Abstract
Objective
We describe a phase II clinical trial of the combination of ribociclib and letrozole for treatment of relapsed oestrogen receptor (ER)-positive ovarian cancer (OC) and endometrial cancer (EC). The primary endpoint was the proportion of patients alive, progression-free survival (PFS), and still on treatment at 12 weeks (PFS12), with 45% or greater considered positive.
Methods
Patients with measurable, relapsed ER-positive OC or EC (platinum-sensitive or resistant) were eligible and treated with 400 mg of oral ribociclib and 2.5 mg of oral letrozole daily. Patient-derived xenografts (PDXs) were created from imaging-guided tumour biopsies.
Results
Forty patients (20 OC and 20 EC) were enrolled. A PFS12 of 55% was observed in the EC cohort and 50% in the OC cohort. A PFS greater or equal to 24 weeks (PFS24) was seen in 20% (4/20) of the OC cohort and 35% (7/20) of the EC cohort. The greatest benefit was seen in low-grade serous OC (LGSOC) (3/3, 100% PFS24) and grades 1 and 2 EC (5/11, 45% PFS24). All three LGSOC patients obtained at least a partial response lasting for over 2 years, with two of the three patients still on treatment. PDX tumour engraftment was feasible in 45% of patients. Positive survival effects of the combination of ribociclib and letrozole were observed in two of three EC PDX models.
Conclusion
Ribociclib and letrozole have promising clinical activity in relapsed ER-positive OC and EC, particularly in LGSOC and relapsed ER-positive grade 1 and 2 EC. Generation of PDX models is feasible with positive survival effects observed in EC models.
Trial registration number
ClinicalTrials.gov registry (NCT02657928).
Keywords: ovarian cancer, endometrial cancer, ribociclib, letrozole
Key questions.
What is already known about this subject?
Aromatase inhibitors (AIs) have limited activity and are used against oestrogen receptor (ER)-positive ovarian cancer (OC) and endometrial cancer (EC).
Cyclin kinase inhibitors (ribociclib, palbociclib and abemaciclib) synergise with AIs in the treatment of ER-positive breast cancer
New treatments are needed for metastatic EC and OC.
What does this study add?
Promising activity of the combination of ribociclib and letrozole in patients with metastatic ER-positive EC and OC.
Most significant effects observed in the OR positive low-grade serous OC (LGSOC) and grade 1 to 2 EC subsets.
Tumour xenograft generation was feasible in 45% of the cases with evidence of activity of the combination of ribociclib and letrozole observed in studied endometrial carcinoma models.
How might this impact on clinical practice?
The combination of ribociclib and letrozole is safe and active in ER-positive OC and EC, particularly in LGSOC, and grade 1 to 2 EC and represent a promising treatment option for these patients, pending results of further confirmatory trials.
Introduction
Oestrogen receptor (ER) positivity is present in 38% to 60% of all ovarian cancers (OCs) and up to 80% of all endometrial cancers (ECs).1 2 Treatment with aromatase inhibitors (AIs) is an acceptable option included in the National Comprehensive Cancer Network treatment guidelines for relapsed OC or EC. Despite the high prevalence of ER expression, limited clinical activity of single-agent AIs have been reported in at least two phase II trials in EC and eight phase II clinical trials in OC.3–13 A Gynecologic Oncology Group trial of anastrozole in chemotherapy-naïve EC showed median progression-free survival (PFS) of 1 month and a 9% response rate.11 In OC, a phase II study of letrozole in 60 patients showed that only 20% were progression-free and still on trial at 12 weeks.3 The activity of these agents might be higher in patients with ER-positive tumours and those without previous exposure to tamoxifen.
Strategies to improve the efficacy of AIs in metastatic breast cancer have been extensively studied. Cyclin-dependent kinase (CDK) 4 and 6 are important downstream targets of OR. In conjunction with cyclin D1 binding partner, a combination of CDK 4/6 and cyclin D1 holoenzyme acts on critical cell cycle checkpoints that allow cell cycle progression. Alterations in cell cycle checkpoint regulation occur in nearly all malignancies14 and contribute to endocrine therapy resistance in breast cancer.15 CDK inhibitors (palbociclib, ribociclib and abemaciclib) have been developed and shown on clinical trials to significantly prolong PFS (palbociclib, ribociclib and abemaciclib) and overall survival (OS) (ribociclib and abemaciclib) when combined with AIs or fulvestrant in the treatment of metastatic ER-positive breast cancer, leading to approval by the Food and Drug Administration.16–18 In addition, a patient with refractory high-grade serous OC with a homozygous CDKN2 deletion had a significant response to palbociclib and letrozole lasting over 12 months.19 An unpublished report of a phase II trial of palbociclib and letrozole in OC, showed a PFS of 3.7 months and a Partial Remission (PR) rate of 4% with 60% stable disease.20 Given these findings, we decided to explore if the possibility that combination ribociclib and letrozole could improve outcomes against relapsed OR-positive OC and EC.
