaustion of the Adult Neural Stem Cell Pool. Cell stem cell 23, 444–452 e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell F, Dehler S, Martin-Villalba A, Marciniak-Czochra A, 2018. Revealing age-related changes of adult hippocampal neurogenesis using mathematical models. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]