ABSTRACT
Cryptococcal urease is believed to be important for the degradation of exogenous urea that the yeast encounters both in its natural environment and within the human host. Endogenous urea produced by the yeast's own metabolic reactions, however, may also serve as a substrate for the urease enzyme. Using wild-type, urease-deletion mutant and urease-reconstituted strains of Cryptococcus neoformans H99, we studied reactions located up- and downstream from endogenous urea. We demonstrated that urease is important for cryptococcal growth and that, compared to nutrient-rich conditions at 26°C, urease activity is higher under nutrient-limited conditions at 37°C. Compared to cells with a functional urease enzyme, urease-deficient cells had significantly higher intracellular urea levels and also showed more arginase activity, which may act as a potential source of endogenous urea. Metabolic reactions linked to arginase were also affected, since urease-positive and urease-negative cells differed with respect to agmatinase activity, polyamine synthesis, and intracellular levels of proline and reactive oxygen species. Lastly, urease-deficient cells showed higher melanin levels at 26°C than wild-type cells, while the inverse was observed at 37°C. These results suggest that cryptococcal urease is associated with the functioning of key metabolic pathways within the yeast cell.
Keywords: Cryptococcus, HIV, virulence factor, urease, endogenous urea, yeast metabolism
The urease enzyme, a major virulence factor of Cryptococcus neoformans, is linked to several key metabolic pathways within the yeast cell.
INTRODUCTION
The urease enzyme of Cryptococcus neoformans is commonly known for its role in cryptococcal virulence during human host infection (Cox et al. 2000; Olszewski et al. 2004; Shi et al. 2010; Singh et al. 2013). This enzyme catalyses the degradation of urea, resulting in the formation of ammonia and carbamic acid, with the latter undergoing spontaneous hydrolysis to carbonic acid and a second molecule of ammonia (Rutherford 2014). During infection, the production of ammonia is thought to result in alkalisation of surrounding tissue and ultimately host tissue damage. In murine infection models of cryptococcosis, the presence of a urease enzyme was found to influence blood–brain barrier traversal (Olszewski et al. 2004; Shi et al. 2010) and pulmonary immune responses (Osterholzer et al. 2009). In a more recent study, urease was found to increase phagolysosomal pH, which consequently enhanced the fitness of yeast cells within murine macrophages (Fu et al. 2018). Outside the mammalian host, cryptococcal urease can degrade environmental urea to produce a readily assimilable nitrogen source in the form of ammonia (Steenbergen and Casadevall 2003). This is believed to be useful for the growth and survival on pigeon guano as C. neoformans can use urea as its sole nitrogen source. These studies, however, focused on extracellular sources of urea, without considering the critical role of this molecule as a key product of essential intracellular metabolic pathways, such as the urea cycle and purine catabolism.
In all living organisms, purine catabolism begins with the enzymatic conversion of xanthine into uric acid (Barsoum and El-Khatib 2017). In humans and some animal species, uric acid is the final product of purine metabolism and is excreted in urine. In other organisms, however, uric acid is first degraded into a more soluble compound known as allantoin. Cryptococcus neoformans, like most other fungi, has an extensive uric acid degradation pathway and can degrade allantoin further with additional catabolic enzymes (Lee et al. 2013). Allantoicase and ureidoglycolate hydrolase, in particular, are the enzymes responsible for the formation of urea, which can then be converted to ammonia by urease during the final step of the degradation pathway.
The urease enzyme may, however, also hydrolyse intracellular urea that originates as a by-product of a catabolic urea (ornithine) cycle (Mendz and Hazell 1996; Weyman et al. 2015). Although a urea cycle is yet to be described for C. neoformans, putative genes encoding urea cycle proteins in C. neoformansH99 are available on GenBank, namely that of carbamoyl phosphate synthase (CNAG_00976 and CNAG_07373), ornithine carbamoyltransferase (CNAG_02812), argininosuccinate synthase (CNAG_00930), argininosuccinate lyase (CNAG_02825) and arginase (CNAG_03542). The generally accepted role of the urea cycle is to facilitate excretion of excess nitrogen, derived from amino acids during intracellular protein degradation (Nebes and Morris 1988; Morris 2002). Amino acid catabolism results in the formation of ammonia (Vylkova et al. 2011), which enters the urea cycle as carbamoyl phosphate by the action of carbamoyl phosphate synthetase (Bromke 2013). Reactions of the urea cycle result in the formation of intermediates important for other cellular metabolic processes (Morris 2002; Allen et al. 2011). This is particularly true for the second last reaction of the cycle, whereby l-argininosuccinate lyase converts l-argininosuccinate to fumarate and l-arginine (Eastwood et al. 2007).
The amino acid l-arginine is known to act as a precursor in a number of intracellular pathways leading to the synthesis of molecules, such as nitric oxide, proteins and polyamines (Jenkinson et al. 1996). The latter are a group of small aliphatic polycations (putrescine, spermidine and spermine) with vital roles in gene expression, cell proliferation and cellular stress (Miller-Fleming et al. 2015). The stress protectant nature of polyamines stems from their ability to scavenge reactive oxygen species (ROS), bind to membrane proteins and regulate stress-related gene expression. Although polyamines are yet to be implicated in stress tolerance of C. neoformans, spermidine was found to be vital for cryptococcal growth (Pfaller, Riley and Gerarden 1990). In addition, the absence of this polyamine in spermidine synthase mutants of C. neoformans resulted in a reduction in capsule and melanin biosynthesis, both of which are well-studied virulence factors of pathogenic cryptococci (Kingsbury et al. 2004). To produce spermidine, however, yeast cells first require the presence of another polyamine, putrescine, which is produced from l-arginine via two different pathways (Rocha and Wilson 2019). In the first case, the enzyme arginine decarboxylase can convert l-arginine to agmatine with subsequent conversion to putrescine and urea via agmatinase (Kumar, Saragadam and Punekar 2015). Alternatively, the urea cycle enzyme arginase can hydrolyse l-arginine to the amino acid l-ornithine (Morris 2004), which is either decarboxylated to putrescine for polyamine biosynthesis (Valdés-Santiago and Ruiz-Herrera 2014) or used as a metabolite for the synthesis of other cellular compounds, such as the stress-protectant molecule l-proline (Townsend et al. 1996; Takagi 2008). Like agmatinase, arginase activity also results in the production of intracellular urea (Morris 2004), which in turn can serve as a substrate for the urease enzyme.
The aim of this study was to test the hypothesis that cryptococcal urease is linked to different intracellular metabolic pathways involved in growth and survival of C. neoformans. Therefore, C. neoformans H99 was first used to compare growth and viability of a urease-deficient mutant to that of the wild-type and a urease-reconstituted strain, in a nutrient-rich medium, as well as in a nutrient-limited medium devoid of exogenous urea, at both 26 and 37°C. Then, the effect of the selected experimental conditions on cryptococcal urease was investigated by quantifying urease activity in crude enzyme extracts prepared from cells of the wild-type and urease-reconstituted strains of C. neoformans H99. All three strains (wild-type, urease-deficient and urease-reconstituted) were subsequently screened for enzymatic activity and intracellular metabolite levels relating specifically to pathways potentially involved in or influenced by endogenous urea metabolism. Melanin production was also assessed to determine whether urease may influence the expression of another survival mechanism commonly used by the fungus. The results of this study extend our understanding of cryptococcal urease beyond the mere use of environmental urea as a nitrogen source.
MATERIALS AND METHODS
Strains and maintenance
All experiments in this study were performed using wild-type (H99), urease-mutant (ure1Δ) and urease-reconstituted (ure1Δ::URE1) strains of C. neoformans var. grubii H99 (Cox et al. 2000). These strains were kindly donated by Dr John Perfect (Duke University Medical Center, Durham, NC, USA) and were routinely maintained on yeast extract–malt extract (YM) agar (pH 5; Yarrow 1998) at 26°C.
