Abstract
Background
While patient blood management (PBM) principles are not specific to cancer patients, their application contains the pathophysiological premises that could also benefit this patient population. In this study, we assessed the effects of implementing a PBM bundle for cancer patients in the postoperative period.
Materials and methods
The Azienda USL-IRCCS of Reggio Emilia implemented a two-step PBM bundle for the postoperative period of cancer patients hospitalised in the semi-intensive post-surgery (SIPO) ward. Step 1 included seminars and lessons specifically targeting SIPO personnel; Step 2 introduced Points of Care (POCs) for the continuous monitoring of haemoglobin (Radical7, Masimo Corp, Irvine, CA, USA). We conducted 3 audits on 600 cancer patients recruited between 2014 and 2017: Audit 1 on 200 patients before the application of our PBM bundle; Audit 2 after Step 1 on 200 patients; Audit 3 after Step 2 on 200 patients monitored with POCs. Red blood cell (RBC) transfusion appropriateness in the postoperative period was evaluated using the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) recommendations.
Results
RBC transfusion appropriateness in the postoperative period of cancer patients rose from 38% to 75% after seminars, and reached 79% after the introduction of POC. The mean number of RBC units each patient received remained unchanged after training sessions (1.8 units/patient) while the introduction of POCs saw a simultaneous decrease in the number of prescribed units (1.3 units/patient).
Discussion
Our PBM bundle positively impacted RBC transfusion appropriateness in postsurgical cancer patients, both in terms of quality and quantity. A structured PBM programme specifically dedicated to surgical oncology should cover the entire perioperative period and might further improve transfusion appropriateness in these patients. The publication of guidelines on the management of anaemia in surgical oncology should be a priority.
Keywords: patient blood management, red blood cell transfusion, cancer patients
INTRODUCTION
Patient blood management (PBM) is an evidence-based, multidisciplinary approach aimed at optimising the care of patients who might need blood transfusions. The application of PBM principles is recommended for surgical patients, and might have specific beneficial effects on surgical oncology patients for whom perioperative blood transfusions may negatively affect disease recurrence and overall survival, possibly because of an immunosuppressive effect of transfusions1,2.
The primary goal of PBM is to improve the patient’s management (both medical and surgical) in ways that boost and conserve his/her own blood3. This approach usually leads to an improvement in patient outcome and a reduction in transfusion episodes, which in turn leads to a reduction in transfusion-associated reactions.
Anaemia is a common complication in cancer patients and its correct management has been associated with improvement in clinical outcome and favourable response to chemotherapy4.
As the number of cancer patients is increasing worldwide, improving blood product utilisation, specifically that of RBC, is a challenge that needs to be met. Italian and international indications for PBM have historically focused mainly on orthopaedic surgery in adults5, and even in the countries were PBM is consolidated6, its importance in surgical oncology is still under-rated. Greater attention to blood management of cancer patients should include not only the pre- and intraoperative period, but also the postoperative period, since we recently observed that a structured PBM policy could significantly improve transfusion appropriateness during this time7.
The aim of this study was to assess whether the gradual introduction of our PBM bundle could affect the appropriateness of postoperative transfusion therapy in cancer patients, given that transfusions imply additional risks in this specific population8,9.
MATERIALS AND METHODS
The study was carried out at the Azienda USL-IRCCS of Reggio Emilia and approved on 30th September 2015 by the Ethics Committee of Reggio Emilia (protocol n. 2015/0021926). Written informed consent was obtained from all study participants according to the Declaration of Helsinki.
Study design
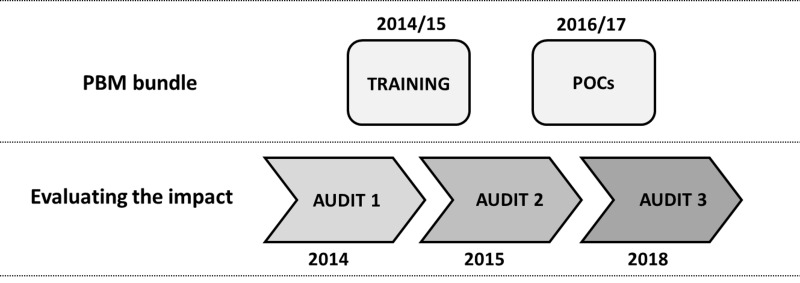
The study was conducted on cancer patients admitted to the semi-intensive postoperative (SIPO) ward after major cancer surgery, and was designed as follows (Figure 1):
Figure 1.
