Abstract
Thrombotic complications of the novel coronavirus (COVID-19) are a concerning aspect of the disease, due to the high incidence in critically ill patients and poor clinical outcomes. COVID-19 predisposes patients to a hypercoagulable state, however, the pathophysiology behind the thrombotic complications seen in this disease is not well understood. Several mechanisms have been proposed and the pathogenesis likely involves a host immune response contributing to vascular endothelial cell injury, inflammation, activation of the coagulation cascade via tissue factor expression, and shutdown of fibrinolysis. Treatments targeting these pathways may need to be considered to improve clinical outcomes and decrease overall mortality due to thrombotic complications. In this review, we will discuss the proposed pathophysiologic mechanisms for thrombotic complications in COVID-19, as well as treatment strategies for these complications based on the current literature available.
Keywords: venous thromboembolism, coagulopathy, embolism
Introduction
The first cases of COVID-19 began in Wuhan, Hubei Province, China, in December 2019.1 Since then, this disease caused by a highly pathogenic virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been labeled as a global pandemic. It is believed that the primary means of viral spread is via respiratory droplets during close-range, human-to-human contact.2 Currently, over 26 million cases have been confirmed across the globe.3 Thrombotic complications are a concerning aspect of this disease because of poor outcomes.4,5 The first case of COVID-19-induced pulmonary embolism (PE), in association with acute heart failure, was reported on March 16, 2020.6 During the SARS-CoV-1 epidemic between 2002-2004, the reported incidence of deep vein thrombosis (DVT) and PE was 20% and 11%, respectively.7 The incidence of thrombotic complications in patients with COVID-19 has been reported to be as high as 79%.8 With this in mind, the rising incidence of thrombosis during the current pandemic warrants further investigation into the proposed mechanisms and current treatment modalities being utilized.
Coronaviridae comprises a family of enveloped, positive-sense, single-stranded, RNA viruses.9 There are 4 genera of Coronaviridae: alpha, beta, gamma, and delta. The alpha and beta genera are known to only infect mammals.10 Human coronavirus strains causing the SARS-CoV-1 epidemic and COVID-19 pandemic belong to the genus of beta coronaviruses.9 Transmission occurs primarily via the exchange of respiratory droplets; however, it is suspected that fomites may also be involved in viral transmission, as the virus can remain viable in air droplets for hours and on surfaces for days.11
There are 4 proteins involved in the structure of coronaviruses. These include the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins.9 Upon entry into the human respiratory tract, the spike (S) protein binds to the angiotensin converting enzyme 2 (ACE2) receptor, which is highly expressed by pulmonary type 2 pneumocytes, cardiac myocytes, and vascular endothelial cells.9,10,12 Binding of the S protein to the ACE2 receptor results in proteolytic cleavage of the ACE2 receptor and activation of the S protein by type 2 transmembrane protease (TMPRSS2), facilitating entry of the viral particles into the cell.10,13 Subsequent entry of SARS-CoV-2 into a cell then triggers apoptosis via ectopic expression of the E protein and interaction between the C-terminal region of E protein and Bcl-xL, a member of the anti-apoptotic Bcl-2 family.14 Ultimately, phagocytosis of the apoptotic cell by antigen presenting cells (APCs) occurs, specifically macrophages and dendritic cells.9 Presentation by APCs leads to activation of the immune response and an increase in the release of inflammatory mediators, including IL-6, IL-10, G-CSF, and TNF-alpha.9
Patients with COVID-19 typically present with symptoms of fever, cough, and dyspnea.15–17 Less common symptoms include myalgias/fatigue, rhinorrhea, sore throat, headache, and diarrhea.15 Common laboratory findings include lymphocytopenia and elevated CRP, while other lab abnormalities in cases complicated by coagulopathy include elevated D-dimer, thrombocytopenia, prolonged PT, elevated fibrinogen, elevated lactate dehydrogenase, and elevated ferritin.16,18 Typical imaging findings include bilateral ground glass opacities, as well as bilateral multiple lobular and subsegmental areas of consolidation.19 Complications include respiratory failure, septic shock, and multiple organ failure in severe cases.20
Thrombosis in COVID-19 Infections