Growth studies
A loopful of cryptococcal cells obtained from growth on YM agar plates was inoculated into 25 mL YM broth and incubated on an orbital shaker [130 revolutions per minute (rpm)] for 16 h at 26°C. An aliquot of 4 mL was subsequently removed from the resulting suspension and washed twice in distilled water (dH2O) via centrifugation (10 000 × g for 2 min). Following resuspension in 1 mL dH2O, yeast cells were counted using a haemocytometer (Improved Neubauer, Marienfeld Superior, Germany) and 1 × 106 cells/mL were inoculated into 1 L conical flasks each containing 100 mL brain heart infusion (BHI) broth (Sigma-Aldrich, St Louis, MO). The inoculated flasks were subsequently incubated with shaking (130 rpm) at the respective temperature (26 or 37°C) for 28 h and growth was monitored every 2 h by measuring optical density at 600 nm. Maximum specific growth rate was calculated from the slope of the linear portion of the natural log-transformed optical density readings versus time curve (Tripathi et al. 2010). The same curve was used to calculate the duration of the lag phase by extrapolating the linear portion to the x-axis.
Viability under conditions of nutrient and temperature stress
The viability of all three strains was assessed following exposure to different nutrient and temperature stress conditions. Following 16 h of growth in BHI broth (100 mL in 1 L conical flasks) at 26 and 37°C, respectively, cells were counted haemocytometrically and inoculated into a nutrient-limited medium [Lerm et al. 2017; pH 6.8; 0.1% (w/v) glucose, 0.91% (w/v) monopotassium phosphate and 0.95% (w/v) dipotassium phosphate] to obtain a final cell concentration of 1 × 107 cells/mL. Inoculated flasks were incubated under shaking conditions (130 rpm) at the respective temperature and samples were harvested at regular intervals. At each time interval, samples were serially diluted, plated onto YM agar and incubated at 26°C for 3 days. Cell viability was expressed as the percentage of colonies relative to that of the initial cell number.
Experimental conditions during enzyme and metabolic analyses
All three cryptococcal strains were cultured for 5 days on YM agar plates at 26°C. Then, two loopfuls of yeast cells from each culture were inoculated into each of two 1 L conical flasks containing 100 mL BHI broth. One of the flasks was incubated at 26°C, while the other was cultured at an elevated temperature of 37°C. After 16 h of growth with shaking at 130 rpm, yeast cells (∼1 × 109 cells) from the respective cultures were transferred to 1 L conical flasks containing 100 mL nutrient-limited medium and incubated at the respective temperature of 26 or 37°C for 3 h with shaking (130 rpm). Unless otherwise stated, all experiments in this study were performed on biomass from both the BHI broth and the nutrient-limited medium. In each case, cells were washed three times in dH2O via centrifugation prior to conducting further analyses. All experiments were carried out with at least three biological repeats.
Effect of different nutrient and temperature conditions on urease activity
Crude protein was extracted from cryptococcal cells and assayed for urease activity according to the methods described by Lerm et al. (2017). In brief, cells were lysed by vigorous mixing in the presence of glass beads, whereafter extracted protein was quantified by the Bradford method (Bradford 1976) using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA, USA). Urease activity assays for experiments conducted at 26 and 37°C were performed in reaction mixtures consisting of 100 and 50 µg of crude protein extracts, respectively. All reactions were performed in a 50 mM HEPES buffer supplemented with 20 g/l urea in a final reaction volume of 1 mL. Reaction mixtures were incubated at the same temperature at which the cells were cultured, except when determining the effect of temperature on urease enzyme reaction rate. For this purpose, crude extracts from cryptococcal cells cultured in BHI broth were assayed for urease activity at both 26 and 37°C. To measure urease activity, ammonia production was recorded at regular intervals using a phenol–hypochlorite assay (Weatherburn 1967). Enzyme activity was expressed as nanomoles (nmol) ammonia produced per minute per µg protein and all experiments were conducted in triplicate.
Detection of intracellular urea
Cryptococcal cells were tested for the presence of intracellular urea as a starting point for further investigations into the possibility that urease might be required for the hydrolysis of urea from endogenous sources. Crude extracts were first prepared from cells of the three C. neoformans strains and then deproteinised with an equal volume of 8% perchloric acid. Following centrifugation (4°C, 13 793 ×g, 5 min) the supernatant was harvested and neutralised with 2 M potassium hydroxide. A final centrifugation step (4°C, 13 793 × g, 15 min) was then carried out and the deproteinised supernatant tested for the presence of urea according to the method of Rahmatullah and Boyde (1980). The chromogenic reagent for urea detection was prepared from two different solutions (reagent 1 and reagent 2). Reagent 1 (1 L) was prepared by adding 100 mL concentrated phosphoric acid to 300 mL concentrated sulphuric acid and 600 mL dH2O, followed by the addition of 0.1 g ferric chloride. Reagent 2 consisted of 0.5 g diacetylmonoxime (Sigma-Aldrich) and 0.01 g thiosemicarbazide (Sigma-Aldrich) dissolved in dH2O to a final volume of 100 mL. The chromogenic reagent was prepared immediately before use by mixing two parts of reagent 1 with one part of reagent 2. A volume of 3 mL was then added to 600 µL of the deproteinised sample in a spectrophotometer tube. The resulting suspension was mixed vigorously and boiled in a water bath for 5 min with subsequent cooling to room temperature. The absorbance was measured spectrophotometrically at 525 nm against a blank composed of distilled water and the chromogenic reagent. The urea content of each sample was then determined using a standard curve prepared with known amounts of urea in the range of 0 to 20 nmol.
Arginase assay
Arginase activity in crude enzyme extracts of C. neoformans was measured as the rate of urea production according to the modified Shimke's method (Corraliza et al. 1994), with further adaptations for the purpose of this study. Cells were cultured overnight in BHI broth with subsequent transfer to a nutrient-limited medium for 3 h at the respective temperatures of 26 and 37°C. After washing via centrifugation, crude enzyme extract was prepared and total protein content measured as described above. An aliquot of crude extract equivalent to 200 µg protein was then added to a reaction mixture consisting of 1 mM manganese chloride, 250 mM l-arginine (Sigma-Aldrich) and 25 mM Tris–HCl (pH 8) in a final volume of 1 mL. The reaction mixture was incubated at 37°C for 60 min, whereafter an aliquot of 100 µL was transferred to a new microcentrifuge tube and the reaction stopped with the addition of 800 µL acid mixture [H2SO4/H3PO4/H2O (1:3:7, v/v/v)]. To measure arginase-derived urea, 50 µL of a 9% α-isonitrosopropiophenone (Sigma-Aldrich) solution (dissolved in 95% ethanol) was added, mixed vigorously and incubated in a boiling water bath for 45 min in the dark to allow colour development. Immediately thereafter, the reaction absorbance was measured spectrophotometrically at 540 nm. A control reaction without crude protein was included in the experimentation and served as the blank for all absorbance readings. The amount of urea was calculated from a urea standard curve and arginase activity was recorded as micromoles (µmol) urea produced per hour per µg protein.
Agmatinase assay
For all experimental conditions, crude extracts from cryptococcal cells were tested for agmatinase enzyme activity using a method adapted from Carvajal et al. (2004) and Iyer et al. (2002). Once the protein content was determined, an aliquot of crude extract containing 200 µg protein was added to a reaction mixture (final volume 300 µL) consisting of 50 mM Tris–HCl, 2 mM MnCl2 and 10 mM acetohydroxamic acid (Sigma-Aldrich; Burnat and Flores 2014). The latter is a urease inhibitor, which prevents the hydrolysis of urea that is produced during the agmatinase reaction. Following an initial incubation period of 30 min at 37°C to allow for urease inhibition, the assay mixture was supplemented with 200 mM agmatine sulphate (Sigma-Aldrich) and incubated for a further 60 min at the same temperature. A reaction without agmatine sulphate was included as a control. After incubation, a sample aliquot of 200 µL was transferred to a glass spectrophotometer tube, followed by the addition of an equal volume of acid mixture [H2SO4/H3PO4/H2O (1:3:7, v/v/v)]. To measure urea present within the sample acid mixture, 15 µL of a 9% α-isonitrosopropiophenone solution (prepared in 95% ethanol) was added and the tubes subsequently incubated in a boiling water bath in the dark for 1 h. The resulting absorbances were measured spectrophotometrically at 540 nm and the urea concentration determined using a standard curve prepared with urea concentrations ranging from 0 to 60 nmol. Agmatinase enzyme activity was expressed as the nmol of urea produced per hour per µg protein.
