Abstract
Emerging evidence shows that the recent pandemic of coronavirus disease 19 (COVID-19) is characterized by coagulation activation and endothelial dysfunction. This increases the risk of morbidity, mortality and economic loss among COVID-19 patients. Therefore, there was an urgent need to investigate the extent and risk factors of thromboembolism among COVID-19 patients. English-language based databases (PubMed, Cumulative Index to Nursing and Allied Health Literature, EMBASE, and Cochrane library) were exhaustively searched to identify studies related to prevalence of thromboembolism among hospitalized COVID-19 patients. A random-effects model was employed to estimate the pooled prevalence of thromboembolism. The pooled prevalence of thrombotic events was computed using STATA 16.0 software. Heterogeneity analysis was reported using I2. A total of 19 studies with 2,520 patients with COVID-19 were included. The pooled prevalence of thrombotic events of hospitalized patients with COVID-19 was 33% (95% CI: 25-41%, I2 = 97.30%, p < 0.001) with a high degree of heterogeneity across studies. Elevated D-dimer hospitalized in the intensive care unit and being under mechanical ventilation were the most frequently associated factors for the development of thrombotic events. The pooled prevalence of thrombotic events in COVID-19 patients was 33%. The prevalence of thrombotic event is variables on the basis of study design and study centers. Several risk factors such as, elevated D-dimer, hospitalized in the intensive care unit and being under mechanical ventilation, were the most frequently reported risk factors identified. Therefore, healthcare professionals should consider these risk factors to optimally manage thromboembolism in COVID-19 patients.
Keywords: prevalence, risk factors, thromboembolism, COVID-19
Background
Thromboembolism, the third leading vascular disease, constitutes a major global burden of disease, and its annual incidence is 1 to 2 cases per 1000 people.1 Thromboembolism is a condition that includes deep vein thrombosis (DVT) and pulmonary embolism (PE), and annual incidence of DVT and PE is approximately 60-100 per 100,000 and 23–107 per 100,000 people worldwide, respectively.2 The risk of thromboembolism in the general population remains high.3 Thromboembolism is often associated with significant morbidity,3,4 mortality,3,5 high rates of hospital readmissions,6 poor health-related quality of life,7,8 and had a considerable economic impact.9 Thromboembolic conditions were estimated to account for 1 in 4 deaths worldwide in 2010 and are the leading cause of mortality.10 In addition, the risk of dying within the first year of diagnosis was higher.11 Further, thromboembolism costs $7–12 billion per year in the United States of America.12
Surgical procedures, oral contraception use, cancer, and prolonged immobilization are associated with risk of thromboembolism. In addition, emerging evidence shows that the recent pandemic COVID-19 is characterized by endothelial dysfunction and activation of coagulation.13 COVID-19 predisposes patients to thrombotic disease due to excessive inflammation, platelet activation, endothelial dysfunction and stasis. Studies reported that patients who died of COVID-19 had a higher D-dimers, associated with venous thromboembolism,14 on admission compared with those who survived.15,16 The high incidence of thromboembolic events suggests an important role of COVID-19 on coagulopathy.17 Hence, coagulopathy is a common abnormality in patients with COVID-19.18 Risk of morbidity, mortality and economic loss is even more worsened among patients with COVID-19. To reduce these undesirable consequences, the extent and associated risk factors should be known. Therefore, there is an urgent need to summarize the prevalence and risk factors of thromboembolism among COVID-19 patients.
Methods
A systematic review with meta-analysis was performed following the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)19 (Additional file 1: Table S1). The review protocol was registered in PROSPERO (ID = CRD42020201562), and the published methodology is available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020201562.20
Data Sources and Search Strategy
A systematic search was conducted by visiting important databases; PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus and EMBASE (Ovid). Other supplementary sources such as Google Scholar and Cochrane library were also used. The search was conducted with the aid of carefully selected keywords and indexing terms. These include keywords related to “COVID-19,” “prevalence,” and “thromboembolism.” Boolean operators (“OR,” “AND”) and truncation were used to identify relevant articles and records that meet our research question. The search was conducted from 20th June–10th August 2020. All published and unpublished articles regarding the prevalence of thromboembolism among patients with COVID-19 were included until the end of the data extraction period. Bibliography lists from all eligible articles were also hand-searched to identify additional papers potentially relevant for inclusion.
Inclusion and Exclusion Criteria
All observational studies published in English language addressing thromboembolism among patients with COVID-19 were included in the review. Nonetheless, articles with insufficient information, records with missing outcomes of interest, findings from personal opinions, case reports and series, systematic reviews, and qualitative studies were excluded.
Article Screening Process
Records identified from various electronic databases, indexing services, and directories were saved and exported to Covidence software. Duplicate records were removed using Covidence software. The initial title and abstract screening were done by 2 authors (BK and GT). Three categories (yes, no, maybe) were used during the selection process. The full text of studies considered ‘yes’ or ‘maybe’ during the screening was assessed based on the eligibility criteria by 2 authors (GT and BK). Then, full-text screening was conducted by 2 authors (BK and AD). In each case, the third author (MT) played a critical role in solving discrepancies that arose between 2 authors.