Study design. Patient blood management (PBM) bundle implementation (upper row) and evaluation of PBM impact (lower row)
POCs: Point of Care.
a preliminary audit on 200 cancer patients (Audit 1, from January to May 2014) aimed at evaluating RBC transfusion appropriateness after major cancer surgery before PBM implementation;
a PBM training programme (seminars and training sessions) was organised to ensure SIPO personnel (both physicians and nurses) followed Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) recommendations. An intermediate audit (Audit 2, from January to July 2015) was conducted on 200 cancer patients after the training programme to verify its impact on RBC transfusion appropriateness;
introduction of Points Of Care (POCs) for the continuous non-invasive monitoring of haemoglobin (Hb) levels (Radical-7 Pulse CO-Oximeter device, Masimo Corp., Irvine, CA, USA) in the SIPO ward. POCs were intentionally used in support of and not in place of the routine analyses. A final audit (Audit 3, from May 2016 to October 2017) was conducted on 200 cancer patients monitored with POCs.
Patient recruitment was carried out on consecutive patients admitted to SIPO after major onco-surgery who signed informed consent, in order to reach a total of 200 patients for each of the three Audits. POC monitoring lasted a mean of 72 hours for each patient recruited for Audit 3; this interval is based on the mean duration of admission to SIPO. Nurses applied the POCs on consecutive patients who specifically signed informed consent after surgery.
The physician responsible for ordering RBC was the surgeon caring for that patient, as established by our hospital plan. The transfusion medicine personnel did not interfere with the prescribers’ decisions.
Patient blood management training programme
In our hospital, the SIPO ward is managed by nurses: every day there is also an on call physician of each specialty who can examine SIPO patients. The training programme was attended by most of the SIPO nurses and by at least one physician from each post-surgical ward. Training programmes lasted 3 hours and were organised in two sessions:
a transfusion medicine physician talked about the importance of managing anaemia in cancer patients, shared the SIMTI recommendations for appropriate RBC transfusion therapy, and explained how to interpret the Hb trend registered by POCs;
a Masimo specialist then described how to use POCs and how to apply POC sensors.
At the end of the training programme, we carried out a survey of participant’s satisfaction with the event, the results of which will be published separately.
Radical-7 Masimo POCs can monitor up to 5 parameters (pO2, Hb, heartbeat, perfusion index, pleth variability index), but for this study we focused on Hb trend only.
Patient population
The Azienda USL-IRCCS of Reggio Emilia currently applies the PBM bundle to the following major cancer surgeries (for which transfused patients are, on average, over 15% of the total): colorectal resection, lung resection, liver resection, radical prostatectomy, nephrectomy, bladder resection.
Consecutive cancer patients ≥18 years old who had undergone any of the above-listed surgeries and who were subsequently admitted to the SIPO ward were recruited. Patients undergoing multiple surgeries during the recruitment period were assessed only on first admittance. To avoid overlaps, patients who underwent multiple surgeries during the study period were included only at their first hospital stay. None of the recruited patients received more than one transfusion during their stay in SIPO. Patients with haematologic diseases and patients who received massive transfusion therapy due to postoperative complications were not included in the study.
Surgical procedures were classified according to the Italian surgery and procedure classification, based on the International Classification of Procedures in Medicine. For each patient, the following data were collected from our electronic database: age, sex, diagnosis (oncologic or non-oncologic), comorbidities, type of surgery (intestinal, pancreatic, stomach, hepatobiliary, lung, bladder and kidney, prostate, other-mainly adrenal, breast, gynaecological, oesophagus), date and time of surgery, date and time of admittance and discharge from SIPO post surgery, Hb level measured with routine laboratory test immediately prior and after transfusion therapy, number of RBC units transfused postoperatively (Table I).
Table I.
Demographic data and surgical indications of the recruited patients
| Audit 1 | Audit 2 | Audit 3 | |
|---|---|---|---|
| Patient population (n) | 200 | 200 | 200 |
| Age (mean years±SD) | 69 ± 12 | 70 ± 12 | 68 ± 12 |
| Males (n) | 135 | 126 | 124 |
| Females (n) | 65 | 74 | 76 |
| Type of surgery (n, % total) | |||
| Intestinal | 54 (27.0%) | 66 (33.0%) | 72 (36.0%) |
| Pancreatic | 4 (2.0%) | 10 (5.0%) | 10 (5.0%) |
| Stomach | 14 (7.0%) | 22 (11.0%) | 20 (10.0%) |
| Hepatobiliary | 12 (6.0%) | 17 (8.5%) | 14 (7.0%) |
| Lung | 41 (20.5%) | 51 (25.5%) | 38 (19.0%) |
| Bladder and kidney | 34 (17.0%) | 19 (9.5%) | 17 (8.5%) |
| Prostate | 17 (8.5%) | 0 (0.0%) | 4 (2.0%) |
| Other | 24 (12.0%) | 15 (7.5%) | 25 (12.5%) |
n: number; SD: standard deviation.