Qualitative determination of polyamine synthesis
To obtain an indication of possible polyamine production, the three cryptococcal strains were screened for the ability to decarboxylate the amino acids l-arginine, l-ornithine and l-lysine on Long Ashton decarboxylase (LAD) agar medium (Cloete et al. 2009). It should be noted that glucose was not included in the LAD agar medium as its absence resulted in a more pronounced reaction. A loopful of 5-day-old cells from growth on YM agar plates at 26°C was spot inoculated, in triplicate, onto the centre of LAD agar plates containing either 2 g/l l-arginine monohydrochloride (Sigma-Aldrich), 3.2 g/l l-ornithine monohydrochloride (Sigma-Aldrich) or 3.36 g/l l-lysine (Sigma-Aldrich). Papiliotrema laurentii (syn. Cryptococcus laurentii) CAB 578 was included as a positive control due to its known ability to produce polyamines from l-arginine (Cloete et al. 2009). Each strain was also inoculated onto negative control plates of LAD agar without any added amino acids. All plates were incubated for 3 days at 26 and 37°C, respectively, with daily inspection for the presence of pink haloes. The observed colour change from an otherwise yellow background was indicative of a decarboxylation reaction and thus the potential ability to produce polyamines.
Intracellular proline levels
Crude extracts were analysed for the presence of proline using a method based on that described by Shabnam et al. (2016). Once protein concentrations were determined, 200 µL of lysate was deproteinised with an equal volume of 3% sulphosalicylic acid. Samples were then centrifuged at 13 793 × g for 5 min and the resulting supernatant (300 µL) transferred to a glass spectrophotometer tube. Lastly, 600 µL of ninhydrin reagent (1.25% prepared in glacial acetic acid) was added to each tube and incubated in a boiling water bath for 30 min. After cooling to room temperature, absorbances were measured at 508 nm against a blank that contained all the reagents except crude extract. Proline levels, expressed as nmol proline per mg of protein, were calculated using a standard curve with proline concentrations in the range of 0–150 nmol.
Detection of reactive oxygen species
Cryptococcal cells exposed to the different nutrient and temperature stress conditions were screened for the presence of intracellular ROS using the fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFDA; Nair et al. 2016). Cells were washed once in phosphate buffered saline (PBS) and resuspended in 20 µM DCFDA (prepared in PBS) to a final cell concentration of 8 × 106 cells/mL. The resulting cell suspensions were incubated at 37°C for 1 h in the dark, whereafter 300 µL aliquots were transferred to the wells of a black-bottom 96-well microplate. The fluorescence was measured in a Fluoroskan Ascent 96‐well microplate reader (Labsystems, Helsinki, Finland) set at excitation and emission wavelengths of 485 and 528 nm, respectively. The negative control consisted of cells without dye treatment and hydrogen peroxide-treated cells were included as a positive control. Results obtained for ure1Δ and ure1Δ::URE1 were represented as the percentage difference in fluorescence intensity relative to H99. Fluorescence was also visualised using a Nikon Eclipse E400 epifluorescence microscope equipped with appropriate filters for excitation and emission at 485 and 538 nm, respectively. Images were captured with a Nikon DS-Fi2 camera and a Nikon Digital Sight DS-U3 camera controller (Nikon, Tokyo, Japan).
Melanin production
Qualitative screening of melanin production was carried out on l-3,4-dihydroxyphenylalanine (l-DOPA) agar plates using a method described by Polacheck et al. (1982), who referred to the procedure of Chaskes and Tyndall (1978). The agar medium consisted of (per litre): 1 g glucose, 1 g glycine, 1 g glutamine, 1 g asparagine, 4 g KH2PO4, 2.5 g MgSO47H20, 10 mg thiamine hydrochloride and 20 µg biotin. l-DOPA was also added to the medium at a final concentration of 0.5 mM. Yeast cells cultured in YM broth for 24 h at 26°C were washed three times in physiological saline solution via centrifugation and counted using a haemocytometer. Agar plates were then spot inoculated with 1 × 106 cells in a volume of 20 µL and incubated at the respective temperatures of 26 and 37°C with regular inspection over 5 days for pigment production.
Melanin synthesis was also assessed quantitatively using a method based on that described by Ngamskulrungroj and Meyer (2009) with slight modifications. Yeast cells were inoculated into 5 mL YM tubes and incubated for 24 h at 30°C on a tissue culture roller drum set at 60 rpm. Resulting cultures were washed in physiological saline solution and inoculated into 5 mL l-DOPA media (Hicks et al. 2004) to obtain a final concentration of 1 × 107 cells/mL. After incubation at the respective temperatures of 26 and 37°C for 48 h, melanised cultures were centrifuged (10 000 × g for 3 min) and the supernatant absorbance measured at 475 nm.
Statistical analyses
All experiments were repeated at least three times and results analysed using XLSTAT software (Version 2019.4.1, Addinsoft, Paris, France). Data were tested for normality using Shapiro–Wilk's test and Levene's test was used to assess homogeneity of variances. For normally distributed data with equal variances, significant differences were determined using one-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) post-hoc test. Data with a normal distribution but unequal variances were analysed using a Welch's ANOVA with Games–Howell post-hoc analysis. For non-normally distributed data, the non-parametric Kruskal–Wallis test was used, followed by Dunn's multiple comparison test. Statistical significance was set at P < 0.05.
RESULTS
Growth studies
Growth of the three cryptococcal strains in BHI broth at 26 and 37°C is depicted in Fig. 1. At both tested temperatures, the maximum specific growth rate of the urease-deletion mutant tended to be slightly lower than that of the wild-type (26°C, P = 0.064; 37°C, P = 0.157) and urease-complemented strains (26°C, P = 0.061; 37°C, P = 0.186), but no significant differences were detected (Table 1). The absence of a functional urease gene did, however, result in a significantly longer lag phase at both of the tested temperatures (Table 1).
Figure 1.
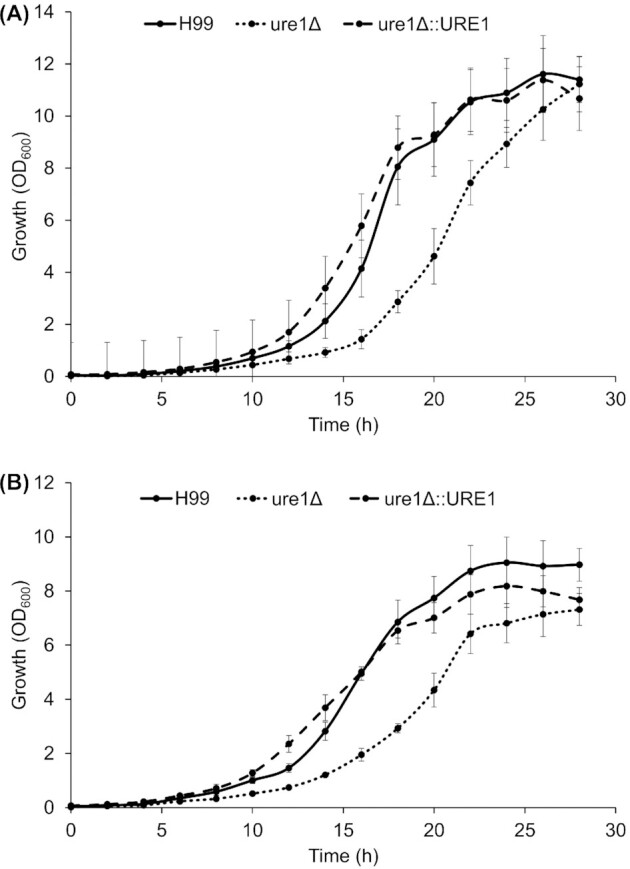
Growth of C. neoformans H99, ure1Δ and ure1Δ::URE1 in BHI broth at 26°C (A) and 37°C (B). Optical density measurements (OD600) were recorded every 2 h over a period of 28 h for both tested temperatures. Each data point represents the mean ± standard error of the mean of three biological replicates.
Table 1.