Data Extraction and Synthesis
Two authors (BK and GT) independently extracted relevant data using a standardized data abstraction format prepared in Microsoft Excel. The extracted data include study characteristics (country and study setting, first author, publication year, study design, population characteristics, and sample size) and the result of studies (the prevalence of thrombotic event and risk factors). Any disagreements were resolved by discussion with the third author (DT) by crosschecking the papers.
Methodological Quality Assessment of Studies
The methodological quality and risk of bias of the included studies were independently assessed by 2 authors (BK and AD) using the Newcastle-Ottawa scale,21 which rate study quality out of 10 points (stars). For ease of evaluation, the tool included important indicators categorized into 3 major domains. The first section assesses the methodological quality of a study, which has a maximum of 5 stars. The second section considers the comparability of the study and takes 2 stars, and the remaining section assesses the outcomes of studies related to the statistical analysis. This critical assessment was conducted to assess the internal and external validity of the included studies. The mean score of 2 authors (BK and AD) was taken for the final decision and studies with a score of 5 and above points/stars were included.
Outcome Measurements and Data Analysis
The outcome measurements of this systematic review were reported in terms of the prevalence of thrombotic events of hospitalized patients with COVID-19. The pooled prevalence of thrombotic events was computed using STATA/MP 16.0 software. Heterogeneity analysis was reported using I2. In addition, sub-group analysis was performed on patient characteristics (admitted at the intensive care unit (ICU) vs. both ICU and medical ward vs. medical ward), study design and study country. Further, contributing factors were also summarized in a table as clinical factors and other characteristics.
Results
Study Selection
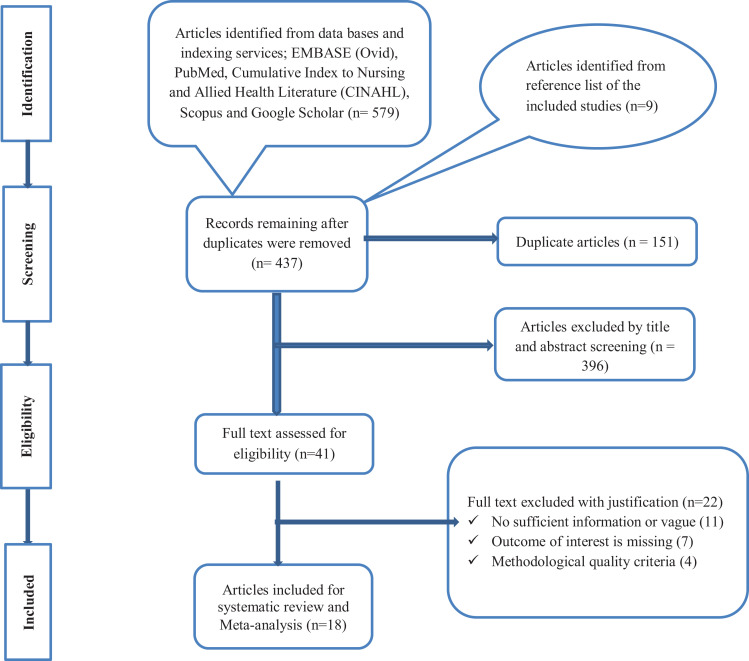
The literature search identified a total of 588 records from several sources. After the removal of duplicate records with Covidence software, the remaining 437 records were screened using their titles and abstracts, and 396 of them were excluded. The full-text of 41 records was then evaluated as per the predetermined eligibility criteria for inclusion. Twenty-two articles were also excluded as the outcome of interest was missing, insufficient and unacceptable methodological quality. Finally, 19 articles met the eligibility criteria and quality assessment in the present systematic review (Figure 1).
Figure 1.
PRISMA flow chart showing article-screening process.
Characteristics of the Included Studies
The characteristics of the articles included in the present systematic review and meta-analysis were summarized in Table 1. A total of 19 studies with 2,520 hospitalized patients with COVID-19 were included for this systematic review and meta-analysis. Thirteen studies (22-34) employed a retrospective cohort study design. Studies were published in the year 2020 and targeted a wide range of population characteristics. The adjusted sample size ranged from 2618 in France to 38813 hospitalized patients in Italy. Eight studies22,23,24,18,25-28 were conducted on ICU patients, whereas 8 studies13,29,30,31-33,34,35 were conducted in both ICU and non-ICU patients, 2 studies36,37 were in non-ICU patients. Although Tsaplin et al.38 reported the prevalence of thromboembolism among hospitalized COVID-19 patients, the study participant of was not clear. The pooled prevalence of thromboembolism in total and ICU studies ranged from 2.9%30 to 85.4%28 in China and 16.8%27 in the Netherlands to 85.4% in China, respectively. Regarding geographic distribution, all studies were conducted in Europe and Asia. Eight studies were conducted in France,36,32,33,24,34,18,25,26 5 studies in China,30,22,23,28,35 2 studies in the Netherlands29,27 and 1 study in Italy,13 Russia,38 United Kingdom31 and Spain.37
Table 1.
