The hallmark of HIV infection is a gradual depletion of CD4 T cells, with a progressive decline of host immunity. How CD4 T cells are depleted in individuals with active and virus-suppressed HIV infection remains unclear. In this study, we employed a cellular model of HIV infection to characterize the mechanisms underlying CD4 T-cell destruction by analyzing the chromosome end (telomere) DNA damage response (DDR) and cellular apoptosis in a T-cell line (highly permissive SupT1 cells), as well as in primary CD4 T cells with active or drug-suppressed HIV infection. We demonstrated that HIV-induced telomeric DDR plays a critical role in inducing telomere loss, premature cell aging, and CD4 T-cell apoptosis or depletion via dysregulation of the PI3K/ATM pathways. This study sheds new light on the molecular mechanisms of telomeric DDR and its role in CD4 T-cell homeostasis during HIV infection.
KEYWORDS: ATM, apoptosis, DNA damage response, HIV, telomere, T-cell homeostasis, ATM kinases, telomere, apoptosis
ABSTRACT
CD4 T-cell depletion is a hallmark of HIV/AIDS, but the underlying mechanism is still unclear. We have recently shown that ataxia-telangiectasia-mutated (ATM) deficiency in CD4 T cells accelerates DNA damage, telomere erosion, and cell apoptosis in HIV-infected individuals on antiretroviral therapy (ART). Whether these alterations in ART-treated HIV subjects occur in vitro in HIV-infected CD4 T cells remains unknown. In this study, we employed a cellular model of HIV infection to characterize the mechanisms underlying CD4 T-cell destruction by analyzing the telomeric DNA damage response (DDR) and cellular apoptosis in highly permissive SupT1 cells, followed by the validation of our observations in primary CD4 T cells with active or drug-suppressed HIV infection. Specifically, we established an in vitro HIV T-cell culture system with viral replication and raltegravir (RAL; an integrase inhibitor) suppression, mimicking active and ART-controlled HIV infection in vivo. We demonstrated that HIV-induced, telomeric DDR plays a pivotal role in triggering telomere erosion, premature T-cell aging, and CD4 T-cell apoptosis or depletion via dysregulation of the PI3K/ATM pathways. This in vitro model provides a new tool to investigate HIV pathogenesis, and our results shed new light on the molecular mechanisms of telomeric DDR and CD4 T-cell homeostasis during HIV infection.
IMPORTANCE The hallmark of HIV infection is a gradual depletion of CD4 T cells, with a progressive decline of host immunity. How CD4 T cells are depleted in individuals with active and virus-suppressed HIV infection remains unclear. In this study, we employed a cellular model of HIV infection to characterize the mechanisms underlying CD4 T-cell destruction by analyzing the chromosome end (telomere) DNA damage response (DDR) and cellular apoptosis in a T-cell line (highly permissive SupT1 cells), as well as in primary CD4 T cells with active or drug-suppressed HIV infection. We demonstrated that HIV-induced telomeric DDR plays a critical role in inducing telomere loss, premature cell aging, and CD4 T-cell apoptosis or depletion via dysregulation of the PI3K/ATM pathways. This study sheds new light on the molecular mechanisms of telomeric DDR and its role in CD4 T-cell homeostasis during HIV infection.
INTRODUCTION
The hallmark of HIV/AIDS pathogenesis is a gradual depletion of CD4 T cells with a progressive decline of host immunity, along with an increased incidence of opportunistic infections, malignancies, and ultimately, death (1, 2). Disease progression from HIV to AIDS in untreated individuals often occurs over 8 to 10 years and was originally thought to be a result of slow but inexorable, virus-mediated CD4 T-cell destruction. However, massive CD4 T-cell destruction is now known to occur quite early during active HIV infection (1, 2). In most HIV-infected individuals, this initial destruction is counteracted by CD4 T-cell regeneration and compensatory responses that preserve CD4 T-cell numbers and functions above the threshold (200 cells/μl) associated with overt immunodeficiency. However, T-cell regeneration, occurring with immune activation and dysregulation, does not always restore all functionally important cell populations and is not durable over time. Ultimately, CD4 T-cell homeostasis fails, and critical effector/memory T cells and even naive populations decline below the threshold necessary to prevent opportunistic infections or malignancies and to generate new cells that can respond to neoantigens (vaccines).
In the era of antiretroviral therapy (ART), HIV replication is suppressed, allowing for CD4 T-cell recovery. However, complete CD4 T-cell recovery does not always occur despite successful control of HIV replication by ART. Even in ART-controlled individuals with long-term HIV suppression and reasonable CD4 T-cell numbers, a profound immune dysregulation often remains (3–8). These changes expose the immune system to unique challenges that lead to T-cell exhaustion and senescence, which is characterized by shortened telomeres, impaired interleukin 2 (IL-2)/gamma interferon (IFN-γ) production, exhausted cell proliferation, and blunted vaccine responses (9–14). These alterations accelerate the decline of CD4 T-cell competency during latent HIV infection, leading to premature “inflammaging,” in which chronic inflammation induces an immune-aged phenotype similar to that observed in the elderly. Thus, the onset of overt immunodeficiency appears to be directly linked to CD4 T-cell dynamics (overproliferation, differentiation, and resistance or sensitivity to apoptosis) due to the interplays between direct viral cytopathogenicity and indirect inflammatory stimulation, resulting in chronic immune activation and, ultimately, inflammaging.
Telomeres are repeating hexameric sequences of DNA at chromosome ends associated with a complex of shelterin proteins. Telomere integrity is a key feature of linear chromosomes that preserves genome stability and function, whereas telomere attrition is a hallmark of cellular senescence that drives cell dysfunction or apoptosis (15, 16). Notably, in healthy CD4 T cells, telomeres undergo shortening at a rate of 50 to 100 bp (bp) per cell division (17, 18). However, telomere loss can have 2- to 5-fold increases during viral infection (18–22), triggering cell cycle arrest when progressive telomere loss reaches a critical point known as senescence (a quiescent, nonreplicative state with anergic activities). Because telomeric DNA damage causes telomere loss that leads to cell senescence and/or apoptosis, we hypothesized that progressive CD4 T-cell depletion and failure of immune recovery during HIV infection could be due, at least in part, to increased telomeric DNA damage response (DDR) driving accelerated telomere erosion. Indeed, we have recently shown that CD4 T cells from patients with HIV, hepatitis C virus (HCV), or hepatitis B virus (HBV) infection are vulnerable to apoptosis due to the accumulation of unrepaired telomeric DNA damage (19–24). Thus far, the precise mechanisms controlling telomeric DNA damage and T-cell homeostasis during active and virus-suppressed HIV infection remain unclear.
While the life cycle of HIV and its impacts on infected cells have been extensively studied (25), the mechanisms involved in CD4 T-cell depletion due to virus-host interactions, in particular the DDR-related signaling pathways, warrant further investigations. We have recently demonstrated that ataxia-telangiectasia-mutated (ATM) deficiency accelerates DNA damage, telomere erosion, premature T-cell aging, and apoptosis in HIV-infected individuals on ART (22). Whether these findings in ART-controlled HIV patients can be recapitulated in an HIV-infected, ART-treated cell model in vitro remains unknown. Currently, there are limited data regarding telomeric DDR and apoptosis in CD4 T cells with active and virus-suppressed HIV infection. Such studies are hampered by the heterogeneity of the HIV infection systems, as the signaling of telomeric DDR may be differentially regulated by active and drug-controlled HIV infection. In this study, we characterized the impact of HIV on the telomeric DDR and phosphoinositide 3-kinase (PI3K) pathways, and on apoptosis in a highly permissive T-cell line (SupT1) as well as in primary CD4 T cells with active or drug-suppressed HIV infection. We introduced an in vitro cell culture system with HIV replication and suppression by raltegravir (RAL), an integrase inhibitor that effectively blocks HIV genomic integration into the cellular genome and affects the early stage of the HIV life cycle, mimicking active and ART-controlled HIV infection in vivo. We demonstrated that HIV-induced telomeric DDR plays a pivotal role in triggering CD4 T-cell apoptosis and depletion via the dysregulation of the PI3K/ATM pathways, thus contributing to the pathogenesis of HIV infection.