Lag phase length and maximum specific growth rate (µmax) of wild-type, urease-mutant and urease-reconstituted strains of C. neoformans H99. Values with superscript letters indicate significant differences (P < 0.05). Only letters of the same prime are comparable. All statistical analyses were carried out using one-way ANOVA with Fisher's LSD post-hoc comparisons.
| 26°C | 37°C | |||
|---|---|---|---|---|
| Lag phase (h)a | µ max (h−1) | Lag phase (h)a | µ max (h−1) | |
| H99 | 12bc′ | 0.314 ± 0.047a″ | 10cd′ | 0.276 ± 0.010ab″ |
| ure1Δ | 14a′ | 0.238 ± 0.022ab″ | 13ab′ | 0.219 ± 0.014b″ |
| ure1Δ::URE1 | 10cd′ | 0.315 ± 0.034a″ | 9d′ | 0.272 ± 0.003ab″ |
Rounded to the nearest hour.
Viability under conditions of nutrient and temperature stress
When viability in a nutrient-limited medium at 26°C was assessed, both the wild-type and urease-reconstituted strains were found to proliferate over time, whereas the urease-deletion mutant merely maintained its original cell number (Fig. 2A). The same trend was evident at 37°C; however, less pronounced growth was observed for strains with a urease enzyme compared to 26°C (Fig. 2B).
Figure 2.
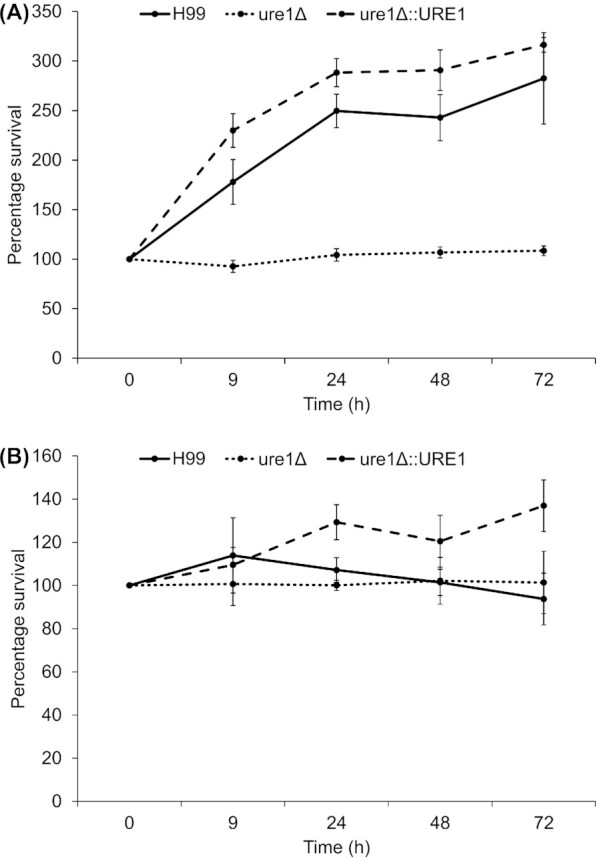
Percentage survival of C. neoformans H99, ure1Δ and ure1Δ::URE1 under nutrient-limited conditions at 26°C (A) and 37°C (B).
Effect of different nutrient and temperature conditions on urease activity
For both the wild-type and urease-reconstituted strains, the transfer of yeast cells from BHI broth to a nutrient-limited medium resulted in notably elevated urease activity levels at 26 and 37°C (Fig. 3). Urease activities at 37°C were also found to be higher than at 26°C for both tested strains under the two different nutrient conditions. In the nutrient-limited medium at 26°C, however, urease activity of ure1Δ::URE1 was significantly lower than that of H99, while the inverse was true for the BHI broth at 37°C. When determining the effect of reaction temperature on the urease reaction rate of H99, no significant differences were observed between 26 and 37°C (Figure S1, Supporting Information). Growth at 37°C had the most significant effect, resulting in consistently higher levels of urease activity compared to 26°C.
Figure 3.
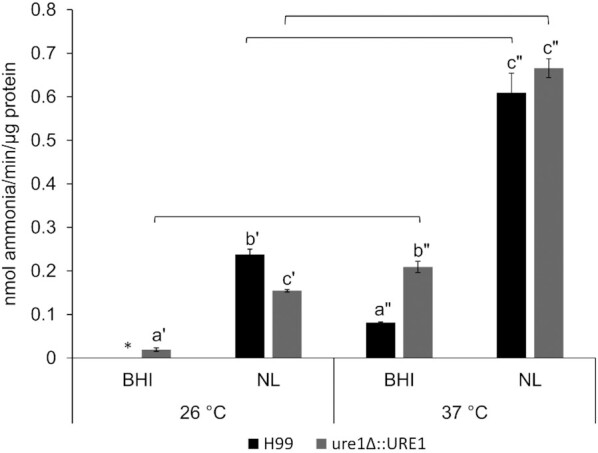
Urease activities of C. neoformans H99 and ure1Δ::URE1 before (from BHI cultures) and after incubation in a nutrient-limited medium (NL) at 26 and 37°C. Error bars represent the standard error of the mean of three biological repeats. Lettering with the same prime illustrates significant differences between different nutrient conditions at the respective temperatures (P < 0.05). Only letters with the same prime are comparable. Significant differences in urease activity between temperatures, but subjected to the same nutrient condition, are indicated by horizontal lines (P < 0.05). *Absent or below assay detection limit.
Detection of intracellular urea
Under all experimental conditions, crude extracts from cells of the urease-deletion mutant tended to have higher urea levels compared to the wild-type and urease-reconstituted strains (Fig. 4). At each respective temperature, urea levels from nutrient-limited cells of the ure1Δ mutant were notably higher than those observed with cells from BHI media. A significant difference, however, was only evident at 26°C. For the urease-producing strains, cells cultured in BHI media were found to have undetectable levels of intracellular urea at both tested temperatures. Urea was only detected in wild-type cryptococci when cells were exposed to a nutrient-limited medium at 26°C. For the urease-reconstituted strain, however, urea was observed in crude extracts from nutrient-limited cells at both 26 and 37°C.
Figure 4.
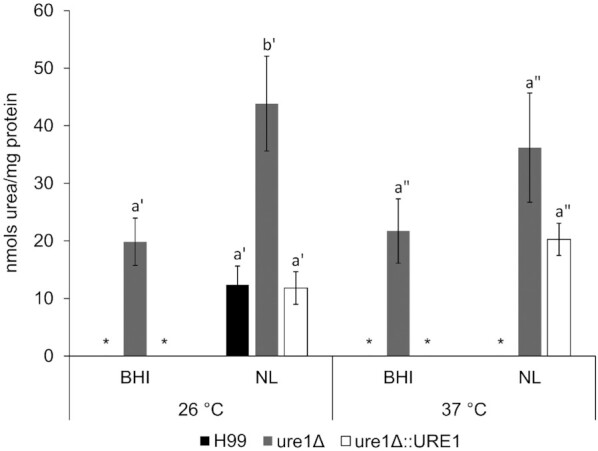
Urea levels in crude enzyme extracts prepared from C. neoformans H99, ure1Δ and ure1Δ::URE1. Statistical comparisons were performed separately for the different temperatures. Significant differences (P < 0.05) are indicated by lettering and only letters with the same prime are comparable. Bars denote the mean of at least three biological repeats and error bars represent standard error of the mean. *Absent or below assay detection limit.
Arginase enzyme activity
The detection of arginase enzyme activity was achieved for all three strains at each of the tested nutrient and temperature conditions (Fig. 5). The levels of activity, however, were found to be notably higher in crude extracts of the urease-mutant and urease-reconstituted strains. This was especially evident after exposure to a nutrient-limited medium at 37°C, where arginase levels of ure1Δ and ure1Δ::URE1 were found to be more than double of that observed for H99.
Figure 5.
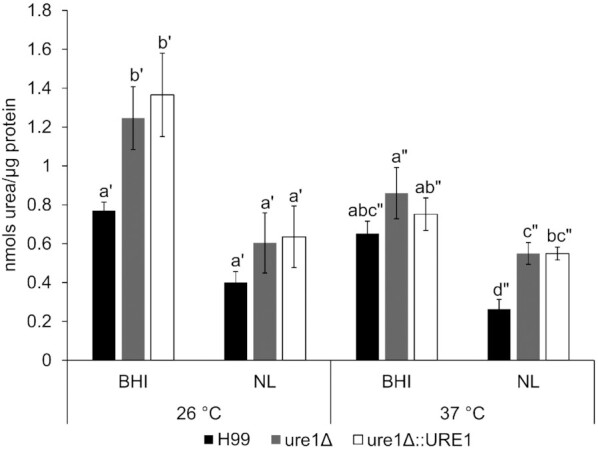
Arginase enzyme activity of H99, ure1Δ and ure1Δ::URE1 strains of C. neoformans exposed to BHI and nutrient-limited (NL) media at 26 and 37°C. Enzyme activity is expressed as the nmol of urea produced from l-arginine per µg of protein. Bars represent the average of at least three biological replicates and error bars depict the standard error of the mean. Different letters indicate statistically significant differences (P < 0.05) and prime lettering indicates comparisons within the same temperature treatment.
