Human cytomegalovirus (HCMV) infection is endemic throughout the world, with a seroprevalence of 40 to 100% depending on geographic location. HCMV infection is generally asymptomatic, but can cause severe inflammatory organ diseases in immunocompromised individuals. The broad array of organ diseases caused by HCMV is directly linked to the systematic spread of the virus mediated by monocytes. Monocytes are naturally programmed to undergo apoptosis, which is rapidly blocked by HCMV to ensure the survival and dissemination of infected monocytes to different organ sites. In this work, we demonstrate infected monocytes also initiate necroptosis as a “trap door” death pathway in response to HCMV subversion of apoptosis. HCMV then activates cellular autophagy as a countermeasure to prevent the execution of necroptosis, thereby promoting the continued survival of infected monocytes. Elucidating the mechanisms by which HCMV stimulates monocyte survival is an important step to the development of novel anti-HCMV drugs that prevent the spread of infected monocytes.
KEYWORDS: cytomegalovirus, monocytes
ABSTRACT
Key to the viral dissemination strategy of human cytomegalovirus (HCMV) is the induction of monocyte survival, where monocytes are normally short-lived cells. Autophagy is a cellular process that preserves cellular homeostasis and promotes cellular survival during times of stress. We found that HCMV rapidly induced autophagy within infected monocytes. The early induction of autophagy during HCMV infection was distinctly required for the survival of HCMV-infected monocytes, as repression of autophagosome formation led to cellular death of infected cells but had no effect on the viability of uninfected monocytes. The inhibition of caspases was insufficient to rescue cell viability of autophagy-repressed infected monocytes, suggesting that autophagy was not protecting cells from apoptosis. Accordingly, we found that HCMV blocked the activation of caspase 8, which was maintained in the presence of autophagy inhibitors. Necroptosis is an alternative form of cell death triggered when apoptosis is impeded and is dependent on RIPK3 phosphorylation of MLKL. Although we found that HCMV activated RIP3K upon infection, MLKL was not activated. However, inhibition of autophagy removed the block in RIPK3 phosphorylation of MLKL, suggesting that autophagy was protecting infected monocytes from undergoing necroptosis. Indeed, survival of autophagy-inhibited HCMV-infected monocytes was rescued when MLKL and RIPK3 were suppressed. Taken together, these data indicate that HCMV induces autophagy to prevent necroptotic cell death in order to ensure the survival of infected monocytes and thus facilitate viral dissemination within the host.
IMPORTANCE Human cytomegalovirus (HCMV) infection is endemic throughout the world, with a seroprevalence of 40 to 100% depending on geographic location. HCMV infection is generally asymptomatic, but can cause severe inflammatory organ diseases in immunocompromised individuals. The broad array of organ diseases caused by HCMV is directly linked to the systematic spread of the virus mediated by monocytes. Monocytes are naturally programmed to undergo apoptosis, which is rapidly blocked by HCMV to ensure the survival and dissemination of infected monocytes to different organ sites. In this work, we demonstrate infected monocytes also initiate necroptosis as a “trap door” death pathway in response to HCMV subversion of apoptosis. HCMV then activates cellular autophagy as a countermeasure to prevent the execution of necroptosis, thereby promoting the continued survival of infected monocytes. Elucidating the mechanisms by which HCMV stimulates monocyte survival is an important step to the development of novel anti-HCMV drugs that prevent the spread of infected monocytes.
INTRODUCTION
Human cytomegalovirus (HCMV) is a member of the betaherpesvirus family and a major worldwide public health burden. Viral seroprevalence has been estimated to be between 40 and 90% in the world, with some countries reporting seroprevalence of 90% and above (1, 2). Infection is generally self-limiting in immunocompetent individuals, but has been associated with substantial morbidity and mortality in neonates and immunocompromised patients (3–5). In these patients, such as those with AIDS, transplant recipients, and those undergoing chemotherapy, HCMV disease is characterized by widespread viral dissemination and inflammation with severe end-organ damage (6).
Monocytes are believed to serve as a key cog in the HCMV dissemination strategy (7, 8). In support, monocytes are the primary carriers of the virus in transplant patients and leukocyte depletion of blood donations sharply reduces HCMV transmission (9). The importance of monocytes was further corroborated using an in vivo murine cytomegalovirus (MCMV) murine model, which showed monocytes to be the predominant cell type responsible for viral dissemination during an acute infection (10–12). A recently developed humanized mouse model also found the source of HCMV in peripheral organs was from human monocyte-derived macrophages (13). However, circulating monocytes are naturally short-lived cells with an average life span of 48 h, making these blood cells ill-suited to mediate HCMV spread (14, 15). Furthermore, monocytes are not permissive for viral replication or gene expression (quiescent infection) until differentiated into long-lived macrophages, a process that can take up to 3 weeks in culture (8, 16, 17). To overcome the biological limitations of monocytes, HCMV must stimulate the survival and differentiation of monocytes into macrophages.
Autophagy is a process responsible for maintaining homeostasis and conservation of energy during times of stress through the recycling of unnecessary or dysfunctional cellular components. Beyond its general role in controlling cellular homeostasis, autophagy also regulates cellular survival pathways. A number of autophagic and apoptotic proteins directly interact, which allows for autophagy to directly impact cell viability (18). Particularly in times of stress, when apoptosis might otherwise become activated due to a buildup of toxic proteins within the cytoplasm, autophagy upregulation acts as a measure of last resort to stave off cellular death. This ability to ward off death appears to play a pivotal function in driving the differentiation of monocytes, which are preprogramed to undergo apoptosis in the absence of differentiation stimuli (19). Inhibition of differentiation-induced autophagy blocks monocyte-to-macrophage maturation and pushes monocytes into apoptosis, highlighting the fundamental need of differentiating monocytes for the prosurvival activity of autophagy.
The role of autophagy during HCMV infection remains complicated. Early studies showed that inhibition of autophagy was crucial to a productive viral infection, which is consistent with the known antiviral activity of autophagy (20). In support, autophagy agonists decrease virus production (21, 22). During lytic infection, HCMV encodes two specific inhibitors in order to suppress autophagy (20, 23). However, before inhibiting autophagosome maturation and formation, HCMV also directly stimulates autophagy during early infection (23, 24). The upregulation of early autophagy appears to be an important source of membrane for nascent virions, as HCMV-mediated arrest of autophagosomal maturation allows for an abundant supply of membrane for progeny virus (25). These studies suggest that whether autophagy has an anti- or proviral function during HCMV infection appears largely to be dependent on cell type. What role autophagy plays during a quiescent monocyte infection, where there is a delayed onset of replication, remains unknown.
In this study, we report that HCMV infection rapidly activated AMP kinase (AMPK), a positive regulator of autophagy, and stimulates the formation of autophagosomes in human primary monocytes. We hypothesized that increased autophagy was contributing to the survival of infected monocytes by inhibiting apoptosis. We found that inhibition of autophagy decreased survival of infected cells, while having minimal effect on uninfected cells. Surprisingly, the inhibition of autophagy did not lead to apoptotic cell death of infected monocytes. Instead, impeding autophagy resulted in cell death via necroptosis, a caspase-independent form of cell death. HCMV infection stimulated the upregulation of receptor-interacting protein kinase 3 (RIPK3), a key member in the necroptotic pathway, but had no effect on the activity of the executioner kinase mixed lineage kinase domain like pseudokinase (MLKL). However, MLKL became activated when autophagy was compromised, leading to death of infected monocytes that could be rescued through the inhibition of RIPK3 or MLKL. Together, these data demonstrate that HCMV upregulation of autophagy in primary monocytes prevents necroptosis; therefore, the induction of autophagy is essential to the viral persistence strategy.
RESULTS
HCMV rapidly stimulates autophagy within infected monocytes.
