Abstract
Introduction
Evidence-based smoking cessation treatments are effective but underutilised, accentuating the need for novel approaches to increase use. This trial investigates the effects of active referral combined with a financial incentive to use smoking cessation services on smoking abstinence among community smokers.
Methods and analysis
This ongoing study is a two-arm, assessor-blinded, pragmatic, cluster randomised controlled trial with follow‐ups at 1, 2, 3 and 6 months after randomisation. We aim to enrol 1134 daily smokers from 70 community sites (clusters) in Hong Kong. All participants receive Ask, Warn, Advise, Refer, Do-it-again (AWARD) guided advice and a self-help booklet at baseline. Additionally, participants in the intervention group receive an offer of referral to smoking cessation services at baseline and a small financial incentive (HK$300≈US$38) contingent on using any of such services within 3 months. The primary outcomes are bioverified abstinence (exhaled carbon monoxide <4 ppm and salivary cotinine <10 ng/mL) at 3 and 6 months. Secondary outcomes include self-reported 7-day point prevalence of abstinence, smoking reduction rate, quit attempts and the use of smoking cessation services at 3 and 6 months. Intention-to-treat approach and regression models will be used in primary analyses.
Ethics and dissemination
This protocol has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB reference number: UW 18-318). The results of this trial will be submitted for publication in peer-reviewed journals, and the key findings will be presented at national and international conferences.
Trial registration number
ClinicalTrials.gov Registry NCT03565796.
Keywords: public health, protocols & guidelines, clinical trials
Strengths and limitations of this study.
This trial examines the effectiveness of active referral combined with a financial incentive to increase the use of smoking cessation services in promoting abstinence in the community.
A proactive approach is used to recruit smokers from a broader, community-based population, who are mostly undetermined to quit in the short term.
Using biochemically verified abstinence as the primary outcome increases scientific rigour and decreases misreporting.
The findings of this trial may be less generalisable to other countries lacking accessible and affordable smoking cessation services.
Introduction
Smoking cessation counselling and medications are cost-effective in reducing tobacco-related morbidity and mortality.1–3 Effective smoking cessation treatments are readily available, yet service utilisation is low, as 70% of the world’s population does not have access to cessation services.4 Publicly funded services for smoking cessation are widely available in Hong Kong5–7; however, very few daily smokers (2.7%) use existing treatments that are proven to be effective.8
To increase the use of smoking cessation services, we designed sequential trials of active referral approaches that proactively connect community smokers with smoking cessation service providers, yielding promising results. Call-back referral (CBR), which assists smokers to book their preferred service provider by calling them back to arrange an appointment for smoking cessation treatment, showed a significantly higher bioverified abstinence at 6 months than did a control condition in which participants received advice according to the Ask, Warn, Advise, Refer, Do-it-again (AWARD) model (9.0% vs 5.0%; odds ratio (OR) 1.85, 95% confidence interval (CI) 1.06 to 3.23, p=0.04).9 We sequentially proposed two active referral approaches with different intensities: onsite referral (OSR), which assists smokers to book appointments with preferred service providers during onsite recruitment, and text messaging referral (TMR), which uses mobile text messaging to promote the use of smoking cessation services. The two modified approaches showed significantly higher bioverified abstinence at 6 months than AWARD-guided advice (7.6% and 7.8%, vs 3.9%; OR for OSR vs control=2.02, 95% CI 1.07 to 3.81; OR for TMR vs control=2.07, 95% CI 1.10 to 3.92; both p<0.050).10 Active referral approaches were effective but adherence was suboptimal, as less than 27% of participants used the smoking cessation service within the 6-month period after receiving active referrals (25.1% in OSR, 26.8% in CBR and 8.1% in TMR).