RESULTS
HIV-infected SupT1 cells—an in vitro culture system that mimics HIV infection of CD4 T cells in vivo.
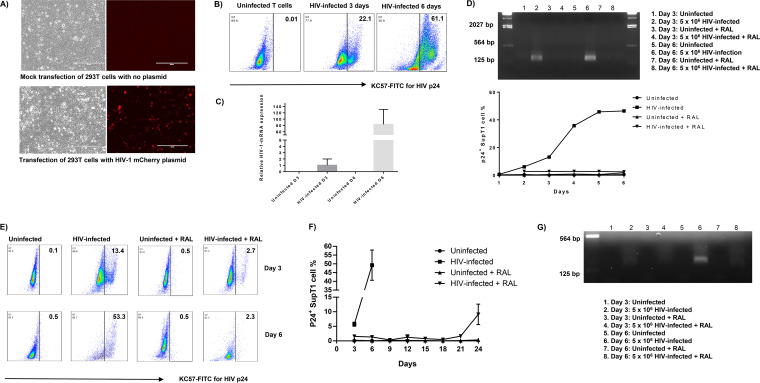
Immunodeficiency due to progressive loss of CD4 T cells is a hallmark of HIV infection (1, 2). To study the mechanisms of CD4 T-cell loss during HIV infection, we employed an in vitro HIV-infected SupT1 cell culture system by employing a pNL4-3 plasmid that contains a full-length HIV viral genome (26). We transfected the pNL4-3 plasmid into human embryonic kidney 293 cell line transformed with SV40 large T antigen (HEK293T) cells to generate infectious HIV particles. An NL4-3 mCherry luciferase plasmid was used as a positive control for transfection efficiency (27). Fluorescence microscopy showed the HIV mCherry cassette (red) in HEK293T cells, whereas mock-transfected cells had a negative fluorescence signal (Fig. 1A). In addition, HIV-RNA and p24 antigen were detected in the supernatant of transfected HEK293T cells by real-time reverse transcription-PCR (RT-PCR) and enzyme-limited immunosorbent assay (ELISA), respectively (data not shown). Since 0.1 ng/ml of p24 protein is equivalent to ∼1 × 106 HIV viral particles (28), culture supernatant aliquots containing 1 × 106 to 5 × 106 HIV virions were used to infect 1.5 × 106 human SupT1 or CD4 T cells using the spinoculation approach (29). As shown in Fig. 1B, p24 antigen was detected in HIV-infected SupT1 cells by flow cytometry, with the frequency of p24+ cells increasing in a time-dependent manner. Real-time RT-PCR analysis showed that HIV-1 mRNA levels also increased in HIV-infected SupT1 cells in a time-dependent manner (Fig. 1C).
FIG 1.
HIV infection of SupT1 cells. (A) Bright and fluorescence microscopic examinations of HEK293T cells with mock transfection (top) and transfected for 48 h with HIV NL4-3 mCherry plasmid (bottom). (B) Flow cytometry analysis of p24 expression in SupT1 cells with HIV infection. (C) Reverse transcription-PCR (RT-PCR) analysis of HIV-1 mRNA levels in SupT1 cells with or without HIV infection on day 3 and day 6. (D) RT-PCR detection of HIV gene expression in SupT1 cells with HIV infection and raltegravir (RAL) treatment (added 2 h post HIV infection). (E) Flow cytometry analysis of p24 expression in SupT1 cells with HIV infection and RAL treatment (added 24 h post HIV infection). (F) Time-dependent p24 expression in SupT1 cells with HIV infection and RAL treatment (added 24 h post HIV infection). (G) RT-PCR detection of HIV gene expression in SupT1 cells with HIV infection and RAL treatment (added 24 h post HIV infection).
HIV transcription starts within 2 h of infection, with one viral replication cycle completing approximately 24 h after infection (30). To mimic clinical HIV infection on ART, we cultured SupT1 cells with or without HIV infection in the presence or absence of antiviral (RAL, 66 nM) treatment after 2 h of spinoculation and removal of HIV medium. Then, HIV replication was measured by real-time RT-PCR and p24 expression levels by flow cytometry at various time points. As shown in Fig. 1D (upper), HIV-RNA was detected in HIV-infected cells without RAL treatment (lanes 2 and 6) at days 3 and 6 after infection, but was undetectable at the same time points in HIV-infected cells treated with RAL at 2 h postinfection. Concurrently, p24 antigen was detected in HIV-infected SupT1 cells by flow cytometry, and the frequency of p24+ cells increased over time but was inhibited in HIV-infected and RAL-treated cells (Fig. 1D, lower). These results indicate that SupT1 cells can be infected by HIV and that RAL potently inhibits HIV replication.
We expected that adding RAL at 2 h post HIV infection would be too early for the virus to complete even one replication cycle. We thus set up the experiments by adding RAL at 24 h post HIV infection and measuring p24 production at various time points by flow cytometry. As shown in Fig. 1E, p24 antigen was detected in HIV-infected SupT1 cells, with the percentage of p24+ cells increasing over time, whereas p24 was inhibited in HIV-infected, RAL-treated cells. To further characterize the effect of RAL on HIV replication, we changed the medium every 3 days and maintained each passage at a fixed number (1.5 × 106) of cells in the culture with or without RAL treatment. Due to the cytopathogenicity of HIV, we could not detect p24 after day 6 in HIV-infected cells without treatment, as the majority of SupT1 cells had died. Nevertheless, HIV-infected cells with RAL treatment could survive beyond 1 week, similarly to uninfected cells with RAL alone. Notably, RAL significantly suppressed HIV replication in SupT1 cells during the first 3 weeks. By the third week, however, the level of p24+ cells gradually increased, likely due to viral mutation and breakthrough of the resistance threshold when RAL was used alone (Fig. 1F). Also, HIV-RNA was detected by RT-PCR (lanes 2 and 6) in the supernatants of HIV-infected SupT1 cells at days 3 and 6, respectively, after infection, but not in the supernatants of uninfected cells (lanes 1, 3, 5, and 7) or HIV-infected, RAL-treated cells (lanes 4 and 8) (Fig. 1G). Taken together, these results demonstrate that SupT1 cells can be infected by HIV and that RAL potently inhibits HIV replication. Thus, this cell culture system represents an in vitro model that mimics active and virus-suppressed HIV infection of CD4 T cells in vivo.
HIV infection induces apoptosis and CD4 T-cell depletion that are reversible by the RAL treatment.
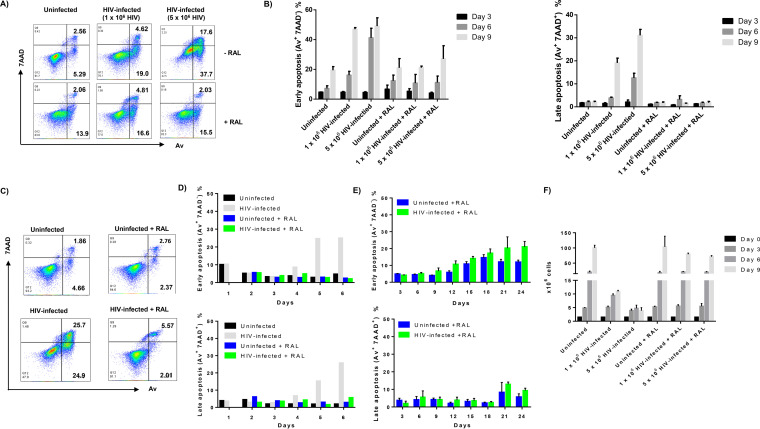
We further investigated whether HIV infection in vitro, with or without RAL treatment, causes T-cell apoptosis. We first measured apoptosis of SupT1 cells with RAL added at 2 h post-HIV infection. Notably, T-cell apoptosis, measured by the annexin V-PE (Av)/7-aminoactinomycin D (7AAD) staining of phosphatidylserine (PS) on the cell surface of apoptotic and dead cells, was not observed in the early phase (days 1 to 3) of HIV infection but was detected at day 6 post HIV infection. Figure 2A shows representative dot plots of Av and 7AAD levels in SupT1 cells, with or without HIV infection and RAL treatment at day 6. Figure 2B shows summary data of Av+ 7AAD− early apoptotic cells and Av+ 7AAD+ late apoptotic cells at days 3, 6, and 9 after infection. Notably, uninfected SupT1 cells cultured for 6 days showed baseline levels of early apoptosis (Av+ 7AAD−, ∼8.0%) as well as late apoptosis (Av+ 7AAD+, ∼2%). HIV infection increased the number of early apoptotic (Av+ 7AAD−) and late apoptotic cells (AV+ 7AAD+) in a dose-dependent manner. The presence of RAL had no significant effect on apoptotic death in uninfected cells. However, compared to the infected cells without RAL, RAL treatment significantly reduced HIV-induced apoptotic cell death, as evidenced by a reduction in the numbers of Av+ 7AAD− early apoptotic cells and Av+ 7AAD+ late apoptotic cells after infection.