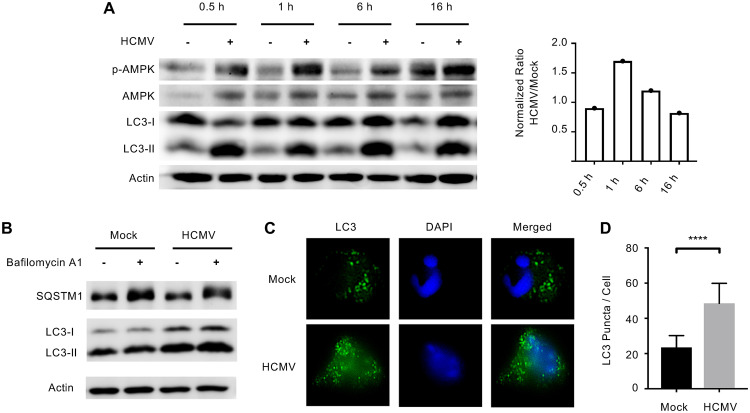
Autophagy is activated in response to cellular insults, such as nutrient deprivation and viral infections (26). The role of autophagy during HCMV infection is largely described during lytic infection of fibroblasts, where autophagy exhibits antiviral activity that is rapidly suppressed by several HCMV gene products (20–24). Here, we sought to elucidate the role of autophagy during quiescent infection of monocytes. To test whether autophagy is induced following HCMV infection of human primary monocytes, we first measured levels of activated AMPK, which induces autophagy when cellular levels of ATP are low. We found that AMPK was rapidly activated in infected monocytes at 30 min postinfection (mpi) and remained elevated through 16 h postinfection (hpi) compared to uninfected cells (Fig. 1A), suggesting the rapid induction of autophagy following HCMV infection. In support, we found LC3-II, a cytoplasmic protein that is lipidated and attached to autophagosomes during their maturation, was rapidly formed by 30 mpi and was maintained through 16 hpi (Fig. 1A). Further, we found that HCMV infection did not inhibit autophagic flux, as treatment with bafilomycin A1 increased levels of SQSTM1, an autophagosomal cargo protein degraded by autophagy, compared to baseline (Fig. 1B). Immunofluorescence analysis of LC3-II demonstrated a 117% increase in the number of puncta within HCMV-infected monocytes relative to mock-infected cells at 6 hpi (Fig. 1C and D), confirming the increased formation of autophagosomes. Taken together, these data indicate that HCMV rapidly stimulates AMPK activity shortly after infection to induce autophagosome formation without inhibiting autophagic flux.
FIG 1.
HCMV infection induces autophagy in monocytes. (A) Human peripheral blood monocytes were mock or HCMV infected for 30 min or 1, 6, or 16 h. Levels of phosphorylated AMPK (p-AMPK), total AMPK, LC3-I, LC3-II, or actin were detected by immunoblotting from whole-cell lysates. Levels of p-AMPK were normalized to actin and then total AMPK for each treatment and time point. The ratio of HCMV to mock of these values was then plotted for each time point (right). (B) Mock- or HCMV-infected monocytes were treated with 200 nM bafilomycin A1 for 6 h and the levels of SQSTM1 and LC3 were determined by immunoblot. (C and D) Monocytes were mock or HCMV infected for 6 h, followed by immunofluorescent analysis with anti-LC3 antibody (green) or DAPI (blue). Results are representative of at least 3 independent experiments using monocytes from different blood donors. (D) LC3 puncta per cell were counted using FIJI and the average puncta per cell were plotted with the mean and the 95% confidence interval (CI); ****, P < 0.0005.
Autophagy contributes to HCMV-induced survival.
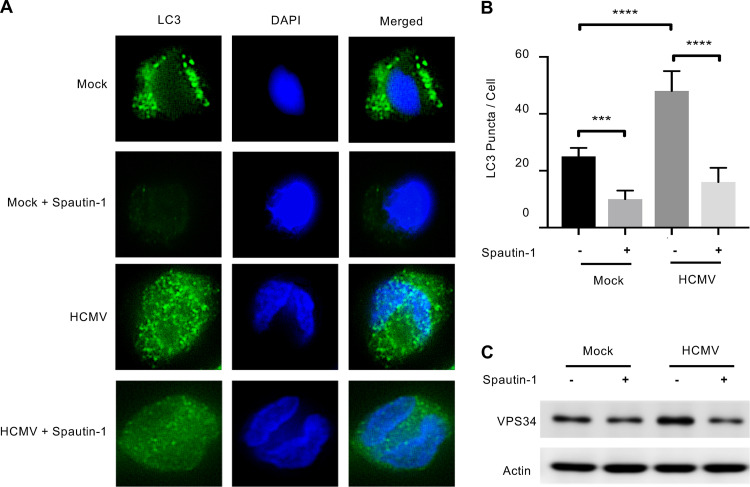
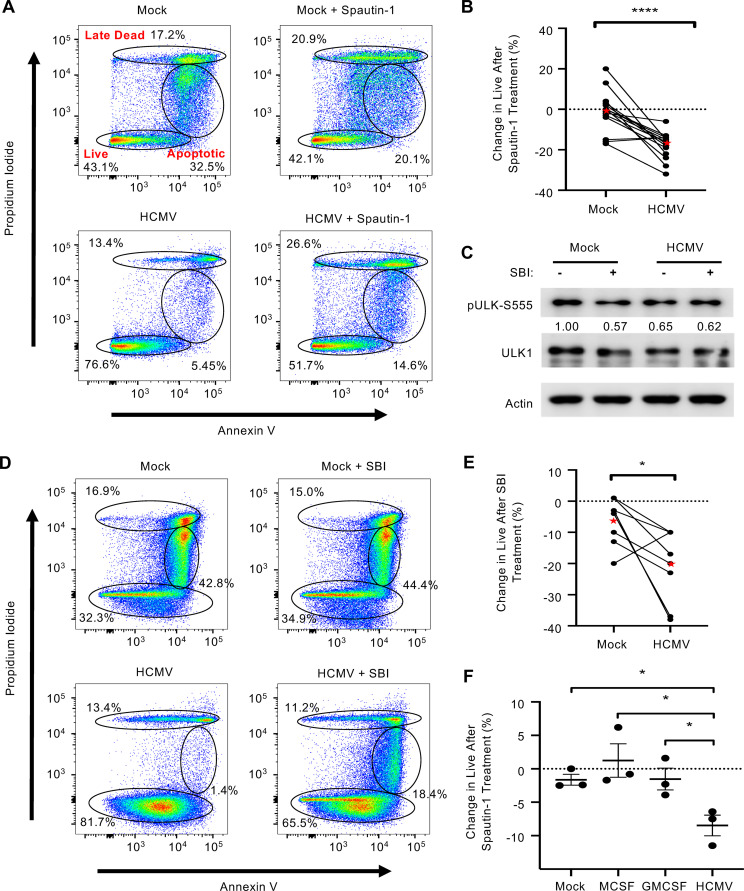
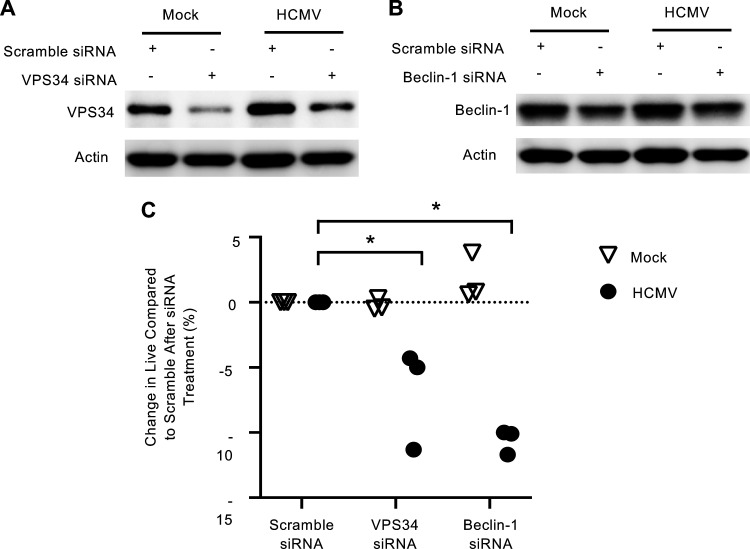
Autophagy is essential to the monocyte-to-macrophage differentiation process mediated by normal myeloid growth factors (19). In addition, autophagy regulates cellular survival during periods of stress through the recycling of cellular components and through cross talk with the apoptosis pathway (27). Thus, we asked whether autophagy was contributing to monocyte survival during early HCMV infection. A specific and potent inhibitor of autophagy (Spautin-1) inhibits the deubiquinating activity of USP10 and USP13, leading to the selective degradation of the class III P3K/Vps34 complex that controls autophagy initiation (28). Immunofluorescence of LC3 puncta showed Spautin-1 reduced autophagosome formation in infected monocytes to that of uninfected cells (Fig. 2A and B). We confirmed activity of Spautin-1 against the VPS34 complex by immunoblotting (Fig. 2C). Next, we examined if HCMV-induced autophagy was required for the survival of infected monocytes. Indeed, infected monocytes treated with Spautin-1 showed a 24.9% reduction in “live” cells concomitant with a 9% and 13.2% increase in “early apoptotic” and “late dead” cells, respectively (Fig. 3A). Importantly, Spautin-1 had no significant effect on uninfected monocytes, suggesting that autophagy-mediated survival was specific to infected monocytes. Despite donor variability inherent to primary blood monocytes, autophagy-induced survival of HCMV-infected monocytes was consistent over 15 independent blood donors, with an average decrease in survival of 18% in HCMV-infected cells and 1% in uninfected cells in the presence of the inhibitor (Fig. 3B). Although Spautin-1 is a highly selective autophagy inhibitor and shows limited off-target effects compared to other autophagy inhibitors, we utilized a second unique autophagy inhibitor to ensure that our results were not due to off-target effects (28, 29). SBI-0206965 (SBI) inhibits autophagy in an independent fashion from Spautin-1 via the suppression of ULK1 phosphorylation, a serine/threonine kinase that promotes autophagy (Fig. 3C). Treatment with SBI led to an average decrease in survival of 21% in HCMV-infected monocytes compared to a 6% decrease in survival in uninfected controls (Fig. 3D and E). To show that this effect was unique to HCMV infection, we also treated monocytes with myeloid growth factors MCSF or GMCSF before inhibition with Spautin-1. We found that inhibition of autophagy significantly reduced survival in HCMV-infected monocytes compared to both MCSF and GMSF, indicating that there is a distinct role of autophagy in promoting monocyte survival during viral infection (Fig. 3F). Because Spautin-1 and SBI inhibit autophagy through distinct