Financial incentives are external motivators and may increase intervention adherence and service attendance.11 12 Financial incentives increased service enrolment13 and use of tobacco-dependent treatment (medications, nicotine replacement therapies and counselling)14–17 and service providers have offered them effective treatments to increase abstinence.12 Our previous trial revealed that time constraints and low interest are the main barriers to use smoking cessation services.9 Although smoking cessation services in Hong Kong are mostly free or charge minimal fees, proactive models that offer referral assistance with a small financial incentive may increase smokers’ motivation to overcome perceived barriers. However, incentive-based trials to increase both service use and abstinence have shown mixed findings. Our previous community-based trial suggested that offering a cash incentive (HK$500≈US$64) for successful quitting increased quit attempts but did not increase service use or abstinence.18 Recent trials have shown that referral assistance (eg, proactive calls, patient navigation) combined with a financial incentive increased treatment engagement and abstinence among smokers of low socioeconomic status.14 19 20 Based on previous trials, it seems more effective to offer a financial incentive to increase the use of smoking cessation services among population-based, community-recruited smokers.
In this trial, we aim to investigate whether a small financial incentive (HK$300≈US$38) combined with active referral (CBR model) and brief (eg, AWARD-guided) advice in the community will increase bioverified abstinence at 6 months. We anticipate that the financial incentive will enhance smokers’ motivation to use the services.
Methods and analysis
Study design
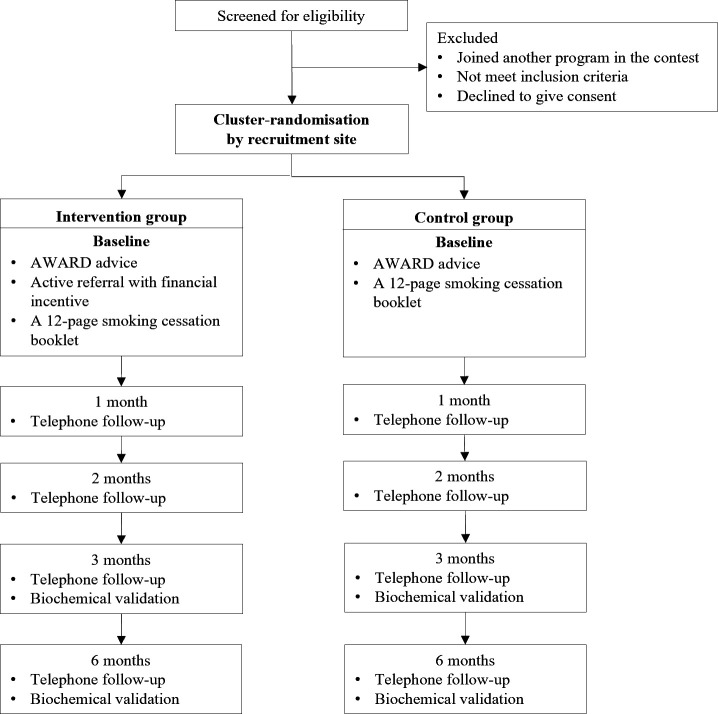
This is a two-arm, assessor-blinded, pragmatic, cluster randomised controlled trial nested within the ninth ‘Quit to Win’ (QTW) Smoke-Free Community Campaign. The QTW campaign9 10 18 21–24 is a community-based smoking cessation contest organised annually by the Hong Kong Council on Smoking and Health. Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Figure 1.
CONSORT flow diagram. AWARD, Ask, Warn, Advise, Refer, Do-it-again; CONSORT, Consolidated Standards of Reporting Trials.
Recruitment and participants
Similar to the previous QTW campaign9 10 18 21–24 recruitment activities are conducted in community sites (n=70) (eg, shopping malls and public areas) of all 18 Hong Kong districts. Using the ‘foot-in-the-door’ approach,25 trained smoking cessation advisors proactively approach smokers at smoking hotspots in the vicinity of recruitment booths, explain the QTW contest and invite smokers to participate. Smokers are informed that the intervention involves a baseline assessment of their exhaled carbon monoxide level, brief questions on past smoking behaviours (baseline questionnaire) and further telephone interviews (follow‐up questionnaires at 1, 2, 3 and 6 months). Eligible participants are Hong Kong residents aged ≥18 years, currently smoking ≥1 cigarette per day during the past 3 months, with an exhaled carbon monoxide level ≥4 ppm, able to communicate in Cantonese or read Chinese, and motivated to quit or reduce smoking. Exclusion criteria are either having physical or cognitive difficulties in communication or currently participating in other smoking cessation programmes.