Methods
Patient population
This investigator-initiated concomitant phase II clinical trial (separate trials for OC and EC) was sponsored by Novartis and performed at Mayo Clinic in Rochester in Minnesota, Phoenix in Arizona and Jacksonville in Florida. Patients were eligible if they had biopsy-proven, relapsed and measurable ER-positive ovarian, fallopian tube or primary peritoneal carcinomas (OCs) or relapsed ER-positive EC; had not been previously treated with ribociclib or AIs; and had an Eastern Cooperative Oncology Group performance status between 0 and 2. Central review of ER or pathology was not required. Given the pilot nature of this study, patients with platinum-resistant, platinum-refractory or platinum-sensitive ovarian tumours were included. Patients had to have measurable disease and willing to undergo tumour biopsy. Tumours had to be ER-positive, defined as 10% or greater staining on immunohistochemistry, and had to stain positive for retinoblastoma protein. Protocol was modified to not require retinoblastoma protein positivity for eligibility to facilitate patient accrual, since retinoblastoma protein positivity was observed in the majority of cases (12/13 (92.3%) initial cases). Patients with brain metastasis, clear cell or mucinous histology, or substantial liver or gut dysfunction were not eligible.
Treatment and evaluation
Treatment
Patients received 400 mg of oral ribociclib and 2.5 mg of oral letrozole daily, without interruption, on an every 4-week cycle, until the development of progressive disease or serious toxicity (both agents provided by Novartis). The ribociclib and letrozole doses were decreased in the event of grade 3 or higher drug-related toxicity (liver toxicity for letrozole), with a maximum of two dose reductions allowed. Patients requiring further dose reductions were taken off study. The Response Evaluation Criteria in Solid Tumors (RECIST) were used to assess treatment response every 12 weeks after the initiation of treatment. Staging studies included CT and serum cancer antigen 125 (CA125) levels. The trial was registered with the National Institute of Health. Mayo Clinic Institutional Review Board approval was obtained.
Statistics
The primary endpoint was the percentage of patients (OC and EC analysed separately) without evidence of progression and still on treatment at 12 weeks (PFS12). Progression was defined using RECIST 1.1 criteria.21 A one-stage design with the first eligible 19 patients with OC and 19 patients with EC was used to determine whether the PFS12 was at least 45% (ie, clinically active) versus 20% or less (ie, likely not clinically active). The 20% PFS12 value for determining lack of significant clinical activity was selected based on the phase II study of letrozole for OC reported by Bowman et al3 and the 1-month PFS in a phase II trial in patients with EC.11 An 83% power was calculated to detect a PFS12 of 45%, with a 7% level of significance. We estimated that, if seven or more patients from each 19-patient cohort were alive, without disease progression, and on treatment at 12 weeks, the treatment could be considered to have enough clinical activity to warrant further evaluation.
Secondary endpoints included confirmed response rates, serum CA125 response, adverse events (AEs), PFS and OS. AEs are presented in part in tabular form. AE data was collected for up to 14 days following the off-study date. PFS was defined as the time from study registration to the first of either disease progression or death. OS was defined as the time from study registration to death from any cause. Time-to-event distributions were estimated using the Kaplan-Meier method,19 and SAS V.9.4 (SAS Institute), was used for statistical analysis.22
Patient-derived xenografts
Establishment of patient-derived xenograft models
The protocol for patient-derived xenograft (PDX) establishment has been previously described by our team.23 24 All patients signed consent for imaging-guided biopsies of tumour specimens at baseline. Five to six cores of tumour samples were collected under sterile conditions. Tumour specimens were implanted intraperitoneally into one or two female severe combined immunodeficient (SCID)-beige mice (C.B.-17/IcrHsd-Prkdcscid Lystbg; Envigo) as previously described21 and in accordance with the Mayo Clinic Institutional Animal Care and Use Committee (IACUC).
In vivo efficacy of ribociclib and letrozole
The clinical characteristics of EC and OC PDX models for in vivo studies are reported in accordance with the minimal information standards (online supplemental table S1).25 Fresh tumour slurry (0.1 to 0.2 cc) was prepared and mixed in 1:1 ratio with McCoy’s media before intraperitoneal injection into female SCID-beige mice. Each tumour model was assigned a ‘U1561’ or ‘O1561’ prefix according to the histology (U=uterine, O=ovarian) followed by a unique # identifier to protect patient confidentiality in accordance with the Health Insurance Portability and Accountability Act. For experiments, low passages (≤5 to avoid genetic drift) were established in up to 40 female oophorectomised SCID mice. Tumour diameter and cross-sectional area were measured by transabdominal ultrasound using an S Series Ultrasound System (Fujifilm SonoSite) with on-board measurement tools as previously validated.26–28 When tumour area reached 0.3 to 0.5 cm2, mice were randomised by tumour size to treatment arms. Ribociclib was provided by Novartis and administered daily by oral gavage in 0.5% methylcellulose (75 mg/kg) as previously described,29 while letrozole was administered by subcutaneous injections at 10 μg daily.30 During the study period, weekly ultrasounds measured the largest tumour cross-sectional area through day 56. Mice were euthanised individually when moribund or as a cohort on day 56. The primary endpoint was change in tumour area by ultrasound, normalised to the day 0 area of the same tumour and plotted as a ratio (relative to day 0). Two-group comparisons were performed using student’s t-test, and a p value less than 0.05 was considered significant. A secondary endpoint was OS, for which Kaplan-Meier curves were created using GraphPad Prism 7 (GraphPad Software). Censored observations were defined as mice euthanised due to planned experimental endpoints or for meeting humane endpoints. Humane endpoints were defined as: tumour burden equal to 10% of the original body weight; ascites equal to 10% of the original body weight; weight loss greater than or equal to 20% of body weight; ulcerated tumours; tumours that interfere with vital functions, such as ambulation, eating or drinking; poor body condition; and behavioural score of 5 or less using the IACUC-approved scoring system (score items: appearance (scored 0 to 2), natural behaviour (scored 0 to 3), provoked behaviour (scored 0 to 3), and body condition (scored 1 to 5)) according to previously published literature.31
esmoopen-2020-000926supp001.pdf (79.2KB, pdf)
PDX models tissue processing and immunohistochemistry
Tissue collected from mice were fixed overnight in buffered formalin (Cat#23-011-120; Thermo Scientific) and processed in the tissue core facility at Mayo Clinic in Phoenix, Arizona. Deparaffinised and rehydrated 5 μm to 6 μm sections were unmasked for 15 min in ethylenediaminetetraacetic acid (EDTA) buffer (1 mM EDTA, 0.05% Tween 20, pH 8.0) at 95°C to 99°C. Primary antibodies to determine ER and Ki67 expression were purchased from Ventana (ER clone SP1 at 1: 100) and Dako North America (Ki67 clone MIB1 at 1:100, human specific) and incubated overnight at 4°C. Secondary antibodies (Cat#8125S and #8114; SignalStain Boost IHC detection system; Cell Signaling Technology) were applied for 30 to 60 min at room temperature. Chromogenic detection of protein expression was determined in the presence of 3,3’-diaminobenzidine (Cat#DS900H, Betazoid DAB Chromogen Kit; Biocare Medical) and visualised under light microscopy. Digital images were captured using Image Scope (Aperio).
