Abstract
The prevalence of “toxic stress” and huge downstream consequences in disease, suffering, and financial costs make prevention and early intervention crucial, say Charles A Nelson and colleagues
Today’s children face enormous challenges, some unforeseen in previous generations, and the biological and psychological toll is yet to be fully quantified. Climate change, terrorism, and war are associated with displacement and trauma. Economic disparities cleave a chasm between the haves and have nots, and, in the US at least, gun violence has reached epidemic proportions. Children may grow up with a parent with untreated mental illness. Not least, a family member could contract covid-19 or experience financial or psychological hardship associated with the pandemic.
The short and long term consequences of exposure to adversity in childhood are of great public health importance. Children are at heightened risk for stress related health disorders, which in turn may affect adult physical and psychological health and ultimately exert a great financial toll on our healthcare systems.
Growing evidence indicates that in the first three years of life, a host of biological (eg, malnutrition, infectious disease) and psychosocial (eg, maltreatment, witnessing violence, extreme poverty) hazards can affect a child’s developmental trajectory and lead to increased risk of adverse physical and psychological health conditions. Such impacts can be observed across multiple systems, affecting cardiovascular, immune, metabolic, and brain health, and may extend far beyond childhood, affecting life course health.1 2 3 These effects may be mediated in various direct and indirect ways, presenting opportunities for mitigation and intervention strategies.
Defining toxic stress
It is important to distinguish between adverse events that happen to a child, “stressors,” and the child’s response to these events, the “toxic stress response.”4 A consensus report published by the US National Academy of Sciences, Engineering, and Medicine (2019) defined the toxic stress response as:
Prolonged activation of the stress response systems that can disrupt the development of brain architecture and other organ systems and increase the risk for stress related disease and cognitive impairment, well into the adult years. The toxic stress response can occur when a child experiences strong, frequent, and/or prolonged adversity—such as physical or emotional abuse, chronic neglect, caregiver substance abuse or mental illness, exposure to violence, and/or the accumulated burdens of family economic hardship—without adequate adult support. Toxic stress is the maladaptive and chronically dysregulated stress response that occurs in relation to prolonged or severe early life adversity. For children, the result is disruption of the development of brain architecture and other organ systems and an increase in lifelong risk for physical and mental disorders.
What is childhood adversity?
A large number of adverse experiences (ie, toxic stressors) in childhood can trigger a toxic stress response.4 5 6 These range from the commonplace (eg, parental divorce) to the horrific (eg, the 6 year old “soldier” ordered to shoot and kill his mother7).
Adversity can affect development in myriad ways, at different points in time, although early exposures that persist over time likely lead to more lasting impacts. Moreover, adversity can become biologically embedded, increasing the likelihood of long term change. Contextual factors are important.
Type of adversity—Not all adversities exert the same impact or trigger the same response; for example, being physically or sexually abused may have more serious consequences for child development than does parental divorce.8 9
Duration of adversity—How long the adversity lasts can have an impact on development. However, it is often difficult to disentangle duration of adversity from the type of adversity (eg, children are often born into poverty, whereas maltreatment might begin later in a child’s life).
Developmental status and critical period timing—The child’s developmental status at the time he or she is exposed to adversity will influence the child’s response, as will the timing of when these adversities occur.10
Number of adversities and the interaction among them——The Adverse Childhood Experiences (ACE) study11 12 and subsequent body of ACE research provide compelling evidence that the risk of adverse health consequences increases as a function of the number of categories of adversities adults were exposed to in childhood. Although this seems intuitive, it belies the fact that, when it comes to severe adversity (eg, maltreatment), few children are exposed to only a single form of adversity at a single point in time. In addition, the effects of exposure to multiple adversities is likely more than additive. Thus, multiple forms of adversity may act in complex and synergistic ways over time to affect development.
Exacerbating factors—Children with recurrent morbidities, concurrent malnutrition, key micronutrient deficiencies, or exposure to environmental toxicants may be more sensitive to the adverse effects of other forms of toxic exposures.13
Supportive family environments—Children develop in an environment of relationships,14 15 16 and supportive relationships can buffer the response to toxic stress. Safe, stable, and nurturing relationships and environments are associated with reduced neuroendocrine, immunologic, metabolic, and genetic regulatory markers of toxic stress, as well as improved clinical outcomes of physical and mental health.17 18
Pre-existing characteristics—Many of the adversities being considered are not distributed at random in the population. They may occur more commonly in children and families with pre-existing vulnerabilities linked to genetic or fetal influences that lead to cognitive deficits.19 20 21 Infants who are more vulnerable to adverse life events (eg, stigma) include those born very early (eg, at 25 weeks’ gestation) or very small (eg, <1500 g), those born with substantial perinatal complications (eg, hypoxic-ischaemic injury), infants exposed prenatally to high levels of alcohol, or those born with greater genetic liability to develop an intellectual or developmental disability (eg, fragile X syndrome) or impairments in social communication (eg, autism).