Randomisation and blinding
Randomisation occurs at the community level. Participants within the same recruitment session are cluster randomised in a 1:1 ratio to the intervention or control group. The randomisation sequence (random permuted blocks of 2, 4 and 6) is generated using a web-based system (www.sealedenvelope.com). One investigator who is not involved in participant enrolment implements the allocation sequence and notifies the recruitment staff 1 day prior to the recruitment session. Because of the nature of the intervention, the recruitment staff delivering the interventions cannot be blinded to participant allocation but participants are not informed about the treatment in the other group. Outcome assessors and statistical analysts are blinded to the group allocation.
Sample size
Our previous trial showed that bioverified abstinence at 6 months was about 9.0% in the CBR group and 5% in the control group.9 Full financial coverage of the costs of smoking cessation treatment had an effect size of 1.77 on abstinence when compared with no incentive in a healthcare setting.12 We conservatively estimate an effect size of 1.25 for a small financial incentive to use smoking cessation services combined with CBR in a community-based trial. Validated abstinence at 6 months is therefore expected to be 11.0% in the intervention group and 5% in the control group. Using G*Power software, in order to achieve a 95% CI (alpha=0.05) and 80% power (1‐beta=0.80), the required sample size was calculated to be 286 per group. Assuming an intracluster correlation coefficient of 0.01522 with an average cluster size of 17 and a retention rate of 70% at 6-month follow-up,9 10 the overall sample size of the study should be 1134 for the two groups (320×2 groups×1.24 design effect/70% retention rate).
Treatment integrity
Smoking cessation advisors are recruited through university mass emails and advertising posters. They include university students (with an hourly rate of HK$66≈US$8.5) and volunteers of non-governmental organisations. All smoking cessation advisors are required to attend a full-day workshop (6 hours) before participant recruitment. The contents of the workshop include: (1) overview of QTW contest, intervention contents (eg, AWARD-guided advice, active referral and financial incentives) and recruitment demonstration (eg, foot-in-the-door approach, test on exhaled carbon monoxide); (2) knowledge of smoking harms and quitting benefits; (3) smoking cessation methods and counselling techniques and (4) sharing sessions of ex-smokers. We conduct a pre-test and post-test to assess advisors’ knowledge, attitudes and practice regarding smoking cessation.
An experienced research staff provides supervision and assistance at each recruitment session. To ensure the accurate delivery of the intervention, all advisors are instructed to follow a standardised recruitment script and complete an adherence checklist outlining each of the intervention components. Eligible smokers who decline to participate are asked to provide a reason for refusing. Information on the number of approached smokers is gathered and smokers’ declining reasons are recorded verbatim by smoking cessation advisors.
Interventions
AWARD-guided advice
Well-trained smoking cessation advisors deliver advice based on the AWARD model to both intervention and control groups on site. AWARD-guided advice lasts 3–5 min and includes five steps: (1) ask about the smoking history, (2) warn smokers about the harms of smoking (using the result of exhaled carbon monoxide level), (3) advise to quit or reduce smoking as soon as possible, (4) refer to existing cessation services and (5) do it again if smokers fail to quit. Participants also receive a 12-page generic self-help booklet used in our previous trials.9 10 18 21–24 The contents of the self-help booklet include smoking harms, benefits and methods of quitting, relapse prevention and existing smoking cessation services.
CBR to smoking cessation services
Participants in the intervention group receive intensified interventions based on Refer and Do it again, which is a more tailored and personalised approach than that of the control group.
