Structured Abstract
Background:
Morbidity following pancreatectomy is commonly due to leakage of exocrine secretions resulting in abscess or pancreatic fistula (PF). Previously, we authored a double-blind randomized controlled trial demonstrating perioperative pasireotide administration lowers abscess or PF formation by over 50%. Accordingly, we adopted pasireotide usage as standard practice following pancreatectomy in October 2014 and hypothesized a similar PF/abscess rate reduction would be observed.
Study Design:
A prospectively maintained database was queried for all patients who underwent pancreatectomy between October 2014 and July 2017. Pasireotide was administered preoperatively and twice daily for 7 days postoperatively or until discharge. The primary outcome was clinically-relevant PF/abscess requiring procedural intervention, identical to the prior trial outcome. Logistic regression was utilized to compare outcomes to the placebo arm of the prior randomized trial and to control known PF risk factors.
Results:
During the 34-month study period, 652 patients underwent pancreatectomy (211 distal pancreatectomy, 441 pancreaticoduodenectomy). Compared to the historical placebo group (N=148), the observational group had an increased prevalence of higher ASA scores (69% vs. 54%, p<0.001) and high-risk cases (small duct and soft gland, 47% vs. 36%, p=0.030). The primary outcome occurred in 13.3% of patients receiving pasireotide versus 20.9% in the placebo arm of the prior trial (OR 0.58 [95% C.I. 0.37–0.92], p=0.020). Biliary leakage was lower in those receiving pasireotide (0.6% versus 3.4%, p=0.014) while other morbidity was unchanged. No subpopulation was identified more likely to benefit from pasireotide.
Conclusion:
At our center, adoption of pasireotide has allowed us to achieve a clinically significant abscess or pancreatic leak rate of 13.3%, approximating the effect observed in the randomized trial of pasireotide during routine surgical practice.
Keywords: Pancreas, Pancreatectomy, Morbidity, Somatostatin receptor antagonist, Octreotide, Pasireotide
Precis.
Pasireotide has been shown to decrease leak-related morbidity after pancreatectomy in a randomized trial, but subsequent case series haven’t fully duplicated this benefit elsewhere. This study of 652 patients undergoing pancreatectomy with routine pasireotide usage observed a durable long-term decrease in pancreatic leak and abscess formation.
Introduction.
In the modern era, pancreatectomy is safely performed at major centers with a mortality of less than 2%. However, major morbidity continues to occur in over 30% of patients, many of whom experience pancreatic leakage and resultant sequelae(1, 2). While many operative techniques or other adjuncts purporting to decrease fistula- or other leak-related morbidity have been described, no one technique has proven widely effective and interest in novel strategies to mitigate leakage following pancreatectomy remains high(3, 4). The prototype somatostatin analogue, octreotide, was first reported in 1982 and remains in widespread clinical usage(5, 6). Octreotide has been demonstrated to decrease the volume and potency of both pancreatic exocrine secretions and hormone production (7). As the major etiology of morbidity following pancreatectomy is leakage of pancreatic exocrine secretions, this finding provided a rationale for perioperative administration of octreotide to decrease leakage-related complications such as pancreatic fistula or abscess formation(8, 9). While some European trials identified a benefit associated with octreotide administration following pancreatic resection, Western studies and meta-analyses have largely demonstrated that the incidence of clinically-relevant pancreatic leakage is not significantly altered(10–14).
Octreotide has demonstrated activity at only one of the five (sst2) endogenous somatostatin receptors present in humans. This may possibly explain the lack of reduction in postoperative pancreatic fistula or abscess formation following administration of octreotide after pancreatic resection. The novel analogue, pasireotide, has activity at four somatostatin receptors (sst1-3, sst5), a longer half-life, and demonstrated reduction in exocrine pancreatic secretion in animal models(15, 16). We evaluated the effectiveness of pasireotide to reduce the incidence of pancreatic leak, fistula, and abscess formation following pancreatic resection in a double-blinded randomized controlled trial reported in 2014(17). The primary outcome was limited to clinically-relevant morbidity requiring procedural intervention and demonstrated a statistically significant absolute risk reduction of 12%, from 21% in the placebo group to 9% in those receiving pasireotide.
Since this trial was reported, our center adopted routine perioperative pasireotide usage following all pancreatic resections. Other single-institution non-randomized experiences describing the results of perioperative pasireotide administration have not duplicated the results of the randomized trial(18, 19). As such, the primary aim of this study was to assess the incidence of clinically-relevant pancreatic leak, fistula, and abscess formation in patients undergoing pancreatic resection since the initiation of routine perioperative pasireotide administration at our institution. To best inform this effort, the placebo-receiving arm of the previous randomized trial served as a comparison cohort. Secondary aims included evaluating trends and adherence with the treatment regimen and checking for interactions to assess if any subgroups derived differential benefit from pasireotide.
Methods.
Patient selection.