Individual variation—Finally, children may have different physiological reactions to the same stressor. For example, Boyce,22 has proposed that by virtue of temperament, some children (such as those who are particularly shy and behaviourally inhibited) are highly sensitive to their environments and unless the environment accommodates such children, the risk of developing serious lifelong psychopathology is greatly increased; conversely, some children thrive under almost any conditions.
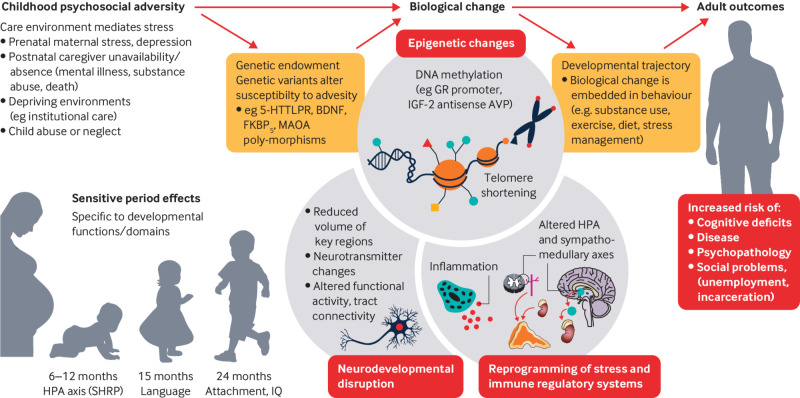
Figures 1 and 2 illustrate how duration and type of adversity interact with family environments and pre-existing characteristics to affect development (fig 1), and how early adversity may become biologically embedded (fig 2).
Fig 1.
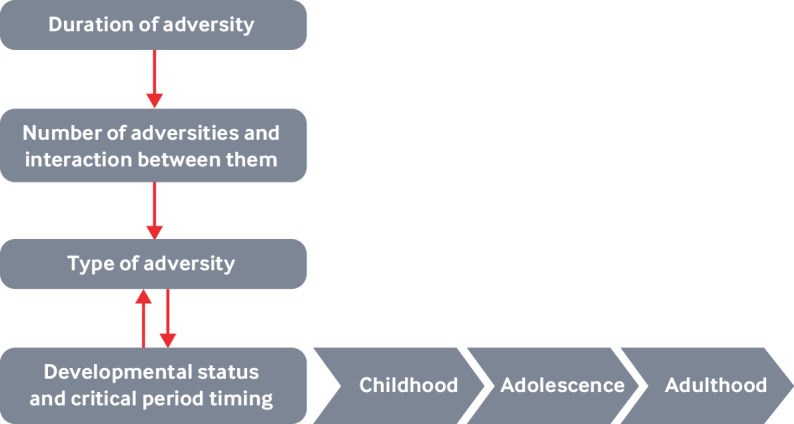
The interplay of adversities, context, and human development
Fig 2.
Some of the pathways that mediate exposure to early adversity and adult outcomes. Exposure to adversity early in life interacts with a child’s genetic endowment (eg variations in genetic polymorphisms), which in turn leads to a host of biological changes across multiple levels. These changes, in turn, influence adult outcomes (adapted from Berens et al23). HPA axis (SHRP)=hypothalamic pituitary adrenal axis (stress hyporesponsive period)
Consequences of exposure to adversity
Behavioral consequences—Childhood exposure to adversity may result in a variety of behavioral and emotional problems7—for example, increased risk taking, aggressive behaviour, involvement in violence (home, school, and neighbourhood), and difficulties in relationships with others.24 25 Of great concern is the development of post-traumatic stress disorder (PTSD).9 26
Children experiencing trauma (eg, witnessing the murder of a family member; sexual assault) are also at elevated risk of several other psychiatric disorders, including depression, PTSD, conduct problems, substance abuse, self-harm, and suicidal thoughts and attempts.8 25 Some forms of physical and psychological abuse in early childhood can be associated with eating disorders and mental health issues affecting typical development and education.