FIG 2.
HIV infection induces SupT1 cell apoptotic death, which is reversible by antiviral treatment. (A and B) Representative dot plots and summary data of flow cytometry analysis of early (Av+ 7AAD−) and late (Av+ 7AAD+) apoptosis of SupT1 cells with HIV infection and RAL treatment (added 2 h post HIV infection). (C to E) Representative dot plots and summary data of flow cytometry analysis of early (Av+ 7AAD−) and late (Av+ 7AAD+) apoptosis of SupT1 cells with HIV infection and RAL treatment (added 24 h post HIV infection). (F) Manual count of the number of SupT1 cells with HIV infection and RAL treatment (added 24 h post HIV infection).
Additionally, we measured the apoptosis of SupT1 cells with RAL added 24 h post HIV infection. Figure 2C shows representative dot plots of Av and 7AAD staining in SupT1 cells, with or without HIV infection and RAL treatment after 6 days of culture. Figure 2D shows summary data of early apoptotic (Av+ 7AAD−) cells and late apoptotic (Av+ 7AAD+) cells after 1 to 6 days of infection. Notably, T-cell apoptosis was not observed in the early phase (days 1 to 3) of HIV infection, and HIV infection increased the number of early apoptotic and late apoptotic cells thereafter (days 4 to 6). However, compared to infected cells without treatment (gray bar), RAL significantly reduced HIV-induced apoptosis, as shown by the remarkable decrease in both early and late apoptotic cells (green bar).
We next kinetically assessed the effect of RAL on HIV-infected SupT1 cell apoptosis. To this end, we changed the medium every 3 days and maintained a certain number (1.5 × 106) of cells at each passage with or without RAL addition (66 nM), in order to maintain similar growth advantages between groups. As shown in Fig. 2E, RAL alone (blue bar) increased Av levels in uninfected cells after 2 weeks of exposure but did not increase late apoptosis (7AAD levels) over the 3-week period, suggesting that long-term exposure of SupT1 cells to the HIV drug (RAL) results in early apoptosis but not cell death. Although early apoptosis was observed starting at day 9 in HIV-infected SupT1 cells with RAL treatment (green bar), RAL clearly prevented the death of infected cells until the virus breakthrough at day 21. These data support our conclusions that HIV increases T-cell apoptosis and that RAL treatment rescues cell death.
In parallel, we performed manual cell counting for cultures with or without HIV infection and/or RAL treatment. To this end, SupT1 cells were cultured at 1.5 × 106 cells/3 ml and counted every 3 days during each passage. Figure 2F represents the exponential growth of the total cell population, which was calculated according to the observed cell growth from day 0 to day 3 and the cell counts seeded following discarding of some cells at each passage. The number of SupT1 cells increased rapidly in the uninfected group, and HIV infection clearly reduced the cell proliferation and killed the cells in an HIV dose- and time-dependent manner. However, no such effects were observed in RAL-treated cells. These data further confirm that HIV infection induces T-cell apoptotic death and that RAL treatment reduces the death rate of HIV-infected cells, although they remain in an early apoptotic state. Therefore, this in vitro HIV-infected, RAL-treated cell model recapitulates our findings in CD4 T cells from HIV-infected individuals ex vivo, which show that the degree of apoptosis within HIV-specific CD4 T cells correlates with viral load and disease progression and that ART abrogates these differences (31).
HIV infection in primary CD4 T cells induces apoptosis, which is reversible by ART.
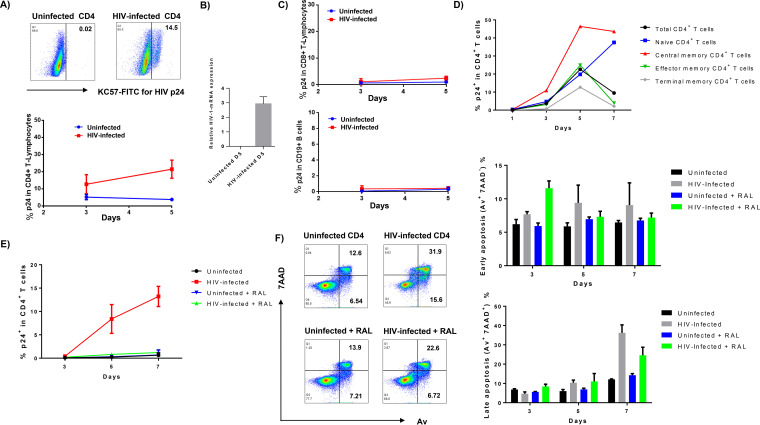
Given the disparity in the cell biology of immortalized cells and primary cells, we also established an in vitro HIV-infected primary CD4 T-cell culture system using the same approach described above, in order to validate the data obtained with the highly permissive SupT1 cells. Since activated T cells are more susceptible to HIV infection, we stimulated peripheral blood mononuclear cell (PBMCs) with the mitogenic agent phytohemagglutinin (PHA; 2.5 μg/ml) and CD4 T cells (purified from healthy subjects) with anti-CD3/CD28 (1 μg/ml) for 24 h prior to HIV infection. As shown in Fig. 3A, activated human CD4 T cells could be infected by HIV, as evidenced by a time-dependent increase in HIV p24+ CD4 T cells. HIV-1 mRNA was also detected by real-time RT-PCR in primary CD4 T cells at day 5 after infection (Fig. 3B). However, CD8 T cells or B cells could not be infected by HIV (Fig. 3C). We then measured the sensitivity of different subsets of CD4 T cells to HIV infection. The results showed that central memory CD4 T cells (TCM; CD4+ CD45RA− CCR7+) were more susceptible to HIV infection than effector and terminal memory CD4 T cells (Fig. 3D). This is in line with our previous observation that TCM are vulnerable to apoptotic death and thus are preferentially depleted in the peripheral blood of HIV patients (22). To mimic the clinical setting of HIV-infected patients on ART, we cultured HIV-infected and uninfected CD4 T cells with or without adding RAL at 24 h after infection and measured p24 expression at various time points by flow cytometry. As shown in Fig. 3E, while the percentage of p24+ CD4 T cells increased over time, RAL potently suppressed HIV replication to the extent that p24 was undetectable. Thus, this cell culture system represents an in vitro model that mimics active and virus-suppressed HIV infection of CD4 T cells in vivo.
FIG 3.
HIV infection of primary CD4 T cells induces apoptosis, which is reversed by the RAL treatment. (A) Flow cytometry analysis of p24 expression in phytohemagglutinin (PHA)-activated CD4+ T cells with or without HIV infection at the indicated time points (n = 3). (B) RT-PCR analysis of HIV-1 mRNA levels in CD4+ T cells at day 5 after infection. (C) Flow cytometry analysis of p24 expression in PHA-activated CD8+ and CD19+ lymphocytes with or without HIV infection at the indicated time points (n = 3). (D) p24 expression in different subsets of CD4+ T cells with HIV infection: naive CD4 T cells (CD4+ CD45RA+ CCR7+), central memory CD4 T cells (CD4+ CD45RA− CCR7+), effector memory CD4 T cells (CD4+ CD45RA− CCR7−), and terminal effector CD4 T cells (CD4+ CD45RA+ CCR7−) following activation with anti-CD3/CD28 for indicated times. (E) p24 expression in T-cell receptor (TCR)-activated CD4 T cells with HIV infection and RAL treatment. (F) Representative dot plots and summary data of flow cytometry analysis (n = 6) of early (Av+ 7AAD−) and late (Av+ 7AAD+) apoptosis of primary CD4 T cells with HIV infection and RAL treatment (added 24 h post HIV infection).