At baseline, smoking cessation advisors assist participants to choose their preferred services using a threefold pocket-sized referral card, which outlines the five major smoking cessation services in Hong Kong, together with available therapies, opening hours and branch locations (see online supplemental appendix 1). For participants who agree to be referred, research staff email participants’ name and telephone number to the chosen service providers within 1 week (Refer). The providers call-back participants within 1–2 weeks and arrange an appointment for telephone counselling or a smoking cessation clinic visit. Research staff monitor the use of smoking cessation services at each follow-up (1, 2, 3 and 6 months) and encourage and assist participants to book or rebook the services if they fail to quit (Do it again).
bmjopen-2020-038351supp001.pdf (855.2KB, pdf)
Incentives for promoting smoking cessation service use
Participants in the intervention group are informed that they will receive a small financial incentive for using any of the smoking cessation services within 3 months. The incentive is a HK$300 (≈US$38) coupon for a popular local supermarket. Participants who agree to book the smoking cessation services sign two copies of the referral form stating that they are willing to use the selected services (see online supplemental appendix 2). Participants keep one copy as information/reminder; research staff retain one copy for the records. The conditions for receiving the incentive are also outlined in the referral form. The incentive has no restrictions on the type of smoking cessation treatments used, which include pharmacotherapy (eg, nicotine replacement therapy), behavioural support (eg, face-to-face/phone counselling, group therapy) or a combination thereof. Postpayment financial incentives are distributed to participants in the intervention group who self-report using the smoking cessation service at 1-month, 2-month and 3-month follow-ups. The mailing procedure is standardised. The incentive is sent by registered mail with an accompanying cover letter explaining the purpose of the incentive.
Procedures
Participants are assessed at baseline, 1, 2, 3 and 6 months after treatment initiation (table 1). The baseline questionnaire measures participants’ smoking behaviour (eg, daily cigarette consumption, age of starting smoking, time of first cigarette on waking up in the morning, attempts to quit or reduce, methods used in past quit attempts), intention to quit, perceived self-efficacy regarding quitting (importance, difficulties and confidence) and sociodemographic characteristics. Participants are informed that they may withdraw from the study at any time without giving a reason. Participants are followed up at 1, 2, 3 and 6 months by trained smoking cessation counsellors with a maximum of seven telephone calls at different times. Participants who self-report abstinence for more than 7 days at 3 and 6 months are invited for a biochemical validation. Exhaled carbon monoxide samples are collected by research staff with a piCO Smokerlyzer (Bedfont Scientific) and saliva cotinine samples are measured using a NicAlert test strip (Nymos Pharmaceutical Corporation). To increase participation, participants receive a cash incentive of HK$500 (≈US$64) for passing the biochemical validation at 3 and 6 months.
Table 1.
Schedule of baseline and follow‐up assessments
| Assessments | Time‐point | ||||
| Baseline | 1 month | 2 months | 3 months | 6 months | |
| Informed consent (see online supplemental appendix 3) | × | ||||
| Eligibility screen | × | ||||
| Randomisation | × | ||||
| Intervention/control initiation | × | ||||
| Sociodemographic characteristics* | × | ||||
| Smoking behaviour | × | × | × | × | × |
| Quit attempts | × | × | × | × | × |
| Use of smoking cessation services | × | × | × | × | × |
| Self-efficacy of quitting | × | × | × | ||
| Biochemically validated abstinence | × | × | |||
| Qualitative evaluation | × | ||||
*Sociodemographic characteristics include age, sex, education level, marital status and household income.
Outcomes
The primary outcomes are bioverified abstinence at 3 months (end of treatment) and 6 months after treatment initiation confirmed by an exhaled carbon monoxide level <4 ppm and salivary cotinine level <10 ng/mL.26 27
Secondary outcomes include the following:
Self-reported 7-day point-prevalence abstinence.
Smoking reduction, defined by at least 50% reduction in daily cigarette consumption compared with that at baseline.
Quit attempts.
Cumulative use of smoking cessation services, defined by using at least one treatment session (eg, face-to-face/phone counselling, nicotine replacement therapy, acupuncture).