Neurobiological consequences—Many studies have identified structural and functional differences in brain development associated with environmental stressors, such as low socioeconomic status,27 28 29 30 31 physical abuse,32 and care giving neglect.33 34 For example, exposure to maternal stress in infancy has been associated with reduced brain activity, as inferred from electroencephalogram testing35, and profound psychosocial deprivation has been associated with differences in overall brain volume along with reductions in white and grey matter volume in several brain areas36 37 and reduced brain electrical activity.38 39 Differences in brain development have also been associated with decreases in several cognitive functions,40 and particularly executive functions,41 and distally, in educational achievement.42
Physical consequences—Early exposure to adversities, especially poverty, is associated with linear growth failure and wasting, and has recently been shown to be associated with reduced brain volume43 and altered functional connectivity.44 Children exposed to higher psychological stress have been shown to have higher cortisol levels and greater risk of common diseases of childhood, including otitis media, viral infections, asthma, dermatitis, urticaria, intestinal infectious diseases, and urinary tract infections.45
Childhood adversities have also been associated with greater risk of adult chronic conditions, including cardiovascular disease, stroke, cancer (excluding skin cancer), asthma, chronic obstructive pulmonary disease, kidney disease, diabetes, overweight or obesity, and depression, as well as increased health risk behaviours.46 47
Tables 1 and 2 show many of the physical and psychological harms observed among children and adults exposed to adversity early in life.
Table 1.
Health conditions in children associated with adverse childhood experiences (ACE)
| Symptom or health condition | For ≥ x ACEs (compared with 0) | Odds ratio |
|---|---|---|
| Asthma | 4 | 1.7-2.8 |
| Allergies | 4 | 2.5 |
| Dermatitis and eczema | 3* | 2.0 |
| Urticaria | 3* | 2.2 |
| Increased incidence of chronic disease, impaired management | 3 | 2.3 |
| Any unexplained somatic symptoms(eg, nausea/vomiting, dizziness, constipation, headaches) | 3 | 9.3 |
| Headaches | 4 | 3.0 |
| Enuresis, encopresis | - | - |
| Overweight, obesity | 4 | 2.0 |
| Failure to thrive, poor growth; psychosocial dwarfism | — | — |
| Poor dental health | 4 | 2.8 |
| Increased infections (viral, upper and lower respiratory tract infections and pneumonia, acute otitis media, urinary tract infections, conjunctivitis, intestinal | 3* | 1.4-2.4 |
| Later menarche (≥ 14 years) | 2* | 2.3 |
| Sleep disturbances | 5† | PR† 3.1 |
| Developmental delay | 3 | 1.9 |
| Learning and/or behaviour problems | 4 | 32.6 |
| Repeating a year at school | 4 | 2.8 |
| Not completing homework | 4 | 4.0 |
| High school absenteeism | 4 | 7.2 |
| Graduating from high school | 4 | 0.4 |
| Aggression, physical fighting | For each additional ACE | 1.9 |
| Depression | 4 | 3.9 |
| Attention deficit/hyperactivity disorder (ADHD) | 4 | 5.0 |
| Any of: ADHD, depression, anxiety, conduct/behaviour disorder | 3 | 4.5 |
| Suicidal ideation | For each additional ACE | 1.9 |
| Suicide attempts | 1.9-2.1 | |
| Self-harm | 1.8 | |
| First use of alcohol at <14 years | 4 | 6.2 |
| First use of illicit drugs at <14 years | 5 | 9.1 |
| Early sexual debut (<15-17 years) | 4 | 3.7 |
| Teenage pregnancy | 4 | 4.2 |
Odds ratio represents at least one ACE, but also includes other adversities
Prevalence ratio represents at least one ACE, but also includes other adversities
Table 2.
ACE-associated health conditions in adults associated with adverse childhood experiences (ACE)
| Symptom or health condition | Odds ratio (excluding outliers)* |
|---|---|
| Cardiovascular disease (coronary artery disease, myocardial infarction, ischemic heart disease) | 2.1 |
| Tachycardia | ≥1 ACE: 1.4 |
| Stroke | 2.0 |
| Chronic obstructive pulmonary disease (emphysema, bronchitis) | 3.1 |
| Asthma | 2.2 |
| Diabetes | 1.4 |
| Obesity | 2.1 |
| Hepatitis or jaundice | 2.4 |
| Cancer, any | 2.3 |
| Arthritis, self-reported | 3 ACEs, hazard ratio=1.5 ≥1 ACE, 1.3 |
| Memory impairment (all causes, including dementias) | 4.9 |
| Kidney disease | 1.7 |
| Headaches | ≥ 5 ACEs: 2.1 |
| Chronic pain, any (using trauma z-score) | 1.2 |
| Chronic back pain (using trauma z-score) | 1.3 |
| Fibromyalgia | ≥ 1 ACE: 1.8 |
| Unexplained somatic symptoms, including somatic pain, headaches | 2.0-2.7 |
| Skeletal fracture | 1.6-2.6 |
| Physical disability requiring assistive equipment | 1.8 |
| Depression | 4.7 |
| Suicide attempts | 37.5 |
| Suicidal ideation | 10.5 |
| Sleep disturbance | 1.6 |
| Anxiety | 3.7 |
| Panic and anxiety | — |
| Post-traumatic stress disorder | 4.5 |
| Illicit drug use (any) | 5.2 |
| Injected drug, crack cocaine, or heroin use | 10.2 |
| Alcohol use | 6.9 |
| Cigarette or e-cigarette use | 6.1 |
| Cannabis use | 11.0 |
| Teen pregnancy | 4.2 |
| Sexually transmitted infections, lifetime | 5.9 |
| Violence, victimization (intimate partner violence, sexual assault) | 7.5 |
| Violence perpetration | 8.1 |
Odds ratios compare outcomes in individuals with >4 ACEs with those with 0 ACEs, except where specified.