General Characteristics of the Included Studies Focused on Patients With COVID-19 With Thromboembolism Events.
| Authors | Publication year | Study country |
Study
design |
Study setting | Admission ward/class | Sample size | Prevalence of TE, N (%) | Event rate | SE |
|---|---|---|---|---|---|---|---|---|---|
| Demelo-Rodríguez et al. 37 | 2020 | Spain |
PC | Single-center | Non-ICU | 156 |
23 (14.7) | 0.147 | 0.028 |
| Tsaplin et al. 38 | 2020 | Russia | RC | Single-center | Not clear | 168 | 11 (6.5) | 0.065 | 0.019 |
| Lodigiani, et al. 13 | 2020 | Italy | RC | Single-center | ICU & non-ICU | 388 | 28 (7.7) | 0.077 | 0.014 |
| Zhang et al. 35 | 2020 | China | CS | Single-center | ICU & non-ICU | 143 |
66 (46.1) | 0.461 | 0.042 |
| Artifoni et al. 36 | 2020 | France | RC | 2-centers | Non-ICU | 71 | 23 (32.5) | 0.325 | 0.056 |
| Xu et al. 30 | 2020 | China | RC | Single-center | ICU & non-ICU | 138 |
4 (2.9%) | 0.029 | 0.014 |
| Middeldorp et al. 29 | 2020 | The Netherlands |
RC | Single-center | ICU & non-ICU | 198 | 33 (17%) | 0.170 | 0.027 |
| Nahum et al. 25 | 2020 | France | PC | Single-center | ICU | 34 |
27 (79%) | 0.790 | 0.07 |
| Chen et al. 22 | 2020 | China | RC | Single-center | ICU | 88 |
40 (46%) | 0.460 | 0.053 |
| Llitjos et al. 18 | 2020 | France | RC | 2-centers | ICU | 26 |
18 (69%) | 0.690 | 0.091 |
| Helms et al. 26 | 2020 | France | PC | 2-centers | ICU | 150 | 64 (42.7%) | 0.427 | 0.04 |
| Cui et al. 23 | 2020 | China | RC | Single-center | ICU | 81 | 20 (25%) | 0.250 | 0.048 |
| Klok et al. 27 | 2020 | The Netherlands |
PC | 3-centers | ICU | 184 |
31 (16.8%) | 0.168 | 0.028 |
| Whyte et al. 31 | 2020 | United Kingdom | RC | Single-center | ICU & non-ICU | 214 |
80 (37%) | 0.370 | 0.033 |
| Grillet et al. 32 | 2020 | France | RC | Single-center | ICU & non-ICU | 100 | 23 (23%) | 0.230 | 0.042 |
|
Leonard-Lorant, et al.
33
|
2020 | France | RC | Single-center | ICU & non-ICU | 106 |
32 (30%) | 0.300 | 0.045 |
| Ren et al. 28 | 2020 | China | CS | 2-centers | ICU | 48 | 41 (85.4%) | 0.051 | |
| Fraissé et al. 24 | 2020 | France | RC | Single-center | ICU | 92 |
37 (40%) | 0.051 | |
| Bompard et al. 34 | 2020 | France | RC | 2-centers | ICU & non-ICU | 135 | 32 (23.7%) | 0.037 |
TE: Thromboembolism, ICU: Intensive care unit, CS: Cross-sectional, N: Number, RC: Retrospective cohort, PC: Prospective cohort.
Study Outcome Measures
Prevalence of thrombotic events among hospitalized patients with COVID-19
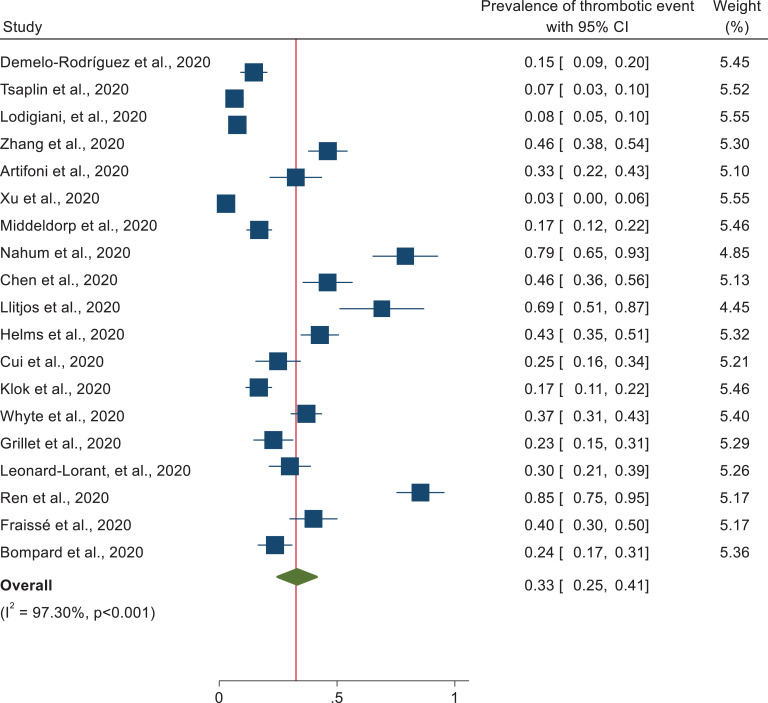
Random effects model with DerSimonian-Laird method was assumed for this meta-analysis. From the 19 studies reporting thrombotic events, the pooled prevalence of thrombotic event of hospitalized patients with COVID-19 was found to be 33% (95% CI: 25, 41). As the I2 (I2 = 97.30%, p < 0.001) statistic revealed, there is a high degree of heterogeneity across studies (Figure 2).