End points and appropriateness of red blood cell transfusion
The primary end point was the appropriateness of RBC transfusions in the postoperative period, which was intended as the duration of patient stay in the SIPO ward (generally three days).
The postoperative transfusion episodes were considered appropriate when in agreement with the SIMTI recommendations10:
Hb<80 g/L;
80<Hb<90 g/L in the presence of risk factors (i.e. cardiac insufficiency, coronary pathology or cerebrovascular pathology);
90<Hb<100 g/L in the presence of symptoms related to hypoxia (hypotension, ischaemia or lactic acidosis).
Secondary end points were the number of transfused RBC units and the percentage of patients with RBC transfusion.
Data management and statistical analysis
Data were extracted from the the electronic database and blood bank registry of our hospital.
Continuous data were expressed as mean±standard deviation (SD), and comparisons were made using an independent t-test. Categorical variables were described using frequencies and percentages, and analysed using a χ2 test. Statistical significance was set at *p≤0.05, **p≤0.001. Analyses and graphs were performed using GraphPad Prism 7.02 (GraphPad Software, San Diego, CA, USA) and OriginPro2018b (OriginLab Corporation, Northampton, Massachusetts, USA) softwares, respectively.
RESULTS
Patient population
A total number of 600 cancer patients were included in the analysis (200 patients per audit). The three groups were similar in terms of sex, age, disease, and type of surgery (see Table I for details). The three populations were also representative of the cancer patients that are generally admitted to our hospital (data not shown).
Of the 600 patients recruited, 90 received RBC units during the postoperative period in the SIPO ward, with similar percentages among the three audits: 14.5% (29/200) in Audit 1, 16.0% (32/200) in Audit 2, and 14.5% (29/200) in Audit 3 (Table II).
Table II.
Demographic data, surgical indications, postoperative hemoglobin (Hb) and number of red blood cells (RBCs) transfused in the postoperative period of the recruited patients
| Audit 1 | Audit 2 | Audit 3 | |
|---|---|---|---|
| Transfused patients (n) | 29/200 (14.5%) | 32/200 (16.0%) | 29/200 (14.5%) |
| Age (mean years ± SD) | 72 ± 10 | 75 ± 9 | 72 ± 10 |
| Males (n) | 19 | 20 | 20 |
| Females (n) | 10 | 12 | 9 |
| Type of surgery (n transfused/total surgeries) | |||
| Intestinal | 13/54 | 9/66 | 6/72 |
| Pancreatic | 2/4 | 3/10 | 4/10 |
| Stomach | 2/14 | 4/22 | 4/20 |
| Hepatobiliary | 3/12 | 3/17 | 3/14 |
| Lung | 1/41 | 351 | 3/38 |
| Bladder and kidney | 5/34 | 8/19 | 9/17 |
| Prostate | 0/17 | 0/0 | 0/4 |
| Other | 3/24 | 2/15 | 0/25 |
| Hb pre-transfusion (g/dL) | 11.4 ± 1.6 | 8.8 ± 1.1 | 9.8 ± 1.7 |
| N. of RBCs transfused (n) | 52 | 58 | 39 |
| RBCs transfused per patient (mean) | 1.8 | 1.8 | 1.3 |
| Transfusion appropriateness (n/total) | 11/29 (37.9%) | 24/32 (75.0%) | 23/29 (79.3%) |
n: number; SD: standard deviation.
Appropriateness of postoperative red blood cell transfusion therapy
Baseline clinical audit (Audit 1) showed that RBC transfusions were appropriate in 38% of patients (11/29 patients), while after the teaching and training activities (Audit 2), appropriateness of transfusions increased to 75% (24/32 patients). We registered 79% appropriateness of RBC transfusion after the introduction of POCs, with 23/29 patients appropriately transfused (Figure 2, Audit 3). We observed a decrease in the total number of RBC units transfused in the postoperative period after the introduction of POCs (Figure 3A). The mean number of RBC units each patient received decreased from 1.8 (Figure 3B, Audits 1 and 2) to 1.3 (Figure 3B, Audit 3).