Next, we asked whether HIV infection in vitro could cause primary CD4 T-cell apoptotic death. Figure 3F shows representative dot plots and summary data of Av and 7AAD staining in CD4 T cells, with or without HIV infection and RAL treatment for 7 days. Notably, while T-cell receptor (TCR)-activated CD4 T cells showed baseline levels of Av and 7AAD staining, apoptosis did not change in the early phase (days 1 to 3) of HIV infection but did increase in a time-dependent manner thereafter (days 5 to 7). Moreover, compared to the infected cells without treatment, RAL reduced HIV-induced cell apoptotic death, as evidenced by the significant decrease in both Av+ 7AAD− and Av+ 7AAD+ cells. These data are consistent with our observations in the T-cell line that HIV infection increases T-cell apoptosis, whereas RAL treatment reduces cell death by inhibiting HIV replication.
HIV-induced apoptosis of CD4 T cells in vitro is independent of the extrinsic death pathways.
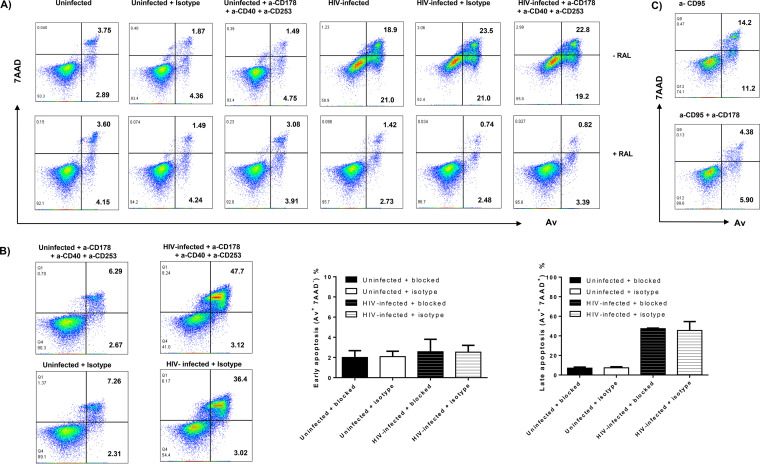
We explored the mechanisms underlying the HIV induction of CD4 T-cell apoptosis. Interactions between the Fas-Fas ligand, the tumor necrosis factor alpha (TNF-α)-TNF receptor, and the TRAIL-TRAIL receptor have been shown to play an important role in cell apoptosis in response to exogenous or endogenous stimuli. However, resting naive T cells usually do not express these receptors on their surface to initiate programmed cell death (20, 21, 32, 33). To determine whether HIV-induced T-cell apoptosis utilizes these extrinsic death pathways, we incubated HIV-infected or uninfected SupT1 and primary CD4 T cells with or without blocking antibodies against Fas (10 μg/ml of anti-human CD178), TNF-α (10 μg/ml of anti-human CD40), and TRAIL (10 μg/ml of anti-human CD253), or IgG1 isotype control antibodies for 5 to 6 days, followed by the Av/7AAD assay to determine cell apoptosis. As shown in Fig. 4A, the rate of cell apoptotic death did not change in SupT1 T cells incubated with the blocking antibodies in the absence of HIV infection and RAL treatment. HIV infection increased T-cell apoptosis and death; however, the combination of all three blocking antibodies did not abrogate the HIV-induced T-cell apoptotic death. RAL treatment remarkably reduced cell apoptotic death, while the blocking antibodies had no effect on these RAL-treated cells. Of note, we were able to reproduce these results using primary CD4 T cells from 3 different subjects (Fig. 4B). As a positive control for the blocking experiments, we treated the SupT1 cells with anti-CD95 (α-CD95) (Fas) in the presence or absence of the α-CD178 (Fas-L) blocking antibodies. As shown in Fig. 4C, the Fas-mediated apoptosis in SupT1 cells was partially blocked by the presence of the α-CD178 antibody. Taken together, these results suggest that blocking the extrinsic death pathways via disrupting interactions of the Fas-Fas ligand, the TNF-α–TNF receptor, and the TRAIL-TRAIL receptor in cultured CD4 T cells does not alter HIV-induced cell apoptotic death. These observations are consistent with published reports showing that naive CD4 T cells are resistant to exogenous apoptotic pathway-mediated cell death but sensitive to endogenous oxidative stress and are particularly vulnerable to reactive oxygen species (ROS)-mediated genotoxicity (20, 21, 31, 32).
FIG 4.
The extrinsic death pathways are not involved in HIV-induced CD4 T-cell apoptosis in vitro. (A) Av and 7AAD levels on SupT1 cells with HIV infection and RAL treatment in the presence of anti-CD178, anti-CD40, and anti-CD253 blocking or isotype control antibodies. (B) Av and 7AAD levels in primary CD4 T cells with or without HIV infection, in the presence of anti-CD178, anti-CD40, and anti-CD253 blocking or isotype control antibodies for 5 days (n = 3). (C) Av and 7AAD levels in SupT1 cells treated with an α-CD95 (Fas) in the presence or absence of α-CD178 blocking antibodies.
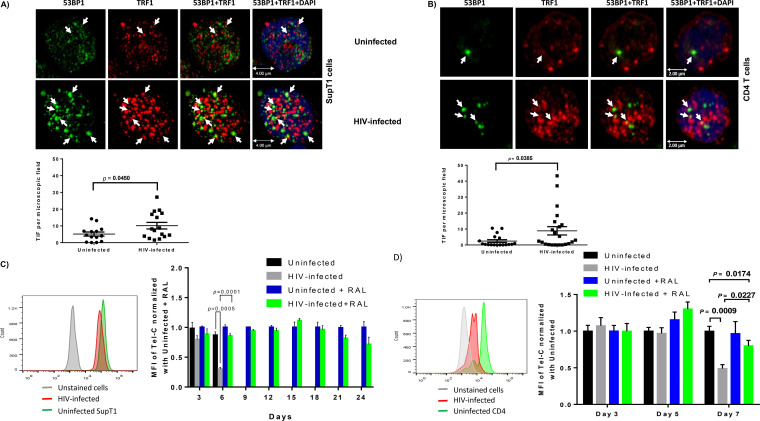
HIV infection induces telomeric DDR, resulting in telomere erosion in CD4 T cells.
Given the importance of telomere integrity for cell survival or death, we determined whether HIV-induced T-cell apoptosis is related to the telomeric DDR. It has been reported that, following genotoxic insult, 53BP1 functions as a docking site for other transducers and DNA repair proteins to form microscopically visible nuclear foci (DNA damage foci) (34). Thus, the presence of dysfunctional telomere-induced 53BP1 foci (TIF) is typically considered a hallmark of telomeric DDR (35). To determine telomeric DDR in HIV-infected SupT1 and primary CD4 T cells, we compared the number of TIF by examining the colocalization of 53BP1 and TRF1 using confocal microscopy. Approximately 50 to 100 cells were counted for TIF. The number of TIF per nucleus was higher in both SupT1 cells (Fig. 5A) and primary CD4 T cells (Fig. 5B) after HIV infection. The confocal microscopy images were analyzed using the confocal software Leica Application Suite X (LAS X), and the summary data for the colocalization of TRF1 and 53BP1 signal in each microscopic frame are shown below the images. These results suggest that telomeric DNA damage may cause cell apoptosis and CD4 T-cell loss in HIV infection, highlighting the role of telomere integrity in securing T-cell survival.
FIG 5.
DNA damage response in HIV-infected SupT1 and CD4 T cells with or without RAL treatment. (A and B) Confocal microscopy analysis of 53BP1 and TRF1 colocalization as a marker for dysfunctional telomere-induced foci (TIF) in SupT1 and primary CD4 T cells with HIV infection. The confocal images were analyzed using the confocal software Leica Application Suite X (LAS X), and summary data for the colocalization of TRF1 and 53B1 signal analyzed in each microscopic frame are shown. (C and D) Representative overlaid histograms and summary data of mean fluorescence intensity (MFI) of Tel-C in SupT1 cells or primary CD4 T cells, with or without HIV infection and RAL treatment, as measured by flow-FISH at the indicated times.