Figure 2.
Forest plot illustrating the pooled analysis of 19 studies reporting thrombotic events in patients with COVID-19.
Sensitivity and Subgroup Analyses
There was no significant change in the degree of heterogeneity even if we attempted to exclude the expected outliers and 1 or more of the studies from the analysis. Therefore, we were subjected to include all the studies for the meta-analysis.
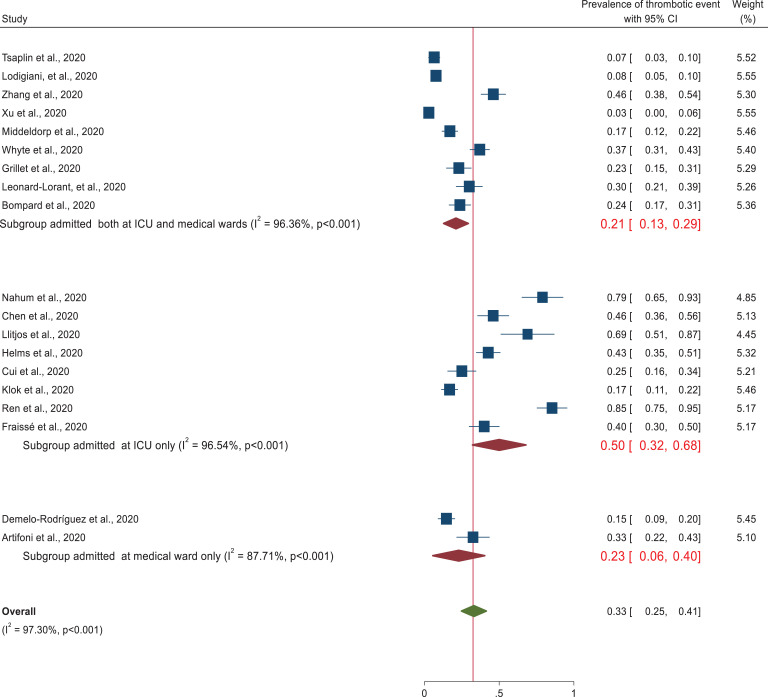
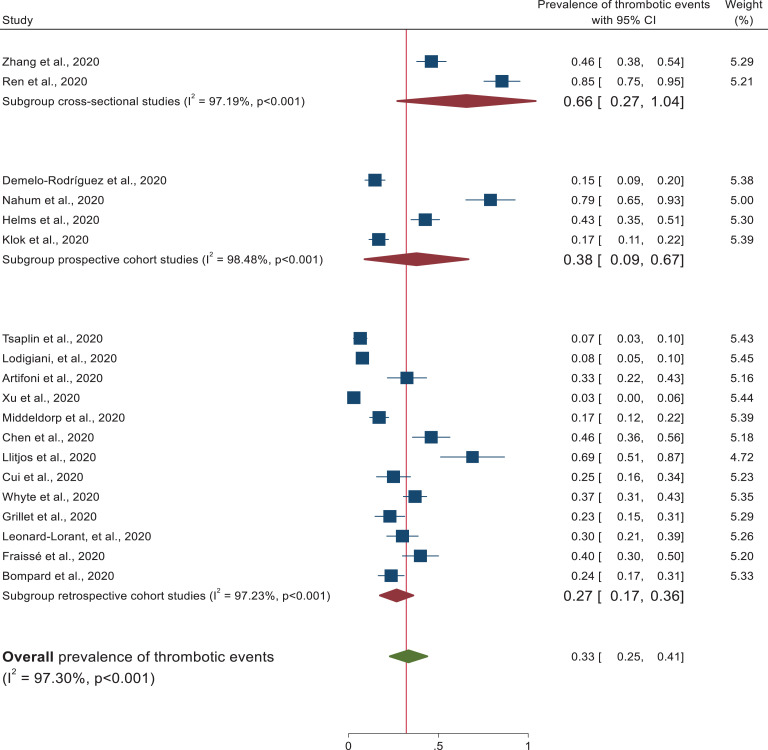
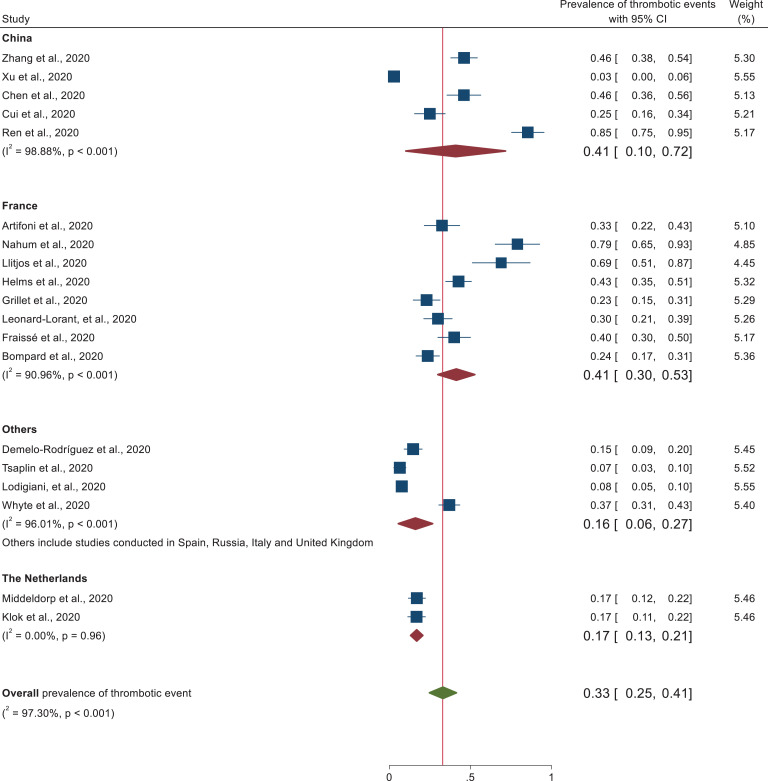
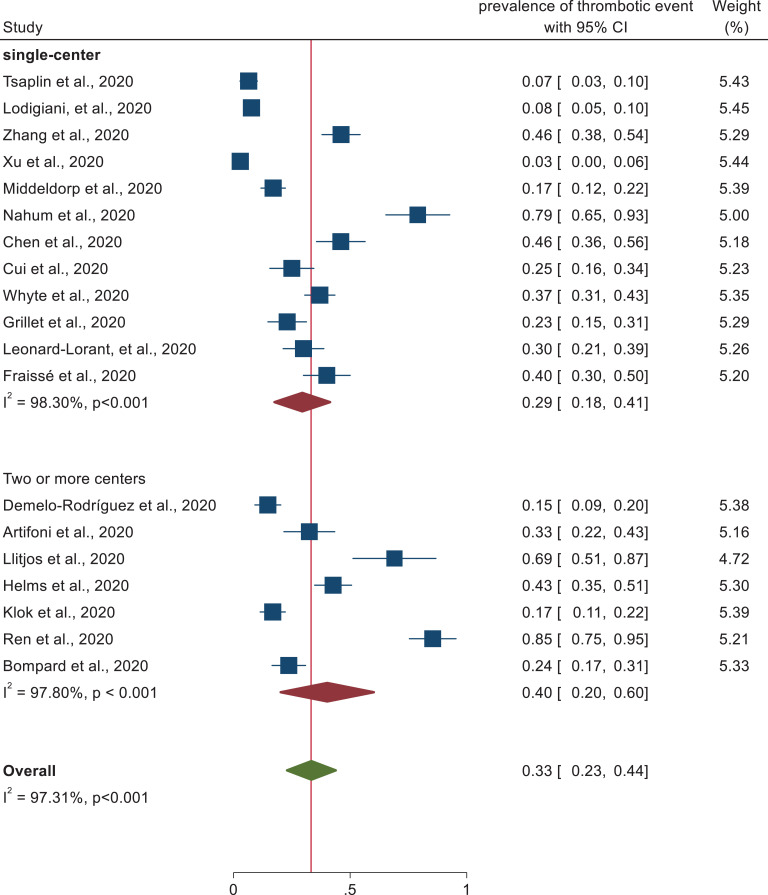
Subgroup analysis was performed on study design, patient characteristics, and geographical distribution. Subgroup analysis based on patient characteristics revealed a higher prevalence of thrombotic events (50%) (95% CI: 32-68) was observed in patients admitted at the intensive care unit than studies conducted in both medical ward and intensive care unit (21%) (95% CI: 13–29) as depicted in the forest plot (Figure 3). In each subgroup, there was higher heterogeneity among studies. In addition, studies conducted with cross sectional study design (66%) (95% CI: 27–104) revealed higher thrombotic events prevalence followed by prospective study design (38%) (95% CI: 9–67) and retrospective cohort design (27%) (95% CI: 17–36). Heterogeneity was higher among each subgroup studies (Figure 4). In addition, despite the higher heterogeneity among each study setting, this systematic review was also revealed that the pooled prevalence of thrombotic events in China and France was approximately 41% (95% CI: 10-72) and 41% (95% CI: 30, 53), respectively (Figure 5). Furthermore, prevalence of thrombotic events was higher among studies conducted in 2 or more centers (40%) (95% CI: 23–57) than single centered studies (29%) (95% CI: 20–38) (Figure 6).