Figure 2.
Appropriateness of postoperative red blood cell (RBC) transfusion, expressed as % of transfused patients in the postoperative period within the selected ward
**p≤0.001.
Figure 3.
Red blood cell (RBC) units transfused in the postoperative period
(A) Total number of transfused RBC units; (B) mean number of RBC units received by each patient.
DISCUSSION
The growing body of data in the literature suggests that transfusions negatively impact cancer patient outcomes by favouring tumour growth and metastases11. The European Society of Medical Oncology (ESMO) recently published new Clinical Practice Guidelines on the management of anaemia and iron deficiency in cancer patients that included recommendations on how to safely manage chemotherapy-induced anaemia with RBC transfusions alone or in combination with drugs (i.e. erythropoiesis-stimulating agents and iron preparations)12. Anaemia and thrombocytopenia are common side effects of chemotherapy and can lead to the need for RBC and platelet transfusions, significantly influencing mortality, morbidity, and quality of life. Nevertheless, ESMO guidelines do not discuss how to manage cancer patients in surgical settings.
Although clinical evidence of the effects of a structured PBM programme on long-term outcomes in surgical oncology is still lacking13, we can expect such a programme to lead to improved long-term outcomes14. The application of transfusion guidelines specifically for cancer patients, therefore, should be a priority. The few PBM studies on cancer patients published so far have shown that the role of PBM on major prognostic oncologic indicators (such as overall survival) is far from being clear15–17.
In a recently published single-centre retrospective study, the implementation of a PBM programme in the perioperative period of cancer patients significantly reduced the need for blood transfusions and resulted in an increase in 2-year overall survival18.
We previously observed that the gradual introduction of a PBM bundle significantly improved RBC transfusion appropriateness in the postoperative period at our institution7. With the present study, we verified the impact of our programme on the surgical cancer patients on the SIPO ward.
Before starting the PBM bundle, RBC transfusion appropriateness was 37.9% (Audit 1). After the seminars specifically targeting all the healthcare professionals, we registered a significant improvement in RBC transfusion appropriateness, which reached 75% in a patient population with comparable characteristics. With the introduction of POCs, which provided immediate and continuous information about Hb levels, appropriateness reached 79%. These data are interesting because, instead of the expected lower performance over time after the training period19, transfusion appropriateness actually increased by further 4%.
Our study lasted approximately three years (2015–2017), during which time our hospital underwent different phases of operational reorganisation. As a consequence, the distribution of surgical patients in the SIPO ward and other postoperative wards slightly changed, as shown in Table I. Since 2015, for example, almost all prostate surgery patients have been admitted to the Urology Unit. However, since prostate cancer patients are rarely transfused in the postoperative period in our hospital, this event did not have any impact on our results.
Another observed consequence of the operational reorganisation was a progressive increase in the SIPO ward of the number of patients who had undergone more complex surgery, for example, intestinal, stomach and pancreatic surgery (Table I, Audits 2 and 3).
This higher number of complex patients resulted, at least in part, in the lower mean pre-transfusion Hb levels registered in Audits 2 and 3 (Table II).
The significant increase in transfusion appropriateness registered between Audits 1 and 2 (Figure 2) is apparently inconsistent with the increased number of transfused RBC units (Figure 3A). Our interpretation, however, is that this is due to the progressive increase in the number of complex surgical patients admitted to the SIPO ward during the study period.
It is worth noting that, with the introduction of POCs (Audit 3), the mean number of RBC units each patient received decreased from 1.8 to 1.3 (Figure 3B). These approaches, therefore, might have helped the physicians select the most appropriate transfusion therapy, in terms of quality and quantity.
This is a single-cohort study in which patients were not selected according to surgical indication or concomitant disease. We are aware that there are factors that can influence POCs accuracy in monitoring Hb. However, in the postoperative setting under study, where the prevalence of hypovolaemia is relatively low, POCs can help select the best therapeutic approach.
CONCLUSIONS
In our experience, the results of implementing a postoperative PBM bundle for cancer patient care are encouraging. A structured PBM approach in surgical oncology involving all three pillars is urgently needed. Anaemia in cancer patients is multifactorial and is present, in varying degrees, in all tumour types. Its management, therefore, should be accurate and multidisciplinary.
Specific attention should be paid to the recruitment of cancer patients onto PBM programmes, which should be carried forward as early as possible to guarantee the highest efficacy of the therapeutic approach aimed at managing perioperative anaemia and minimising the number of transfusions.