Statistical analyses
Data will be analysed according to intention-to-treat principles. Chi-squared and t tests will be used to compare baseline the characteristics of participants to assess balance between the two groups. The intervention effect on primary and secondary outcomes will be analysed using regression models with and without adjustment for imbalanced baseline characteristics. Generalised estimating equation models will be used to adjust for the potential clustering effect of recruitment sessions. Analysis of variance method will be used to calculate intracluster correlation for abstinence outcomes. Sensitivity to missing data will be examined using multiple imputation by chained equations assuming the data will be missing at random.28
We will also examine the association between intervention adherence (eg, received referral, used smoking cessation services, received financial incentive) and the primary outcome within the participants in the intervention group. The intervention effect by subgroups will be assessed, respectively, including age group, sex, education level, household income, previous quit attempts, cigarette dependence and intention to quit. Statistical analyses will be conducted using Stata V.15.1 (Stata Corp, Texas, USA).
Post-trial qualitative evaluation
Qualitative evaluations using a subsample of participants receiving the intervention will be conducted after the end of the study. The semistructured interview aims to explore participants’ experience of the intervention and adherence to it and obtain study feedback. The sample size for the qualitative evaluation will be determined by data saturation. Participants will be sampled purposively based on sociodemographic characteristics, smoking status and intervention adherence. We anticipate that up to 20 participants will be included subject to data saturation. All interviews will be audio-recorded and transcribed verbatim. The transcripts will be organised using a thematic framework29 based on topics specified in the interview guide and emerging themes identified through a process of familiarisation with the transcripts.
Patient and public involvement
Neither patients nor the public are directly involved in the study design or conduct of the study. Study results will be disseminated to the general public.
Ethics and dissemination
The trial is conducted and reported in accordance with the CONSORT statement for clinical trials reporting and has been registered at ClinialTrials.gov Registry (registration number: NCT03565796). Ethical approval has been granted by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB reference number: UW 18-318). Authorship will be determined in accordance with the International Committee of Medical Journal Editors guidelines. Findings will be published in peer-reviewed journals and presented at local, national and international conferences to publicise and explain the research to key audiences.
Discussion
This trial uses active referral plus a financial incentive as a model to increase smoking cessation attendance and abstinence in the community. If the intervention is found to be effective, this will be valuable for decision-makers to prioritise financial support to encourage the use of smoking cessation services, which will ultimately increase smoking abstinence.
This trial is innovative for three main reasons. First, as one of the sequential interventions using active referral, our trial combining an active referral with a financial incentive has important implications for research and practice. We intensified the CBR model by incentivising service use, which is easy to implement in practice. Compared with OSR and TMR models, CBR plus incentive shifts the burden of OSR and uses money (instead of low-intensity text messaging) to motivate service use. Our findings regarding the effectiveness of different models of active referral provide insight into the development of high-quality adaptive trials30 on smoking cessation. Second, a handful of trials used incentives to reward successful cessation;31 however, we provide financial incentives to increase service use. Strategies to increase adherence to smoking cessation treatment are important but understudied.32 Our findings will provide evidence regarding the use of incentives to increase the motivation to use services. Third, the incentive amount in our trial (≈US$38) is much smaller than that in the incentive-based trials (ranging from US$45 to US$1185) included in a recent meta-analysis,31 which showed that the incentive size had no impact on cessation outcomes. Large incentives probably cannot be sustained in real-world practice. Small incentives may be adequate for behavioural change if using an effective approach to deliver the potential health benefits.33
This trial has a number of strengths. We use a proactive approach to recruit smokers from a broader, community-based population, who are not in clinical settings and are mostly undetermined to quit in the short term. The brief intervention mode for promoting smoking cessation is flexible, feasible and low cost. Moreover, we use bioverified abstinence (ie, exhaled carbon monoxide and salivary cotinine tests) as the primary outcome to increase scientific rigour and decrease misreporting.34
This trial also has several potential limitations. First, the trial is pragmatic and cannot completely disentangle the effect of each intervention component (brief advice, active referral, financial incentive). However, we are more interested in the combined effect of the multicomponent trial, which targets several barriers for maintaining abstinence. Future research comparing the effect of different levels of active referral (eg, CBR plus incentive vs CBR only) on abstinence is warranted. Second, we are unable to assess the long-term effects of the intervention (eg, 12 months) because of budget constraints. Nevertheless, four consecutive follow-ups (at 1, 2, 3 and 6 months) allow us to keep track of cessation outcomes, service use and changes in cessation-related factors in the short term (≤6 months). Third, the evidence on the use of smoking cessation services is based on self-reporting. This is done for practical reasons as the records of service utilisation cannot be directly obtained by the research team. Fourth, as women’s smoking prevalence rates are relatively low in Hong Kong,8 we expect a higher proportion of male participants relative to female participants. This may limit the generalisability of our findings to other settings where female smoking is more prevailing (eg, Western countries). Fifth, our findings may be less generalisable to other countries lacking accessible and affordable smoking cessation services.