Figure 3.
Subgroup analysis of thrombotic event by patient characteristics.
Figure 4.
Subgroup analysis of thrombotic events based on study design.
Figure 5.
Subgroup analysis of studies reporting the prevalence of thrombotic event segregated by country.
Figure 6.
Subgroup analysis of studies describing the prevalence of thrombotic event by number of settings.
Factors Associated With the Prevalence of Thrombotic Events Among Hospitalized Patients With COVID-19
In this systematic review, various factors contribute to thrombotic events among patients with COVID-19. Elevated D-dimer,36,29,22,23,31,33,24,34,35,37 ICU admission30,32,33,34 and mechanical ventilation use32,24,34 were the most frequently associated factors for the development of thrombotic event. Furthermore, being older,23,27 delay from onset of symptoms to computerized tomography scan (days),32,33 higher Padua prediction score & CURB-65 score (confusion, urea, respiratory rate, blood pressure, and 65 years of age or older),35 hypoalbuminemia & higher sequential organ failure assessment score,22 lower lymphocyte counts, & longer activated partial thromboplastin time,23 longer median hospitalization duration34 and having chronic renal failure24 were associated factors for the prevalence of thrombotic event (Table 2).
Table 2.
Factors Associated With the Prevalence of Thrombotic Events Among Hospitalized COVID-19 Patients.
| Authors | Sample size | Prevalence of TE, N (%) | Factors affecting the prevalence of TE |
|---|---|---|---|
| Demelo-Rodríguez et al.37 | 156 | 23 (14.7) | Elevated D-dimer |
| Zhang et al.35 | 143 |
66 (46.1) | Higher Padua prediction score, CURB-65 score, elevated D-dimer |
| Artifoni et al.36 | 71 | 23 (32.5) | Elevated D-dimer |
| Xu et al.30 | 138 | 4 (2.9) | ICU hospitalization |
| Middeldorp et al.29 | 198 | 33 (17) | Elevated D-dimer |
| Chen et al.22 | 88 |
40 (46) | Elevated D-dimer, hypoalbuminemia, higher SOFA score and inpatient status |
| Cui et al.23 | 81 | 20 (25) | Elevated D-dimer, older age, lower lymphocyte counts, longer APTT |
| Klok et al.27 | 184 | 31 (16.8) | Older age |
| Whyte et al.31 | 214 | 80 (37) | Elevated D-dimer |
| Grillet et al.32 | 100 | 23 (23) | Invasive mechanical ventilation, ICU hospitalization, delay from onset of symptoms to CT scan (days) |
| Leonard-Lorant, et al.33
|
106 | 32 (30) | Elevated D-dimer, ICU hospitalization, Delay from onset of symptoms to CT scan (days) |
| Fraissé et al.24 | 92 | 37 (40) | Chronic renal failure, invasive mechanical ventilation, elevated D-dimer |
| Bompard et al.34 | 135 | 32 (23.7) | More frequently hospitalized in ICU and under mechanical ventilation, longer median hospitalization duration, elevated D-dimer |
TE: thrombotic event, ICU: Intensive care unit, APTT: activated partial thromboplastin time, CT: computerized tomography, SOFA: Sequential organ failure assessment, CURB-65: Confusion, urea, respiratory rate, blood pressure, and 65 years of age or older.
Publication Bias
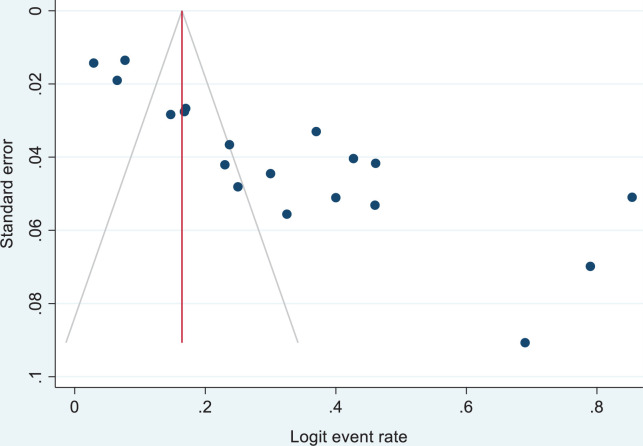
In our estimates, publication bias was assessed using funnel plots under the fixed-effects model, which helped us visualize each funnel plot’s symmetry status. In principle, visual assessment of the effect estimates from larger studies that spread narrowing at the top of the plot, with more broadly dispersed estimates at the bottom of the plot among smaller studies, may inform the existence of bias.39 The exclusion of unpublished research and the inclusion of various individual research with a range of sample sizes in our study may have affected and maximized the risk of publication bias40 (Figure 7).