Results
Patients
Forty patients (20 OC and 20 EC) were recruited for treatment from 22 August 2016, through 4 May 2018. Patients were enrolled at all three sites with 22 in Rochester, Minnesota, nine in Jacksonville, Florida, and nine in Phoenix, Arizona. Patient demographics are summarised in table 1. Most had platinum-resistant or refractory disease, and most were heavily pretreated. Only three patients had not received platinum-based chemotherapy (one OC and two EC). Seventeen (85.0%) of the patients with OC had high-grade serous carcinoma, with three having low-grade serous carcinomas. Eleven (55.0%) of the patients with EC had grade 1 or 2 endometrioid tumours and nine (45.0%) had grade 3 tumours (five high-grade serous and four grade 3 endometrioid).
Table 1.
Clinical characteristics of participating patients (n=40)
| Patients characteristics | Ovarian cancer cohort (n=20) | Endometrial cancer cohort (n=20) |
| Age, years, median (range) | 61.0 (30.0 to 82.0) | 64.5 (52.0 to 75.0) |
| ECOG PS, No. (%) | ||
| 0 | 12 (60.0) | 11 (55.0) |
| 1 | 7 (35.0) | 6 (30.0) |
| 2 | 1 (5.0) | 3 (15.0) |
| Race, No. (%) | ||
| White | 18 (90.0) | 20 (100.0) |
| Asian | 1 (5.0) | 0 (0.0) |
| Not reported | 1 (5.0) | 0 (0.0) |
| Cell type | ||
| Low-grade serous | 3 (15.0) | NA |
| High-grade serous | 17 (85.0) | NA |
| Grade 1 to 2 endometrioid | NA | 11 (55.0) |
| Grade 3 (five serous and four endometrioid) | NA | 9 (45.0) |
| No. of previous chemotherapy regimens median (range) | 3 (0 to 6) | 2 (0 to 6) |
| Platinum resistance, No. (%)* | ||
| Sensitive | 7 (36.8) | NA |
| Resistant/refractory | 12 (63.2) | NA |
*One OC and two EC patients had not received platinum therapy.
EC, endometrial cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not applicable; OC, ovarian cancer.
Efficacy
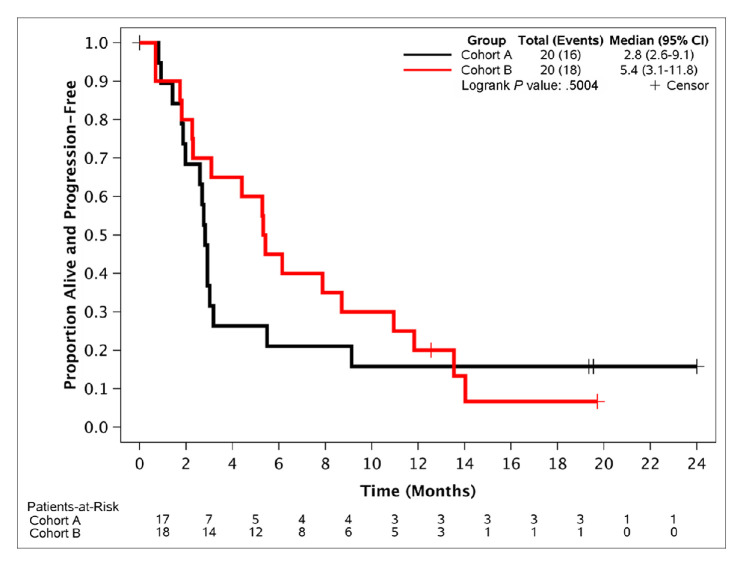
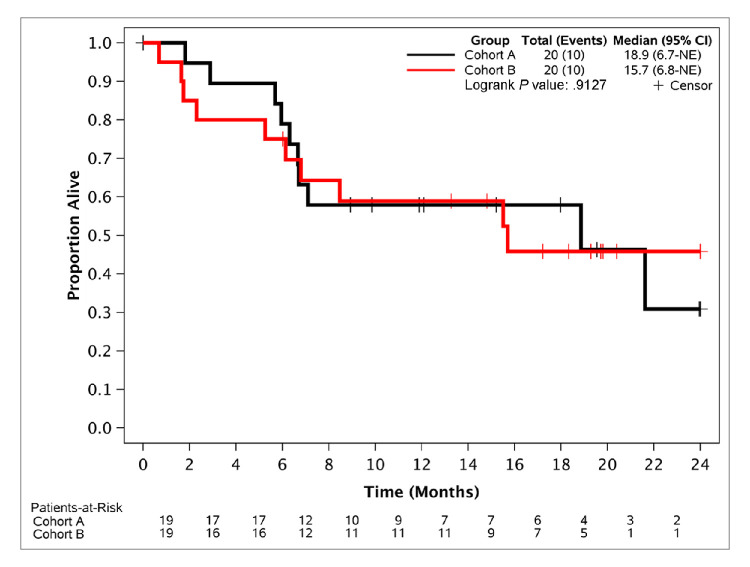
Eleven of 20 EC patients and 10 of 20 OC patients were alive, progression-free and on treatment at 12 weeks, for a PFS12 of 55% and 50%, respectively, meeting the primary endpoint. Median PFS was 5.4 months (95% CI, 3.1 to 11.8) for the EC cohort and 2.8 months (95% CI, 2.6 to 9.1) for the OC cohort (figure 1). Thirty-five per cent of patients in the EC cohort and 20% in the OC cohort were alive, progression-free and on treatment for at least 24 weeks (PFS24), demonstrating significant clinical activity (table 2). Subset analysis showed that the most significant benefit from study participation was observed in patients with low-grade serous OC (LGSOC) and in patients with grade 1 or 2 endometrioid EC (table 2). All three patients with LGSOC obtained durable responses to treatment (one complete response and two partial responses lasting for over 2 years), with two of them still on treatment for periods exceeding 30 months (30.4 months and 36.2 months). Only one of 17 (6%) patients with high-grade serous OC had not progressed and was still on treatment for at least 24 weeks (table 2). No significant differences in PFS24 between the platinum-sensitive versus the platinum-resistant subsets (2/7 (28.5%) vs 2/12 (16.6%)) were observed. Five of 11 patients (45.4%) with grade 1 or 2 endometrioid EC were still on treatment without progression for at least 24 weeks (table 2), while two of nine (22.2%) with high-grade EC tumours were without progression and on treatment for at least 24 weeks. Median OS for the OC and EC cohorts were 18.9 months (95% CI, 6.7 months to not reached) and 15.7 months (95% CI, 6.8 months to not reached), respectively (figure 2). A confirmed response was observed in five patients (three OC and two EC); all responses were partial, except one patient with low-grade serous carcinoma of the ovaries who had a complete response. Of the 29 patients with CA125 levels collected over time and which were elevated at baseline, nine (31%; five OC and four EC) had a decrease in these levels and five (17%; four OC and one EC) had at least a 50% decrease in these levels from baseline.