The study period began when routine perioperative pasireotide usage was initiated in October 2014 and continued until June 2017. Adult patients undergoing either pancreaticoduodenectomy or distal pancreatectomy with or without splenectomy were included. Exclusion criteria were limited to patients that did not receive a single dose of pasireotide during their hospital stay and those who did not ultimately undergo resection. All patients underwent appropriate cardiac risk stratification for major abdominal surgery, but patients were not excluded based on ECG criteria as the previous trial demonstrated no adverse cardiac events. This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSKCC) (17).
Study design and outcome assessment.
A prospectively-maintained database of all consecutive patients undergoing pancreatic resection at MSKCC was queried. The primary outcome measure was aggregate incidence of grade 3 or higher postoperative pancreatic fistula, leak, or abscess at 60 days, as defined by the MSKCC Surgical Secondary Events (SSE) system. This system has been validated and approximates the National Cancer Institute Common Terminology Criteria for Adverse Events and other published metrics for surgical morbidity (Table 1) (20, 21). Pancreatic leak or fistula required the presence of amylase-rich drain effluent while diagnosis of abscess required positive microbial cultures. Grade 3 or higher events were defined as those that required postoperative procedural intervention, either percutaneous drain placement/adjustment or re-exploration in the operating room. Patients with operative drains who met the definition of fistula, leak, or abscess as above were considered as having met the primary endpoint. These definitions mirror those of the International Study Group for Pancreatic Surgery (ISGPS) for clinically-relevant (grade B/C) pancreatic fistula (22). Secondary outcomes of interest were similarly defined using the MSKCC SSE or defined clinically (such as readmission).
Table 1.
MSKCC Surgical Secondary Events reporting system definition and grading for postoperative pancreatic fistula, leak, and abscess formation. POD, postoperative day; TPN, total parenteral nutrition.
| Complication | Definition | Grade |
|---|---|---|
| Pancreatic fistula | Clinical signs and symptoms of pancreatic fistula, with amylase-rich drainage of >50mL per day after POD 10 | 1. Oral medication or bedside medical care required 2. Intravenous medical therapy with resolution or antibiotics or TPN required 3. Radiologic, endoscopic, or operative intervention required 4. Chronic deficit or disability associated with the event 5. Death associated with sequelae of this event |
| Pancreatic anastomotic leak | Clinical signs and symptoms or radiologic confirmation of pancreatic anastomotic leak, with amylase-rich drainage of >50mL per day after POD 5, without the development of a fistula | |
| Intraabdominal abscess | Clinical signs and symptoms or radiologic diagnosis of intraabdominal abscess or peritonitis | |
For comparison, we utilized the placebo-receiving group in the randomized controlled trial as a control group as the criteria for inclusion were identical and the surgical technique, ancillary caregivers, and perioperative management were essentially unchanged(17). Moreover, the primary outcome measure – incidence of clinically-relevant (grade III or above) pancreatic leak, fistula, or abscess development – was carefully scrutinized and prospectively recorded. Briefly, these patients underwent resection between October 2009 and July 2013 and were assigned to receive placebo via randomly sized permuted blocks to stratify group assignments according to type of procedure (distal pancreatectomy or pancreaticoduodenectomy) and absence or presence of a dilated pancreatic duct (defined as >4mm). All members of the clinical team were blinded to the group assignments during the initial study.
Operative technique and perioperative care.
Surgical care was provided by any one of seven experienced pancreatic surgeons; technique was individual to each surgeon. In general, gastro-/duodenojejunostomy was performed in an end-to-side manner in the antecolic position. Pancreaticojejunostomy was performed in a two-layer end-to-side fashion utilizing duct-to-mucosa reconstruction (Blumgart technique). Preservation of the pylorus or classic (Kausch-Whipple) pancreaticoduodenectomy was at the discretion of the surgeon. Operative drains were selectively employed. During distal pancreatectomy, transection was either performed using a stapling device or sharp transection and oversewing of the pancreatic remnant. All patients were cared for on a hospital ward specific to hepatopancreatobiliary surgical care with dedicated nursing and ancillary staff. Nasogastric tubes were used selectively and removed on postoperative day (POD) 1 if employed; dietary progression to clear liquids was initiated on POD 2 and advanced as tolerated. If employed, operative drains were removed at the discretion of the treating surgeon, but generally occurred when the output had amylase concentration of less than 300 units/L or the volume was less than 100 mL/day.
All patients in the study group received at least one dose of 900 µg subcutaneous pasireotide. The first dose was administered either in the presurgical ward on the day of operation or in the operating room at the time of induction. Pasireotide was continued twice daily for seven days postoperatively or until discharge, whichever occurred first; these patients were considered to have received a ‘full dose’. For patients with an adverse reaction to pasireotide, dosage was reduced or therapy discontinued; these patients were considered to have received a ‘partial dose’ and the total number of doses received was recorded. Cross-sectional imaging was performed when concern existed for intraabdominal morbidity and appropriate interventions were sought as needed.
Statistical analysis.