Footnotes
FUNDING AND RESOURCES
This study was conducted in the Azienda USL-IRCCS di Reggio Emilia, approved by our Local Ethical Committee with protocol n. 2015/0021926 in 30/09/2015, and funded by “Bando Sangue Plasma 2015” of the Centro Regionale Sangue Emilia Romagna.
AUTHORSHIP CONTRIBUTIONS
LM performed the audits and data analysis. CM contributed to data analysis and wrote the manuscript. EDB recruited patients and performed the audits. MTM organised seminars and training sessions. TAP and RB designed the study and contributed to manuscript preparation. AB designed the study, performed the audits, and contributed to manuscript preparation.
The authors declare no conflicts of interest.
REFERENCES
- 1.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Luo L, Huang H, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97:1827–37. doi: 10.1016/j.athoracsur.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 3.National Blood Authority Australia. Patient Blood Management (PBM) [Accessed on 28/01/2020]. Available at: https://www.blood.gov.au/patient-blood-management-pbm#whatispbm.
- 4.Clarke H, Pallister CJ. The impact of anaemia on outcome in cancer. Clin Lab Haematol. 2005;27:1–13. doi: 10.1111/j.1365-2257.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaglio S, Prisco D, Biancofiore G, et al. Centro Nazionale Sangue. 1st ed. [in italian] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmer SL, Towler SC, Leahy MF, et al. Drivers for change: Western Australia Patient Blood Management Program (WAPBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA) Best Pract Res Clin Anaesthesiol. 2013;27:43–58. doi: 10.1016/j.bpa.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Di Bartolomeo E, Merolle L, Marraccini C, et al. Patient Blood Management: transfusion appropriateness in the postoperative period. Blood Transfus. 2019;17:459–64. doi: 10.2450/2019.0035-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopewell S, Omar O, Hyde C, et al. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ Open. 2013;3:e002154. doi: 10.1136/bmjopen-2012-002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med. 2017;377:1261–72. doi: 10.1056/NEJMra1612789. [DOI] [PubMed] [Google Scholar]
- 10.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party: Recommendations for the transfusion management of patients in the peri-operative period. II. The post-operative period. Blood Transfus. 2011;9:320–35. doi: 10.2450/2011.0076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goubran HA, Elemary M, Radosevich M, et al. Impact of transfusion on cancer growth and outcome. Cancer Growth Metastasis. 2016;9:1–8. doi: 10.4137/CGM.S32797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv96–110. doi: 10.1093/annonc/mdx758. [DOI] [PubMed] [Google Scholar]
- 13.Ecker BL, Simmons KD, Zaheer S, et al. Blood transfusion in major abdominal surgery for malignant tumors: a trend analysis using the National Surgical Quality Improvement Program. JAMA Surg. 2016;151:518–25. doi: 10.1001/jamasurg.2015.5094. [DOI] [PubMed] [Google Scholar]
- 14.Keding V, Zacharowski K, Bechstein WO, et al. Patient Blood Management improves outcome in oncologic surgery. World J Surg Oncol. 2018;16:159. doi: 10.1186/s12957-018-1456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson MJ, Koopman-van Gemert AWMM, Harlaar JJ, et al. Patient blood management in colorectal cancer patients: a survey among Dutch gastroenterologists, surgeons, and anesthesiologists. Transfusion. 2018;58:2345–51. doi: 10.1111/trf.14807. [DOI] [PubMed] [Google Scholar]
- 16.Leahy MF, Trentino KM, May C, et al. Blood use in patients receiving intensive chemotherapy for acute leukemia or hematopoietic stem cell transplantation: the impact of a health system-wide patient blood management program. Transfusion. 2017;57:2189–96. doi: 10.1111/trf.14191. [DOI] [PubMed] [Google Scholar]
- 17.Gross I, Trentino KM, Andreescu A, et al. Impact of a Patient Blood Management program and an outpatient anemia management protocol on red cell transfusions in oncology inpatients and outpatients. Oncologist. 2016;21:327–32. doi: 10.1634/theoncologist.2015-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keding V, Zacharowski K, Bechstein WO, et al. Patient blood management improves outcome in oncologic surgery. World J Surg Oncol. 2018;16:159. doi: 10.1186/s12957-018-1456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tippett J. Nurses’ acquisition and retention of knowledge after trauma training. Accid Emerg Nurs. 2004;12:39–46. doi: 10.1016/s0965-2302(03)00064-x. [DOI] [PubMed] [Google Scholar]