There is a striking amount of evidence emerging that suggests the presence of a hypercoagulable state in patients with COVID-19. This is particularly important because of the poor clinical outcomes associated with this complication in critically-ill patients.4,5 In fact, the incidence of pulmonary thrombosis in COVID-19 infected patients in some studies has been reported to be as high as 79% (Table 1).4,8,21–25 Table 1 further summarizes the incidence of thrombotic events reported in the literature thus far in patients with COVID-19, and even summarizes the incidence of thrombotic events in ICU versus non-ICU patients. It should be noted that in studies that specifically looked at ICU patients, incidence of thrombosis ranged from 31% to 79% and were higher than non-ICU patients.4,8,22,25 Studies that reported incidences in non-ICU patients showed rates of thrombosis from 9.2% to 15%.22,25 Furthermore, in one study, autopsy findings of 12 COVID-19 patients revealed that 58% had undiagnosed deep vein thromboses, with the direct cause of death being massive pulmonary embolism in 4 of these patients.23 These findings are of particular significance, as the incidence of pulmonary thromboembolism in other common causes of bacterial or viral pneumonia is reported to be only around 1% to 2.6%.26 Furthermore, from Table 1, it can be concluded from multiple studies that critically ill patients have a considerably higher risk of thrombosis than non-ICU patients.4,8,22,24,25 The reason for this may be due to the increased pro-inflammatory and anti-fibrinolytic state seen in patients with more severe infection.27 In a multi-center, retrospective cohort study by Zhou et al., it was found that the 54 patients who had died due to COVID-19 infection were more likely to have severe lymphopenia, as well as elevated D-dimer, cardiac troponin, ferritin, lactate dehydrogenase, and IL-6 levels, providing support for this notion.27
Table 1.
Incidence of Thrombotic Events in Patients Diagnosed With COVID-19 Infections.
| Study | Sample size | Thrombotic event reported | Confirmatory diagnostic test | Incidence |
|---|---|---|---|---|
| Klok et al.4 | N = 184 ICU patients | Venous or arterial thrombosis | CTPA or Ultrasound | 31% |
| Leonard-Lorant et al.21 | N = 106 (48 ICU and 58 non-ICU) | Acute PE | CTPA | 30% of all COVID-19 patients developed PE irrespective of ICU status |
| Helms et al.22 | N = 150 ICU patients | Clinically significant thrombosis | CTPA | 43% |
| Wichmann et al.23 | N = 12 (5 ICU and 7 non-ICU) | DVT | Autopsy | 58% of all COVID-19 patients autopsied had evidence of PE, irrespective of ICU status |
| Demelo-Rodríguez et al.24 | N = 156 non-ICU patients | DVT | Ultrasound | 15% |
| Nahum et al.8 | N = 34 ICU patients | DVT | Ultrasound | 79% |
| Middeldorp et al.25 | N = 198 (123 non-ICU and 75 ICU) | VTE in non-ICU vs ICU | Ultrasound | 9.2% in non-ICU vs 59% in ICU |
CTPA, computed tomography pulmonary angiogram; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
As mentioned above, elevated D-Dimer is a lab abnormality commonly seen in patients with COVID-19 complicated by coagulopathy.16,18 However, until now, D-Dimer data has not been compiled and reviewed in a manner that examines if elevated levels can predict severity of thrombotic complications (determined by severity of disease, ICU stay, and mortality) in patients with COVID-19. Two studies stratified data based on disease severity in patients with COVID-19, finding D-Dimer levels to be significantly higher in patients with severe disease phenotype compared to patients with ordinary or mild disease.28,29 Han et al. found that patients with ordinary disease (n = 49) had D-dimer values with a range of 2.14 ± 2.88 ug/mL, while patients with severe disease (n = 35) had values of 19.11 ± 35.48 ug/mL.28 Further, Gao et al. found that patients with mild disease had a median D-dimer value of 0.21 ug/mL (range:0.19-0.27 ug/mL), while patients with severe disease had a median value of 0.49 ug/mL (range:0.29-0.91 ug/mL).29 Ordinary disease was defined as the disease process that does not result in acute respiratory distress syndrome (ARDS), ICU admission, or high oxygen requirements, such as high flow nasal cannula. Severe disease was defined as the development of acute respiratory distress syndrome (ARDS), ICU admission, and higher oxygen requirements.29 Another study found median D-Dimer levels to be twice as high in critically ill patients when compared to non-ICU patients diagnosed with COVID-19.30 Moreover, multiple studies found an association between elevated D-Dimer levels and increased mortality, with survivor median D-Dimer levels ranging from 0.5 mcg/mL to 0.6 mcg/mL and non-survivor D-dimer levels ranging from 2.1 mcg/mL to 5.2 mcg/mL (normal < 0.4 mcg/mL).5,27,31 Tang et al. found that elevations of both D-Dimer and fibrinogen degradation product levels were strongly associated with poor outcomes in patients with COVID-19.5 It was observed that 71% of non-survivors met criteria for overt disseminated intravascular coagulation (DIC), whereas only 0.6% of survivors met criteria for DIC throughout the course of the study.5 The results of these studies are significant in that they demonstrate that D-dimer elevation is a frequent finding in patients with more severe disease and that these levels may be predictive of disease severity and poor outcomes in COVID patients, however due to the fact that the units of measurement vary between institutions, it is difficult to provide a true summary of the D-dimer data across multiple studies.