Fisher’s Exact test and the Wilcoxon Rank Sum test were used to compare characteristics between cohorts. Rates of complications were reported with exact 95% confidence intervals and compared with Fisher's Exact test. Univariable and multivariable logistic regression was used to assess the relationship between cohort and primary outcome complications grade≥3 (pancreatic fistula, leak, or abscess). Known or suspected confounding factors, including age, body mass index, gender, American Society of Anesthesiologists (ASA) score, resection type, and pancreatic duct size were controlled for in the multivariable model. Because of the collinearity between pancreatic duct size and gland texture, only gland texture was included in the multivariable model. The relationship between pasireotide administration, known risk factors and primary complication rate was assessed via logistic regression.
The proportion with exact 95% confidence interval of overall primary complications and grade≥3 primary complications were stratified by risk factors (bile duct size, texture, risk score, and resection type) and visualized with bar charts. The interaction between each risk factor with the overall primary outcome was assessed with logistic modelling including the two main effects and interaction term. Two-sided p-values less than 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 (The SAS Institute, Cary, NC).
Results.
Patient Characteristics.
During the nearly three-year study period, 662 patients underwent pancreaticoduodenectomy or distal pancreatectomy. Of these, 652 received at least one dose of pasireotide (98.6%) and formed the current study sample. Resection was performed for pancreatic ductal adenocarcinoma in 66.3% of cases. The group that received placebo in the prior randomized trial of pasireotide consisted of 148 patients and were utilized for comparison (Supplemental Table 1). The current experimental and historical control groups were balanced in age and no significant differences were seen for gender or BMI as shown in Table 2, although operative blood loss was higher in the placebo group (median 300 mL versus 250 mL, p < 0.001). The ASA score was significantly higher in the treatment group with 69.2% (451/652) having ASA 3 or 4 scores, compared to 54.1% (79/146) in the placebo group (p < 0.001) (Table 2).
Table 2.
Demographic and surgical characteristics of the study population compared to the historical placebo-receiving control population. Risk profile is a composite estimate of baseline risk for pancreatic leakage based on gland texture and pancreatic duct size (soft gland and smaller duct considered higher risk). BMI, body mass index; EBL, estimated blood loss; ASA, American Society of Anesthesiologists.
| Characteristic | Received Pasireotide (n=652) | Received Placebo (n=148) | p-value |
|---|---|---|---|
| Age, median (years) (range) | 66 (17-90) | 66 (31-89) | 0.56 |
| Gender | 0.24 | ||
| Female | 320 (49.1%) | 81 (54.7%) | |
| Male | 332 (50.9%) | 67 (45.3%) | |
| EBL, median (mL) (range) | 250 (0-4500) | 300 (25-2400) | < 0.001 |
| BMI, median (kg/m2) (range) | 26.2 (15.5-52.2) | 27.8 (18.7-47.3) | 0.12 |
| ASA Score | < 0.001 | ||
| 1-2 | 201 (30.8%) | 67 (45.9%) | |
| 3-4 | 451 (69.2%) | 79 (54.1%) | |
| Operative Drain | 0.67 | ||
| Yes | 150 (23.1%) | 37 (25%) | |
| No | 500 (76.9%) | 111 (75%) | |
| Resection | > 0.95 | ||
| Distal pancreatectomy | 211 (32.4%) | 48 (32.4%) | |
| Pancreaticoduodenectomy | 441 (67.6%) | 100 (67.6%) | |
| Duct Size | |||
| ≤ 4 mm | 368 (56.4%) | 77 (52%) | 0.14 |
| 4 - 8 mm | 254 (39.1%) | 59 (39.9%) | |
| ≥ 8 mm | 28 (4.3%) | 12 (8.1%) | |
| Gland Texture | 0.09 | ||
| Soft | 392 (60.2%) | 75 (52.4%) | |
| Firm | 259 (39.8%) | 68 (47.6%) | |
| Risk Profile | |||
| Low (>4 mm duct, firm) | 198 (30.5%) | 47 (32.9%) | 0.030 |
| Moderate (≤4 mm or soft) | 144 (22.2%) | 44 (30.8%) | |
| High (≤4 mm and soft) | 308 (47.4%) | 52 (36.4%) | |
Percentages are derived from cohort totals with available data excluding unknown data points, which are not displayed
The proportion of patients that underwent distal pancreatectomy (32.4% in each group) versus pancreaticoduodenectomy (67.6% in each group) and had operative drains placed (23.1% vs. 25%) were nearly identical. Gland texture and pancreatic duct size were also evaluated. A similar fraction was noted to have small (≤4 mm) ducts in each group, and although the study group had a higher proportion of soft gland texture (60.2% [392/651] versus 52.4% [75/143]), this difference was not statistically significant(p=0.09). A composite ‘Risk Profile’ is also shown in Table 2, aggregating these two inherent gland characteristics known to be associated with pancreatic leak into low, moderate, and high-risk groups based on the coincidence of soft glands with small ducts. In the aggregate risk score, treatment patients had a higher proportion of high risk glands (soft gland and small duct, 47.4%, 308/650) compared to the placebo patients (36.4%, 52/143, p=0.030).