What mediates the effects of adversity?
The link between exposure to adversity early in life and physical and psychological development are thought to be mediated through several direct and indirect pathways. We first talk about the effects on physical development, then turn our attention to psychological development.
Effects mediated directly may include altering the regulation of stress-signalling pathways and immune system function48; changing brain structure and function49; and changing the expression of DNA and by accelerating cellular ageing.50 51For example, abuse or neglect might directly lead to physical injury or undernutrition or malnutrition. Similarly, stress can directly lead to dysregulation of the hypothalamic-pituitary-adrenal axis and associated neuro-endocrine-immune19 as well as epigenetic effects.52
Effects mediated indirectly might include changing the quality of the care giving environment (eg, less responsive care3) or the surrounding distal environment (eg, neighbourhood violence, which in turn will affect child development across several levels53); or building dysfunctional cognitions about the self and the world.25 54 55The effects of food insecurity (leading to undernutrition or malnutrition) and unsafe or substandard housing (resulting in exposure to asthmagens or environmental toxicants such as lead) can lead to social disparities in health.4 Distal effects of adversity include the early adoption of health damaging behaviors (eg, smoking, poor food choices) that later in life lead to diabetes, heart disease, and metabolic syndrome.47
On the psychological side, early adversity can lead to the development of psychopathology early in life (eg, disruptive behavior) that later in life manifests in more severe forms (eg, antisocial personality). Furthermore, it can lead to the development of dysfunctional cognition about self and others.54 The interplay of these different mediation mechanisms remains largely unclear.
Modelling the effects of adversity must take into consideration the type of adversity, the duration and timing of the adversity, the synergistic effects of multiple forms of adversity with the child’s genetic endowment (fig 2), and the social supports and interventions on which the child can depend (such as caregivers to whom the child is attached).
What can we do now?
If we wish for today’s youth to inherit a world that is safe and conducive to healthy development, we must do all we can to create such a world, by preventing disorders from developing and intervening once they are apparent.
Even for children living in adverse circumstances, much can be done now to make a difference by preventing such disorders from developing and intervening once they have surfaced. For example, we can screen children experiencing adverse life events, and once screened refer such children to early intervention services, as California is doing (see elsewhere in this collection).
Intervention strategies have been developed to help children manage their toxic stress response7 56 and to help families cope with adversity. Many children are resilient, and physician-community partnerships can help foster resilience.26
Recommendations for research
Much of the evidence has depended on the use of self- or parent-report measures, which are relatively easy to score, can be scaled at population level, and can be used (with modification) across cultures. However, such measures are inherently subjective and prone to biases (eg, recall bias). Other measures, such as official court or child protection records, provide a more objective assessment but often underestimate the prevalence of adversity.
Objective and subjective measures of childhood adversity identify largely non-overlapping groups of individuals57 and, thus, may be associated with health outcomes through different pathways. Subjective experience is particularly important for psychopathology, over and above objective experience.54
A challenge in examining the effects of adversity on development is how to compare children growing up in different cultures. For example, one study58 reported that a questionnaire on bullying used in different cultures and countries did not generalize well (eg, how one culture interpreted bullying differed from another). Adversity and trauma should be considered in context, and investigators in different cultures may need to develop different assessments.
To move away from subjective evaluations of toxic stress (eg, self- or other-report), and to gain insight into the neural and biological mechanisms that mediate the toxic stress response, several investigators have started to develop more objective biomarker panels for screening for toxic stress that use markers of neurological, immunological, metabolic, and genetic regulatory derangements.59 60 61 As this work continues, issues to consider include how much better (eg, as predictors) such measures are than behavior, how early in life they can be used, and whether they are scalable.