mechanisms, their effects on monocyte survival is likely due to the inhibition of autophagy rather than an off-target effect. Importantly, in both cases, the respective inhibitors did not decrease uninfected cell viability substantially, indicating that autophagy-induced survival appears to be specific to HCMV-infected monocytes. We also undertook a complementary genetic approach using small interfering RNA (siRNA) knockdown of both VPS34 and Beclin-1, a key regulator of autophagy induction (Fig. 4A and B). Knockdown of either VPS34 or Beclin-1 decreased survival in HCMV-infected, but not mock-infected, monocytes, affirming the effects seen with both Spautin-1 and SBI are not likely due to off-target effects (Fig. 4C). These data demonstrate the central role of autophagy to the survival of HCMV-infected monocytes, but not uninfected or growth factor-treated cells.
FIG 2.
Spautin-1 reduces autophagosome formation in monocytes. (A) Human peripheral blood monocytes were pretreated with Spautin-1 for 1 h and then mock or HCMV infected for 4 h, followed by immunofluorescent analysis with anti-LC3 antibody (green) or DAPI (blue). Results are representative of at least 3 independent experiments using monocytes from different blood donors. (B) LC3 puncta per cell were counted using FIJI and the average puncta per cell were plotted with the median and the 95% CI; ****, P < 0.0005; ***, P < 0.005. (C) Levels of VPS34, a class III PI3K complex, were determined by immunoblotting in the presence or absence of Spautin-1 at 2 h posttreatment in monocytes.
FIG 3.
HCMV-induced autophagy stimulates survival of infected monocytes. (A and D) Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by treatment with either 50 μM Spautin-1, 10 μM SBI-0206965 (SBI), or vehicle control for an additional 24 h. Viability was measured by flow cytometry using propidium iodide (PI) and annexin V staining. Gates represent live, apoptotic, and late dead cells. (B, E, and F) Change in survival after the addition of Spautin-1 or SBI was quantified and plotted for mock- and HCMV-infected monocytes (B and E) or myeloid growth factors MCSF and GMCSF (F). Lines connect donor-matched data points from the same experiment. Red stars in B and E indicate the mean result for each group. Results in E are plotted as the mean ± SEM. (C) Levels of pULK1-S555 and ULK1 were determined by immunoblotting in the presence or absence of SBI in HCMV- or mock-infected monocytes after 6 h. All results are representative of at least 3 independent experiments using different donors; ****, P < 0.0005; *, P < 0.05.
FIG 4.
VPS34 and Beclin-1 knockdown reduces the survival of infected monocytes. (A to C) Mock- or HCMV-infected monocytes were transfected 24 h after infection with scramble, VPS34, or Beclin-1 siRNA for 48 additional hours as indicated. (A and B) Levels of VPS34 and Beclin-1 were determined by immunoblotting. Results are representative of at least 3 independent experiments. (C) Survival of monocytes was determined by annexin V and PI staining. Change in live cells relative to scramble siRNA treatment was quantified and plotted for 3 independent donors; *, P < 0.05.
Autophagy does not block apoptosis.
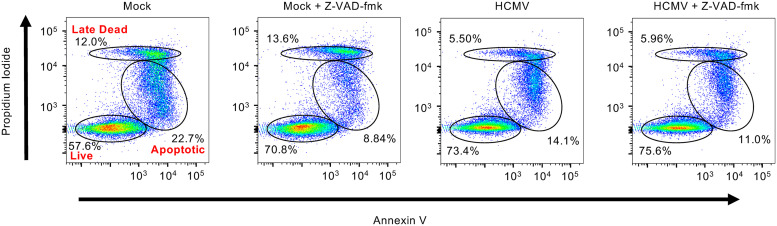
Monocytes are preprogramed to rapidly undergo apoptosis in the absence of a differentiation stimulus (30). In line with this, treatment of uninfected monocytes with Z-VAD-FMK, a pan-caspase inhibitor, increased live cells by 14% while simultaneously decreasing apoptotic cells by 14% (Fig. 5). In contrast, Z-VAD-FMK did not increase cell survival of HCMV-infected monocytes, which is consistent with our previous findings that HCMV infection stimulates the survival of monocytes by inhibiting apoptosis itself (31–33). There is extensive cross talk between apoptosis and autophagy, with several autophagy proteins able to directly repress apoptosis (18, 27, 34). As autophagy appears to be contributing to monocyte survival during HCMV infection, we investigated if autophagy was preventing caspase-mediated apoptosis. As expected, Z-VAD-FMK treatment resulted in an average decrease of 18% in apoptosis of uninfected monocytes treated with Spautin-1, which alone had no effect on the viability of uninfected cells (Fig. 3A and Fig. 6A), among 4 independent blood donors (Fig. 6A and B). Conversely, Z-VAD-FMK did not decrease the number of late dead cells of uninfected monocytes, suggesting commitment to apoptosis was beyond a rescuable level or that these cells may have undergone a different form of cell death. Surprisingly, we found that Z-VAD-FMK did not reverse Spautin-1-induced cell death of HCMV-infected monocytes, indicating autophagy was not functioning to inhibit apoptosis during infection (Fig. 6A and B). To ensure there were no off-target effects, the experiments were repeated using SBI. Similar to the results with Spautin-1, Z-VAD-FMK reduced apoptosis of SBI-treated uninfected monocytes but had no effect on SBI-treated HCMV-infected monocytes, confirming that autophagy was not blocking apoptosis (Fig. 6C). These data indicate that the inhibition of autophagy leads to a caspase-independent form of cell death in infected monocytes, while death of uninfected monocytes occurs via apoptosis.
FIG 5.
Uninfected monocytes rapidly undergo caspase-dependent apoptosis. Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by treatment with 5 μM Z-VAD-FMK or vehicle control for an additional 24 h. Viability was measured by flow cytometry using PI and annexin staining. Results are representative of at least 3 independent experiments using different blood donors.
FIG 6.