Study Outcomes.
The primary outcome of aggregate grade 3 or higher pancreatic leak, fistula, and abscess formation occurred in 13.3% (95% C.I., 10.8-16.2%) of those receiving pasireotide compared to 20.9% (95% C.I., 14.7-28.4%) of those in the placebo-receiving control group (OR: 0.58, 95% C.I.: 0.37-0.92, p=0.020). The absolute risk reduction of 7.6% reflected a number needed to treat with pasireotide of 13.2 patients to prevent one occurrence of the primary outcome. As shown in Table 3, in univariable analyses patients with pancreatic duct size greater than 4mm were at lower risk of primary outcome (OR: 0.60, 95% C.I.: 0.40-0.91, p=0.015) and patients with a higher BMI had higher odds of primary outcome (OR: 1.05, 95% C.I.: 1.01-1.09, p=0.007). Soft gland texture was marginally associated with primary outcome (OR: 1.48, 95% C.I.: 0.98-2.24, p=0.06).
Table 3.
Analysis of risk factors for the primary outcome measure (aggregate incidence of pancreatic fistula/leak/abscess) across all study participants (N = 800) via univariable logistic regression. ASA, American Society of Anesthesiologists; BMI, body mass index; C.I, confidence interval; Ref., reference statistics.
| Characteristic | Incidence/Number at Risk (%) | Odds Ratio | 95% C.I. | p-value |
|---|---|---|---|---|
| Treatment Cohort | ||||
| Pasireotide | 87/652 (13.3) | 0.58 | [0.37 – 0.92] | 0.020 |
| Placebo | 31/148 (20.9) | |||
| ASA Score | ||||
| 1-2 | 36/268 (13.4) | Ref. | ||
| 3 | 79/507 (15.6) | 1.19 | [0.78 – 1.82] | 0.71 |
| 4 | 3/23 (13.0) | 0.97 | [0.27 – 3.42] | |
| Gender | ||||
| Female | 69/401 (17.2) | 1.48 | [1.00 – 2.21] | 0.050 |
| Male | 49/399 (12.3) | Ref. | ||
| Resection | ||||
| Distal pancreatectomy | 40/259 (15.4) | 1.08 | [0.72 – 1.64] | 0.70 |
| Whipple | 78/541 (14.4) | Ref. | ||
| Gland Texture | ||||
| Soft | 78/467 (16.7) | 1.48 | [0.98 – 2.24] | 0.06 |
| Firm | 39/327 (11.9) | Ref. | ||
| Duct Size | ||||
| > 4 mm | 40/353 (11.3) | 0.60 | [0.40 – 0.91] | 0.015 |
| ≤ 4 mm | 78/445 (17.5) | Ref. | ||
| Age at Surgery, years | 118/800 (14.8) | 0.99 | [0.98 – 1.01] | 0.20 |
| BMI, kg/m2 | 107/745 (14.4) | 1.05 | [1.01 – 1.09] | 0.007 |
Table 4 displays morbidity related to secondary events of interest. The incidence of biliary leak/fistula was substantially decreased in those receiving pasireotide (0.6% [95% C.I.: 0.2-1.6%] versus 3.4% [95% C.I.: 1.1-7.7%], p=0.014), while the rates of delayed gastric emptying or enteric leaks were similar. Of the individual components of the primary outcome, pancreatic leak or fistula incidence decreased from 14.2% to 8.0% (p=0.02) while abscess incidence decreased from 10.1% to 6.3% (p=0.11). Grade 1 or 2 pancreatic leak/fistula events (which would be analogous to ISGPF Grade A or biochemical leaks) occurred in 4.8% of patients. Postoperative hemorrhage, for which grade 2 complications were considered meaningful (grade 2 being indicative of need for transfusion) was decreased from 8.8% to 4.6% in those receiving placebo and pasireotide, respectively. This trend did not reach significance (p= 0.07). Overall mortality at 90 days was 1.1% and did not differ between groups (p>0.95).
Table 4.
Comparison of secondary outcome measures from patients in the study population versus those that received placebo. Only events of grade 3 or higher (those requiring procedural intervention) were considered meaningful, except where indicated. C.I., confidence interval.
| Secondary Outcome | Pasireotide Group (n=652) | 95% C.I. | Placebo Group (n=148) | 95% C.I. | p-value |
|---|---|---|---|---|---|
| Secondary Outcomes | |||||
| Biliary leak/fistula | 4 (0.6%) | [0.2 - 1.6] | 5 (3.4%) | [1.1 – 7.7] | 0.014 |
| Delayed gastric emptying | 30 (4.6%) | [3.1 - 6.5] | 6 (4.1%) | [1.5 – 8.6] | >0.95 |
| Enteric leak/fistula | 3 (0.5%) | [0.1 - 1.3] | 2 (1.4%) | [0.2 – 4.8] | 0.23 |
| Other morbidity ≥ grade 3 | 45 (6.9%) | [5.1 - 9.1] | 18 (12.2%) | [7.4 – 18.5] | 0.041 |
| Hemorrhage (≥ grade 2) | 30 (4.6%) | [3.1 - 6.5] | 13 (8.8%) | [4.8 – 14.6] | 0.07 |
| Mortality (90 day, any cause) | 8 (1.2%) | [0.5 - 2.4] | 1 (0.7%) | [0.0 – 3.7] | >0.95 |
As mentioned in the methods, a multivariate logistic regression model was built, but multi-collinearity prevented inclusion of both gland texture and pancreatic duct size as independent variables; the resultant model is also demonstrated in Supplemental Table 1 with an odds ratio for patients receiving pasireotide of 0.65 (95% C.I. 0.38-1.13; p=0.13)
Analysis of Pasireotide-Only Cohort.