The study of toxic stress and the toxic stress response needs to move away from correlational and cross-sectional studies and deploy designs that are amenable to drawing causal inference. This would include longitudinal studies and ideally studies that involve interventions. An advantage of the latter includes the ability to shed light on mechanism.
More attention also needs to be paid to individual differences. Different people respond differently to the same stressors. For example, only a minority of children who experience trauma or maltreatment go on to develop enduring psychiatric disorders; and some children develop physical health disorders such as asthma whereas others will not.62 In addition, individual differences exist in biological sensitivity to stressors: for example, children identified as shy or inhibited early in life may be more vulnerable to stressors than children with more robust temperaments and who are less fearful of novelty63 64 65 and are more predisposed to anxiety as adults.66
Recommendations for policy
Policy is only as good as the underpinning evidence, and these recommendations have sufficient evidence to support them.
Careful consideration should be given to implementing evidence-informed policies for optimizing health, nutrition, and early child development,67 which in turn can be expanded to include older children and adolescents. Although the first three years of life are generally emphasized, older children exhibit remarkable plasticity in molding their personalities and behaviors.27 68 Effective interventions exist to treat and possibly prevent psychopathology emerging after childhood trauma, but implementation needs to be scaled up.7
Linking and optimizing preventive child health and education initiatives early in life are key to successful intervention69 and need to be done at the appropriate level in the health and education systems. The development of the nurturing care framework70 has been a welcome step in this direction, engaging platforms such as community health workers and pre-schools .71
Community, school, and after-school based interventions can reduce the effects of traumatic events among children and adolescents living in adverse circumstances.25 72
Public health strategies for primary, secondary, and tertiary prevention of childhood maltreatment and adversity include both universal and targeted interventions, ranging from home visiting programs to parent training programs, routine screening for adversity, and cognitive behavioral therapy.73 74
Key recommendations.
Researchers should consider both objective and subjective measures of childhood adversity
Researchers should broaden assessment of interventions beyond mental health measures to more regularly include health outcomes such as asthma, infection, inflammation, and insulin resistance
Adversity and trauma should be considered in context, and investigators in different cultures may need to develop different assessments
Researchers should consider how much better (eg, as predictors) objective biomarker panels are than behavior, how early in life they can be used, and whether they are scalable
Researchers should move towards longitudinal studies and ideally studies that involve interventions
Researchers should pay more attention to individual differences
Governments should implement evidence-informed policies for optimizing health, nutrition, and early child development
Health and education systems should link and optimize preventive child health and education initiatives early in life at the appropriate level
Use community, school, and after-school based interventions
Consider public health strategies for primary, secondary, and tertiary prevention of childhood maltreatment and adversity
Acknowledgments
We thank Lee Anglin and Lily Breen for proofing the manuscript.
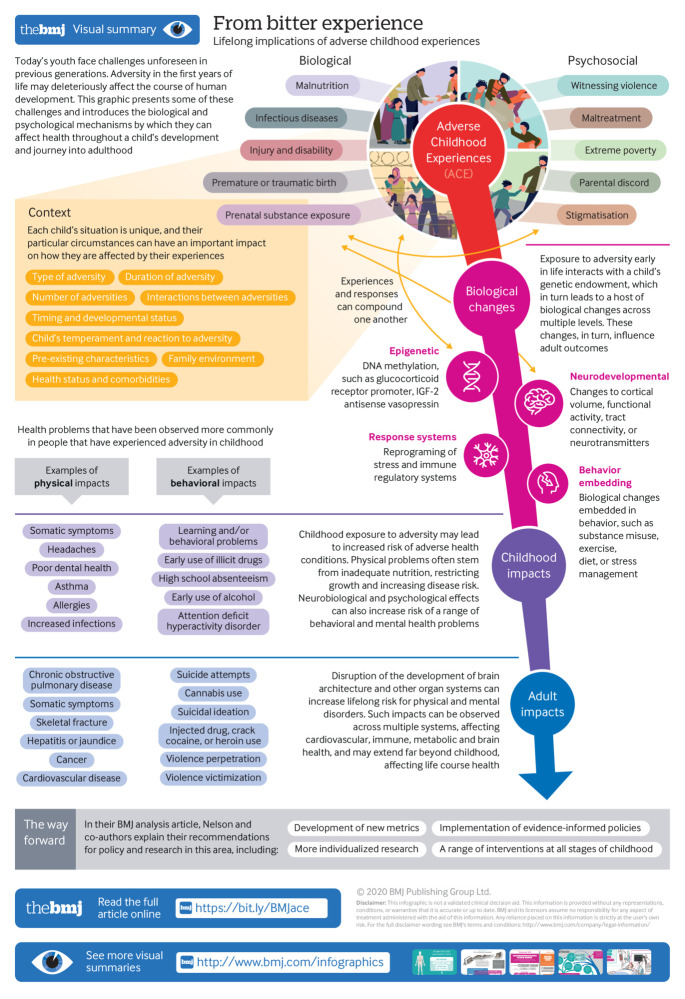
Web extra.