Autophagy does not protect HCMV-infected cells from apoptosis. (A) Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by treatment with 5 μM Z-VAD-FMK for 1 h and then either 50 μM Spautin-1 or vehicle control for an additional 24 h. Viability was measured by flow cytometry using PI and annexin V staining. Results are representative of 3 to 4 independent experiments using different blood donors. (B and C) Pooled results from 3 to 4 independent blood donors were plotted by percentage of cells in either the “apoptotic” or “late dead” gates following treatment with Spautin-1 or SBI with or without Z-VAD-FMK.
HCMV-induced autophagy inhibits necroptosis.
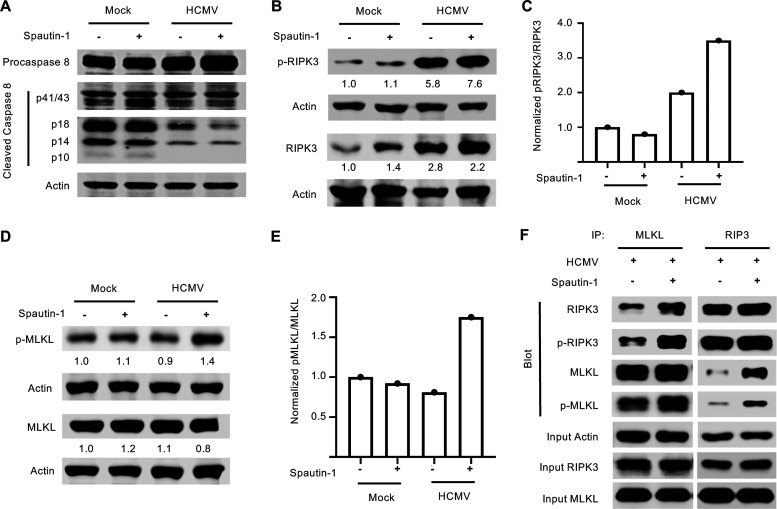
Necroptosis is a programmed form of necrosis independent of caspase activity (35, 36). Necroptosis is mediated through the sequential phosphorylation of RIPK1, RIPK3, and MLKL, which leads to poration of the cellular membrane (37). There are several triggers that can initiate necroptosis, including tumor necrosis factor receptor (TNFR), Toll-like receptors (TLRs), or cytoplasmic nucleic acid sensor DAI/ZBP1 (38–40). Regardless of the trigger, caspase 8 cleavage must be inhibited for necroptosis to proceed, thus it is often referred to as a “trap door” death pathway (35, 36). We found that HCMV reduced the cleavage/activation of procaspase 8 through 48 hpi, while caspase 8 was rapidly activated in uninfected monocytes, consistent with their naturally short life span (Fig. 7A). The prolonged inhibition of caspase 8 suggested that the necroptosis pathway could be activated in HCMV-infected monocytes.
FIG 7.
Inhibition of autophagy leads to activation of the necroptotic pathway in HCMV-infected monocytes. (A, B, and D) Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by treatment with 50 μM Spautin-1 or vehicle control for 1 h. Levels of procaspase 8, caspase 8, total RIPK3, p-RIPK3, total MLKL, p-MLKL, and actin were detected by immunoblotting from whole-cell lysates. (C and E) The normalized ratios of p-RIPK3/RIPK3 and p-MLKL/MLKL, respectively, were quantified and plotted. (F) Monocytes were HCMV infected for 24 h, then treated with Spautin-1 or vehicle control for 2 h and immunoprecipitated with antibodies recognizing MLKL or RIP3. Western blot analysis was performed to determine the presence of total RIPK3, p-RIPK3, total MLKL, and p-MLKL in the immunoprecipitated samples. Input controls were blotted for actin to confirm homogeneous loading of the samples. All results are representative of at least 3 independent experiments using different blood donors.
We next examined RIPK3 expression and found total protein expression increased by 24 hpi in infected monocytes compared to uninfected cells (Fig. 7B). Furthermore, RIPK3 was phosphorylated at 24 hpi in HCMV-infected cells, indicating that the first steps of the necroptosis pathway were activated during HCMV infection (Fig. 7B and C). However, MLKL, the “executioner” kinase of the necroptotic pathway, was not phosphorylated above mock levels in HCMV-infected monocytes despite RIPK3 phosphorylation, suggesting a block between RIPK3 phosphorylation and MLKL phosphorylation (Fig. 7D). To test if autophagy was mediating the block between RIPK3 and MLKL, monocytes were treated with Spautin-1 and examined for MLKL activation. Indeed, MLKL phosphorylation was increased in HCMV-infected monocytes at 24 hpi when treated with Spautin-1 (Fig. 7D and E). There also appeared to be an increase in the proportion of phosphorylated RIPK3 (p-RIPK3) to total RIPK3 in infected, but not control, monocytes upon treatment with Spautin-1 (Fig. 7C). Because MLKL is a direct target of RIPK3, these data suggest that either MLKL, RIPK3, or both are being sequestered away or degraded by autophagosomes, thereby preventing their interaction. In support, we found enhanced protein-protein interaction between RIPK3 and MLKL in the presence of Spautin-1 (Fig. 7F). Specifically, we observed increased coimmunoprecipitation (co-IP) of p-RIPK3 with MLKL in infected monocytes, regardless of whether the complex was immunoprecipitated by RIPK3 or MLKL antibodies, in the presence of autophagy inhibition (Fig. 7F). Taken together, these data suggest that the viral induction of autophagy short circuits the necroptotic death pathway within HCMV-infected monocytes.
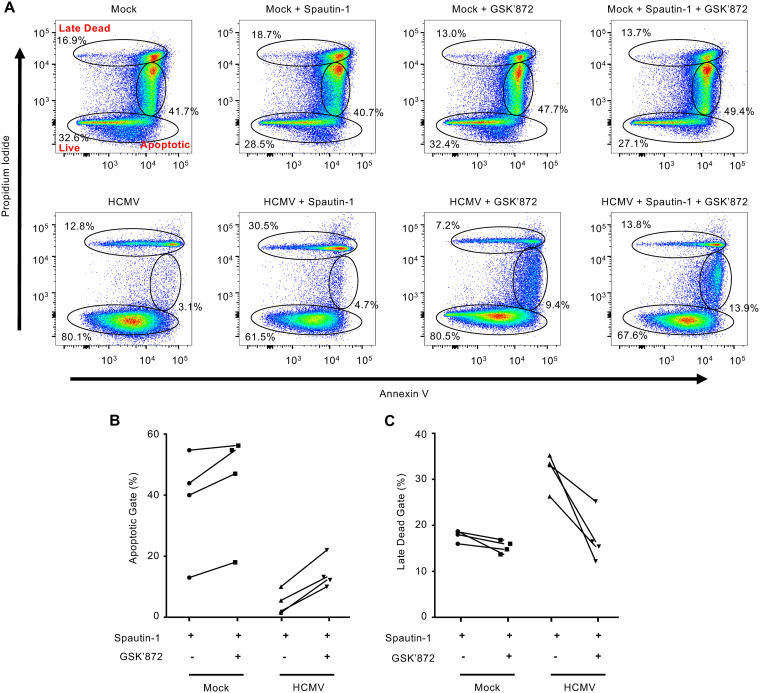
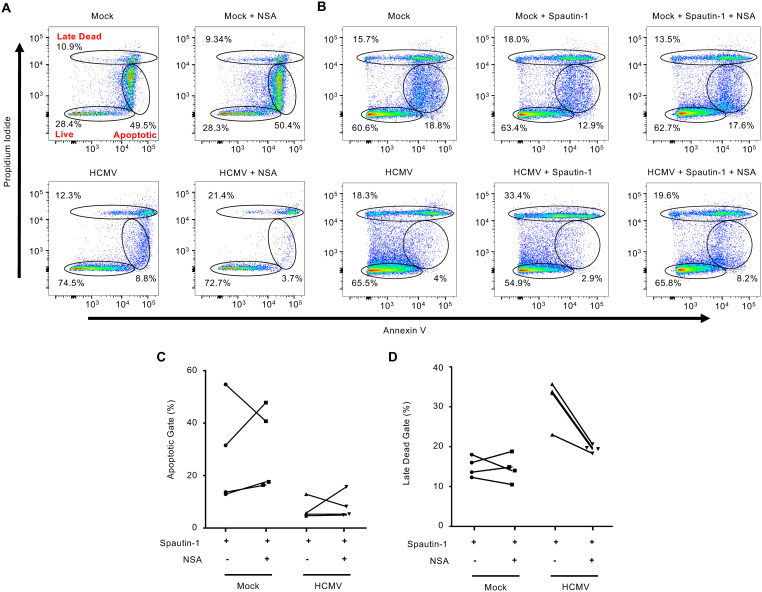
Since RIPK3 is upregulated and activated during HCMV infection, we next examined if inhibiting RIPK3 could rescue survival of autophagy-inhibited infected monocytes. Following treatment with GSK’872, a highly selective RIPK3 inhibitor, we examined the frequency of cells found in the late dead gate as cells undergoing necroptosis could be either PIhigh/Annexinhigh or PIhigh/Annexinlow, in contrast to PIlow/Annexinhigh early apoptotic cells. GSK’872 treatment of Spautin-1-treated HCMV-infected monocytes reduced the percentage of late dead cells by 17%, back to levels exhibited by HCMV-infected monocytes, while having no effect on reducing the frequency of early apoptotic cells (Fig. 8A). These changes were consistent over 4 independent blood donors (Fig. 8B and C). The reversal of the levels of late dead cells back to that of untreated HCMV-infected monocytes demonstrates a RIPK3-dependent cell death pathway is being initiated upon inhibition of autophagy. To determine if the RIPK3-dependent cell death pathway inhibited by autophagy was necroptosis, we blocked MLKL activity using necrosulfonamide (NSA), which alone had no effect on the cell viability of mock- or HCMV-infected monocytes (Fig. 9A). As expected, NSA did not affect the levels of cells undergoing early apoptosis (Fig. 9B). However, NSA reduced the levels of late dead Spautin-1-treated cells to similar levels as HCMV-infected monocytes while simultaneously increasing the number of live cells (Fig. 9B and D), consistent with autophagy blocking necroptosis of infected monocytes. Overall, these data indicate that HCMV infection triggers necroptosis within infected monocytes, but is stalled by the simultaneous viral-mediated initiation of autophagy.