Agmatinase enzyme activity
Wild-type and urease-reconstituted strains demonstrated similar levels of agmatinase enzyme activity across all experimental conditions (Fig. 6). For the urease-mutant strain, however, agmatinase activity could only be detected following growth in BHI broth at 26°C. In this case, activity levels observed for ure1Δ did not significantly differ to those observed for H99 and ure1Δ::URE1.
Figure 6.
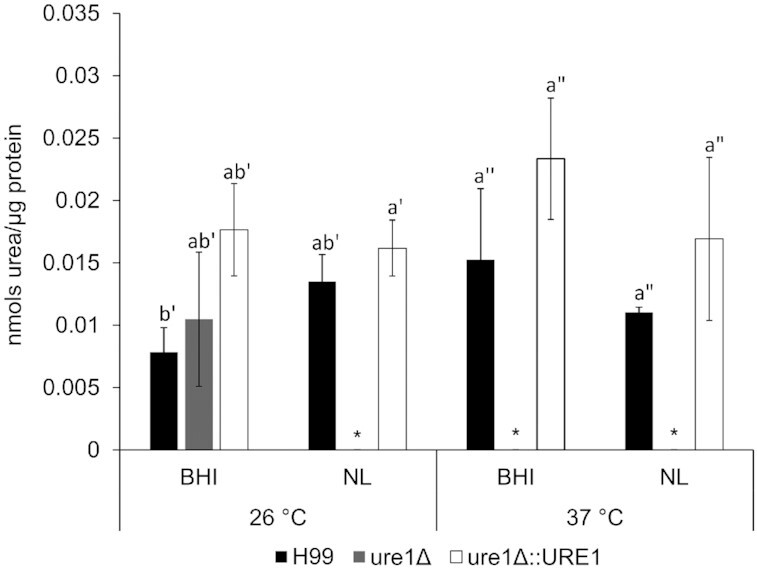
Agmatinase enzyme activity of C. neoformans H99, ure1Δ and ure1Δ::URE1 after exposure to BHI and nutrient-limited media (NL) at 26 and 37°C. The bars and whiskers represent the mean ± standard error of the mean for at least three biological repeats. Statistical comparisons were carried out to determine significant differences between strains exposed to the same temperature (P < 0.05). *Absent or below assay detection limit.
Qualitative determination of polyamine synthesis
At both tested temperatures, H99, ure1Δ and ure1Δ::URE1 tested positive for polyamine synthesis in the presence of the amino acid l-ornithine. A positive reaction was also observed for all three strains on l-arginine plates at 26°C. In contrast, when inoculated l-arginine plates were incubated at 37°C, a notably weaker reaction was observed for the urease-deficient strain compared to that obtained for H99 and ure1Δ::URE1 (Figure S2, Supporting Information). None of the cryptococcal strains showed signs of polyamine synthesis from l-lysine at both 26 and 37°C.
Intracellular proline levels
Crude extracts of the three cryptococcal strains were found to contain similar levels of proline under all experimental conditions, except after nutrient limitation at 37°C (Fig. 7). When cells of the urease-mutant strain were exposed to a nutrient-limited medium at 37°C, proline levels were significantly elevated compared to H99 and the urease-reconstituted strain. In general, nutrient limitation seemed to result in lower levels of proline within crude extracts of H99, ure1Δ and ure1Δ::URE1.
Figure 7.
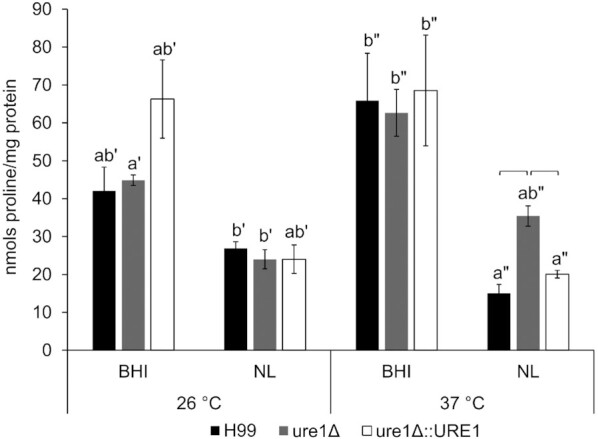
Intracellular proline levels of H99, ure1Δ and ure1Δ::URE1 strains of C. neoformans exposed to BHI and nutrient-limited (NL) media at 26 and 37°C. Proline content is represented as the nanomoles of proline present per milligram of protein. Bars with error bars represent means with standard error of the mean for at least three biological repeats. Lettering of the same prime denotes significant differences within a temperature treatment (P < 0.05). Short horizontal lines indicate significant differences after exposure to nutrient-limited conditions at 37°C (P < 0.05).
Detection of intracellular ROS
As is evident from literature, many of the above factors were found to play a role in the eukaryotic metabolism of ROS (Chattopadhyay, Tabor and Tabor 2006; Takagi 2008; Caldwell et al. 2015). At 37°C in both BHI broth and nutrient-limited media, ROS-induced fluorescence levels of the urease-mutant strain tended to be higher than the wild-type and urease-complemented strains (Fig. 8). This phenomenon was visualised microscopically for cells exposed to nutrient-limited media at 37°C (Fig. 9). Unlike H99 and ure1Δ::URE1, fluorescence levels obtained for ure1Δ at 37°C were also notably elevated compared to that obtained at 26°C.
Figure 8.
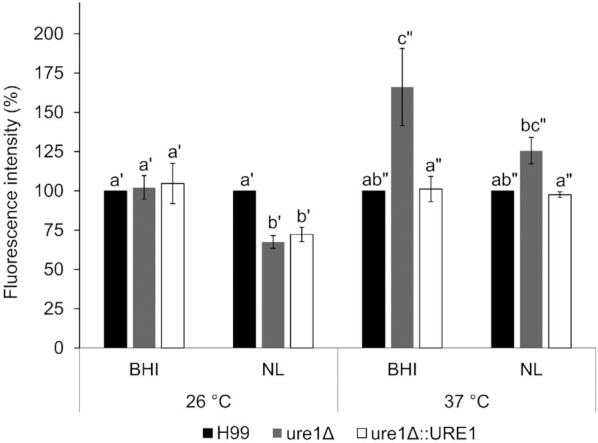
Fluorescence intensity of cells stained with the ROS indicator dye DCFDA after exposure to BHI and nutrient-limited (NL) media at 26 and 37°C. Results are expressed as the percentage fluorescence intensity relative to wild-type cryptococcal cells. Bars denote the mean of at least three biological repeats and error bars represent standard error of the mean. Statistical analyses were performed separately for the two different temperatures as indicated by letters with the same number of primes (significance set at P < 0.05 in all cases). Results obtained for nutrient-limited conditions at 37°C were also analysed separately for significance, as indicated by brackets (P < 0.05).
Figure 9.
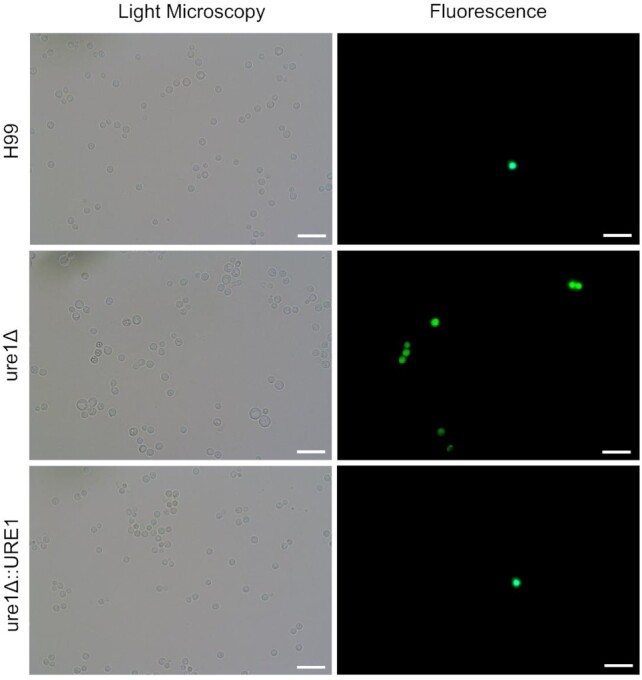
Fluorescence microscopy images of cryptococcal cells stained with the ROS indicator dye DCFDA after exposure to nutrient-limited conditions at 37°C for 3 h. Light microscopy images of stained yeast cells are on the left and corresponding fluorescence microscopy images on the right. All scale bars represent 20 μm.