For patients in the pasireotide-receiving cohort, potential risk factors for postoperative pancreatic leak, fistula, or abscess formation were assessed via logistic regression in a separate analysis. A full dose of pasireotide, defined as twice-daily pasireotide for 7 days postoperatively or until discharge (whichever occurs first), was received by 83.4% (544/652, Table 5). The median time of pasireotide administration in those who received partial doses (N=108) was 5 days (range, 1-6 days). As shown in Table 5, the primary outcome risk did not differ between those receiving a full or partial dose (p=0.18). Conversely, soft gland texture (OR: 1.87, 95%CI: 1.14-3.09, p=0.014) demonstrated statistically significant increased risk of the primary outcome and pancreatic duct size >4mm demonstrated decreased risk of primary outcome (OR: 0.61, 95%CI: 0.38-0.99, p=0.044). Patients with moderate- or high-risk glands had higher odds of primary outcome compared to low-risk patients, although the magnitude of increased risk was similar (odds ratio 2.32/2.31 for moderate-/high-risk (p = 0.019). The most common treatment-limiting toxicity leading to partial dose administration was nausea. No life-threatening adverse events were attributed to pasireotide.
Table 5.
Analysis of risk factors for the primary outcome measure (aggregate incidence of pancreatic fistula/leak/abscess) amongst patients in the study arm alone (received pasireotide, N = 652) via univariable logistic regression. A full dose of pasireotide is considered 14 total doses given twice daily or continued administration until discharge, whichever occurs earlier. C.I., confidence interval.
| Characteristic | Incidence/Number at Risk (%) | Odds Ratio | 95% C.I. | p-value |
|---|---|---|---|---|
| Received Full Course (14 Doses) | ||||
| Yes | 77/544 (14.2) | 1.62 | [0.81 – 3.23] | 0.18 |
| No | 10/108 (9.3) | |||
| Incremental Days on Pasireotide | 87/652 (13.3) | 1.07 | [0.86 – 1.32] | 0.55 |
| Gland Texture | ||||
| Soft | 63/392 (16.1) | 1.87 | [1.14 – 3.09] | 0.014 |
| Firm | 24/259 (9.3) | Ref. | ||
| Duct Size | ||||
| > 4 mm | 29/282 (10.3) | 0.61 | [0.38 – 0.99] | 0.044 |
| ≤ 4 mm | 58/368 (15.8) | Ref. | ||
| Risk Profile | ||||
| High (≤4 mm and soft) | 49/308 (15.9) | 2.31 | [1.26 – 4.24] | |
| Moderate (≤4 mm or soft) | 23/144 (16.0) | 2.32 | [1.16 – 4.62] | 0.019 |
| Low (>4 mm duct, firm) | 15/198 (7.6) | Ref. | ||
Subgroup Analysis.
Figure 1 demonstrates the incidence of the primary outcome stratified by the above risk factors in addition to type of resection (distal pancreatectomy versus pancreaticoduodenectomy) and compared to the equivalent group in the placebo-receiving control group. None of the interactions between pancreatic duct size, gland texture, complication risk, or resection time with treatment cohort were found to be significant (p=0.12-0.69, Supplemental Table 2), which mirrors the similar primary outcome rates presented in Supplementary Figure 1. Although no individual subgroup demonstrated a statistically-significant decrease, an absolute risk reduction was observed across all subgroups, with the greatest reduction seen in patients with firm gland texture (12.8%).
Figure 1.