Hypercoagulability Pathophysiology
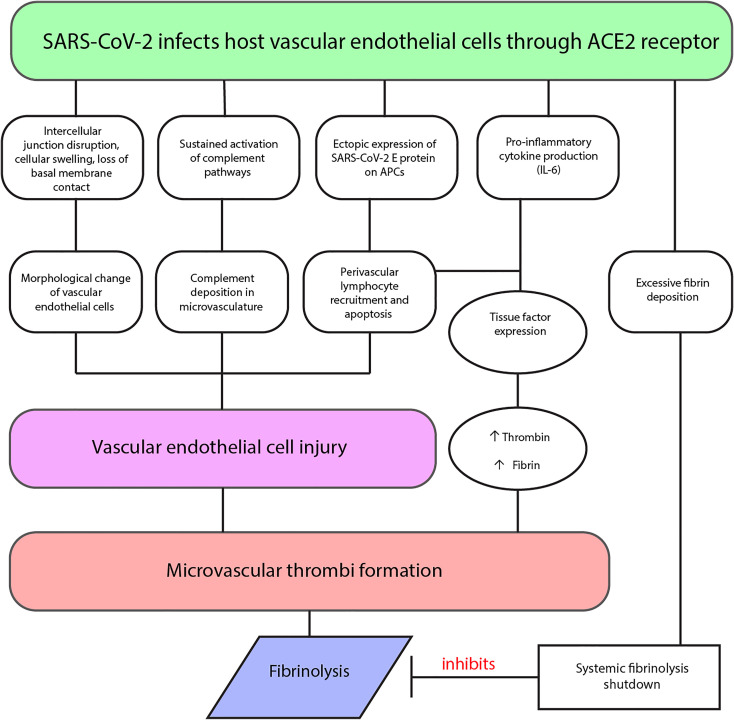
There are several proposed mechanisms for the hypercoagulable state seen in COVID-19 patients, which are described below and summarized in Figure 1. These mechanisms, alone or in combination, may lead to the severe thrombotic complications causing significant mortality.
Figure 1.
Proposed mechanisms of thrombosis in COVID-19 diagnosed patients.
Vascular endothelial cells are critical to the regulation of vascular permeability, maintaining hemostasis, and regulating hemolysis.32 Thus, it comes as no surprise that they likely play a significant role in COVID-19 vasculopathy and thrombosis. Vascular endothelial injury appears to be an important observation in the lungs of patients with COVID-19. Whereas it was originally assumed clots in the lungs in COVID-19 patients were the result of pulmonary emboli, it is now known that a hallmark of COVID-19-related clots include a process where primary thrombosis formation occurs directly in the lungs.33 As described previously, SARS-CoV-2 infects host endothelial cells through the integral membrane protein, ACE2.12 When comparing autopsy lung tissue samples of patients with COVID-19 to H1N1 influenza patients and healthy controls, greater numbers of ACE2-positive endothelial cells were found in the COVID-19 patients.33 Additionally, significant endothelial cell morphological change was seen, including intercellular junction disruption, cellular swelling, and loss of basal membrane contact, in addition to the presence of intracellular virus and disrupted cell membranes.33 Both COVID-19 and influenza groups demonstrated diffuse alveolar damage and infiltrating perivascular lymphocytes, however COVID-19 lungs revealed 9-fold greater alveolar capillary microthrombi.33 This capillary microthrombi can lead to a disseminated intravascular coagulopathy (DIC)-like clinical picture, where D-dimer and fibrin/fibrinogen breakdown products are significantly elevated, similar to classical DIC, but prothrombin time, partial thromboplastin time, and platelet counts are unchanged on initial presentation.34 These findings confirm that the intracellular presence of SARS-CoV-2 may lead to direct endothelial injury.