Melanin production
Cryptococci cope with oxidative stress by producing the pigment melanin, which, among other functions, is known for its free radical scavenging properties (Casadevall, Rosas and Nosanchuk 2000). On l-DOPA agar plates at 26°C, all three strains showed similar levels of pigmentation (Fig. 10). In liquid media at 26°C, however, urease-deficient cells produced significantly more melanin than that of the wild-type and urease-complemented strains at 26°C (Fig. 11). A different pattern was observed at 37°C, where colonies of ure1Δ and ure1Δ::URE1 appeared to be less melanised than that of H99 within 1 day of incubation on agar media (Fig. 10). Except in the case of ure1Δ::URE1, colonies showed the same degree of pigmentation after 2 days of incubation (Figure S3, Supporting Information). Similar results were observed with the quantitative method at 37°C, where urease-deficient and urease-reconstituted strains produced significantly lower levels of melanin compared to H99 (Fig. 11).
Figure 10.
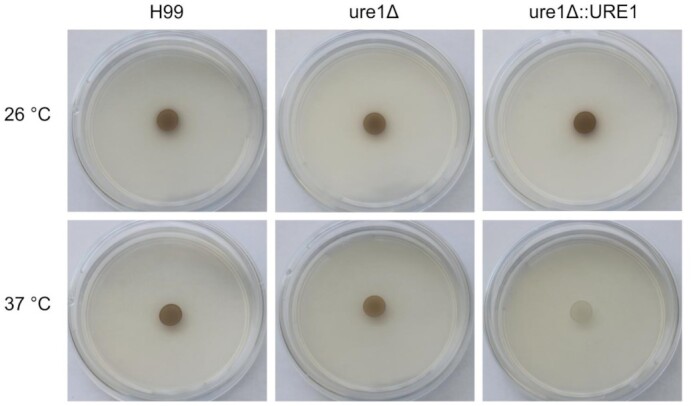
Melanin production by H99, ure1Δ and ure1Δ::URE1 strains of C. neoformans on l-DOPA agar plates within 1 day of incubation at 26 and 37°C, respectively.
Figure 11.
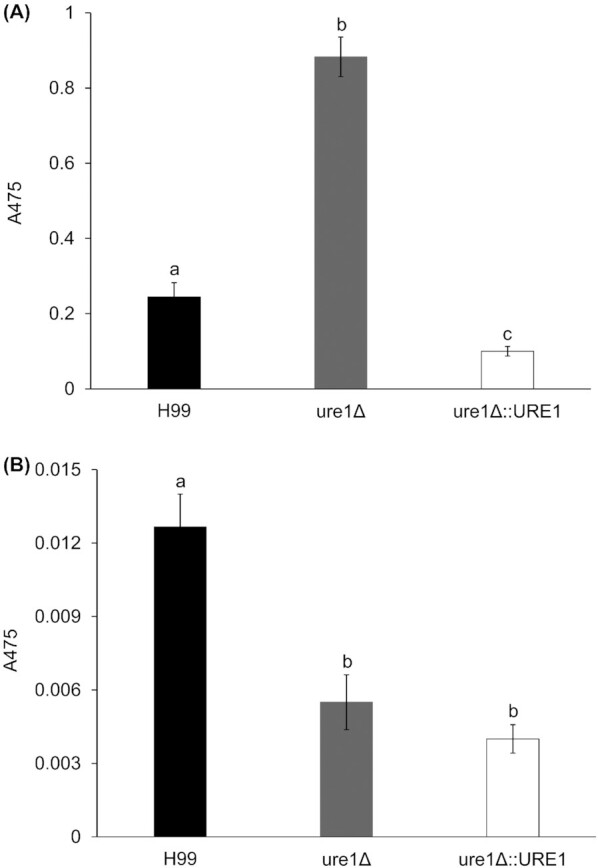
Melanin content of C. neoformans H99, ure1Δ and ure1Δ::URE1 after incubation in l-DOPA media at 26°C (A) and 37°C (B) for 48 h. Different letters indicate statistically significant differences (P < 0.05).
DISCUSSION
The presence of a urease enzyme was found to be important for cryptococcal growth at 26 and 37°C in BHI and nutrient-limited media. For the latter at 37°C, however, the urease-mutant behaved in a similar manner to H99 and ure1Δ::URE1, possibly due to the combined effect of temperature and nutrient stress that adversely affected the strains even with a urease enzyme. Our finding of a growth defect in a urease-deficient mutant of C. neoformans is in agreement with Fu et al. (2018), who observed a similar phenomenon while investigating growth of the same mutant in a minimal medium. Compared to that observed with BHI broth, the nutrient-limited conditions used in our study also resulted in the highest levels of urease activity in crude enzyme extracts of wild-type and urease-reconstituted strains of C. neoformans. Similar results were previously obtained by Lerm et al. (2017) while studying the urease activity of another C. neoformans clinical strain (CAB 1055), cultured in the same media at 37°C. As is evident from Fig. 3, differences in urease activities were observed between H99 and ure1Δ::URE1. The reason for this is unclear, but it may be a result of differences in urease expression levels, as was also suggested by Fu et al. (2018). In general, our results also showed that urease activities were higher at 37 than at 26°C, especially under nutrient-limited conditions. This is interesting to note as the nutrient-limited medium was devoid of urea and most literature to date notes the importance of cryptococcal urease for the use of available urea in the external environment and within the human host (Cox et al. 2000; Steenbergen and Casadevall 2003). Based on our findings, however, it appears that under conditions of nutrient and temperature stress, cryptococci regulate intracellular levels of active urease even in the absence of extracellular urea.
Although it is believed that cryptococci frequently encounter urea in their surroundings (Cox et al. 2000; Steenbergen and Casadevall 2003; Rutherford 2014; Fu et al. 2018), the possibility that urea from endogenous sources could serve as a substrate for urease is rarely considered. During this study, we found notably higher levels of urea in crude extracts from cells of the urease-mutant strain across all experimental conditions. In most cases, urea was either absent or below the assay detection limit in crude extracts of the wild-type and urease-reconstituted strains of C. neoformans. The differences in urea levels observed between H99 and ure1Δ::URE1 may be a result of the differences in urease metabolism discussed above for Fig. 3. Overall, these findings therefore suggest that urea accumulates intracellularly in the absence of a urease enzyme. Moreover, seeing that urea levels of the urease-mutant were higher in nutrient-limited media than BHI, this is the first indication that endogenous urea could serve as a substrate for the urease enzyme of C. neoformans.
Urea that originates from inside the yeast cell can result from intracellular arginase activity that catalyses the hydrolysis of l-arginine to produce l-ornithine and urea (McGee et al. 1999). Interestingly, this reaction was studied in C. neoformans >30 years ago as a possible explanation for canavanine resistance (Polacheck and Kwon-Chung 1986). When we screened crude extracts of cryptococcal cells for arginase enzyme activity, the urease-mutant and urease-reconstituted strains tended to have higher arginase levels compared to the wild-type strain across all tested nutrient and temperature conditions. It therefore appears that cryptococcal urease affects arginase activity within the cells of C. neoformans.