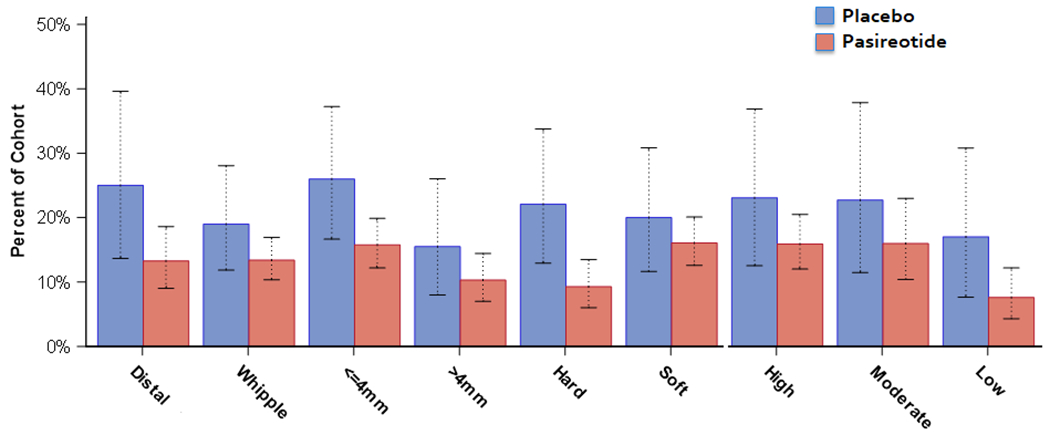
Incidence of the primary outcome (aggregate incidence of pancreatic fistula/leak/abscess) in the current study population routinely receiving pasireotide versus the control population that received placebo. Error bars indicate 95% confidence interval.
Discussion.
The primary aim of this study was to assess the incidence of clinically-relevant pancreatic leak, fistula, or abscess formation following pancreatic resection with routine perioperative administration of the somatostatin analogue pasireotide. 652 patients were evaluated who underwent either distal pancreatectomy or pancreaticoduodenectomy and 13.3% developed the primary outcome. We instituted a program of routine pasireotide administration after its efficacy in reducing these complications was demonstrated in a randomized, placebo-controlled study conducted at our center in 2014. This trial reported a statistically-significant absolute risk reduction of 11.7% in those receiving pasireotide, but we have not reported the impact of pasireotide in routine usage outside of a trial setting. For the current study, the placebo group for the prior trial served as a historical comparison group as the setting and outcome measures are identical. During the nearly three-year period examined for this study, an absolute risk reduction of 7.6% was observed. While the overall incidence of fistula or abscess formation was higher than the incidence observed in those patients receiving pasireotide in the trial setting (9.2%), the decrease shown here remained significantly lower than the prior placebo cohort. Moreover, this occurred despite the current study group having more cases at high risk for pancreatic leakage (coexisting soft gland/small duct) compared to the placebo group (Table 2). The current group also had more comorbidities than the placebo group, as evidenced by higher ASA scores.
Postoperative pancreatic leak and related complications a major source of postoperative morbidity and continue to defy innumerable attempts at mitigation (1, 3). Interest in a pharmacologic solution employing somatostatin analogues has been longstanding, with octreotide being intensively studied over the past three decades (10, 13, 14). Because meta-analyses have suggested the efficacy of octreotide is limited, its routine usage is not widespread (12, 23) at most institutions. However, notable exceptions exist. In a 2016 study, over 80% of German surgeons reported using somatostatin analogues either some or all of the time following pancreatectomy, although only 5% of these included pasireotide (23). Pasireotide has different pharmacologic properties with a broader binding profile and longer half-life compared to octreotide, and because of this we initiated a randomized placebo-controlled trial. Utilizing the risk reduction data from that trial, three papers (including one from our group) suggested routine pasireotide usage would be cost-effective or cost-neutral(24–26); a fourth paper has suggested costs would be further optimized if pasireotide was limited to those at highest risk for fistula (27). Due to the efficacy results from our trial and these data regarding cost, we implemented a standard practice of giving perioperative pasireotide following all pancreatic resections at MSKCC.
In early 2018, two similar studies first reported the effects of pasireotide at institutions beyond MSKCC. Elliot and colleagues at UCLA gave pasireotide to 111 consecutive patients and compared the rate of clinically-relevant pancreatic fistula to 168 historical controls; no difference in fistula prevalence was noted (19). At Washington University, Dominguez-Rosado, et al conducted a similar study with 127 consecutive patients receiving pasireotide compared to pre- and post-pasireotide cohorts that did not receive the drug and similarly failed to demonstrate a significant reduction in overall or clinically-relevant fistula incidence (18). A subgroup of 112 patients was propensity-matched due to considerable differences in patients who did or did not receive pasireotide with similar findings. Although the results of these two studies appear to conflict with the data reported here, several important differences exist. All define pancreatic fistula as persistent amylase-rich drainage after surgery, approximating the consensus definition favored by the ISGPF, but the primary outcome measure differs. In the current study, and original randomized trial, the primary outcome aggregates pancreatic leakage and intraabdominal abscesses, whereas the UCLA study adheres to ISGPF grade B/C events only and the Washington University study favors severe morbidity classified by a modified accordion grading system. These differences magnify the dissimilar baseline event rate seen between the studies. While the MSKCC Surgical Secondary Events morbidity reporting system (shown in Table 1) does not replicate the ISGPF definitions when evaluating pancreatic leak/fistula, our primary outcome of Grade 3 events of higher essentially mirrors grade B/C events from the ISGPF – so-called ‘clinically-relevant’ postoperative pancreatic fistulas. Also, important disparities exist in the study samples and institutional practice patterns. For example, in the Washington University study 95% of those who received pasireotide received operative drains compared to 25% of patients in the current study. Defining a grade B leak in the setting of an operative drain has some subjectivity, as it is determined not only by the presence of amylase in the drain but also by a “clinically-relevant condition” related to the fistula. Whereas some could suggest simply having any drain for 10 days would be clinically significant, others may consider that a biochemical leak only and thus a grade A fistula. This emphasizes the importance of randomization and blinded assessment. The impact of these differences may explain the differential findings between our studies, but the data here clearly demonstrate a durable reduction in pancreatic leaks, fistulae, and intraabdominal abscesses at our institution during the three-year study period in over 650 patients undergoing pancreatic resection.