Supplementary Material
Acknowledgments
The authors would like to thank the participants, helpers from the universities and non-governmental organisations and research assistants who involved in this study.
Footnotes
Contributors: MPW, XW and THL participated in study concept and design. MPW, XW and CYL participated in conducting the study. XW drafted the manuscript. MPW, HCWL, YTDC, ACSK, VWYL and SSCC provided critical comments. All authors have read and approved the final manuscript.
Funding: This study is funded by the Hong Kong Council on Smoking and Health. The funders have no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclaimer: The funding source do not have any role in the study design, data collection, analysis and interpretation, writing of the report or the decision to submit the paper for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Ronckers ET, Groot W, Ament AJHA. Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness. Med Decis Making 2005;25:437–48. 10.1177/0272989X05278431 [DOI] [PubMed] [Google Scholar]
- 2.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017;3:CD001292. 10.1002/14651858.CD001292.pub3 [DOI] [PubMed] [Google Scholar]
- 3.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2017;3:CD001007. 10.1002/14651858.CD001007.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO report on the global tobacco epidemic, 2019: offer help to quit tobacco use. Geneva: World Health Organization, 2019. [Google Scholar]
- 5.Wang Y-Y, Liu Z, Wu Y, et al. Acupuncture for smoking cessation in Hong Kong: a prospective multicenter observational study. Evid Based Complement Alternat Med 2016;2016:1–8. 10.1155/2016/2865831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S, Leung D, Chan H, et al. An evaluative study of the integrated smoking cessation services of Tung Wah group of hospitals, 2011. [Google Scholar]
- 7.Li WHC, Chan SSC, Wang MP, et al. An evaluation of the youth quitline service young Hong Kong smokers. J Adolesc Health 2017;60:584–91. 10.1016/j.jadohealth.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 8.Census and Statistics Department Thematic household survey, report No.70: pattern of smoking. Hong Kong SAR: Hong Kong SAR Government, 2020. [Google Scholar]
- 9.Wang MP, Suen YN, Li WH-C, et al. Intervention with brief cessation advice plus active referral for proactively recruited community smokers: a pragmatic cluster randomized clinical trial. JAMA Intern Med 2017;177:1790–7. 10.1001/jamainternmed.2017.5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng X, Luk TT, Suen YN, et al. Effects of simple active referrals of different intensities on smoking abstinence and smoking cessation services attendance: a cluster-randomized clinical trial. Addiction 2020;115:1902–12. 10.1111/add.15029 [DOI] [PubMed] [Google Scholar]
- 11.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med 2009;360:699–709. 10.1056/NEJMsa0806819 [DOI] [PubMed] [Google Scholar]
- 12.van den Brand FA, Nagelhout GE, Reda AA, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database Syst Rev 2017;9:CD004305. 10.1002/14651858.CD004305.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpp KG, Gurmankin Levy A, Asch DA, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev 2006;15:12–18. 10.1158/1055-9965.EPI-05-0314 [DOI] [PubMed] [Google Scholar]
- 14.Fraser DL, Fiore MC, Kobinsky K, et al. A randomized trial of incentives for smoking treatment in Medicaid members. Am J Prev Med 2017;53:754–63. 10.1016/j.amepre.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker TB, Fraser DL, Kobinsky K, et al. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: effects on post-birth abstinence. J Consult Clin Psychol 2018;86:464–73. 10.1037/ccp0000278 [DOI] [PubMed] [Google Scholar]