Figure 1.
Progression-free survival. Cohort A is the ovarian cancer cohort and cohort B is the endometrial cancer cohort.
Table 2.
Subset analysis of PFS
| Total Patients PFS ≥24 weeks | 11/40 (27.5%) |
| Ovarian group | 4/20 (20.0%) |
| Low-grade serous | 3/3 (100.0%)* |
| High-grade serous | 1/17 (5.9%) |
| Endometrial group | 7/20 (35.0%) |
| Grade 1 to 2 | 5/11 (45.5%) |
| High-grade | 2/9 (22.2%) |
*Patients on treatment for 36+, 30+ and 27 months.
PFS, progression-free survival.
Figure 2.
Overall survival. Cohort A is the ovarian cancer cohort and cohort B is the endometrial cancer cohort. NE, not estimated.
Treatment and toxicity
Only the two patients with LGSOC remain on treatment. The other 38 patients ended treatment for the following reasons: disease progression (76%), AEs (19%), patient refusal (3%) and death on study (3%). The median (range) number of treatment cycles received was three (1 to 28) for patients who completed treatment. online supplemental table S2 summarises the observed grade 3 or higher AEs, regardless of attribution. In all, 24 (60%) patients had at least one grade 3 or worse AE and six (15%) had at least one grade 4 or 5 AE. Three patients had grade 5 AEs unrelated to treatment and it was felt to be related to their primary progressing malignancy (one grade 5 sepsis; one grade 5 neoplasms; and one grade 5 acute kidney injury). The total grade 3 or higher toxicity observed in this trial is similar to the toxicity rates observed in the MONALEESA-2 trial of ribociclib and letrozole versus letrozole, in which 221/334 (66.2%) of patients in the combination arm experienced a grade 3 toxicity and 50/334 (15%) experienced a grade 4 toxicity.18 The most common potentially drug-related grade 3 or worse AEs, happening in at least three patients each (8%) included: leucopenia (23%), lymphocytopenia (23%), neutropenia (15%), fatigue (13%), dehydration (8%), abnormal liver function tests (8%) and acute kidney injury (8%). The prevalence of grade 3 or higher neutropenia in this trial (6/40, 15%) was lower than that observed in the MONALEESA-2 trial (198/334, 59.3%).18
esmoopen-2020-000926supp002.pdf (202.5KB, pdf)
PDX coclinical trial
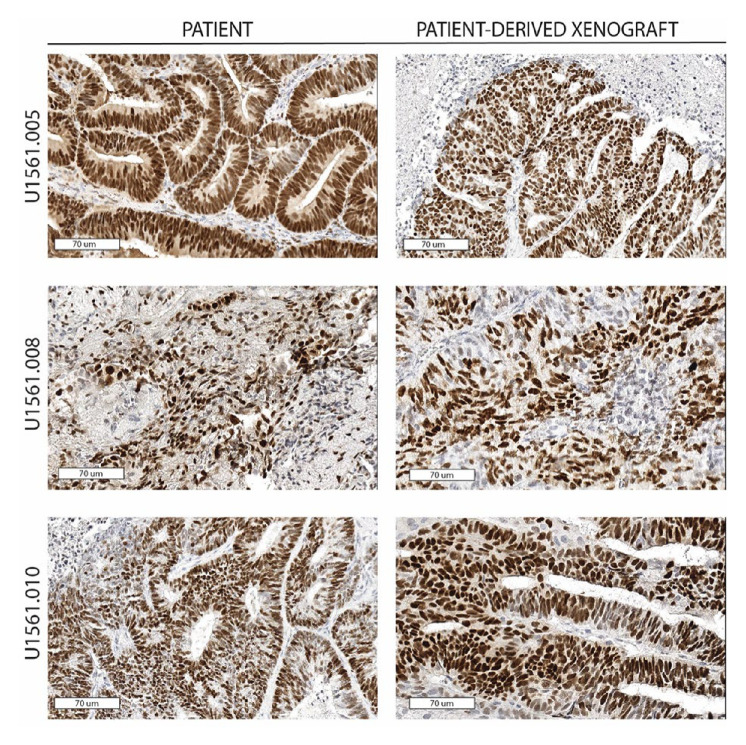
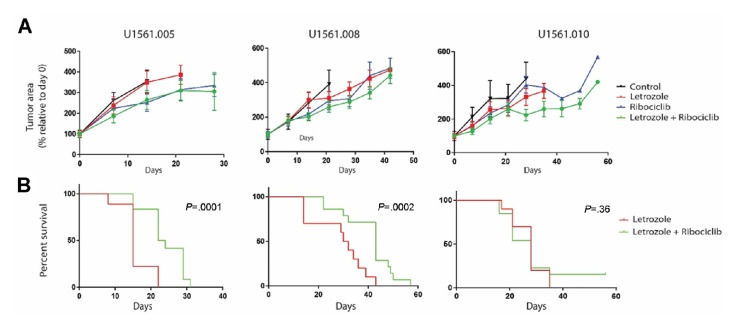
Of 40 consenting patients, 34 underwent successful biopsies of metastatic lesions for PDX creation and 16 (47%) were successfully engrafted. The engraftment rate was 69% (11/18) among the EC cohort, 31% (5/16) among the OC cohort, 38% (5/13) among those with high-grade serous OC, and 0% (0/3) among those with LGSOC. All four cases of high-grade endometrioid EC engrafted (100%), while only 40% (2/5) of those of high-grade serous EC engrafted and 55% (5/9) with grade 1 or 2 endometrioid EC engrafted. Across all models, the average time from tumour injection to first tumour harvest was 180 days (196 days for EC and 135 days for OC) (online supplemental figure S1). Considering that the patients on this clinical trial remained on treatment for an average of 162 days (EC) and 63 days (OC), the time required to create a PDX commonly exceeded the patients’ time on study. Indeed, only one model (U1561.010) engrafted before the corresponding patient discontinued the trial. Due to this time limitation, it was not feasible to use the PDX models to determine mechanisms of resistance and influence therapeutic options as hoped.32 Nevertheless, in vivo studies were performed to better understand the benefits of combination therapy over single-agent letrozole. To ensure the continued expression of ER throughout xenotransplantation and propagation, tissue sections were stained from three original patient tumours and tumours from the corresponding PDX models collected at the completion of in vivo efficacy studies. ER expression was preserved without changes in tumour histological phenotype, thus supporting the rationale for using letrozole in these models (figure 3). Since the EC PDX models had a higher engraftment rate and the EC cohort responded better overall than those with high-grade OC, three EC PDX models (U1561.005, U1561.008 and U1561.010) were tested for clinical effectiveness of combination ribociclib and letrozole compared with letrozole as a single agent. Time from biopsy to completion of animal studies included tumour expansion to generate sufficient mass for efficacy studies: 594 days for model U1561.005, 522 days for model U1561.008 and 361 days for U1561.010. For comparison, the three corresponding patients remained on the clinical trial for 161, 57 and 265 days, respectively. Although tumour regression below baseline was not observed in the three models that underwent in vivo studies (figure 4A), tumours treated with combination ribociclib and letrozole showed a slower progression compared with standard treatment (letrozole). However, differences between these groups with regard to tumour area change from baseline was only observed in model U1561.010 (p=0.017 at 35 days; this time point was chosen because mice in the comparator arm, letrozole, did not survive beyond day 35) and not for U1561.005 or U1561.008 (p=0.23 at 21 days and p=0.92 at 42 days, respectively). Another measure of therapeutic efficacy is animal survival, since strict criteria are followed for humane endpoints of tumour burden and tumour area. Since animal dropout is not easily visualised by standard tumour growth curves, survival analysis was performed to determine if ribociclib added to the efficacy of letrozole. A statistically significant difference in survival was observed in models U1561.005 and U1561.008 for the combination arm when compared with standard treatment (letrozole) (figure 4B). However, the improved outcome seen in U1561.005 was largely influenced by ribociclib alone, which provided a similar change in tumour area (figure 4A) and animal survival compared with combination therapy and untreated controls (online supplemental figure S2). Consistent with the observation that combination of ribociclib and letrozole slowed tumour growth, assessment of tumour proliferation determined by qualitative assessment of Ki67 expression showed reduced staining in tissues exposed to the combination therapy when compared with untreated controls and single-arm letrozole in models U1561.005 and U1561.008 (online supplemental figure S3). No qualitative differences in terms of Ki67 expression were observed in model U1561.010 among the different treatment arms. However, it is notable that this model showed a lower expression of Ki67 in treatment-naïve tissue samples when compared with the other two models, indicating a lower proliferative state at baseline compared with the other models.