Reductions in biliary leak/fistula and postoperative hemorrhage were notable findings in the analysis of secondary outcomes. Large historical databases suggest biliary leaks complicate approximately 3% of pancreaticoduodenectomies (2, 20). In this study, only four patients developed biliary leakage (0.6%, or 0.9% when limited to those undergoing pancreaticoduodenectomy). The known decrease in bile secretion after administration of somatostatin analogues may be the physiologic rationale for this observed decrease. The rate of grade 2 or higher (requiring transfusion) postoperative hemorrhage also decreased by nearly half amongst those who received pasireotide (8.8% to 4.6%), a trend that neared statistical significance (p = 0.07). Interestingly, the group from Washington University reported a decrease in postoperative anemia or hemorrhage of an even greater magnitude in their study; this was statistically significant in their analysis. Furthermore, the decrease persisted in the propensity-matched subgroup analysis as well (18). This decrease may be due to reduction in extraluminal late post-pancreatectomy hemorrhage secondary to concomitant reduction in pancreatic leak- or fistula-mediated vascular erosion and bleeding, a phenomenon well-known to pancreatic surgeons. Alternatively, somatostatin-mediated decreases in portal and splanchnic blood flow may be causative. These findings merit further study as outcomes independent of pancreatic leakage in any future trials examining pasireotide following pancreatectomy.
Pasireotide remains a routine component of pancreatic resection at our institution. While the price of its use is not inconsequential, the lasting decrease in fistula, leak, and abscess formation shown here supports the cost-effectiveness models of previous reports from our center (24–26). Nausea remains the most prominent treatment-related toxicity and is treatment-limiting in approximately 15% of patients. Over the study period, we have noted a significant improvement in pasireotide tolerance when co-administered with parenteral ondansetron, which mitigates severe nausea in many of our patients. Intriguingly, no difference in event rate was observed in the study population who received partial (less than 14) dose regimens. This suggests the duration of administration could possibly be reduced without loss of effect and a potential cost savings.
As in the studies from Washington University and UCLA, this study is limited by its retrospective nature, even though data on morbidity was collected prospectively. Moreover, despite a large number of patients over a prolonged period, this remains a single-center study and confirmatory of practices tested in a clinical trial at our institution. The placebo-receiving comparison population was smaller than the study population that received pasireotide; however, this was considered preferable to a ‘historical’ group of larger size given the careful evaluation of outcomes and granular data available for participants in the pasireotide clinical trial. Despite interesting secondary findings (i.e. reduction in hemorrhage), the role of pasireotide in conclusively reducing leak-related complications following pancreatectomy at other institutions remains unconfirmed. It has been suggested that the lack of efficacy of somatostatin analogues may be due to their inability to act on a ‘stunned’ pancreas in a postoperative state (28), but this is contradicted by the available physiologic data. Studies have consistently shown a decrease in volume and potency of pancreatic exocrine secretions with somatostatin analogue therapy, with the average maximum drain amylase concentration with pasireotide in the Washington University study being 60-70% reduced compared to untreated patients (7, 18). It seems that the exocrine function of the postoperative pancreas is undoubtedly affected by somatostatin analogues, but translation of this effect into reducing clinically-relevant morbidity following pancreatectomy appears different between institutions and available studies. The etiology of this phenomenon remains unclear. Certainly, any future studies examining pasireotide should be multi-institutional and ideally stratified by variables such as duct size and operative drain to maximize their impact. Such a multi-institutional trial would serve to conclusively address the lack of uniformity seen amongst the single-institution studies currently available.
Conclusions.
After three years of routinely administering pasireotide to all patients undergoing pancreatic resection, we have found the aggregate incidence of clinically-relevant pancreatic leakage, fistula, and abscess formation to be 13.3%, a significant decrease from those not receiving pasireotide in our previous study population. Incidence of biliary leaks and postoperative hemorrhage have also decreased. We continue to routinely employ pasireotide in the perioperative care of patients undergoing pancreatectomy.
Supplementary Material
Supplemental Figure 1. Schematic of study design noting enrollment and randomization from prior controlled trial evaluating pasireotide in patients undergoing pancreatectomy(17) compared to the current study as indicated.
Abbreviations:
- MSKCC
Memorial Sloan Kettering Cancer Center
- SSE
Surgical Secondary Events reporting system
- POD
postoperative day
- ISGPS
International Study Group for Pancreatic Surgery
- ASA
American Society of Anesthesiologists
- BMI
body mass index
Footnotes
Meeting Information:
Presented at the Americas Hepatopancreatobiliary Association Annual Meeting on March 11, 2018 by Dr. Kunstman at the podium.