Another proposed pathophysiologic mechanism includes the formation of microvascular microthrombi triggering active tissue factor expression on macrophages and endothelial cells. This results in increased blood borne tissue factor present in the pulmonary vasculature.32 Increases in tissue factor, in combination with local hypoxia from COVID-19 induced ARDS, creates a positive thromboinflammatory feedback loop, otherwise known as a cytokine storm.32 Elevated levels of the proinflammatory cytokines tumor necrosis factor-α, interleukin-1, and interleukin-6 have been associated with severe COVID-19 inflammatory reactions as well.15 This likely has great implications on the development of a procoagulant state, as IL-6 is a known inducer of tissue factor expression on mononuclear cells.18 Tissue factor complexes with factor VIIa, initiating the extrinsic pathway of the coagulation cascade, and ultimately leading to the formation of thrombin and fibrin.35 The presence of proinflammatory cytokines and activation of the coagulation cascade, in addition to thin vessel walls, triggers immunothrombosis with resultant vessel wall tissue damage, pulmonary infarction, and hemorrhaging.32 These pathologic findings are consistent with the idea that primary pulmonary thrombosis, or thrombosis originating in the pulmonary vasculature, occurs in addition to the high rate of pulmonary embolus in COVID-19 patients. In fact, embolism originating outside of the pulmonary vasculature (secondary thrombosis) has been shown to occur in as many as 58% of patients autopsied after death due to COVID-19.23 Both microvascular and macrovascular thrombosis have been observed in COVID-19 patients with platelet-fibrin micro-thrombi seen in microvasculature and both red and white thrombi seen in macrovasculature.36
Other, less-supported proposed mechanisms for the thrombotic complications of COVID-19 may lie within the complement pathway. One study of 5 patients with severe COVID-19 infection found the lungs to be relatively spared of the diffuse alveolar damage with hyaline membranes, type II pneumocyte hyperplasia, and inflammation classically seen in ARDS.37 However, sustained activation of the alternate and lectin-based complement pathways was evidenced by findings of significant complement deposition in the microvasculature.37 Thus, complement-mediated microvascular injury may explain at least a subset of complications seen in this disease.
COVID-19 related thrombosis does not appear to be limited to the venous circulation. A single-center cohort study found significantly increased rates of acute limb ischemia in patients with COVID-19 (16.3%) from January 2020 to March 2020, compared to patients without COVID-19 (1.8%) presenting from January 2019 to March 2019.38 Generally, young age and female gender are risk factors associated with hypercoagulable disorders, however, in patients with COVID-19, native arterial occlusions were observed in both younger and older individuals, and more often in males.38 Additionally, the macroscopic appearance of thrombosis specimens in these patients was quite different than pre-COVID-19 specimens, as they were much more gelatinous and longer than typical thrombi, an observed hallmark of COVID-19 thrombi.38 Both observations suggest that the increased rates of acute limb ischemia are likely not related to well-known hypercoagulability disorders, but due to COVID-19 infection. Coronary thrombosis also has been reported in patients with COVID-19.39 Coronary angiography in this particular case revealed multivessel thrombotic stenosis believed to be in situ thrombosis, since no significant atheroma was found and thrombus of the left anterior descending was non-occlusive (60%) making a coronary embolus unlikely.39 These findings imply a COVID-19 coagulopathy as the cause of thrombosis and provide further evidence that thrombotic complications of COVID-19 are not limited to the venous vasculature.