- 16.Anderson CM, Cummins SE, Kohatsu ND, et al. Incentives and patches for Medicaid smokers: an RCT. Am J Prev Med 2018;55:S138–47. 10.1016/j.amepre.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 17.Tong EK, Stewart SL, Schillinger D, et al. The medical incentives to quit smoking project: impact of statewide outreach through health channels. Am J Prev Med 2018;55:S159–69. 10.1016/j.amepre.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 18.Cheung YTD, Wang MP, Li HCW, et al. Effectiveness of a small cash incentive on abstinence and use of cessation AIDS for adult smokers: a randomized controlled trial. Addict Behav 2017;66:17–25. 10.1016/j.addbeh.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 19.Lasser KE, Quintiliani LM, Truong V, et al. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety-net Hospital: a randomized clinical trial. JAMA Intern Med 2017;177:1798–807. 10.1001/jamainternmed.2017.4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks MJ, Hughes KD, Keller PA, et al. Financial incentives and proactive calling for reducing barriers to tobacco treatment among socioeconomically disadvantaged women: a factorial randomized trial. Prev Med 2019;129:105867. 10.1016/j.ypmed.2019.105867 [DOI] [PubMed] [Google Scholar]
- 21.Chan SSC, Wong DCN, Cheung YTD, et al. A block randomized controlled trial of a brief smoking cessation counselling and advice through short message service on participants who joined the quit to WIN contest in Hong Kong. Health Educ Res 2015;30:609–21. 10.1093/her/cyv023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SSC, Cheung YTD, Wong YMB, et al. A brief smoking cessation advice by youth counselors for the smokers in the Hong Kong quit to WIN contest 2010: a cluster randomized controlled trial. Prev Sci 2018;19:209–19. 10.1007/s11121-017-0823-z [DOI] [PubMed] [Google Scholar]
- 23.Wang MP, Li WH, Cheung YT, et al. Brief advice on smoking reduction versus abrupt quitting for smoking cessation in Chinese smokers: a cluster randomized controlled trial. Nicotine Tob Res 2017;20:67–72. 10.1093/ntr/ntx026 [DOI] [PubMed] [Google Scholar]
- 24.Wang MP, Luk TT, Wu Y, et al. Chat-based instant messaging support integrated with brief interventions for smoking cessation: a community-based, pragmatic, cluster-randomised controlled trial. Lancet Digit Health 2019;1:e183–92. 10.1016/S2589-7500(19)30082-2 [DOI] [PubMed] [Google Scholar]
- 25.Freedman JL, Fraser SC. Compliance without pressure: the foot-in-the-door technique. J Pers Soc Psychol 1966;4:195–202. 10.1037/h0023552 [DOI] [PubMed] [Google Scholar]
- 26.Javors MA, Hatch JP, Lamb RJ. Cut-Off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction 2005;100:159–67. 10.1111/j.1360-0443.2004.00957.x [DOI] [PubMed] [Google Scholar]
- 27.Cooke F, Bullen C, Whittaker R, et al. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res 2008;10:607–12. 10.1080/14622200801978680 [DOI] [PubMed] [Google Scholar]
- 28.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J 2009;9:466–77. 10.1177/1536867X0900900308 [DOI] [Google Scholar]
- 29.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 30.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016;375:65–74. 10.1056/NEJMra1510061 [DOI] [PubMed] [Google Scholar]
- 31.Notley C, Gentry S, Livingstone-Banks J, et al. Incentives for smoking cessation. Cochrane Database Syst Rev 2019;7:CD004307. 10.1002/14651858.CD004307.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollands GJ, Naughton F, Farley A, et al. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database Syst Rev 2019;8:CD009164. 10.1002/14651858.CD009164.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland K, Christianson JB, Leatherman S. Impact of targeted financial incentives on personal health behavior: a review of the literature. Med Care Res Rev 2008;65:36S–78. 10.1177/1077558708324235 [DOI] [PubMed] [Google Scholar]
- 34.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res 2020;22:1086–97. 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038351supp001.pdf (855.2KB, pdf)