References.
- 1.Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2016. February;113(2):188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006. November;10(9):1199–210; discussion 210-1. [DOI] [PubMed] [Google Scholar]
- 3.Kitahata Y, Kawai M, Yamaue H. Clinical trials to reduce pancreatic fistula after pancreatic surgery-review of randomized controlled trials. Transl Gastroenterol Hepatol. 2016;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshmuminov D, Schneider MA, Tschuor C, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford). 2018. May 25. [DOI] [PubMed] [Google Scholar]
- 5.Bloom SR, Mortimer CH, Thorner MO, et al. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet. 1974. November 9;2(7889):1106–9. [DOI] [PubMed] [Google Scholar]
- 6.Bauer W, Briner U, Doepfner W, et al. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982. September 13;31(11):1133–40. [DOI] [PubMed] [Google Scholar]
- 7.Williams ST, Woltering EA, O'Dorisio TM, Fletcher WS. Effect of octreotide acetate on pancreatic exocrine function. Am J Surg. 1989. May;157(5):459–62. [DOI] [PubMed] [Google Scholar]
- 8.Ahren B, Tranberg KG, Bengmark S. Treatment of pancreatic fistula with the somatostatin analogue SMS 201-995. Br J Surg. 1988. July;75(7):718. [DOI] [PubMed] [Google Scholar]
- 9.Miller BM, Traverso LW, Freeny PC, Abumrad NN. Failure of somatostatin or an analog to promote closure of end pancreatic fistulae. Int J Pancreatol. 1989. February;4(1):65–72. [DOI] [PubMed] [Google Scholar]
- 10.Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992. January;163(1):125–30; discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 11.Droeser RA, Jeanmonod P, Schuld J, Moussavian MR, Schilling MK, Kollmar O. Octreotide prophylaxis is not beneficial for biochemical activity and clinical severity of postoperative pancreatic fistula after pancreatic surgery. Dig Surg. 2012;29(6):484–91. [DOI] [PubMed] [Google Scholar]
- 12.Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013. April 30(4):CD008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997. November;226(5):632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000. September;232(3):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golor G, Hu K, Ruffin M, et al. A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug Des Devel Ther. 2012;6:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002. May;146(5):707–16. [DOI] [PubMed] [Google Scholar]
- 17.Allen PJ, Gonen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014. May 22;370(21):2014–22. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez-Rosado I, Fields RC, Woolsey CA, et al. Prospective Evaluation of Pasireotide in Patients Undergoing Pancreaticoduodenectomy: The Washington University Experience. J Am Coll Surg. 2018. February;226(2):147–54 e1. [DOI] [PubMed] [Google Scholar]
- 19.Elliott IA, Dann AM, Ghukasyan R, et al. Pasireotide does not prevent postoperative pancreatic fistula: a prospective study. HPB (Oxford). 2018. February 2. [DOI] [PubMed] [Google Scholar]
- 20.Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007. March;204(3):356–64. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. August;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017. March;161(3):584–91. [DOI] [PubMed] [Google Scholar]
- 23.Volk A, Nitschke P, Johnscher F, et al. Perioperative application of somatostatin analogs for pancreatic surgery-current status in Germany. Langenbecks Arch Surg. 2016. November;401(7):1037–44. [DOI] [PubMed] [Google Scholar]
- 24.Abbott DE, Sutton JM, Jernigan PL, et al. Prophylactic pasireotide administration following pancreatic resection reduces cost while improving outcomes. J Surg Oncol. 2016. June;113(7):784–8. [DOI] [PubMed] [Google Scholar]
- 25.Goyert N, Eeson G, Kagedan DJ, et al. Pasireotide for the Prevention of Pancreatic Fistula Following Pancreaticoduodenectomy: A Cost-effectiveness Analysis. Ann Surg. 2017. January;265(1):2–10. [DOI] [PubMed] [Google Scholar]
- 26.Ma LW, Dominguez-Rosado I, Gennarelli RL, et al. The Cost of Postoperative Pancreatic Fistula Versus the Cost of Pasireotide: Results from a Prospective Randomized Trial. Ann Surg. 2017. January;265(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denbo JW, Slack RS, Bruno M, et al. Selective Perioperative Administration of Pasireotide is More Cost-Effective Than Routine Administration for Pancreatic Fistula Prophylaxis. J Gastrointest Surg. 2017. April;21(4):636–46. [DOI] [PubMed] [Google Scholar]
- 28.Yeo CJ. Invited Commentary: Pasireotide and the Prevention of Pancreatic Fistula After Pancreatectomy: “The Continued Search for Harry Potter’s Liquid Luck”. Ann Surg. 2017. January;265(1):17–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic of study design noting enrollment and randomization from prior controlled trial evaluating pasireotide in patients undergoing pancreatectomy(17) compared to the current study as indicated.