Laboratory Findings
Trends in laboratory abnormalities of COVID-19 patients with increased coagulopathy are being observed as time progresses. These lab abnormalities include elevated D-Dimer, modest thrombocytopenia, and prolongation of the prothrombin time.18 However, other laboratory abnormalities may shed light on the hypercoagulable state as well. Elevated serum levels of lactate dehydrogenase and serum ferritin were associated with increased risk of death.27 Interestingly, these abnormalities resemble findings seen in thrombotic microangiopathy, further supporting the notion of a microangiopathic cause of the thrombotic complications of COVID-19.
Other serologic observations may help explain this massive prothrombotic state. Several patients with significant coagulopathy were found to be positive for anticardiolipin IgA antibodies and anti–β2-glycoprotein I (β2GPI) IgA and IgG antibodies.40 The emergence of these antibodies has been seen in a variety of other viral infections and can result in thrombotic events via similar mechanisms of complex formation seen in heparin-induced thrombocytopenia and antiphospholipid syndrome.41,42 Prothrombotic complexes lead to the activation of platelets by their F(ab)2 region leading to micro-thrombi and macro-thrombi.42
In addition to the well-documented procoagulant state, research has indicated COVID-19 is causing an anti-fibrinolytic state through a phenomenon called fibrinolysis shutdown.43 Notably, fibrinolysis shutdown, measured by decreased clot lysis during a 30-minute time span on thromboelastogram assay (TEG LY30), and elevated D-dimer heightens the risk of venous thromboembolism (VTE), renal failure, and thrombotic events.43 Lack of clot lysis at 30 minutes was seen in 57% of patients and was a significant predictor of VTE, while a D-dimer greater than 2600 ng/mL was a significant predictor of need for hemodialysis.43 Patients with both of these coagulation risk factors had a 50% rate of VTE, compared to 0% in patients with neither risk factor.43 Hemodialysis was needed in 80% of patients with both risk factors, compared to 14% of patients with neither.43 This elevation in D-dimer is likely to be reflective of an excessive amount of intravascular polymerized fibrin, burdening systemic fibrinolysis.43 Fibrinolysis shutdown has also recently been correlated with increased morbidity and mortality in early sepsis, indicating its presence in COVID-19 may be used as a predictor of poor prognosis.44
Hypercoagulability Management
Treatment guidelines for thromboembolism in COVID-19 have been published by the World Health Organization (WHO).45 These include treatment of adolescents and adults hospitalized with COVID-19 using prophylactic low molecular weight heparin (LMWH) to prevent VTE.45 In addition to heparin’s anticoagulant effects, LMWH has also been shown to have anti-inflammatory properties, which may be beneficial in combating the pro-inflammatory state caused by the novel coronavirus.46 Heparin also has suppressive effects against IL-6 and IL-8 expression from pulmonary epithelial cells, which may help reduce the thrombotic complications associated with cytokine storm in COVID-19 pneumonia.47,48 Furthermore, heparin has been shown to have competitive binding activity to previous strains of coronavirus, possibly resulting in reduced pathogenic activity of SARS-CoV-2.38 For patients with contraindications to anticoagulation therapy, VTE prophylaxis is recommended via the use of intermittent pneumatic compression devices.45 Despite these recommendations, clinical trials regarding the efficacy of prophylactic LMWH in hospitalized patients with COVID-19 are scarce, and the findings are mixed. One study found no reduction in the incidence of pulmonary embolism in hospitalized COVID-19 patients treated with prophylactic LMWH.49 Likewise, a study found no difference in 28-day mortality between hospitalized COVID-19 patients receiving prophylactic heparin and those who did not receive prophylactic heparin.50 This may be explained by low anti-thrombin levels, commonly seen in patients with COVID-19, rendering prophylactic heparin or LMWH ineffective.51 It may also indicate a possible use for anti-thrombin supplementation in this setting.51 However, Tang et al. found a benefit of prophylactic heparin in patients with D-Dimer levels >3.0 μg/mL (6 times the upper limit of normal), as there was approximately a 20% reduction in mortality.50 Furthermore, since the activation of coagulation compartmentalizes pathogens and prohibits their invasion, Tang et al. proposed anticoagulation therapy in patients without significant coagulopathy may put them at potentially increased risk of worsening infection.50 Therefore, their findings suggest that only patients with severe coagulopathy may benefit from treatment with prophylactic LMWH and may help establish guidelines for which patients should receive anticoagulation therapy. Similarly, Thachil proposed higher doses of LMWH may be needed in patients with a high body mass index, D-dimer concentration 6-8 times greater than normal, ARDS, or increasing oxygen requirement.52 Thus, the need for additional clinical trials to evaluate the benefit of prophylactic heparin treatment in hospitalized patients with COVID-19 is evident.