We then employed flow-FISH (a telomere measurement technique that combines the use of fluorescence in situ hybridization [FISH] with flow cytometry) to measure the telomere length in SupT1 cells with or without HIV infection. Figure 5C shows representative overlaid histograms and the summary data from 6 independent experiments. SupT1 cells with HIV infection cultured for 3 to 6 days displayed significantly shortened telomeres compared to uninfected cells, especially at day 6. RAL treatment abrogated the HIV-induced telomere attrition at day 3, and particularly at day 6 after infection. We were unable to measure telomere length after day 6 because the majority of HIV-infected SupT1 cells without treatment were dead at that time. In contrast, we were able to measure telomere lengths in RAL-treated SupT1 cells for up to 3 weeks after infection, and we detected slightly shortened telomeres in HIV-infected, RAL-treated cells compared to those in uninfected cells on RAL. Notably, telomere attrition was accelerated (Fig. 5C) following the HIV breakthrough of RAL monotherapy and rebound on days 21 to 24 (Fig. 1C). We also measured telomere length in primary CD4 T cells with or without HIV infection and RAL treatment. As shown in Fig. 5D, telomere shortening was detected in primary CD4 T cells 7 days post HIV infection. While RAL alone did not alter telomere lengths, RAL partially blocked or restored telomere lengths in HIV-infected CD4 T cells. These results are consistent with our findings of telomere shortening in CD4 T cells derived from HIV-infected patients on ART (22) and support the notion that telomere erosion may be associated with cell apoptosis in HIV infection.
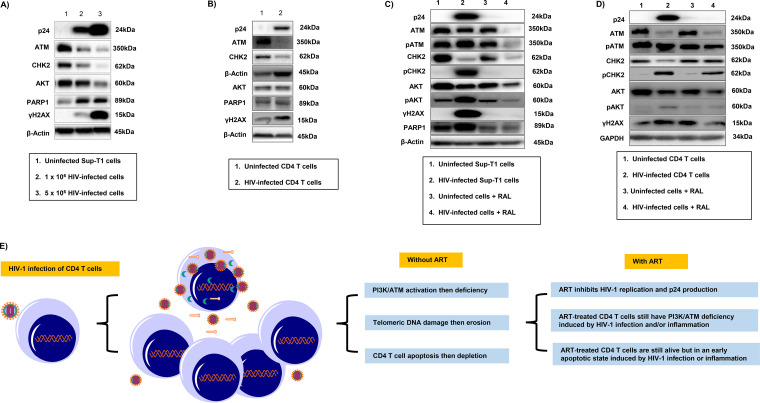
The PI3K/ATM pathway is dysregulated in HIV-infected CD4 T cells in vitro, promoting DDR and cell apoptosis.
Cells are equipped with DNA damage surveillance and repair machineries to prevent cell death associated with genomic instability (36–38). Accumulation of damaged DNA in CD4 T cells indicates an impairment of DNA damage repair machinery during HIV infection. DNA damage can cause telomere erosion and lead to cell senescence or apoptosis. We hypothesized that progressive CD4 T-cell loss and failure of immune recovery during HIV infection could be due, at least in part, to dynamic alterations in the DNA damage and repair kinases, which include ATM and its downstream checkpoint kinase 2 (CHK2) that belongs to the PI3K family, potentially leading to disruption of telomere integrity. To determine whether telomeric DNA damage and cell apoptosis were associated with the PI3K/ATM dynamics induced by HIV infection, we measured ATM, CHK2, and AKT levels, along with the DNA damage marker γH2AX and the apoptosis marker PARP1, in SupT1 cells infected with different concentrations of HIV for 6 days. As shown in Fig. 6A, Western blot analysis revealed HIV dose-dependent changes in p24 expression in SupT1 cells. Interestingly, ATM and its downstream signaling kinase CHK2 were suppressed by HIV infection. PI3K/AKT had a similar pattern to that of ATM/CHK2. Correspondingly, γH2AX and caspase 3-dependent cleavage of PARP1, which plays an important role in DNA damage-mediated cell apoptosis (39), increased along with HIV infection. We also measured ATM, CHK2, AKT, γH2AX, and PARP1 levels by Western blotting in primary CD4 T cells following HIV infection and observed a similar pattern, i.e., p24 was detected in HIV-infected CD4 T cells (Fig. 6B). Levels of ATM, CHK2, and AKT were suppressed by the HIV infection, whereas levels of the DNA damage and cell apoptosis markers, γH2AX and cleaved PARP1, were increased along with HIV infection (Fig. 6B). We were able to reproduce these results in CD4 T cells isolated from different subjects and confirmed the data by flow cytometry and gene array analysis (data not shown).
FIG 6.
The PI3K/ATM pathways are dysregulated in HIV-infected T cells and promote DDR and cell apoptosis. (A) Western blot analysis of p24, ATM, CHK2, AKT, PARP-1, and γH2AX expressions in SupT1 cells at day 6 of HIV infection. (B) Western blot analysis of p24, ATM, CHK2, AKT, PARP-1, and γH2AX expressions in primary CD4 cells at day 5 of HIV infection. (C) Western blot analysis of p24, ATM, pATM, CHK2, pCHK2, AKT, pAKT, γH2AX, and PARP-1 expressions in SupT1 cells at day 6 of HIV infection with or without RAL treatment. (D) Western blot analysis of p24, ATM, pATM, CHK2, pCHK2, AKT, pAKT, and γH2AX levels in primary CD4 T cells at day 5 of HIV infection with or without RAL treatment. (E) A model of HIV infection in CD4 T cells. The PI3K/ATM pathways are suppressed, causing telomeric DNA damage and erosion and CD4 T-cell apoptosis and depletion during HIV infection. ART can partially reverse this process by inhibiting HIV replication. However, PI3K/ATM pathways will remain dysregulated in these virus-suppressed, ART-treated CD4 T cells, and thus the cells will survive but exist in an early apoptotic state in this setting of latent HIV infection and/or inflammation.
Based on the above observations, we exposed SupT1 cells with or without HIV infection and RAL treatment to gain further insights into the role of the PI3K/ATM pathway in the DDR and double-strand break (DSB) repair during HIV infection. As shown in Fig. 6C, p24 was only detected in HIV-infected SupT1 cells but not in HIV-infected, RAL-treated cells. Intriguingly, compared to the uninfected controls (lane 1), HIV-infected cells (lane 2) exhibited a slight decrease in total ATM but an increase in pATM, likely reflecting HIV-induced DDR. In parallel, levels of total CHK2 and AKT proteins were decreased, but levels of pCHK2 and pAKT were significantly increased by HIV infection, along with elevation of the DDR-mediated apoptotic markers γH2AX and PARP1. RAL treatment alone (lane 3) did not alter ATM, pATM, CHK2, pCHK2, AKT, pAKT, γH2AX, and PARP1 protein levels in SupT1 cells compared to those in the uninfected cells without RAL treatment (lane 1). However, RAL treatment of HIV-infected cells (lane 4) inhibited p24 expression and resulted in lower levels of ATM, pATM, CHK2, pCHK2, AKT, pAKT, γH2AX, and PARP1 compared to those in HIV-infected cells without RAL treatment. We also observed decreasing expressions in total ATM, CHK2, and AKT, but increases in pATM, pCHK2, and pAKT protein levels in primary CD4 T cells at day 5 post HIV infection and RAL treatment (Fig. 6D). Taken together, these results suggest that the PI3K/ATM signaling pathways are dysregulated in CD4 T cells in HIV infection and are associated with DDR-mediated CD4 T-cell apoptosis and depletion.