Extra material supplied by authors
Infographic: From bitter experience: lifelong implications of adverse childhood experiences
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is part of a series commissioned by The BMJ for the World Innovation Summit for Health (WISH) 2020. The BMJ peer reviewed, edited, and made the decisions to publish. The series, including open access fees, is funded by WISH.
References
- 1. Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health 2019;4:e517-28. 10.1016/S2468-2667(19)30145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nurius PS, Green S, Logan-Greene P, Borja S. Life course pathways of adverse childhood experiences toward adult psychological well-being: A stress process analysis. Child Abuse Negl 2015;45:143-53. 10.1016/j.chiabu.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shonkoff JP, Levitt P, Bunge S, et al. Supportive relationships and active skill-building strengthen the foundations of resilience: working paper 13. 2015. www.developingchild.net
- 4. Shonkoff JP, Garner AS, Siegel BS, et al. Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012;129:e232-46. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- 5. Burke Harris N. The deepest well: healing the long-term effects of childhood adversity. Houghton Mifflin Harcourt, 2018. [Google Scholar]
- 6. Hertzman C. The significance of early childhood adversity. Paediatr Child Health 2013;18:127-8. 10.1093/pch/18.3.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danese A, McLaughlin K, Muthanna S. Epidemiological and treatment research on trauma-related psychopathology in children pinpoints barriers to clinical implementation. BMJ [forthcoming]. [Google Scholar]
- 8. Afifi TO, Boman J, Fleisher W, Sareen J. The relationship between child abuse, parental divorce, and lifetime mental disorders and suicidality in a nationally representative adult sample. Child Abuse Negl 2009;33:139-47. 10.1016/j.chiabu.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 9. Lewis SJ, Arseneault L, Caspi A, et al. The epidemiology of trauma and post-traumatic stress disorder in a representative cohort of young people in England and Wales. Lancet Psychiatry 2019;6:247-56. 10.1016/S2215-0366(19)30031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson CA, 3rd, Gabard-Durnam LJ. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci 2020;43:133-43. 10.1016/j.tins.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245-58. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 12. Hillis SD, Anda RF, Felitti VJ, Nordenberg D, Marchbanks PA. Adverse childhood experiences and sexually transmitted diseases in men and women: a retrospective study. Pediatrics 2000;106:E11. 10.1542/peds.106.1.e11 [DOI] [PubMed] [Google Scholar]
- 13. McDonald CM, Olofin I, Flaxman S, et al. Nutrition Impact Model Study The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr 2013;97:896-901. 10.3945/ajcn.112.047639 [DOI] [PubMed] [Google Scholar]
- 14. Estrada P, Arsenio WF, Hess RD, Holloway SD. Affective quality of the mother-child relationship: longitudinal consequences for children’s school-relevant cognitive functioning. Dev Psychol 1987;23:210-5 10.1037/0012-1649.23.2.210 [DOI] [Google Scholar]
- 15. Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Dev Psychobiol 1996;29:191-204. [DOI] [PubMed] [Google Scholar]
- 16. Traub F, Boynton-Jarrett R. Modifiable resilience factors to childhood adversity for clinical pediatric practice. Pediatrics 2017;139:e20162569. 10.1542/peds.2016-2569 [DOI] [PubMed] [Google Scholar]
- 17. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171-9. 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- 18. McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33-44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- 19. Danese A, Baldwin JR. Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol 2017;68:517-44. 10.1146/annurev-psych-010416-044208 [DOI] [PubMed] [Google Scholar]
- 20. Danese A, van Harmelen A-L. The hidden wounds of childhood trauma. Eur J Psychotraumatol 2017;8(supp 5):137584. 10.1080/20008198.2017.1375840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danese A, Moffitt TE, Arseneault L, et al. The origins of cognitive deficits in victimized children: implications for neuroscientists and clinicians. Am J Psychiatry 2017;174:349-61. 10.1176/appi.ajp.2016.16030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyce, WT. The orchid and the dandelion: why some children struggle and how all can thrive. Knofp Doubleday 2019.