Kidney injury is a common manifestation of COVID-19 and adjusted thromboprophylaxis in patients with renal failure needs to be considered. The proposed mechanisms of kidney injury are similar to those of the pulmonary thrombotic complications: including cytokine storm, angiotensin II pathway activation, complement dysregulation, hypercoagulation, and microangiopathy.53 The Swiss Society of Hematology recommends that patients with creatinine clearance >30 ml/min should receive LMWH and patients with creatinine clearance <30 ml/min should receive unfractionated heparin subcutaneously 2-3 times per day, with an increased dose in both settings if the patient is overweight (100 kg).54 Since LMWH is less appealing for renal dosing and more difficult to manage if bleeding occurs, unfractionated heparin is more appropriate in patients with renal failure.51
More aggressive thromboprophylaxis has been trialed with the use of antiplatelet therapy. A case series of 16 critically ill patients used 6,000 IU LMWH twice daily (8000 IU if body mass index >35) with clopidogrel at a loading dose of 300 mg plus 75 mg/d if platelet count >400 000 cells/µL.53 No thromboembolic events were reported in this group, however mortality rates remained high (44%) due to hypoxia and multiorgan failure.55 The utility of antiplatelet therapy for thromboprophylaxis in COVID-19 is unclear and complicated by the role of platelets in host defense against infection through thrombus formation and pulmonary neutrophil recruitment.56 Furthermore, with diffuse alveolar hemorrhage being a commonly reported finding in COVID-19, inability to deposit fibrinogen and repair damaged pulmonary vasculature is a concern in patients on anti-platelet therapy.32,56 Additionally, as thrombocytopenia is a reported manifestation of COVID-19 and associated with increased risk of pneumonia and ARDS, proactive measures should be taken in patients on antiplatelet therapy with platelet counts <100 000/μL.56 Antiplatelet therapy should be discontinued in patients with platelet counts <50 000/μL.56 It is important for clinicians to be aware of the risks and benefits of antiplatelet therapy in patients with COVID-19 and the role of antiplatelet therapy in thromboprophylaxis needs to be evaluated further.
Full-dose anticoagulation is another area of consideration in COVID-19. Patients with signs and symptoms suggestive of thromboembolism, such as deep vein thromboses and pulmonary embolisms, should be managed with the same diagnostic and treatment pathways as the general population, unless contraindicated.45 Though therapeutic anticoagulation in certain COVID-19 patients without pulmonary embolisms or deep vein thromboses has been advocated for as well, this topic remains controversial due to the lack of data showing improved outcomes.57 The American Society of Hematology currently recommends empiric therapeutic anticoagulation in individuals clinically suspected to have emboli, thromboses, or respiratory failure with elevated D-dimer and/or fibrinogen, when there is no possibility of performing imaging to confirm diagnosis.57 Additionally, in a case series of 15 critically ill patients, resistance to therapeutic unfractionated heparin was found in 80% of patients, further indicating the need for studies to determine optimal thromboprophylaxis and treatment in COVID-19 patients.58
Hypercoagulability refractory to therapeutic anticoagulation has also been reported due to hyperviscosity, a known risk factor for thrombosis due to endothelial damage.59,60 In one study, 15 patients received anticoagulation according to institutional protocol derived from findings suggesting increased rates of VTE with D-Dimer levels ≥3 μg/mL.59,50 Therapeutic anticoagulation was given to patients with D-Dimer ≥3 μg/mL and known (or highly suspected) thrombosis. Intermediate (subtherapeutic) dosing was given for D-Dimer ≥3 μg/mL without known thrombosis, and low-dose prophylactic LMWH or subcutaneous heparin was given for D-Dimer <3 μg/mL. These 15 patients had plasma viscosity exceeding 95% of normal ranging from 1.9–4.2 centipoise (cP), measured by capillary viscometry.59 All patients with plasma viscosity greater than 3.5 cP experienced a thrombotic complication.59 As a result, plasma exchange may have a therapeutic role in COVID-19, as it is a highly effective therapy in other symptomatic hyperviscosity conditions such as hypergammaglobulinemia.61