FIG 8.
HCMV-induced autophagy prevents RIPK3-dependent monocyte cell death. (A) Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by either treatment with 5 μM GSK’872 or cotreatment with GSK’872 and 50 μM Spautin-1 or vehicle control for an additional 24 h. Viability was measured by flow cytometry using PI and annexin staining. Results are representative of 4 independent experiments using different donors. (B and C) Pooled results from 4 independent blood donors were plotted by percentage of cells in either the “apoptotic” or “late dead” gates following treatment with Spautin-1 with or without GSK’872.
FIG 9.
MLKL-dependent cell death is initiated upon inhibition of autophagy in HCMV-infected monocytes. (A and B) Human peripheral blood monocytes were mock or HCMV infected for 24 h, followed by either treatment with 0.5 μM necrosulfonamide (NSA) or cotreatment with NSA and Spautin-1 or vehicle control for an additional 24 h. Viability was measured by flow cytometry using PI and annexin staining. All experiments are representative of 4 independent experiments using different donors. (C and D) Pooled results from 4 independent blood donors were plotted by percentage of cells in either the “apoptotic” or “late dead” gates following treatment with Spautin-1 with or without NSA.
DISCUSSION
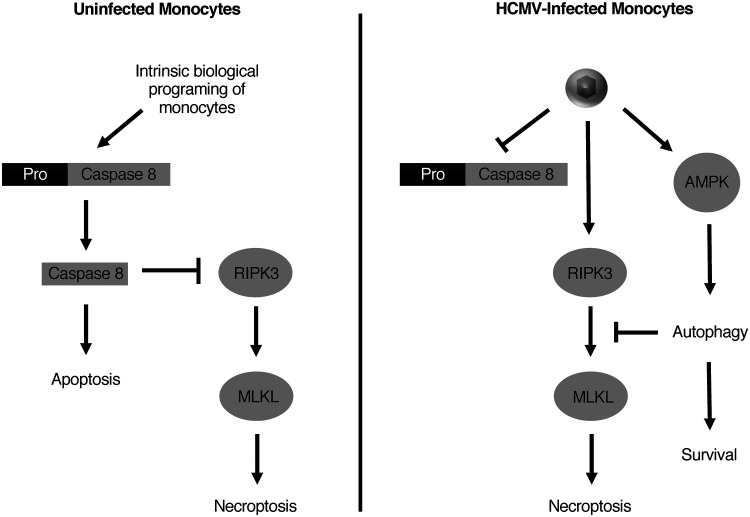
Peripheral blood monocytes are a primary target of HCMV in vivo and are believed to be responsible for the hematogenous dissemination of the virus to distant organ systems and bone marrow (7, 8). As monocytes have a naturally limited life span of 48 h in circulation and are nonpermissive for viral replication, these blood cells seem ill-suited to mediate viral dissemination (14). However, HCMV stimulates the survival of infected monocytes to ensure their differentiation into virus replication-permissive macrophages (31, 32, 41–44). The molecular processes responsible for mediating HCMV-induced monocyte-to-macrophage differentiation are not completely understood. In the current study, we demonstrate that the early induction of autophagy following HCMV infection of monocytes is required to circumvent the intrinsic biological programming of monocytes to undergo cell death, which is consistent with growth factor-induced late autophagy being also essential for monocyte-to-macrophage differentiation (19). However, although inhibition of growth factor-induced autophagy leads to apoptosis, we found that inhibition of early HCMV-induced autophagy led to death of infected monocytes via necroptosis, a caspase-independent form of cell death (19). Our data demonstrated that HCMV infection initiated the early steps of the necroptotic pathway but that the concurrent induction of autophagy prevented the final execution of necroptosis by blocking the activation of MLKL (Fig. 10).
FIG 10.
Proposed model for HCMV regulation of autophagy and survival. Upon entering circulation from the bone marrow, peripheral blood monocytes undergo apoptosis within 48 h in the absence of myeloid differentiation factors. In addition to the intrinsic proapoptotic programing of monocytes, HCMV infection triggers host antiviral self-death pathways resulting in an exceedingly proapoptotic environment within the infected cell. To circumvent apoptosis, HCMV induces the rapid upregulation of several cellular prosurvival proteins that leads to a persistent inactivation of caspase 8 (31, 32, 41, 43, 44). Consequently, necroptosis is triggered as a “trap door” mechanism of cell death within infected monocytes. However, the concomitant activation of AMPK by HCMV leads to the upregulation of autophagosomal production and maturation that then blocks necroptotic cell death prior to MLKL activation.
As many pathogens encode anti-apoptotic proteins, necroptosis likely evolved as a secondary cellular defense mechanism against infections. Necroptosis is a programmed form of necrosis that is canonically activated by death signals in the absence of caspase 8 activity (37, 45). In monocytes, HCMV induces host anti-apoptotic proteins to block apoptosis (32, 41, 44, 46), which leads to a sustained inhibition of caspase 8, enabling the initiation of necroptosis. However, the necroptosis pathway must also be triggered by means additional to the absence of caspase 8 activity. How HCMV activates the necroptosis pathway in monocytes is unclear. TNF-α is considered the canonical activator of necroptosis and requires both RIPK3 and RIPK1 (47–49). HCMV stimulates the production of TNF-α following infection of monocytes (50), suggesting TNF-α may be responsible for stimulating necroptosis. Alternatively, both MCMV and HCMV have also been shown to stimulate DAI/ZBP1, a cytoplasmic nucleic acid detector able to directly activate RIPK3 in the absence of RIP1 activity (40, 51, 52). Although inhibition of RIPK3 completely reversed the levels of late dead HCMV-infected monocytes when autophagy was blocked, we cannot exclude the possibility of both canonical and noncanonical pathways being initiated during HCMV infection of monocytes. We are currently investigating the mechanism by which HCMV stimulates the necroptotic pathway during infection of monocytes. Regardless, it seems clear that HCMV triggers necroptosis in monocytes, which is simultaneously blocked by infection at the step prior to MLKL activation.