Figure 3.
ER expression in primary patient samples and the corresponding PDX. ER expression was detected by immunohistochemistry techniques in primary patient samples (left side) and the corresponding PDX models (right side). Three examples of endometrial carcinomas (U1561.005, U1561.008 and U1561.010) are shown, demonstrating both the ER and histological pattern preservation across samples. Digital images are captured at 70 μm (30×) using ImageScope. PDX, patient-derived xenograft; ER, oestrogen receptor.
Figure 4.
In vivo experiments testing the efficacy of ribociclib and letrozole in three PDX models of recurrent endometrial cancer. Three PDX models were tested in vivo for efficacy studies. (A) Tumour growth curves showed that tumour regression below baseline was not observed in any of the study arms, although a slower proliferation was observed in the combination arm when compared with standard treatment (letrozole) and untreated controls. (B) An overall survival benefit was observed in the combination arm when compared with standard therapy (letrozole) in two of three models. PDX, patient-derived xenograft.
esmoopen-2020-000926supp003.pdf (32.2KB, pdf)
esmoopen-2020-000926supp004.pdf (61.7KB, pdf)
esmoopen-2020-000926supp005.pdf (62.2KB, pdf)
esmoopen-2020-000926supp006.pdf (511.1KB, pdf)
Discussion
Our study demonstrates the potential synergism between ribociclib and letrozole in patients with relapsed ER-positive OC or EC. The greatest benefit was seen in LGSOC and grade 1 and 2 EC. Previously reported data have shown a median PFS of 7 months from AI therapy in LGSOC, which supports the possibility of synergism between ribociclib and letrozole observed in our three patients.33 These three patients obtained a partial or complete response that is ongoing for two of them, and all three were progression-free and on treatment for at least 27 months. Previous reports have described a median duration of clinical benefit of anastrozole in patients with LGSOC of 9.5 months (in the PARAGON trial of 36 patients) and an overall response rate of only 9% in a retrospective study by Gershenson et al.33 34 These findings, together with the low toxicity of the drug combination, makes ribociclib and letrozole in patients with LGSOC a promising treatment, pending confirmatory trials. An ongoing trial of letrozole and ribociclib in patients with recurrent LGSOC (NCT03673124) is evaluating this regimen for this subset of patients.
No significant clinical activity of this combination was observed in the 17 patients with high-grade serous OC given the median PFS of 2.8 months and a PFS24 of only 6%. This may have been due in part to the fact that most of these patients were heavily pretreated, which may had contributed to resistance to this combination.
The PFS24 of 45.4% observed in the ER-positive grade 1 or 2 endometrioid carcinomas, in addition to the PDX study results, suggest that ribociclib and letrozole is a promising treatment for this subset, pending confirmatory trials. The ENGOT-EN3-NSGO/PALEO trial (NCT02730429), a randomised phase II trial in ER-positive ECs, comparing letrozole versus letrozole and palbociclib has completed accrual. The lower PFS24 rate (22.2%) seen in the high-grade EC subset may also reflect the aggressive biology of these tumours and tumour resistance related to previous treatments. Whether greater activity would be seen in high-grade EC patients who were not as heavily pretreated or in those with higher level expression of ER, should be evaluated in future studies.
The selection of patients with tumours with an ER expression of 10% or greater in this trial was arbitrary. ER expression of 1% or greater is considered positive in breast cancer and amenable for aromatase treatment. No established cut-off of ER positivity exists in OCs and ECs. Additional studies are needed to determine whether there is a significant correlation between response to this combination treatment and level of ER expression. A schedule of ribociclib at 400 mg daily without interruption was used instead of the approved schedule used in breast cancer of 600 mg daily for 3 weeks on followed by a week off, in order to obtain additional data on the tolerability of this schedule. The dose intensity of this schedule over a 28-day period is similar to that of the 600 mg schedule (total of 1120 mg ribociclib over 28 days versus 1260 mg over 28 days).
Limitations of our study include the small number of cases of LGSOC, the heavily pretreated nature of most tumours and the non-randomised nature of this trial. Our results suggest that future trials of ECs should stratify for grade, given the marked differences in outcomes observed in these subsets.
Our study also demonstrates a relatively high rate of successful PDX models from biopsy specimens (47%), confirming our previous findings in OC and extending them to EC.35 The success of engraftment is determined largely by the amount of tissue available; therefore, it is higher when tissue is obtained from surgical procedures (74%).21 Coclinical trials with PDX models have been proposed as a tool to discover mechanisms of resistance to therapies that could be used to adjust therapy for the patient from whom the PDX was derived.30 Despite the good engraftment rates, our study highlighted one of the limitations of coclinical trials using ovarian and uterine cancer PDX models; that is, the relatively long time needed to complete in vivo studies. It has been widely described that xenograft models can take long periods of time to grow while the disease in the patient might progress in a relatively short period of time.36 In our study for example, the completion of the three in vivo experiments occurred an average of 492 days after the biopsy, while the three patients remained on trials for an average number of 161 days. As such, a PDX coclinical trial attempting to use PDX response data to impact an individual patient’s treatment would be challenging. However, the coclinical trial strategy still provides an interesting platform for the identification of biomarkers of response and the assessment of therapeutic benefit of novel combinations. In our study, the PDX model data showing a noteworthy prolongation of survival in two of three endometrial models further supports the clinical activity of this combination. PDXs will also allow us to generate resistant tumours and investigate the molecular basis for this resistance.