Additional modalities currently being used to assess the hypercoagulable state of patients with COVID-19 include thromboelastography (TEG) and rotational thromboelastometry (ROTEM).62,63 TEG and ROTEM testing have been shown to be more reliable for the detection of coagulopathy than traditional coagulation tests (platelet count, APTT, PT, D-dimer, fibrinogen), however they are not commonly used.63 TEG and ROTEM are tests that measure shear elastic modulus during clotting and fibrinolysis, which allows for this tests to determine clotting time, clot formation, strengthening of the clot, amplitude of the clot, stability, and clot lysis.64 In a case by Iwasaki et al., an ICU patient with COVID-19 was evaluated with contrast enhanced CT and conventional coagulation laboratory testing which revealed no evidence of hypercoagulable state.63 ROTEM was performed, revealing values of clot firmness and clot formation time suggestive of hypercoagulopathy.63 The patient was subsequently started on unfractioned heparin and was ultimately extubated due to improvement of clinical condition.63 Further research is needed to determine the utility of these testing modalities in recognizing hypercoagulability in patients suffering from COVID-19.
As more proposed mechanisms for the thrombotic complications of COVID-19 arise, further treatment modalities may need to be considered on a case by case basis. Recognizing the immunologic qualities of COVID-19 pneumonia and elevated serum IL-6, the role of anti-cytokine therapy for immunothrombosis deserves further investigation.32 Prior to the COVID-19 pandemic, monoclonal antibody therapy resulting in decreased serum IL-6 has shown benefit in all-cause cardiovascular mortality.65 Given the prolonged activation of the complement pathway in several cases of severe COVID-19, anticomplement therapy may be an area for future consideration as well.37 The human monoclonal antibody, OMS721, inhibits mannan-binding lectin-associated serine protease-2 and has been shown to be efficacious in stem cell transplant-linked thrombotic microangiopathies.66 Another study has considered the use of assessing fibrinolytic shutdown to predict the need for more aggressive anticoagulation measures and the need for hemodialysis.43 Choudhury et al. even predicted that the use of fibrinolytic therapy (tissue plasminogen activator) as salvage therapy in COVID-19 ARDS may improve mortality.67
Conclusion
The presence of a hypercoagulable state and evidence for the development of thrombotic complications in patients with COVID-19 is expanding. Although mechanisms regarding development of these complications are not entirely clear, it is unnervingly apparent that this thrombotic state is in part responsible for the high mortality of the disease. Several mechanisms involving vascular endothelial injury, proinflammatory cytokines, complement, serum procoagulants, and shutdown of anti-coagulation pathways have been discussed, all of which may be vital in developing more specific treatments as this disease evolves. However, data is limited and further investigation is needed. Current treatment recommendations include the prophylactic use of LMWH, however further clinical trials are needed to confirm its efficacy. The hope is that more information will become available as the landscape of the current pandemic continues to evolve.