It is important to note that the arginase enzyme plays a pivotal role in the synthesis of amino acids and polyamines (Jenkinson et al. 1996). With this in mind, we aimed to determine whether the observed changes in arginase activity with the deletion of urease could affect the enzymes and metabolites of closely related biosynthetic pathways. This became apparent when agmatinase activity levels were assessed in crude extracts of the three cryptococcal strains. Except for BHI media at 26°C, agmatinase activity was undetected in crude extracts of cells without a urease enzyme. Although a putative gene encoding cryptococcal agmatinase is available on GenBank, this is the first report of its activity in C. neoformans. The agmatinase enzyme is commonly studied for its role in polyamine biosynthesis (Szumanski and Boyle 1990; Sekowska et al. 1998; Pérez-Mozqueda, Vazquez-Duhalt and Castro-Longoria 2019). Therefore, its undetectable levels in ure1Δ led to the hypothesis that perhaps cryptococcal cells without a urease enzyme are less able to synthesise polyamines compared to their wild-type and urease-reconstituted counterparts. Interestingly, when we screened cryptococcal strains for potential polyamine production from l-arginine, the reaction observed for the urease mutant was almost non-existent, suggesting a reduced ability to synthesise polyamines from l-arginine. The latter finding may provide a possible explanation for the observed growth defect of urease-negative cultures (Fig. 1), as the essential role played by polyamines in cell growth is well documented (Rocha and Wilson 2019).
In addition to its function in polyamine biosynthesis, l-arginine is required for the intracellular production of amino acids such as l-proline (Caldwell et al. 2015; Fichman et al. 2015). The metabolic pathway involved in l-proline synthesis begins with the aforementioned arginase enzyme, the activity of which was altered in the absence of urease during this study. This, however, only seemed to impact proline levels under nutrient-limited conditions at 37°C, where significantly higher levels of this amino acid were observed in crude extracts of urease-deficient cells. Increased intracellular proline levels are commonly observed in response to stress conditions for the maintenance of osmolarity and ROS (Takagi 2008). Interestingly, when stained with the ROS indicator dye DCFDA, urease-mutant cells showed greater fluorescence under the same conditions where increased proline levels were observed. This phenomenon was evident from both the qualitative and quantitative data obtained in this study. Using fluorometry, we also observed significantly higher fluorescence in urease-deficient cells following growth in BHI media at 37°C. Taken together, our findings suggest that cryptococcal urease influences intracellular proline levels, which could lead to additional metabolic changes, such as those relating to oxidative stress. Whether linked to proline levels or not, our results also indicate an association between urease and the intracellular accumulation of ROS under certain conditions.
In C. neoformans, ROS-induced cellular damage is prevented by antioxidant enzyme activity (Narasipura, Chaturvedi and Chaturvedi 2005) and the production of melanin pigments (Wang and Casadevall 1994). The elevated ROS levels observed in urease-deficient cells of C. neoformans therefore led us to question whether melanin synthesis is affected by the presence of a urease enzyme. Similar to Cox et al. (2000), we observed no difference in melanin production of wild-type and urease-mutant strains of C. neoformans H99 on l-DOPA agar plates at 26°C. But when a quantitative method was used, melanisation was found to be significantly higher in urease-deficient cells. With both melanin detection methods (qualitative and quantitative), urease-negative cells were less pigmented at 37°C. For reasons unknown, melanisation of the urease-reconstituted strain at 37°C was barely visible on l-DOPA agar plates, and with the quantitative method, results were similar to those obtained for urease-deficient cells. Taken together, our results indicate that urease influences melanin production in C. neoformans at both 26 and 37°C. The mechanism behind this phenomenon requires further investigation but may be linked to one or more of the other metabolic aspects explored in this study. For example, we found urease-mutant cells to be defective in polyamine synthesis, which was previously linked to melanin formation in a spermidine synthase mutant of C. neoformans (Kingsbury et al. 2004).
In summary, urease was found to be important for cryptococcal growth in BHI and nutrient-limited media. In cells with a urease enzyme, nutrient-limited conditions and a temperature of 37°C resulted in the highest levels of urease activity. Increased urease activity in cellular crude extracts may be a way to metabolise urea from endogenous sources, as cells without a urease enzyme were found to contain significantly higher intracellular urea levels. This study provided the first indications that urea from intracellular sources could serve as a substrate for cryptococcal urease, thereby suggesting that urease activity may also occur in environments where urea is unavailable. Arginase enzyme activity detected in crude extracts of cryptococcal cells could be a potential source of endogenous urea and was found to be higher in urease-deficient cells. Metabolic reactions linked to arginase were also affected as urease-mutant cells differed from wild-type and urease-reconstituted cells with respect to agmatinase enzyme activity, polyamine synthesis, intracellular proline levels and ROS. In addition, it was shown for the first time that urease activity may influence melanin production, an important virulence mechanism of C. neoformans. Lastly, it was found that some of the results obtained for the urease-reconstituted strain differed from those of the wild type. Interestingly, Fu et al. (2018) also reported on differences between these two strains when analysing data on the phagolysosomal pH of macrophages containing live cells of the respective strains. The reason for our observations is at this stage unknown, but taken together, indications are that the urease-reconstituted strain differs from the wild type in more than one aspect of its metabolism.
To conclude, the results of this study provide strong evidence that the urease enzyme of C. neoformans is associated with the regulation of key intracellular metabolic pathways (Fig. 12). This should be considered in all future research conducted on the urease-mutant, as the observed effects may not be a direct result of the urease enzyme itself. With the above in mind, the mechanism by which urease enhances cryptococcal virulence may therefore be more complex than previously thought, extending beyond ammonia production and its toxic effects on the human host. Based on our observations, gene deletion can result in unintended effects on cellular metabolism and without knowledge thereof, one cannot fully understand the enzyme's cellular role in the external environment or within the human host. Interconnectedness of metabolic pathways is a common feature of eukaryotic cells and should therefore be routinely considered in all gene deletion studies for accurate functional analysis.
Figure 12.
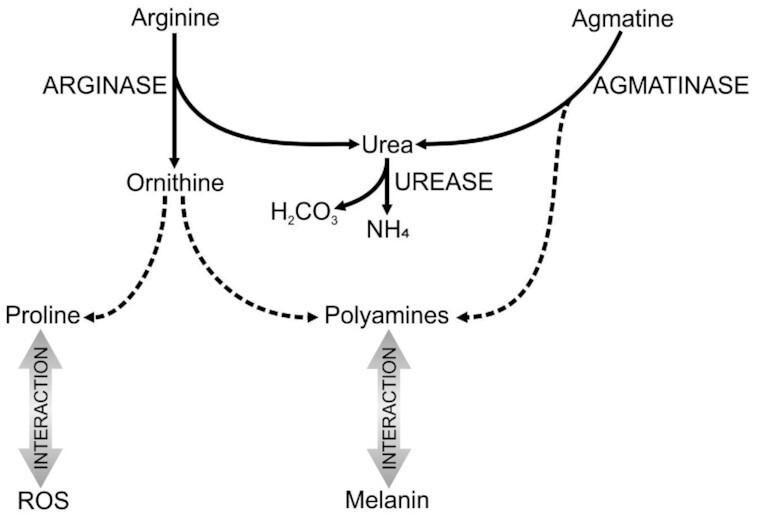
The urease enzyme catalyses the hydrolysis of urea to produce ammonia (NH4) and carbonic acid (H2CO3). The substrate urea can be produced intracellularly as a by-product of respective metabolic reactions catalysed by the enzymes arginase and agmatinase. In the latter case, agmatine that is produced from l-arginine is converted via agmatinase to urea and the polyamine putrescine. Synthesis of putrescine can also occur via the arginase enzyme, which hydrolyses l-arginine to produce urea and l-ornithine. The amino acid l-ornithine can then be converted to putrescine or used for the production of other cellular compounds, such as proline. Both polyamines and proline have been implicated in other aspects of cellular metabolism, including melanin formation and the generation of ROS. It should be noted that putrescine synthesis is vital for the production of the polyamines spermidine and spermine via the enzymes spermidine synthase and spermine synthase, respectively. Solid arrows represent reactions observed during the present study, while the dotted arrows indicate hypothetical pathways obtained from literature. Compiled from our own results and from a review of literature (Wang and Casadevall 1994; Kingsbury et al. 2004; Morris 2004; Takagi 2008; Rutherford 2014; Kumar, Saragadam and Punekar 2015; Rocha and Wilson 2019).
Supplementary Material
FUNDING
Barbra Toplis was funded by the National Research Foundation of South Africa. John R. Perfect is supported by the U.S. Public Health Service Grants AI73896 and AI95327.
Conflict of interest
None declared.