Figure 7.
Publication bias using funnel plot of standard error by Logit event rate.
Discussion
This systematic review provides the overall prevalence of thromboembolism and associated risk factors among COVID-19 patients. Nineteen studies published during the year 2020 were identified to determine the prevalence of thromboembolism among patients infected with the novel coronavirus disease-19.
This is the first systematic review and meta-analysis to provide a comprehensive overview of venous thromboembolism prevalence based on 19 studies involving 2,520 hospitalized COVID-19 patients. Recent studies showed that critically ill COVID-19 patients had an elevated level of D-dimer, fibrinogen and fibrinogen degradation products, which are the most common markers of thrombus formation in the arterial and venous circulation.41 The present study showed that the pooled prevalence of thrombotic events among hospitalized patients with COVID-19 was 33% (95% CI: 25–41, I2 = 97.3). This high heterogeneity could probably be due to the difference in the study participants’ clinical and sociodemographic characteristics, sample size, and study designs. The finding is comparable with other systematic reviews, which revealed a high prevalence of thrombotic events in viral diseases.42–45 This high prevalence of thrombotic events could probably be due to the inflammatory process, cytokine storm, lung injury, and endothelial injury that increase the risk of hypercoagulable state in hospitalized COVID-19 patients.46 Subgroup analysis based on patient characteristics revealed that the highest incidence of thrombotic events was observed in patients admitted to the intensive care unit (50%) (95% CI: 32–68) relative to patients admitted in medical wards. Due to prolonged bed-ridden, sepsis and surgical procedures, ICU patients are more susceptible to coagulation problems.47,48 On the contrary, a lower (21%, 95% CI: 13–29) pooled estimate was observed in studies that include patients admitted at both the medical ward and intensive care unit. Our review also showed that there is higher heterogeneity among studies. The prevalence of thrombotic event was higher among studies conducted in 2 or more centers (40%) and studies which used prospective study design (38%) than single centered studies (29%) and studies performed retrospective design (27%). Further, this systematic review revealed that China (41%) and France (42%) had the highest thrombotic events. This could probably be due to the higher number of aged population and critically ill patients in these countries.
Viral infections, which is associated with problem of coagulation, can affect all aspects of the coagulation cascade, primary hemostasis, coagulation, and fibrinolysis.49 Smeeth et al.50 also found that respiratory viruses raise the risk of developing DVT and PE. Likewise, the derangement of the coagulation cascade and the subsequent development of systemic fibrin clots in coronavirus infections associated with severe coagulation-related disorders.51
Recently, a novel coronavirus strain disease, COVID-19, has emerged in Wuhan, China, and has spread rapidly across China and subsequently around the world.52 Several studies have indicated that thrombotic complications are emerging problems in patients with COVID-19 despite thromboprophylaxis.51,53,54
In this systematic review and meta-analysis, various factors contributing to the prevalence of thrombotic events among patients with COVID-19 were identified. Elevated D-dimer, hospitalized in the ICU and being under mechanical ventilation were the most frequently contributing factors for the prevalence of thrombotic event. Formation and dissolution of fibrin are key events in haemostasis. Coagulation is initiated by the tissue factor VIIa complex, which promotes thrombin generation subsequently converting fibrinogen to fibrin monomers. In addition, factor XIII links with D-dimer of fibrin monomers which facilitates a solid fibrin clot. The level of D-dimer in serum is highly sensitive for coagulation; hence, it is used for a marker of blood clotting.55 In COVID-19 disease, some pathological conditions favor for coagulation. In addition, due to disturbances of primary hemostasis, abnormality of blood plasma and complex coagulopathies among ICU admitted patients make this group of patients susceptible to coagulation.56
Furthermore, being older, longer median duration of hospitalization and having chronic renal failure were associated factors for the prevalence of thrombotic event. In the elderly, higher level of plasma concentration of clotting factors like F I, VII, VIII: C, X, high molecular weight-kininogen and prekallikrein, lower level of antithrombin III and plasminogen activators in the euglobulin fraction were lower.57 Together with blood stasis and vessel wall damage, this shift of the hemostatic balance contributes to a higher incidence of thromboembolic events in the elderly.57 Chronic renal failure patients experience hyper-fibrinogen level and higher level of D-dimer, von Willebrand factor, factor VII, and factor XIII antigens. They also exhibited significant reductions of antithrombin III, free protein S, plasminogen, and tissue-type plasminogen activator concentrations.58 These increase risk of coagulation. Further, prolonged hospitalization result in longer immobility time which increases risk of coagulation.59
The strengths of our systematic review include complete literature search in more than 1 relevant database (PubMed, EMBASE, CINAHL, Scopus, Google, and Google scholar) and proper screening of eligible studies by 2 independent reviewers. In addition, our review has the following limitations; due to heterogeneity among studies, the meta-analysis result should be interpreted cautiously. Finally, we acknowledge that we may not have been able to retrieve unpublished data and gray literature.