Conclusion
Combination ribociclib and letrozole is associated with a promising 50% 12-week PFS rate in relapsed ER-positive OC and a 55% 12-week PFS rate in patients with relapsed ER-positive EC, meeting the primary endpoint of the trial. The benefit of this combination treatment was most noticeable in patients with ER-positive recurrent LGSOC, with three of three patients achieving a partial or complete remission lasting at least 27 months, and two of three patients still on treatment. Also, 45% of the patients with relapsed ER-positive grade 1 or 2 endometrioid EC obtained substantial benefit with no evidence of progression for at least 24 weeks. Patients with high-grade serous OC and grade 3 EC did not obtain clinically significant benefit from this treatment. Mild drug-related toxicity was observed, consisting mainly of reversible myelosuppression. Creation of xenograft tumour models from imaging-guided biopsies of OC and EC tumours was feasible, with evidence of demonstrable survival benefit of the combination treatment in the EC PDX models.
Footnotes
Presented at: Presented at 2019 American Society of Clinical Oncology Annual Meeting, 2 June 2019, Chicago, Illinois.
Contributors: Conceptualisation: GCO, VZ, NRF, EJA, JAC and SJW. Data curation: GCO, VZ, XH, NRF, EJA and SJW. Formal analysis: GCO, VZ, XH, NRF, EJA and SJW. Funding acquisition: GCO and SJW. Investigation: GCO, VZ, XH, NRF, AEWH, AJ, MSB, CLL, GEC, TAD, MWR, JKC, KAB, JAC and SJW. Methodology: GCO, VZ, XH, EJA and SJW. Project administration: GCO, VZ, XH and SJW. Resources: GCO and SJW. Software: NRF, EJA and SJW. Supervision: GCO, VZ, NRF, EJA, AEWH, AJ, MSB, CLL, GEC, TAD, MWR, JKC, KAB, JAC and SJW. Validation: GCO, VZ, XH, NRF, EJA and SJW. Visualisation: GCO. Writing – original draft: GCO, VZ, XH, NRF, EJA, AEWH, AJ, MSB, CLL, GEC, TAD, MWR, JKC, KAB, JAC and SJW. Writing – review and editing: GCO, VZ, XH, NRF, EJA, AEWH, AJ, MSB, CLL, GEC, TAD, MWR, JKC, KAB, JAC and SJW.
Funding: Funding for this study was provided by Novartis as an Investigator Initiated Trial and by the Mayo Clinic Cancer Center (NCI CA15083).
Competing interests: GC-O and SJW report grants from Novartis during the conduct of the study. MSB reports grants from Immune Design, Pharmacyclics, Marker Therapeutics, Merck, Genentech and Bristol Myers Squibb, outside the submitted work. We certify that the other authors have no financial affiliation/interest (eg, employment, stock holdings, consultant arrangements, honoraria in the subject matter, materials or products) mentioned in this manuscript.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data is included in the article.
Author note: Published in abstract form: Colon-Otero G, Weroha SJ, Zanfagnin V, et al. Results of a phase II trial of ribociclib and letrozole in patients with either relapsed estrogen receptor (ER)-positive ovarian cancers or relapsed ER-positive endometrial cancers. J Clin Oncol 2019;37 (suppl):abstr 5510. doi: 10.1200/JCO.2019.37.15_suppl.5510.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Andersen CL, Sikora MJ, Boisen MM, et al. Active estrogen receptor-alpha signaling in ovarian cancer models and clinical specimens. Clin Cancer Res 2017;23:3802–12. 10.1158/1078-0432.CCR-16-1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenta PA, Thomas SG, Judson PL, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol 2009;113:205–9. 10.1016/j.ygyno.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 3.Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res 2002;8:2233–9. [PubMed] [Google Scholar]
- 4.del Carmen MG, Fuller AF, Matulonis U, et al. Phase II trial of anastrozole in women with asymptomatic Müllerian cancer. Gynecol Oncol 2003;91:596–602. 10.1016/j.ygyno.2003.08.021 [DOI] [PubMed] [Google Scholar]
- 5.Gourley C, Smyth JF, Mackean M, et al. Phase II study of letrozole in estrogen receptor (ER) positive relapsed epithelial ovarian cancer (EOC). J Clin Oncol 2006;24:5025 10.1200/jco.2006.24.18_suppl.502517075122 [DOI] [Google Scholar]
- 6.Hirakawa H, Yokoyama Y, Yoshida H, et al. Inhibitory effects of aromatase inhibitor on estrogen receptor-alpha positive ovarian cancer in mice. J Ovarian Res 2014;7:4. 10.1186/1757-2215-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanagh JJ, Hu W, Fu S, et al. Anti-tumor activity of letrozole in patients with recurrent advanced low malignant potential or low-grade serous ovarian tumors. J Clin Oncol 2007;25:5582 10.1200/jco.2007.25.18_suppl.5582 [DOI] [Google Scholar]
- 8.Krasner CN, Debernardo RL, Findley M, et al. Phase II trial of anastrazole in combination with gefitinib in women with asymptomatic mullerian cancer. J Clin Oncol 2005;23:5063 10.1200/jco.2005.23.16_suppl.5063 [DOI] [Google Scholar]
- 9.Li YF, Hu W, Fu SQ, et al. Aromatase inhibitors in ovarian cancer: is there a role? Int J Gynecol Cancer 2008;18:600–14. 10.1111/j.1525-1438.2007.01075.x [DOI] [PubMed] [Google Scholar]