- 23. Berens AE, Jensen SKG, Nelson CA., 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med 2017;15:135. 10.1186/s12916-017-0895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Afifi TO, Taillieu T, Salmon S, et al. Adverse childhood experiences (ACEs), peer victimization, and substance use among adolescents. Child Abuse Negl 2020;106:104504. 10.1016/j.chiabu.2020.104504 [DOI] [PubMed] [Google Scholar]
- 25. El-Khodary B, Samara M. The relationship between multiple exposures to violence and war trauma, and mental health and behavioural problems among Palestinian children and adolescents. Eur Child Adolesc Psych; 2019; 10.1007/s00787-019-01376-8. [DOI] [PubMed] [Google Scholar]
- 26. Samara M, Hammuda S, Vostanis P, El-Khodary B, Al-Dewik N. Rethinking trauma, PTSD, and resilience in the context of political violence: new directions in research and practice. BMJ [forthcoming]. [Google Scholar]
- 27. Jenkins LM, Chiang JJ, Vause K, et al. Subcortical structural variations associated with low socioeconomic status in adolescents. Hum Brain Mapp 2020;41:162-71. 10.1002/hbm.24796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics 2016;137:e20153075. 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luby JL. Poverty’s most insidious damage: the developing brain. JAMA Pediatr 2015;169:810-1. 10.1001/jamapediatrics.2015.1682 [DOI] [PubMed] [Google Scholar]
- 30. Noble KG, Farah MJ. Neurocognitive consequences of socioeconomic disparities: the intersection of cognitive neuroscience and public health. Dev Sci 2013;16:639-40. 10.1111/desc.12076 [DOI] [PubMed] [Google Scholar]
- 31. Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci 2015;18:773-8. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016;17:652-66. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- 33. McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 2014;47:578-91. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci 2014;18:580-5. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierce LJ, Thompson BL, Gharib A, et al. Association of perceived maternal stress during the perinatal period with electroencephalography patterns in 2-month-old infants. JAMA Pediatr 2019;173:561-70. 10.1001/jamapediatrics.2019.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology 2016;41:177-96. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA., 3rd Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A 2012;109:12927-32. 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marshall PJ, Fox NA, Bucharest Early Intervention Project Core Group A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci 2004;16:1327-38. 10.1162/0898929042304723 [DOI] [PubMed] [Google Scholar]
- 39. Vanderwert RE, Marshall PJ, Nelson CA, 3rd, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One 2010;5:e11415. 10.1371/journal.pone.0011415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burneo-Garcés C, Cruz-Quintana F, Pérez-García M, Fernández-Alcántara M, Fasfous A, Pérez-Marfil MN. Interaction between socioeconomic status and cognitive development in children aged 7, 9, and 11 years: a cross-sectional study. Dev Neuropsychol 2019;44:1-16. 10.1080/87565641.2018.1554662 [DOI] [PubMed] [Google Scholar]
- 41. Ursache A, Noble KG, Pediatric Imaging, Neurocognition and Genetics Study Socioeconomic status, white matter, and executive function in children. Brain Behav 2016;6:e00531. 10.1002/brb3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr 2015;169:822-9. 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turesky TK, Jensen SKG, Yu X, et al. The relationship between biological and psychosocial risk factors and resting-state functional connectivity in 2-month-old Bangladeshi infants: A feasibility and pilot study. Dev Sci 2019;22:e12841. 10.1111/desc.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie W, Jensen SKG, Wade M, et al. Growth faltering is associated with altered brain functional connectivity and cognitive outcomes in urban Bangladeshi children exposed to early adversity. BMC Med 2019;17:199. 10.1186/s12916-019-1431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karlén J, Ludvigsson J, Hedmark M, Faresjö Å, Theodorsson E, Faresjö T. Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics 2015;135:e1450-7. 10.1542/peds.2014-2561 [DOI] [PubMed] [Google Scholar]
- 46. Merrick MT, Ford DC, Ports KA, et al. Vital signs: estimated proportion of adult health problems attributable to adverse childhood experiences and implications for Prevention—25 States, 2015-2017. MMWR Morb Mortal Wkly Rep 2019;68:999-1005. 10.15585/mmwr.mm6844e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suglia SF, Koenen KC, Boynton-Jarrett R, et al. American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation 2018;137:e15-28. 10.1161/CIR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elwenspoek MMC, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017;82:140-54. 10.1016/j.psyneuen.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 49. Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr 2015;169:211-9. 10.1001/jamapediatrics.2014.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193-8. 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- 51. Marini S, Davis KA, Soare TW, et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 2020;113:104484. 10.1016/j.psyneuen.2019.104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thaler L, Steiger H. Eating disorders and epigenetics. Adv Exp Med Biol 2017;978:93-103. 10.1007/978-3-319-53889-1_5 [DOI] [PubMed] [Google Scholar]