The mechanism used by HCMV to impede necroptosis appears to be dependent on cell type and whether HCMV establishes a lytic or latent/quiescent infection. The MCMV-encoded anti-necroptotic protein, M45, contains a RIP homotypic interacting motif (RHIM) that allows for direct binding to and inhibition of both RIPK1 and RIPK3 (53, 54). Although HCMV encodes an M45 homolog, the absence of an RHIM domain suggests an inability to block necroptosis (55, 56). Indeed, to date the HCMV M45 homolog has yet to be shown to exhibit anti-necroptosis activity. Instead, an IE1-regulated gene product is responsible for blocking necroptosis in lytic infected fibroblasts ectopically expressing components of the necroptotic machinery (57). Inhibition was not at the level of RIPK1/RIPK3 during MCMV infection, but rather downstream of MLKL. Very recently, pUL36, which inhibits caspase 8 in productive HCMV infection, was shown to inhibit necroptosis through degradation of MLKL (58). In primary monocytes, however, our previous work has established that there is no expression of pUL36 through 72 h of infection (41). The lack of viral gene expression of pUL36 during HCMV infection of monocytes indicates a distinct regulatory mechanism of necroptosis control independent of a viral gene product (8, 41). Inhibition of caspase 8 activation, required for activation of necroptosis, may be controlled by cellular anti-apoptotic proteins like cFLIP in the absence of pUL36, as HCMV has been shown to control many of these anti-apoptotic proteins through manipulation of cellular signaling. The novel finding that pUL36 inhibits necroptosis through degradation of MLKL raises the possibility that the virus achieves the same result using a parallel pathway in autophagy in the absence of pUL36 expression. In support of this, virally induced upregulation of autophagy interrupted necroptosis between RIPK3 phosphorylation and MLKL activation, as HCMV infection inhibited MLKL phosphorylation despite RIPK3 activation. Selective degradation of proteins through autophagy is an emerging field, and some reports have described MCMV-mediated selective degradation of cellular proteins to be detrimental to viral infection (59). Additionally, the literature of cross talk between autophagy and necroptosis continues to strengthen, with Beclin-1 having been recently shown to be a negative modulator of MLKL oligomerization (60). However, we did not observe a similar interaction in our system, as Beclin-1 did not co-IP with either RIPK3 or MLKL (data not shown), though its downregulation did decrease survival in infected monocytes, likely through its function in the autophagy pathway (Fig. 4). Finally, it has been recently reported that autophagy is critical for turnover of RHIM-domain proteins, including RIPK3, and that inhibition of autophagy enhanced necroptotic death upon stimulation by either TNF or TLRs (61). While these specific mechanisms for interaction between necroptosis and autophagy in monocytes remain undetermined, our data suggest that autophagy plays an important role is sequestering MLKL from RIPK3 in order to prevent the execution of necroptosis during a latent or quiescent infection. Further studies will be necessary to elucidate the exact mechanism of control that autophagy exerts over necroptosis in infected monocytes.
It appears that several herpesviruses have convergently evolved mechanisms to modulate autophagy during infection in order to promote the survival of infected cells. Early studies believed that autophagy would be a strictly antiviral immune response, with activation of TLRs leading to envelopment and degradation of cytoplasmic foreign virions. Epstein-Barr virus (EBV), herpes simplex type I (HSV-I), and Kaposi’s sarcoma associated herpesvirus (KSHV) have been shown to inhibit autophagy in order to promote viral replication and egress (62–64). Autophagy during lytic HCMV infection exhibits antiviral activity, as induction of autophagy reduces viral replication and virion release (20–22). In support of this, HCMV encodes a number of anti-autophagic proteins expressed during lytic infection (20, 23, 25). In contrast, our results demonstrate that autophagy serves as a proviral pathway during the early stages of a quiescent infection by promoting the survival of infected monocytes. Additionally, autophagy is essential for the monocyte-to-macrophage differentiation process induced by normal myeloid growth factors (19). As HCMV has been shown to concurrently drive survival and differentiation of infected monocytes, autophagy may serve dual roles by stimulating cellular survival during early infection and transitioning to a prodifferentiation role during a prolonged infection (65). Given that HCMV-infected myeloid cells have been recently described as a heterogeneous population using next-generation sequencing, there may be differing sensitivities to the inhibition of autophagy on survival and differentiation, depending on the chronological age of both cell and virus (66, 67). This heterogeneity in both population and timing may explain why it appears that only particular subsets of HCMV-infected monocytes are particularly sensitive to autophagy inhibition as it relates to survival. Future studies will be required to characterize these interactions in depth.
In summary, we demonstrate that HCMV rapidly induces autophagy in infected monocytes to prevent the activation of necroptosis and subsequent cellular death. This protective effect of autophagy, acting as a shunt against necroptosis, appears to be HCMV specific, as uninfected cells showed no decrease in survival when treated with autophagy inhibitors nor a rescuing of survival when cotreated with necroptosis inhibitors. In contrast, death of short-lived uninfected monocytes was prevented by apoptosis inhibitors. Additionally, autophagy inhibitors have no effect on the viability of growth factor-treated monocytes. These data highlight the possibility that identifying the molecular mechanisms by which HCMV-induced autophagy opposes necroptosis could provide new pharmaceutical targets to selectively eliminate infected cells, thereby preventing viral dissemination in immunocompromised patients at high risk for acquiring a new infection.
MATERIALS AND METHODS
Human peripheral blood monocyte isolation and culture.
Isolation of human peripheral blood monocytes was performed as previously described (8, 41, 68). Briefly, blood was drawn from random donors by venipuncture, diluted in RPMI 1640 media (ATCC, Manassas, VA), and centrifuged through Histopaque 1077 cell separation medium (Sigma-Aldrich) to remove red blood cells and neutrophils. Mononuclear cells were collected and washed with saline to remove the platelets and then separated by centrifugation through a Percoll (GE Healthcare, Wilkes-Barre, PA) gradient (40.48% and 47.7%). More than 95% of isolated peripheral blood mononuclear cells were monocytes, as determined by CD14+ or CD16+ staining. The cells were washed with saline, resuspended in RPMI 1640 medium, supplemented with 0.5% human type AB serum (Lonza) and counted. All experiments were performed in 0.5% to 1% human serum at 37°C in a 5% CO2 incubator, unless otherwise stated. SUNY Upstate Medical University Institutional Review Board and Health Insurance Portability and Accountability Act guidelines for the use of human subjects were followed for all experimental protocols in our study. For the inhibitor studies, the following reagents were used: Spautin-1 (a USP10 and USP13 inhibitor), SBI-0206965 (SBI; a ULK1 inhibitor), Z-VAD-FMK (Z-VAD; a pan-caspase inhibitor), necrosulfonamide (NSA, an MLKL inhibitor), and GSK’872 (an RIPK3 inhibitor) from Selleckchem (Houston, TX). Inhibitors were titrated to the highest concentration that did not result in downregulation of uninfected monocyte survival via flow cytometry.
Virus preparation and infection.
Human embryonic lung (HEL) 299 fibroblasts (CCL-137; American Type Culture Collection, Manassas, VA) were cultured in Dulbecco modified Eagle medium (DMEM) (Lonza) with 2.5 μg/ml Plasmocin (Invivogen, San Diego, CA) and 10% fetal bovine serum (FBS) (Sigma). When the culture reached confluence, the cells were infected with HCMV (strain TB40/E) in DMEM supplemented with 4% FBS. Virus was purified from the supernatant on a 20% sorbitol cushion to remove cellular contaminants and resuspended in RPMI 1640 medium. A multiplicity of infection (MOI) of 5 was used for each experiment, as >99% of monocytes were infected. Mock infection was performed by adding an equivalent volume of RPMI 1640 medium to monocytes. UV-inactivated virus was prepared by incubating virus under a 30W germicidal (UV type C wavelength, 254 nm) UV lamp (G30 T8; GE Lighting, East Cleveland, OH) for 40 min on ice, and was used in the same manner as “live” virus. The UV-inactivated virus did not replicate or produce any detectable levels of immediate early (IE) gene products.
Transient transfection and RNA Silencing.