- 10.Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology 2004;66:112–7. 10.1159/000077436 [DOI] [PubMed] [Google Scholar]
- 11.Rose PG, Brunetto VL, VanLe L, et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a gynecologic Oncology Group study. Gynecol Oncol 2000;78:212–6. 10.1006/gyno.2000.5865 [DOI] [PubMed] [Google Scholar]
- 12.Tchekmedyian NS, Liem AK, Quan ET, et al. Aromatase inhibitor therapy for estrogen receptor positive ovarian cancer. J Clin Oncol 2006;24:15038 10.1200/jco.2006.24.18_suppl.15038 [DOI] [Google Scholar]
- 13.Verma S, Alhayki M, Le T, et al. Phase II study of exemestane (E) in refractory ovarian cancer (ROC). J Clin Oncol 2006;24:5026 10.1200/jco.2006.24.18_suppl.5026 [DOI] [Google Scholar]
- 14.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer 2019;19:326–38. 10.1038/s41568-019-0143-7 [DOI] [PubMed] [Google Scholar]
- 15.Caldon CE, Sergio CM, Kang J, et al. Cyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cells. Mol Cancer Ther 2012;11:1488–99. 10.1158/1535-7163.MCT-11-0963 [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 17.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 18.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 19.Frisone D, Charrier M, Clement S, et al. Durable response to palbociclib and letrozole in ovarian cancer with CDKN2A loss. Cancer Biol Ther 2020;21:197–202. 10.1080/15384047.2019.1685291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konecny GE, Wahner Hendrickson AE, Jatoi A, et al. A multicenter open-label phase II study of the efficacy and safety of palbociclib a cyclin-dependent kinases 4 and 6 inhibitor in patients with recurrent ovarian cancer. J Clin Oncol 2016;34:5557 10.1200/JCO.2016.34.15_suppl.5557 [DOI] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 23.Weroha SJ, Becker MA, Enderica-Gonzalez S, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res 2014;20:1288–97. 10.1158/1078-0432.CCR-13-2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler KA, Hou X, Becker MA, et al. Prevention of human lymphoproliferative tumor formation in ovarian cancer patient-derived xenografts. Neoplasia 2017;19:628–36. 10.1016/j.neo.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meehan TF, Conte N, Goldstein T, et al. PDX-MI: minimal information for patient-derived tumor xenograft models. Cancer Res 2017;77:e62–6. 10.1158/0008-5472.CAN-17-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AlHilli MM, Becker MA, Weroha SJ, et al. In vivo anti-tumor activity of the PARP inhibitor niraparib in homologous recombination deficient and proficient ovarian carcinoma. Gynecol Oncol 2016;143:379–88. 10.1016/j.ygyno.2016.08.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser G, Weroha SJ, Becker MA, et al. Conventional chemotherapy and oncogenic pathway targeting in ovarian carcinosarcoma using a patient-derived tumorgraft. PLoS One 2015;10:e0126867. 10.1371/journal.pone.0126867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres D, Hou X, Bale L, et al. Overcoming platinum resistance in ovarian cancer by targeting pregnancy-associated plasma protein-A. PLoS One 2019;14:e0224564. 10.1371/journal.pone.0224564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Tiedt R, Loo A, et al. The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget 2018;9:35226–40. 10.18632/oncotarget.26215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue W, Wang J, Savinov A, et al. Effect of aromatase inhibitors on growth of mammary tumors in a nude mouse model. Cancer Res 1995;55:3073–7. [PubMed] [Google Scholar]
- 31.Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 2009;59:234–41. [PMC free article] [PubMed] [Google Scholar]
- 32.Clohessy JG, Pandolfi PP. Mouse hospital and co-clinical trial project--from bench to bedside. Nat Rev Clin Oncol 2015;12:491–8. 10.1038/nrclinonc.2015.62 [DOI] [PubMed] [Google Scholar]
- 33.Gershenson DM, Sun CC, Iyer RB, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 2012;125:661–6. 10.1016/j.ygyno.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang M, O'Connell RL, Amant F, et al. PARAGON: a phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol 2019;154:531–8. 10.1016/j.ygyno.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 35.Colon-Otero G, Weroha SJ, Foster NR, et al. Phase 2 trial of everolimus and letrozole in relapsed estrogen receptor-positive high-grade ovarian cancers. Gynecol Oncol 2017;146:64–8. 10.1016/j.ygyno.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 36.Byrne AT, Alférez DG, Amant F, et al. Interrogating open issues in cancer medicine with patient-derived xenografts. Nat Rev Cancer 2017;17:632. 10.1038/nrc.2017.85 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000926supp001.pdf (79.2KB, pdf)
esmoopen-2020-000926supp002.pdf (202.5KB, pdf)
esmoopen-2020-000926supp003.pdf (32.2KB, pdf)
esmoopen-2020-000926supp004.pdf (61.7KB, pdf)
esmoopen-2020-000926supp005.pdf (62.2KB, pdf)
esmoopen-2020-000926supp006.pdf (511.1KB, pdf)