REFERENCES
- Allen AE, Dupont CL, Oborník Met al.. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature. 2011;473:203–7. [DOI] [PubMed] [Google Scholar]
- Barsoum R, El-Khatib M. Uric acid and life on earth. J Adv Res. 2017;8:471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- Bromke MA. Amino acid biosynthesis pathways in diatoms. Metabolites. 2013;3:294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnat M, Flores E. Inactivation of agmatinase expressed in vegetative cells alters arginine catabolism and prevents diazotrophic growth in the heterocyst‐forming cyanobacterium Anabaena. MicrobiologyOpen. 2014;3:777–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RB, Toque HA, Narayanan SPet al.. Arginase: an old enzyme with new tricks. Trends Pharmacol Sci. 2015;36:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal N, Orellana MS, Salas Met al.. Kinetic studies and site-directed mutagenesis of Escherichia coli agmatinase. A role for Glu274 in binding and correct positioning of the substrate guanidinium group. Arch Biochem Biophys. 2004;430:185–90. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Rosas AL, Nosanchuk JD. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol. 2000;3:354–8. [DOI] [PubMed] [Google Scholar]
- Chaskes ST, Tyndall RL. Pigment production by Cryptococcus neoformans and other Cryptococcus species from aminophenols and diaminobenzenes. J Clin Microbiol. 1978;7:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay MK, Tabor CW, Tabor H. Polyamine deficiency leads to accumulation of reactive oxygen species in a spe2Δ mutant of Saccharomyces cerevisiae. Yeast. 2006;23:751–61. [DOI] [PubMed] [Google Scholar]
- Cloete KJ, Valentine AJ, Stander MAet al.. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb Ecol. 2009;57:624–32. [DOI] [PubMed] [Google Scholar]
- Corraliza IM, Campo ML, Soler Get al.. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–5. [DOI] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GTet al.. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood D, Jetten MS, Van Griensven LJet al.. Argininosuccinate synthetase and argininosuccinate lyase: two ornithine cycle enzymes from Agaricus bisporus. Mycol Res. 2007;111:493–502. [DOI] [PubMed] [Google Scholar]
- Fichman Y, Gerdes SY, Kovács Het al.. Evolution of proline biosynthesis: enzymology, bioinformatics, genetics, and transcriptional regulation. Biol Rev. 2015;90:1065–99. [DOI] [PubMed] [Google Scholar]
- Fu MS, Coelho C, De Leon-Rodriguez CMet al.. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog. 2018;14:e1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JK, D'Souza CA, Cox GMet al.. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2004;3:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RK, Kim HK, Tsoa RWet al.. Cloning and characterization of human agmatinase. Mol Genet Metab. 2002;75:209–18. [DOI] [PubMed] [Google Scholar]
- Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–32. [DOI] [PubMed] [Google Scholar]
- Kingsbury JM, Yang Z, Ganous TMet al.. Novel chimeric spermidine synthase-saccharopine dehydrogenase gene (SPE3-LYS9) in the human pathogen Cryptococcus neoformans. Eukaryot Cell. 2004;3:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Saragadam T, Punekar NS. Novel route for agmatine catabolism in Aspergillus niger involves 4-guanidinobutyrase. Appl Environ Microbiol. 2015;81:5593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IR, Yang L, Sebetso Get al.. Characterization of the complete uric acid degradation pathway in the fungal pathogen Cryptococcus neoformans. PLoS One. 2013;8:e64292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerm B, Kenyon C, Schwartz ISet al.. First report of urease activity in the novel systemic fungal pathogen Emergomyces africanus: a comparison with the neurotrope Cryptococcus neoformans. FEMS Yeast Res. 2017;17:fox069. [DOI] [PubMed] [Google Scholar]
- McGee DJ, Radcliff FJ, Mendz GLet al.. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J Bacteriol. 1999;181:7314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendz GL, Hazell SL. The urea cycle of Helicobacter pylori. Microbiol. 1996;142:2959–67. [DOI] [PubMed] [Google Scholar]
- Miller-Fleming L, Olin-Sandoval V, Campbell Ket al.. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427:3389–406. [DOI] [PubMed] [Google Scholar]
- Morris SM Jr. Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–7S. [DOI] [PubMed] [Google Scholar]
- Morris SM Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. [DOI] [PubMed] [Google Scholar]
- Nair SV, Baranwal G, Chatterjee Met al.. Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int J Med Microbiol. 2016;306:237–48. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Chaturvedi V, Chaturvedi S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol. 2005;55:1782–800. [DOI] [PubMed] [Google Scholar]
- Nebes VL, Morris Jr SM. Regulation of messenger ribonucleic acid levels for five urea cycle enzymes in cultured rat hepatocytes. Requirements for cyclic adenosine monophosphate, glucocorticoids, and ongoing protein synthesis. Mol Endocrinol. 1988;2:444–51. [DOI] [PubMed] [Google Scholar]
- Ngamskulrungroj P, Meyer W. Melanin production at 37°C is linked to the high virulent Cryptococcus gattii Vancouver Island outbreak genotype VGIIa. Australas Mycol. 2009;28:9–14. [Google Scholar]
- Olszewski MA, Noverr MC, Chen GHet al.. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol. 2004;164:1761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Surana R, Milam JEet al.. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 2009;174:932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Riley J, Gerarden T. Polyamine depletion and growth inhibition of Cryptococcus neoformans by α-difluoromethylornithine and cyclohexylamine. Mycopathologia. 1990;112:27–32. [DOI] [PubMed] [Google Scholar]
- Polacheck I, Hearing VJ, Kwon-Chung KJ. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982;150:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck IT, Kwon-Chung KJ. Canavanine resistance in Cryptococcus neoformans. Antimicrob Agents Chemother. 1986;29:468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mozqueda LL, Vazquez-Duhalt R, Castro-Longoria E. Role and dynamics of anagmatinase-like protein (AGM-1) in Neurospora crassa. Fungal Genet Biol. 2019;132:103264. [DOI] [PubMed] [Google Scholar]
- Rahmatullah M, Boyde TR. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin Chim Acta. 1980;107:3–9. [DOI] [PubMed] [Google Scholar]
- Rocha RO, Wilson RA. Essential, deadly, enigmatic: polyamine metabolism and roles in fungal cells. Fungal Biol Rev. 2019;33:47–57. [Google Scholar]
- Rutherford JC. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014;10:e1004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekowska A, Bertin P, Danchin A. Characterization of polyamine synthesis pathway in Bacillus subtilis 168. Mol Microbiol. 1998;29:851–8. [DOI] [PubMed] [Google Scholar]
- Shabnam N, Tripathi I, Sharmila Pet al.. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma. 2016;253:1577–82. [DOI] [PubMed] [Google Scholar]
- Shi M, Li SS, Zheng Cet al.. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Panting RJ, Varma Aet al.. Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans. mBio. 2013;4:e00220–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen JN, Casadevall A. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microb Infect. 2003;5:667–75. [DOI] [PubMed] [Google Scholar]
- Szumanski MB, Boyle SM. Analysis and sequence of the speB gene encoding agmatine ureohydrolase, a putrescine biosynthetic enzyme in Escherichia coli. J Bacteriol. 1990;172:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H. Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl Microbiol Biotech. 2008;81:211–23. [DOI] [PubMed] [Google Scholar]
- Townsend DE, Kaenjak A, Jayaswal RKet al.. Proline is biosynthesized from arginine in Staphylococcus aureus. Microbiology. 1996;142:1491–7. [DOI] [PubMed] [Google Scholar]
- Tripathi SA, Olson DG, Argyros DAet al.. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl Environ Microbiol. 2010;76:6591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Santiago L, Ruiz-Herrera J. Stress and polyamine metabolism in fungi. Front Chem. 2014;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Carman AJ, Danhof HAet al.. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2:e00055–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen-and oxygen-derived oxidants. Infect Immun. 1994;62:3004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherburn MW. Phenol–hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–4. [Google Scholar]
- Weyman PD, Beeri K, Lefebvre SCet al.. Inactivation of Phaeodactylum tricornutum urease gene using transcription activator‐like effector nuclease‐based targeted mutagenesis. Plant Biotech J. 2015;13:460–70. [DOI] [PubMed] [Google Scholar]
- Yarrow D. Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman CP, Fell JW (eds). The Yeasts: A Taxonomic Study. Amsterdam: Elsevier, 1998, 77–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