Primary monocytes (3 × 106/transfection) were washed with phosphate-buffered saline (PBS) and resuspended in 100 μl of P3 Primary Cell Nucleofector solution (Lonza) containing either 500 nM validated Silencer Select siRNAs against Vps34, Beclin-1, or Silencer negative-control siRNAs (Ambion-Thermo Fisher Scientific, Carlsbad, CA) and transfected with a 4D-Nucleofector system using program EI-100. Following transfection, monocytes were incubated in RPMI 1640 supplemented with 2% human AB serum at 37°C and allowed to recover for 4 h. Following the 4-h recovery period, transfected monocytes were then mock- or HCMV-infected for 48 h and subjected to immunoblot or flow cytometry analysis.
Western blot analysis.
Monocytes were harvested in a modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 100 mM NaCl, 1% Triton X-100, 0.1% SDS, 10% glycerol) supplemented with protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktails 2 and 3 (Sigma) for 15 min on ice. The lysates were cleared from the cell debris by centrifugation at 4°C (5 min, 21,130 × g) and stored at –20°C until further analysis. Protein samples were solubilized in Laemmli SDS-sample nonreducing (6×) buffer (Boston Bioproducts, Boston, MA) supplemented with β-mercaptoethanol (Amresco, Solon, OH) by incubating at 100°C for 10 min. Equal amounts of total protein from each sample were loaded in each well, separated by SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Blots were blocked in 5% bovine serum albumin (BSA) (Fisher Scientific, Waltham, MA) for 1 h at room temperature (RT) and then incubated with primary antibodies overnight at 4°C. The following antibodies were purchased: anti-LC3, anti-AMPK, anti-phospho (p)-AMPK, anti-VPS34, anti-Beclin-1, anti-ULK1, anti-pULK1-S555, anti-RIPK3, anti-p-RIPK3, anti-MLKL, and anti-p-MLKL antibodies were from Cell Signaling (Danvers, MA). The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling), and chemiluminescence was detected using the Amersham ECL Prime Western Blotting Detection reagent (GE Healthcare).
Flow cytometry.
Monocytes were washed in phosphate-buffered saline (PBS) and incubated in a blocking solution consisting of fluorescence-activated cell sorting buffer, 5% BSA, and human FcR binding inhibitor (eBioscience, San Diego, CA), followed by staining with an allophycocyanin (APC)-anti-CD14 or APC-anti-mouse IgG1 isotype control antibody (BioLegend, San Diego, CA) on ice. The cells were then washed and stained with fluorescein isothiocyanate (FITC)-annexin V (Life Technologies, Carlsbad, CA) and propidium iodide stain (Life Technologies) to detect dead and dying cells. After staining, the cells were analyzed by flow cytometry using an LSRFortessa cell analyzer and BD FACSDiva software (BD Biosciences, Franklin Lakes, NJ). Our gating strategy on forward scatter (FSC)/side scatter (SSC) was set to include both cells in the early stages of apoptosis (decreased FSC and increased SSC compared to those for viable cells) and cells in the late stages of apoptosis (decreased FSC and decreased SSC compared to those of viable cells).
Immunofluorescence.
Cell monolayers prepared on glass coverslips were fixed for 15 min in 4% paraformaldehyde (PFA) (Sigma) in PBS, and then washed twice with PBS. Cell permeabilization was performed by incubating the coverslips with 1% Triton X-100 in PBS for 30 min at 37°C followed by a 10 min incubation at RT. Nonspecific binding was blocked with a 5% BSA, 0.1% Triton X-100 solution. Slides were then incubated overnight at 4°C in a humidified chamber with an anti-LC3 antibody conjugated to Alexa Fluor 488 (Cell Signaling). Coverslips were mounted with ProLong Gold Antifade with DAPI (4′,6-diamidino-2-phenylindole) (Thermo Fisher) before being analyzed on a Nikon Eclipse ti80 epifluorescence microscope (Nikon, Melville, NY). Digitized images were resized, organized, and labeled using ImageJ software, an open Java-based image processing program developed at the National Institutes of Health. Puncta per cell were counted from at least 30 unique cells per donor per treatment. The mean puncta per cell was then calculated.
Statistical Analysis.
All experiments were performed independently a minimum of 3 times using primary monocytes isolated from different blood donors. Survival data sets obtained from primary monocytes inherently have substantial variation due to donor variability. Consequently, data are displayed as matched experimental data points from individual donors in a side-by-side comparison. Displaying a side-by-side comparison allows for consistent trends between the different donors to be identified that may otherwise be missed when presenting only the mean and that may not be statistically significant given the high number of donors needed to achieve significance on smaller changes. Nonetheless, data were analyzed using a two-way Student’s t test comparison with GraphPad Prism software. In experiments determining LC3 puncta, data were expressed as the mean ± the standard error of the mean (SEM) and were analyzed with Prism software (GraphPad) by using a two-way Student’s t test comparison. P values of less than 0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Christine Burrer in the Department of Microbiology and Immunology at SUNY Upstate Medical University for technical support, maintenance of lab operations, and assistance with virus growth and isolation.
This work was supported by grants from the Carol M. Baldwin Breast Cancer Research Fund to G.C.C., National Institute of Allergy and Infectious Diseases (R01AI141460) to G.C.C., and the National Heart, Lung, and Blood Institute (R01HL139824) to G.C.C.
REFERENCES
- 1.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 2.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eid AJ, Razonable RR. 2010. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs 70:965–981. doi: 10.2165/10898540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Emery VC. 1999. Viral dynamics during active cytomegalovirus infection and pathology. Intervirology 42:405–411. doi: 10.1159/000053978. [DOI] [PubMed] [Google Scholar]
- 5.Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, Sgarabotto D, Torre-Cisneros J, Uknis ME. 2007. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant 21:149–158. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 6.Ho M. 2008. The history of cytomegalovirus and its diseases. Med Microbiol Immunol 197:65–73. doi: 10.1007/s00430-007-0066-x. [DOI] [PubMed] [Google Scholar]
- 7.Chan G, Nogalski MT, Stevenson EV, Yurochko AD. 2012. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination. J Leukoc Biol 92:743–752. doi: 10.1189/jlb.0112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MS, Bentz GL, Alexander JS, Yurochko AD. 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol 78:4444–4453. doi: 10.1128/jvi.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson SM, Shepp DH, Match ME, Axelrod FB, Whitbread JA. 2001. Cytomegalovirus infectivity in whole blood following leukocyte reduction by filtration. Am J Clin Pathol 116:52–55. doi: 10.1309/PVFR-DDWE-302T-WFA1. [DOI] [PubMed] [Google Scholar]
- 10.Bale JF Jr, O'Neil ME. 1989. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J Virol 63:2667–2673. doi: 10.1128/JVI.63.6.2667-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins TM, Quirk MR, Jordan MC. 1994. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol 68:6305–6311. doi: 10.1128/JVI.68.10.6305-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES. 2014. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe 15:351–362. doi: 10.1016/j.chom.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, Nelson JA. 2010. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe 8:284–291. doi: 10.1016/j.chom.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitelaw DM. 1966. The intravascular lifespan of monocytes. Blood 28:455–464. doi: 10.1182/blood.V28.3.455.455. [DOI] [PubMed] [Google Scholar]
- 15.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. 2017. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair J, Sissons P. 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39:293–301. doi: 10.1159/000150501. [DOI] [PubMed] [Google Scholar]
- 17.Sinzger C, Eberhardt K, Cavignac Y, Weinstock C, Kessler T, Jahn G, Davignon JL. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J Gen Virol 87:1853–1862. doi: 10.1099/vir.0.81595-0. [DOI] [PubMed] [Google Scholar]