Footnotes
Authors’ Note: Asim Kichloo is credited with substantial contribution to the design of the work, literature review of all the sections discussed, the revision of critically important intellectual content, final approval of the published version, and agreement of accountability for all aspects of the work. Kirk Dettloff, Michael Aljadah, and Michael Albosta are credited with substantial acquisition, analysis, and extraction of the literature reviewed for the manuscript, drafting the manuscript, final approval of the version to be published, and agreement of accountability for all aspects of the work. Shakeel Jamal and Jagmeet Singh are credited with significant contribution to the design of the manuscript and interpretation of the data, the revision of critically important intellectual content, final approval of the version to be published, and agreement of accountability for all aspects of the work. Farah Wani, Akshay Kumar, Srilakshmi Vallabhaneni, and Muhammad Zia Khan are credited with the revision of critically important intellectual content, final approval of the version to be published, and agreement of accountability for all aspects of the work along with taking the submission. The involved institutions do not require ethical approval for narrative reviews. Verbal informed consent was obtained from the patient(s) for their anonymized information.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michael Albosta  https://orcid.org/0000-0003-4187-4911
https://orcid.org/0000-0003-4187-4911
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. World Health Organization. 2020. Accessed October 8, 2020 https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 3. WHO. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. 2020. Accessed October 8, 2020 https://covid19.who.int/
- 4. Klok FA, Kruip MJHA, Van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020;2(9):1379–1382. doi:10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chong PY, Chui P, Ling AE, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128(2):195–204. [DOI] [PubMed] [Google Scholar]
- 8. Nahum J, Morichau-Beauchant T, Daviaud F, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open. 2020;3(5): e2010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabi FA, Al Zoubi MS, Kasasbeh GA, et al. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navratil V, Loïc L, Longhi S, Hardwick M, Combet C, Aouacheria A. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) envelope (E) protein harbors a conserved BH3-like sequence. bioRxiv; 2020. [Google Scholar]
- 15. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6): e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020;10(5):1058–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 21. Leonard-Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020:296(3):E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;73(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi:10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishiguro T, Kagiyama N, Uozumi R, et al. Clinical characteristics of influenza-associated pneumonia of adults: clinical features and factors contributing to severity and mortality. Yale J Biol Med. 2017;90(2):165–181. [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 29. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi:10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatology. 2020;2(7):e437–e445. doi:10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi:10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29(12):1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi:10.1007/s11239-020-02134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi:10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;S0741-5214(20)31080–31086. doi:10.1016/j.jvs.2020.04.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dominguez-Erquicia P, Dobarro D, Raposeiras-Roubín S, et al. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur Heart J. 2020;41(22):2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17): e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263. [DOI] [PubMed] [Google Scholar]
- 42. Vlachoyiannopoulos PG, Routsias JG. A novel mechanism of thrombosis in antiphospholipid antibody syndrome. J Autoimmun. 2010;35(3):248–255. [DOI] [PubMed] [Google Scholar]
- 43. Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203. doi:S1072-7515(20)30400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. WHO. Clinical management of COVID-19: interim guidance. World Health Organization. 2020. Updated May 27, 2020 Accessed June 4, 2020. https://www.who.int/publications-detail/clinical-management-of-covid-19
- 46. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 48. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;56(1):2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: a comment. J Thromb Haemost. 2020;8(8):2060–2063. doi:10.1111/jth.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Casini A, Alberio L, Angelillo-Scherrer A, et al. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19—a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020;150: w20247. [DOI] [PubMed] [Google Scholar]
- 55. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. doi:10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou X, Li Y, Yang Q. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathologic findings. Circulation. 2020;141(22):1736–1738. [DOI] [PubMed] [Google Scholar]
- 57. Lee A, deSancho M, Pai M, et al. COVID-19 and pulmonary embolism: frequently asked questions. American Society of Hematology. COVID-19 Resources Web site. 2020. Accessed October 8, 2020 Updated September 22, 2020 https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism
- 58. White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287–291. doi:10.1007/s11239-020-02145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maier CL, Truong AD, Auld SC, et al. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gertz MA, Kyle RA. Hyperviscosity syndrome. J Intensive Care Med. 1995;10(3):128–141. [DOI] [PubMed] [Google Scholar]
- 61. Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34(3):171–354. [DOI] [PubMed] [Google Scholar]
- 62. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi:10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iwasaki Y, Shiga T, Konno D, et al. Screening of COVID-19-associated hypercoagulopathy using rotational thromboelastometry. J Clin Anesth. 2020;67:109976 doi:10.1016/j.jclinane.2020.109976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015;2015(2):CD010438 doi:10.1002/14651858.CD010438.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39(38):3499–3507. [DOI] [PubMed] [Google Scholar]
- 66. Rambaldi A, Khaled S, Smith M, et al. Improved survival following OMS721 treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy (HCT-TMA). Eur Hematol Assoc Lib. 2018; 215162:PF724. [Google Scholar]
- 67. Choudhury R, Barrett CD, Moore HB, et al. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg. 2020;15(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]