DISCUSSION
HIV/AIDS pathogenesis is characterized by depletion of CD4 T cells; however, the mechanism underlying CD4 T-cell survival or death during HIV infection remains incompletely understood. We have recently shown that ATM deficiency accelerates DNA damage, telomere erosion, premature T-cell aging, and apoptosis in HIV-infected individuals on ART (22–24). Whether these observations in HIV-infected, ART-treated individuals can be recapitulated in HIV-infected CD4 T cells in vitro remains unknown. To establish a potentially useful cellular model of HIV infection and characterize the mechanisms underlying CD4 T-cell depletion, we investigated telomeric DDR and cell apoptosis in a highly permissive SupT1 cell line and validated the findings in primary CD4 T cells with active or virus-suppressed HIV infection. Here, we report a successfully established in vitro cell culture system with viral replication and RAL suppression, mimicking active and ART-controlled HIV infection in vivo. Specifically, we demonstrated that (i) SupT1 cells and primary CD4 T cells can be infected by HIV and that RAL potently inhibits HIV replication, (ii) HIV infection induces apoptosis and CD4 T-cell depletion that is reversible by the RAL treatment, and (iii) in vitro HIV-induced CD4 T-cell apoptosis is independent of the extrinsic death pathways but occurs through intrinsic DDR via the dysregulation of the PI3K/ATM pathways. Based on these novel findings, we propose a working model of HIV infection in CD4 T cells (Fig. 6E), in which the PI3K/ATM pathways are suppressed, causing telomeric DNA damage and telomere erosion that lead to CD4 T-cell apoptosis and depletion during HIV infection. ART can partially reverse this process by inhibiting HIV replication; however, these ART-treated, virus-suppressed CD4 T cells still have dysregulated PI3K/ATM signaling and thus survive in an early apoptotic state in the setting of latent HIV infection and/or inflammation. Our data illustrate a similar mechanism for apoptosis in these cells, to that found in people living with HIV on ART (22), as well as activation-induced apoptosis through the TCR that results in ROS production, mitochondrial damage, and caspase activation (31). The mechanisms involved in maintaining the survival of HIV-infected cells warrant further investigation to understand the phenomenon of viral reservoirs in ART-controlled, latent HIV infection.
The host factors promoting survival or death of HIV-infected CD4 T cells are not clear, but telomere dynamics play a pivotal role in controlling cellular functions and longevity (17, 18). Thus, how telomere dynamics are dysregulated in the setting of chronic viral infection is under intense investigation. An important question is that of why and how CD4 T cells accumulate DNA damage and fail to repair telomere defects during HIV infection. Notably, we have previously shown that the telomere-associated shelterin protein TRF2 is markedly inhibited through ubiquitin degradation during chronic HCV and HIV infections (19). Since the primary function of TRF2 is to protect telomeres from unwanted DNA damage (18), inhibition of TRF2 can lead to telomere attrition and cell apoptosis. Also, we have recently discovered that TRF2 expression and telomerase activity are significantly inhibited in HIV-infected CD4 T cells in vitro and that TRF2 is the only inhibited shelterin protein in CD4 T cells derived from ART-controlled HIV-infected individuals, thus playing a pivotal role in recruiting telomerase to telomeres to maintain their integrity (unpublished observations). Additionally, we have reported that topoisomerases I and IIα (Top1 and Top2α), two enzymes involved in regulating DNA topology, are significantly inhibited and cause topological DNA damage and cell apoptosis in CD4 T cells during chronic viral (HIV, HBV, and HCV) infections (23, 24). Moreover, the current study showed that the DNA repair kinase ATM and its downstream checkpoint kinase 2 (CHK2) were significantly inhibited in CD4 T cells isolated from HCV-infected and HIV-infected individuals (20, 22), resulting in the damaged DNA being left unrepaired, thus promoting cell apoptosis. Thus, it appears that multiple host machineries are dysregulated by HIV infection and are involved in CD4 T-cell depletion.
Results from this study as well as our published reports demonstrate that the expression of total ATM and AKT are suppressed with HIV infection, along with telomeric DNA damage and cell apoptosis in ART-controlled HIV infection. Indeed, it has been reported that the HIV-1 accessory protein Vpr activates DDR and causes cell cycle arrest (40, 41). We have recently reported ATM deficiency in chronically HIV-infected individuals on antiretroviral therapy (22). Pharmaceutical inhibition or genetic silencing of ATM accelerates DNA damage, telomere erosion, and premature T-cell aging, supporting the notion that failure of DNA damage repair due to ATM deficiency is the driving force, rather than the consequence, of cell apoptotic death. ATM kinase has been established as a central player in DNA double-strand break repair, and its deficiency may not occur only in CD4 T cells, but also in other types of immune and nonimmune cells in people living with HIV. Thus, ATM deficiency in other cell types may also affect CD4 T-cell recovery in people living with HIV on ART. These data beg the question of whether it is even feasible to avoid the disruption of telomeric DDR signaling and cell apoptosis in the HIV-infected host without actually targeting the repair machinery itself. Consistent with our findings, Rossiello and Fumagalli et al. (42, 43) reported that telomeric DNA damage in mammalian cells is irreparable and occurs preferentially at telomeres in linear genomes. They suggested a paradigm, in which cellular senescence and organismal aging result from persistent and accumulated, irreparable telomeric DNA damage that could be permanent; i.e., once telomeres are damaged, they remain damaged. Another study suggested that persistent DDR may trigger senescence-associated inflammatory cytokine secretion, facilitating communication of a compromised state to the surrounding cells (44). Similarly, a bystander effect might occur in which senescent cells induce proximate cell DDR within the milieu via cell-cell contacts, a feature of senescence-induced senescence (45). These processes could certainly occur in our model of HIV-infected individuals on ART.
Importantly, latently HIV-infected cells are more susceptible to DDR-inducing agents, and thus are vulnerable to DDR-induced cell apoptosis (46). Indeed, recent studies showed susceptibility of latently infected TCM to drugs targeting telomeres, which are also known to induce DDR (47). We have recently reported on the telomere attrition effect of this group of drugs (21). This unveils the sensitivity of telomere sustenance, DDR, and the unique vulnerability of latently virus-infected cells, a new feature that could potentially be exploited in developing therapeutics to eliminate HIV reservoirs. While many questions remain unanswered in fighting against HIV infection, our results and the model system described here provide new tools for studying HIV pathogenesis and shed more light on the molecular mechanisms of HIV-induced CD4 T-cell destruction and may enhance future investigations of the telomeric DDR, T-cell premature aging, and immune senescence induced by the HIV-host cell interactions.
MATERIALS AND METHODS
HIV plasmid transfection and virus infection.
The pNL4-3 plasmid, which contains a full-length HIV viral DNA inserted into a pUC18 vector, was obtained from the NIH AIDS Reagent Program (originally from Malcolm Martin) (26). Approximately 20 μg of the HIV plasmid was used to transfect the human embryonic kidney 293 cell line transformed with SV40 large T antigen (HEK293T) using the polyethylenimine (PEI) method (29). The supernatants of HIV-transfected HEK293T cells were used to infect human SupT1 cells (NIH AIDS Reagent Program, catalog no. 100; originally from Dharam Ablashi, isolated from an 8-year-old child with non-Hodgkin’s T-cell lymphoma) using the spinoculation method (29). Briefly, 1.5 × 106 SupT1 cells were infected with supernatant containing 1 × 106 to 5 × 106 HIV virions in culture plates using centrifugation at 1,620 × g in a 37°C incubator. After 2 h of spinoculation, the supernatants were removed to discard the unattached viruses. Raltegravir (from the NIH AIDS Reagent Program) was added to some cultures as an antiretroviral drug. The medium was changed every 3 days, and 1.5 × 106 cells were maintained at each passage with or without adding RAL. Cells were harvested at days 3, 6, 9, 12, 15, 18, 21, and 24 for flow cytometry, RT-PCR, and Western blot analysis.
Primary CD4+ T-cell isolation and culture.
Samples from healthy subjects from Physicians Plasma Alliance (Johnson City, TN) were negative for HBV, HCV, and HIV infection. PBMCs were isolated from whole blood of healthy subjects by Ficoll density centrifugation (GE Healthcare, Piscataway, NJ). CD4+ T cells were isolated from PBMCs using the CD4+ T-cell-negative isolation kit and an LS Column (Miltenyi Biotec, Inc., Auburn, CA). The isolated CD4+ T cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA), 100 IU/ml penicillin, 2 mM l-glutamine (Thermo Scientific, Logan, Utah), and 100 IU IL-2 with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28 (BD Bioscience, San Jose, CA) or 2.5 μg/ml phytohemagglutinin (PHA) for 24 h. The CD4+ T cells were infected with HIV using the same conditions as described above. The cells were harvested at days 3, 5, and 7 for analyses. For receptor blocking experiments, Fas ligand, TNF-α, TRAIL, or IgG1 isotype control antibodies (10 μg/ml each) (BD Bioscience, San Jose, CA) were added to the cultures, and the cells were harvested for apoptosis assay.