- 53. Theall KP, Shirtcliff EA, Dismukes AR, Wallace M, Drury SS. Association between neighborhood violence and biological stress in children. JAMA Pediatr 2017;171:53-60. 10.1001/jamapediatrics.2016.2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Danese A, Widom CS. Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nat Hum Behav 2020. 10.1038/s41562-020-0880-3. [DOI] [PubMed] [Google Scholar]
- 55. El-Khodary B, Samara M. Effectiveness of a school-based intervention on the students’ mental health after exposure to war-related trauma. Front Psych, 2019, 10.3389/fpsyt.2019.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jichlinski A. Defang ACEs: end toxic stress by developing resilience through physician-community partnerships. Pediatrics 2017;140:e20172869. 10.1542/peds.2017-2869. [DOI] [PubMed] [Google Scholar]
- 57. Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry 2019;76:584-93. 10.1001/jamapsychiatry.2019.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Samara M, Foody M, Göbel K, Altawil M, Scheithauer H. Do cross-national and ethnic group bullying comparisons represent reality? Testing instruments for structural equivalence and structural isomorphism. Front Psychol 2019;10:1621. 10.3389/fpsyg.2019.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bush NR, Aschbacher K. Immune biomarkers of early-life adversity and exposure to stress and violence—searching outside the streetlight. JAMA Pediatr 2019;174:1-3. [DOI] [PubMed] [Google Scholar]
- 60. Deighton S, Neville A, Pusch D, Dobson K. Biomarkers of adverse childhood experiences: A scoping review. Psychiatry Res 2018;269:719-32. 10.1016/j.psychres.2018.08.097 [DOI] [PubMed] [Google Scholar]
- 61. Shonkoff JP. Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatr 2016;170:1003-7. 10.1001/jamapediatrics.2016.1559 [DOI] [PubMed] [Google Scholar]
- 62. Wing R, Gjelsvik A, Nocera M, McQuaid EL. Association between adverse childhood experiences in the home and pediatric asthma. Ann Allergy Asthma Immunol 2015;114:379-84. 10.1016/j.anai.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 63. Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009;135:885-908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- 64. Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology 2016;41:142-62. 10.1038/npp.2015.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol 2005;17:303-28. 10.1017/S0954579405050157 [DOI] [PubMed] [Google Scholar]
- 66. Tang A, Crawford H, Morales S, Degnan KA, Pine DS, Fox NA. Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc Natl Acad Sci U S A 2020;117:9800-7. 10.1073/pnas.1917376117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaivada T, Gaffey MF, Bhutta ZA. Promoting early child development with interventions in health and nutrition: a systematic review. Pediatrics 2017;140:e20164308. 10.1542/peds.2016-4308. [DOI] [PubMed] [Google Scholar]
- 68. Dow-Edwards D, MacMaster FP, Peterson BS, Niesink R, Andersen S, Braams BR. Experience during adolescence shapes brain development: From synapses and networks to normal and pathological behavior. Neurotoxicol Teratol 2019;76:106834. 10.1016/j.ntt.2019.106834. [DOI] [PubMed] [Google Scholar]
- 69. Britto PR, Lye SJ, Proulx K, et al. Early Childhood Development Interventions Review Group, for the Lancet Early Childhood Development Series Steering Committee Nurturing care: promoting early childhood development. Lancet 2017;389:91-102. 10.1016/S0140-6736(16)31390-3 [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization, United Nations Children’s Fund, World Bank Group. Nurturing care for early childhood development. 2018. https://apps.who.int/iris/bitstream/handle/10665/272603/9789241514064-eng.pdf
- 71. Richter LM, Daelmans B, Lombardi J, et al. Paper 3 Working Group and the Lancet Early Childhood Development Series Steering Committee Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet 2017;389:103-18. 10.1016/S0140-6736(16)31698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Samara M, El Asam A, Khadaroo A, Hammuda S. Examining the psychosocial wellbeing of refugee children in the UK. Br J Educ Psychol 2020;90:301-29. 10.1111/bjep.12282 [DOI] [PubMed] [Google Scholar]
- 73. Macmillan HL, Wathen CN, Barlow J, Fergusson DM, Leventhal JM, Taussig HN. Interventions to prevent child maltreatment and associated impairment. Lancet 2009;373:250-66. 10.1016/S0140-6736(08)61708-0 [DOI] [PubMed] [Google Scholar]
- 74. National Academies of Sciences Engineering and Medicine Vibrant and healthy kids. Nat Acad Press, 2019, 10.17226/25466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infographic: From bitter experience: lifelong implications of adverse childhood experiences