- 18.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. 2013. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG. 2012. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 119:2895–2905. doi: 10.1182/blood-2011-08-372383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaumorcel M, Lussignol M, Mouna L, Cavignac Y, Fahie K, Cotte-Laffitte J, Geballe A, Brune W, Beau I, Codogno P, Esclatine A. 2012. The human cytomegalovirus protein TRS1 inhibits autophagy via its interaction with Beclin 1. J Virol 86:2571–2584. doi: 10.1128/JVI.05746-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belzile JP, Sabalza M, Craig M, Clark E, Morello CS, Spector DH. 2016. Trehalose, an mTOR-independent inducer of autophagy, inhibits human cytomegalovirus infection in multiple cell types. J Virol 90:1259–1277. doi: 10.1128/JVI.02651-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay R, Venkatadri R, Katsnelson J, Arav-Boger R. 2018. Digitoxin suppresses human cytomegalovirus replication via Na+, K+/ATPase alpha1 subunit-dependent AMP-activated protein kinase and autophagy activation. J Virol 92:e01861-17. doi: 10.1128/JVI.01861-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouna L, Hernandez E, Bonte D, Brost R, Amazit L, Delgui LR, Brune W, Geballe AP, Beau I, Esclatine A. 2016. Analysis of the role of autophagy inhibition by two complementary human cytomegalovirus BECN1/Beclin 1-binding proteins. Autophagy 12:327–342. doi: 10.1080/15548627.2015.1125071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarlane S, Aitken J, Sutherland JS, Nicholl MJ, Preston VG, Preston CM. 2011. Early induction of autophagy in human fibroblasts after infection with human cytomegalovirus or herpes simplex virus 1. J Virol 85:4212–4221. doi: 10.1128/JVI.02435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taisne C, Lussignol M, Hernandez E, Moris A, Mouna L, Esclatine A. 2019. Human cytomegalovirus hijacks the autophagic machinery and LC3 homologs in order to optimize cytoplasmic envelopment of mature infectious particles. Sci Rep 9:4560. doi: 10.1038/s41598-019-41029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikic I, Elazar Z. 2018. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Kang J, Fu C. 2018. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther 3:18. doi: 10.1038/s41392-018-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao S, Li S, Qin Y, Wang X, Yang Y, Bai H, Zhou L, Zhao C, Wang C. 2014. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol 44:1661–1668. doi: 10.3892/ijo.2014.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Li Y, Hao Y, Choi A, Ke H, Ma D, Yuan J. 2011. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitelaw DM. 1972. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 5:311–317. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 31.Cojohari O, Peppenelli MA, Chan GC. 2016. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. J Virol 90:6443–6452. doi: 10.1128/JVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peppenelli MA, Arend KC, Cojohari O, Moorman NJ, Chan GC. 2016. Human cytomegalovirus stimulates the synthesis of select Akt-dependent antiapoptotic proteins during viral entry to promote survival of infected monocytes. J Virol 90:3138–3147. doi: 10.1128/JVI.02879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cojohari O, Burrer CM, Peppenelli MA, Abulwerdi FA, Nikolovska-Coleska Z, Chan GC. 2015. BH3 profiling reveals selectivity by herpesviruses for specific Bcl-2 proteins to mediate survival of latently infected cells. J Virol 89:5739–5746. doi: 10.1128/JVI.00236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD, Thorburn A. 2016. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev Cell 37:337–349. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galluzzi L, Kepp O, Chan FK, Kroemer G. 2017. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol 12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinlich R, Oberst A, Beere HM, Green DR. 2017. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C. 2011. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol 195:931–942. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upton JW, Kaiser WJ, Mocarski ES. 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD. 2010. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol 184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan G, Nogalski MT, Yurochko AD. 2012. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J Virol 86:10714–10723. doi: 10.1128/JVI.07129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peppenelli MA, Miller MJ, Altman AM, Cojohari O, Chan GC. 2018. Aberrant regulation of the Akt signaling network by human cytomegalovirus allows for targeting of infected monocytes. Antiviral Res 158:13–24. doi: 10.1016/j.antiviral.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins-McMillen D, Kim JH, Nogalski MT, Stevenson EV, Chan GC, Caskey JR, Cieply SJ, Yurochko AD. 2015. Human cytomegalovirus promotes survival of infected monocytes via a distinct temporal regulation of cellular Bcl-2 family proteins. J Virol 90:2356–2371. doi: 10.1128/JVI.01994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. 2016. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reeves MB, Breidenstein A, Compton T. 2012. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proc Natl Acad Sci U S A 109:588–593. doi: 10.1073/pnas.1114966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. 2012. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. 2014. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriwaki K, Chan FK. 2013. RIP3: a molecular switch for necrosis and inflammation. Genes Dev 27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noriega VM, Haye KK, Kraus TA, Kowalsky SR, Ge Y, Moran TM, Tortorella D. 2014. Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro. J Virol 88:9391–9405. doi: 10.1128/JVI.00934-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. 2010. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol 84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeFilippis VR, Sali T, Alvarado D, White L, Bresnahan W, Fruh KJ. 2010. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J Virol 84:8913–8925. doi: 10.1128/JVI.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upton JW, Kaiser WJ, Mocarski ES. 2008. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem 283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hahn G, Khan H, Baldanti F, Koszinowski UH, Revello MG, Gerna G. 2002. The human cytomegalovirus ribonucleotide reductase homolog UL45 is dispensable for growth in endothelial cells, as determined by a BAC-cloned clinical isolate of human cytomegalovirus with preserved wild-type characteristics. J Virol 76:9551–9555. doi: 10.1128/jvi.76.18.9551-9555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patrone M, Percivalle E, Secchi M, Fiorina L, Pedrali-Noy G, Zoppe M, Baldanti F, Hahn G, Koszinowski UH, Milanesi G, Gallina A. 2003. The human cytomegalovirus UL45 gene product is a late, virion-associated protein and influences virus growth at low multiplicities of infection. J Gen Virol 84:3359–3370. doi: 10.1099/vir.0.19452-0. [DOI] [PubMed] [Google Scholar]
- 57.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES. 2015. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem 290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher-Etherington A, Nobre L, Nightingale K, Antrobus R, Nichols J, Davison AJ, Stanton RJ, Weekes MP. 2020. Human cytomegalovirus protein pUL36: a dual cell death pathway inhibitor. Proc Natl Acad Sci U S A 117:18771–18779. doi: 10.1073/pnas.2001887117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fliss PM, Jowers TP, Brinkmann MM, Holstermann B, Mack C, Dickinson P, Hohenberg H, Ghazal P, Brune W. 2012. Viral mediated redirection of NEMO/IKKgamma to autophagosomes curtails the inflammatory cascade. PLoS Pathog 8:e1002517. doi: 10.1371/journal.ppat.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo J, Seong D, Nam YW, Hwang CH, Lee SR, Lee CS, Jin Y, Lee HW, Oh DB, Vandenabeele P, Song J. 2020. Beclin 1 functions as a negative modulator of MLKL oligomerisation by integrating into the necrosome complex. Cell Death Differ doi: 10.1038/s41418-020-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim J, Park H, Heisler J, Maculins T, Roose-Girma M, Xu M, McKenzie B, van Lookeren Campagne M, Newton K, Murthy A. 2019. Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife 8:e44452. doi: 10.7554/eLife.44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Granato M, Santarelli R, Farina A, Gonnella R, Lotti LV, Faggioni A, Cirone M. 2014. Epstein-Barr virus blocks the autophagic flux and appropriates the autophagic machinery to enhance viral replication. J Virol 88:12715–12726. doi: 10.1128/JVI.02199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santarelli R, Granato M, Pentassuglia G, Lacconi V, Gilardini Montani MS, Gonnella R, Tafani M, Torrisi MR, Faggioni A, Cirone M. 2016. KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression. Autophagy 12:2311–2325. doi: 10.1080/15548627.2016.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Cojohari O, Mahmud J, Altman AM, Peppenelli MA, Miller MJ, Chan GC. 2020. Human cytomegalovirus mediates unique monocyte-to-macrophage differentiation through the PI3K/SHIP1/Akt signaling network. Viruses 12:652. doi: 10.3390/v12060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shnayder M, Nachshon A, Krishna B, Poole E, Boshkov A, Binyamin A, Maza I, Sinclair J, Schwartz M, Stern-Ginossar N. 2018. Defining the transcriptional landscape during cytomegalovirus latency with single-cell RNA sequencing. mBio 9:e00013-18. doi: 10.1128/mBio.00013-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shnayder M, Nachshon A, Rozman B, Bernshtein B, Lavi M, Fein N, Poole E, Avdic S, Blyth E, Gottlieb D, Abendroth A, Slobedman B, Sinclair J, Stern-Ginossar N, Schwartz M. 2020. Single cell analysis reveals human cytomegalovirus drives latently infected cells towards an anergic-like monocyte state. Elife 9:e52168. doi: 10.7554/eLife.52168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yurochko AD, Huang ES. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunology 162:4806–4816. [PubMed] [Google Scholar]