Flow cytometry.
For the apoptosis assay, the cells were stained with the PE annexin V apoptosis detection kit I (BD Bioscience). Briefly, the cells were harvested, washed with Dulbecco’s phosphate-buffered saline (DPBS), labeled with annexin V-PE (Av) and 7-aminoactinomycin D (7AAD) in binding buffer, and analyzed by an AccuriC6 Plus flow cytometer (BD Bioscience, San Jose, CA) and FlowJo software (Tree Star, Inc., Ashland, OR). For nuclear protein staining, the cells were fixed in fixation buffer (BioLegend, San Diego, CA), permeabilized with fixation/permeabilization concentrate and diluent (Thermo Fisher Scientific, MA, USA), and then labeled with conjugated anti-HIV p24 Monoclonal (KC57)-PE (catalog no. 6604667, Beckman Coulter). The cells were analyzed by flow cytometry. Isotype control antibodies (eBioscience) and single staining controls were used to determine background staining levels and to adjust multicolor compensation as a gating strategy.
Microarray gene expression and real-time RT-PCR.
To measure HIV-1 mRNA levels, total RNA was extracted from 1.0 × 106 HIV-infected or uninfected cells with the PureLink RNA minikit (Invitrogen, Carlsbad, CA), and cDNA was synthesized using the High Capacity cDNA Reverse transcription kit (Applied Biosystems; Foster City, CA). The primers used were: HIV mRNA forward 5′-CAGATCCTGCATATAAGCAGCTG-3′, reverse 5′-TTTTTTTTTTTTTTTTTTTTTTTTGAAGCAC-3′; GAPDH 5′-TGCACCACCAACTGCTTAGC-3′, reverse 5′-GGCATGGACTGTGGTCATGAG-3′. The PCR assays were run in triplicate using the following conditions: 1 cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15s; 60°C for 60s; and 72°C for 60s. Gene expression was normalized to GAPDH, and the values were calculated using the 2−ΔΔCT method and are expressed as fold changes.
Western blotting.
The cells were harvested and lysed on ice in RIPA buffer (Boston BioProducts Inc, Ashland, MA) in the presence of protease inhibitors (Thermo Scientific, Rockford, IL). The protein concentrations were measured by the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). The proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, preblocked with 5% nonfat milk in 0.1% Tween 20 in Tris-buffered saline (TBS), and then incubated overnight with the following primary antibodies: p24 (NIH/NIAID AIDS reagent program), ATM, pATM, CHK2, pCHK2, AKT, pAKT, γH2AX, PARP1, and β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody as a loading control (Cell Signaling, Danvers, MA). After washing, the membranes were incubated with appropriate horseradish peroxide-conjugated secondary antibodies (Cell Signaling), and the protein bands were developed using the Amersham ECL Prime Western blotting detection reagent (GE Healthcare Bio-Sciences, Pittsburgh, PA). The bands were captured and quantified using the ChemiDoc MP imaging system (Bio-Rad).
Confocal microscopy.
SupT1 and primary CD4+ T cells were isolated and cultured as described above. The cells were fixed in 2% paraformaldehyde (PFA) for 20 min, permeabilized with 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 10 min, blocked with 5% bovine serum albumin (BSA) in PBS for 1 h, and then incubated at 4°C overnight with rabbit anti-53BP1 and mouse anti-TRF1 antibodies (Cell Signaling). The cells were washed three times with TBS plus 0.1% Tween 20, stained with anti-rabbit IgG-Alexa Fluor 488 and anti-mouse IgG-Alexa Fluor 555 antibodies (Invitrogen) at room temperature for 1 h, and then washed and mounted with 4′,6-diamidino-2-phenylindole (DAPI) Fluoromount-G (SouthernBiotech, Birmingham, AL). Images were acquired with a confocal laser scanning inverted microscope (TCS SP8; Leica, Germany).
Telomere length measurement.
The flow-fluorescence in situ hybridization (FISH) method was used to measure telomere lengths in SupT1 and primary CD4+ T cells as described previously (19). Briefly, T cells were incubated in a hybridization buffer with 0.25 μM fluorescein isothiocyanate (FITC)-peanut agglutinin (PNA) Tel C probe (CCCTAAC repeats; PNA Bio, Thousand Oaks, CA) for 10 min at room temperature. The samples were heated for 10 min at 85°C, chilled on ice, and hybridized in the dark at room temperature overnight. The samples were washed and analyzed immediately by flow cytometry, and lymphocyte telomere lengths are reported as mean fluorescence intensity (MFI).
Statistical analysis.
The data were analyzed using Prism 7 software and are presented as means plus or minus standard error of the mean (SEM) or as medians with interquartile ranges. Differences between two groups were analyzed by independent Student’s t test or the Mann-Whitney test, paired t test, or Wilcoxon matched-pairs signed-rank test. P values of <0.05 or <0.01 were considered statistically significant or very significant, respectively.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01AI114748 and R21AI138598, Veteran Administration Merit Review awards 1I01BX002670 and 1I01BX004281, and Department of Defense award PR170067 (to Z.Q.Y).
We thank the NIH AIDS Reagent Program for providing key reagents. This publication is the result of work supported with resources and use of facilities at the James H. Quillen Veterans Affairs Medical Center.
The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
We declare no competing financial interests.
REFERENCES
- 1.Okoye AA, Picker LJ. 2013. CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayan KV, Karthigeyan KP, Tripathi SP, Hanna LE. 2017. Pathophysiology of CD4+ T-cell depletion in HIV-1 and HIV-2 infections. Front Immunol 8:580. doi: 10.3389/fimmu.2017.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fülöp T, Herbein G, Cossarizza A, Witkowski JM, Frost E, Dupuis G, Pawelec G, Larbi A. 2017. Cellular senescence, immunosenescence and HIV. Interdiscip Top Gerontol Geriatr 42:28–46. doi: 10.1159/000448542. [DOI] [PubMed] [Google Scholar]
- 4.Appay V, Sauce D. 2017. Assessing immune aging in HIV-infected patients. Virulence 8:529–538. doi: 10.1080/21505594.2016.1195536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan-Lewis E, Aberg JA, Lee M. 2017. Aging with HIV in the ART era. Semin Diagn Pathol 34:384–397. doi: 10.1053/j.semdp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Van Epps P, Kalayjian RC. 2017. Human immunodeficiency virus and aging in the era of effective antiretroviral therapy. Infect Dis Clin North Am 31:791–810. doi: 10.1016/j.idc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 7.AGEhIV Study Group. 2016. T-cell activation independently associates with immune senescence in HIV-Infected recipients of long-term antiretroviral treatment. J Infect Dis 214:216–225. doi: 10.1093/infdis/jiw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco JR, Jarrin I, Martinez A, Siles E, Larrayoz IM, Canuelo A, Gutierrez F, Gonzalez-Garcia J, Vidal F, Moreno S. 2015. Shorter telomere length predicts poorer immunological recovery in virologically suppressed HIV-1-infected patients treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 68:21–29. [DOI] [PubMed] [Google Scholar]
- 9.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, Levandowski RA, Mican JM, Fauci AS. 2005. Compromised B cell responses to influenza vaccination in HIV‐infected individuals. J Infect Dis 191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Barradas MC, Alexandraki I, Nazir T, Foltzer M, Musher DM, Brown S, Thornby J. 2003. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis 37:438–447. doi: 10.1086/375841. [DOI] [PubMed] [Google Scholar]
- 11.Landrum ML, Hullsiek KH, O'Connell RJ, Chun HM, Ganesan A, Okulicz JF, Lalani T, Weintrob AC, Crum-Cianflone NF, Agan BK, and Infectious Disease Clinical Research Program HIV Working Group. 2012. Hepatitis B vaccine antibody response and the risk of clinical AIDS or death. PLoS One 7:e33488. doi: 10.1371/journal.pone.0033488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson SB, Landrum ML, Okulicz JF. 2014. Delayed-type hypersensitivity and hepatitis B vaccine responses, in vivo markers of cellular and humoral immune function, and the risk of AIDS or death. Vaccine 32:3341–3344. doi: 10.1016/j.vaccine.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mena G, Llupià A, García-Basteiro AL, Díez C, León A, García F, Bayas JM. 2012. Assessing the immunological response to hepatitis B vaccination in HIV-infected patients in clinical practice. Vaccine 30:3703–3709. doi: 10.1016/j.vaccine.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 14.del Pozo Balado MDM, Leal M, Méndez Lagares G, Mata RC, López‐Cortés LF, Viciana P, Pacheco YM. 2010. Increased regulatory T cell counts in HIV‐infected nonresponders to hepatitis B virus vaccine. J Infect Dis 202:362–369. doi: 10.1086/653707. [DOI] [PubMed] [Google Scholar]
- 15.Carneiro MC, De Castro IP, Ferreira MG. 2016. Telomeres in aging and disease: lessons from zebrafish. Dis Model Mech 9:737–748. doi: 10.1242/dmm.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackburn EH, Greider CW, Szostak JW. 2006. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn EH. 2000. Telomere states and cell fates. Nature 408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 18.Arkus N. 2005. A mathematical model of cellular apoptosis and senescence through the dynamics of telomere loss. J Theor Biol 235:13–32. doi: 10.1016/j.jtbi.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen LN, Zhao J, Cao D, Dang X, Wang L, Lian J, Zhang Y, Jia Z, Wu XY, Morrison Z, Xie Q, Ji Y, Zhang Z, El Gazzar M, Ning S, Moorman JP, Yao ZQ. 2018. Inhibition of TRF2 accelerates telomere attrition and DNA damage in naïve CD4 T cells during HCV infection. Cell Death Dis 9:900. doi: 10.1038/s41419-018-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Dang X, Zhang P, Nguyen LN, Cao D, Wang L, Wu X, Morrison ZD, Zhang Y, Jia Z, Xie Q, Wang L, Ning S, El Gazzar M, Moorman JP, Yao ZQ. 2018. Insufficiency of DNA repair enzyme ATM promotes naive CD4 T-cell loss in chronic hepatitis C virus infection. Cell Discov 4:16. doi: 10.1038/s41421-018-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao D, Zhao J, Nguyan LN, Nguyen LNT, Khanal S, Dang X, Schank M, Chand Thakuri BK, Wu XY, Morrison ZD, El Gazzar M, Zou Y, Ning S, Wang L, Moorman JP, Yao ZQ. 2019. Disruption of telomere integrity and DNA repair machineries by KML001 induces T cell senescence, apoptosis, and cellular dysfunctions. Front Immunol 10:1152. doi: 10.3389/fimmu.2019.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Ngoc L, Nguyen T, Nguyen LN, Dang X, Cao D, Khanal S, Schank M, Thakuri B, Ogbu S, Morrison ZD, Wu XY, Li Z, El Gazzar M, Ning S, Wang L, Wang Z, Moorman JP, Yao ZQ. 2019. ATM deficiency accelerates DNA damage, telomere erosion, and premature T cell aging in HIV-infected individuals on antiretroviral therapy. Front Immunol 10:2531. doi: 10.3389/fimmu.2019.02531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Dang X, Nguyen LNT, Nguyen LN, Zhao J, Cao D, Khanal S, Schank M, Wu XY, Morrison ZD, Zou Y, El Gazzar M, Ning S, Wang L, Moorman JP, Yao ZQ. 2019. Topological DNA damage, telomere attrition and T cell senescence during chronic viral infections. Immun Ageing 16:12. doi: 10.1186/s12979-019-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang X, Ogbu SC, Zhao J, Nguyen LNT, Cao D, Nguyen LN, Khanal S, Schank M, Thakuri BKC, Wu XY, Morrison ZD, Zhang J, Li Z, El Gazzar M, Ning S, Wang L, Wang Z, Moorman JP, Yao ZQ. 2020. Inhibition of topoisomerase IIA (Top2α) induces telomeric DNA damage and T cell dysfunction during chronic viral infection. Cell Death Dis 11:196. doi: 10.1038/s41419-020-2395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilen CB, Tilton JC, Doms RW. 2012. HIV: cell binding and entry. Cold Spring Harb Perspect Med 2:a006866. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. doi: 10.1128/JVI.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lassen KG, Hebbeler AM, Bhattacharyya D, Lobritz MA, Greene WC. 2012. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS One 7:e30176. doi: 10.1371/journal.pone.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suttiprapa S, Rinaldi G, Tsai IJ, Mann VH, Dubrovsky L, Yan HB, Holroyd N, Huckvale T, Durrant C, Protasio AV, Pushkarsky T, Iordanskiy S, Berriman M, Bukrinsky MI, Brindley PJ. 2016. HIV-1 integrates widely throughout the genome of the human blood fluke Schistosoma mansoni. PLoS Pathog 12:e1005931. doi: 10.1371/journal.ppat.1005931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins LJ, Bonczkowski P, Spivak AM, De Spiegelaere W, Novis CL, DePaula-Silva AB, Malatinkova E, Trypsteen W, Bosque A, Vanderkerckhove L, Planelles V. 2016. Modeling HIV-1 latency in primary T cells using a replication-competent virus. AIDS Res Hum Retroviruses 32:187–193. doi: 10.1089/aid.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi P, Desfarges S, Bartha I, Joos B, Zangger N, Muñoz M, Günthard HF, Beerenwinkel N, Telenti A, Ciuffi A. 2015. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog 11:e1005006. doi: 10.1371/journal.ppat.1005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue FY, Kovacs CM, Dimayuga RC, Gu XXJ, Parks P, Kaul R, Ostrowski MA. 2005. Preferential apoptosis of HIV-1-specific CD4+ T cells. J Immunol 174:2196–2204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 32.Fujii H, Shao L, Colmegna I, Goronzy JJ, Yand CM. 2009. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A 106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krammer PH, Arnold R, Lavrik IN. 2007. Life and death in peripheral T cells. Nat Rev Immunol 7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 34.Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, Burdak-Rothkamm S. 2015. DNA damage foci: meaning and significance. Environ Mol Mutagen 56:491–504. doi: 10.1002/em.21944. [DOI] [PubMed] [Google Scholar]
- 35.Takai H, Smogorzewska A, De Lange T. 2003. DNA damage foci at dysfunctional telomeres. Curr Biol 13:1549–1556. doi: 10.1016/S0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 36.Bartek J, Lukas J. 2007. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Dupré A, Boyer-Chatenet L, Gautier J. 2006. Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat Struct Mol Biol 13:451–457. doi: 10.1038/nsmb1090. [DOI] [PubMed] [Google Scholar]
- 38.Awasthi P, Foiani M, Kumar A. 2015. ATM and ATR signaling at a glance. J Cell Sci 128:4255–4262. doi: 10.1242/jcs.169730. [DOI] [PubMed] [Google Scholar]
- 39.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. 1999. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis: caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 40.Iijima K, Kobayashi J, Ishizaka Y. 2018. Structural alteration of DNA induced by viral protein R of HIV-1 triggers the DNA damage response. Retrovirology 15:8. doi: 10.1186/s12977-018-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nodder SB, Gummuluru S. 2019. Illuminating the role of Vpr in HIV infection of myeloid cells. Front Immunol 10:1606. doi: 10.3389/fimmu.2019.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossiello F, Herbig U, Longhese MP, Fumagalli M, d’Adda di Fagagna F. 2014. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr Opin Genet Dev 26:89–95. doi: 10.1016/j.gde.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, Herbig U, Longhese MP, Di Fagagna FDA. 2012. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14:555–555. doi: 10.1038/ncb0512-555b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:1272–1272. doi: 10.1038/ncb1009-1272a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. 2012. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piekna-Przybylska D, Sharma G, Maggirwar SB, Bambara RA. 2017. Deficiency in DNA damage response, a new characteristic of cells infected with latent HIV-1. Cell Cycle 16:968–978. doi: 10.1080/15384101.2017.1312225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piekna-Przybylska D, Maggirwar SB. 2018. CD4+ memory T cells infected with latent HIV-1 are susceptible to drugs targeting telomeres. Cell Cycle 17:2187–2203. doi: 10.1080/15384101.2018.1520568. [DOI] [PMC free article] [PubMed] [Google Scholar]