Conclusions
The pooled prevalence of thrombotic events of hospitalized patients with COVID-19 was 33%. The prevalence of thromboembolism events is different based on study design and admission site. ICU admission is associated with higher risk of TE. Future studies should investigate why ICU admission is more susceptible and we also recommend researchers to find out relevant prevention strategies. Several risk factors such as, elevated D-dimer, hospitalized in the intensive care unit and being under mechanical ventilation, were the most frequently reported risk factor for the development of thrombotic events.
Acknowledgments
We would like to acknowledge Debre Tabor University, College of Health Sciences staffs, who are technically supported us the realization of this review.
Footnotes
Author’s contribution: BK, GTT, and AD contributed to conceptualization, data curation, investigation, formal analysis, methodology, writing original draft, review, and editing of the manuscript. MT and DT were involved in conceptualization, writing original draft, investigation, and methodology. All authors approved the submitted version of the manuscript critically.
Availability of data and materials: The datasets used during the current study are available from the corresponding author on a reasonable request
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Belayneh Kefale  https://orcid.org/0000-0003-4841-0861
https://orcid.org/0000-0003-4841-0861
Amsalu Degu  https://orcid.org/0000-0002-6562-0548
https://orcid.org/0000-0002-6562-0548
Melaku Tadege  https://orcid.org/0000-0002-1970-5102
https://orcid.org/0000-0002-1970-5102
References
- 1. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060–3073. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126(9):832 e13-e21. [DOI] [PubMed] [Google Scholar]
- 4. Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res. 2018;164( Suppl 1):S112–S118. [DOI] [PubMed] [Google Scholar]
- 5. Al-Hameed FM, Al-Dorzi HM, Qadhi AI, et al. Thromboprophylaxis and mortality among patients who developed venous thromboembolism in seven major hospitals in Saudi Arabia. Ann Thorac Med. 2017;12(4):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monreal M, Agnelli G, Chuang LH, et al. Deep vein thrombosis in Europe—health-related quality of life and mortality. Clin Appl Thromb Hemost. 2019;25:1076029619883946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chuang LH, Gumbs P, van Hout B, et al. Health-related quality of life and mortality in patients with pulmonary embolism: a prospective cohort study in seven European countries. Qual Life Res. 2019;28(8):2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruppert A, Steinle T, Lees M. Economic burden of venous thromboembolism: a systematic review. J Med Econ. 2011;14(1):65–74. [DOI] [PubMed] [Google Scholar]
- 10. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. [DOI] [PubMed] [Google Scholar]
- 11. Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829–836. [DOI] [PubMed] [Google Scholar]
- 12. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly J, Hunt BJ. Role of D-dimers in diagnosis of venous thromboembolism. Lancet. 2002;359(9305):456–458. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gans I, Jain A, Sirisreetreerux N, Haut ER, Hasenboehler EA. Current practice of antibiotic prophylaxis for surgical fixation of closed long bone fractures: a survey of 297 members of the orthopaedic trauma association. Patient Saf Surg. 2017;11(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kefale K, Temesgen G, Degu A, Tadeg M, Tesfa D. Prevalence and risk factors of thromboembolism among patients with COVID-19: a protocol for a systematic review and meta-analysis. PROSPERO 2020 CRD42020201562 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020201562 (accessed 15 June 2020). [DOI] [PMC free article] [PubMed]
- 21. File Jr TM. New guidelines for antimicrobial prophylaxis in surgery. Infect Dis Clin Prac. 2013;21(3):185–186. [Google Scholar]
- 22. Chen S, Zhang D, Zheng T, Yu Y, Jiang J. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis. 2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nahum J, Morichau-Beauchant T, Daviaud F, et al. Venous thrombosis among critically Ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open. 2020;3(5):e2010478–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142(2):181–183. [DOI] [PubMed] [Google Scholar]
- 29. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu J-F, Wang L, Zhao L, et al. Risk assessment of venous thromboembolism and bleeding in COVID-19 patients, 25 March 2020, PREPRINT (Version 1) available at Research Square. 2020. doi: 10.21203/rs.3.rs-18340/v1+
- 31. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leonard-Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296(3):E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;56(1):2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China. Circulation. 2020;142(2):114–128. [DOI] [PubMed] [Google Scholar]
- 36. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsaplin S, Schastlivtsev I, Lobastov K, Zhuravlev S, Barinov V, Caprini J. The validation of the original and modified Caprini score in COVID-19 patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53(2):207–216. [DOI] [PubMed] [Google Scholar]
- 41. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 42. Ambrosino P, Tarantino L, Criscuolo L, Nasto A, Celentano A, Di Minno MND. The risk of venous thromboembolism in patients with hepatitis C. Thromb Haemost. 2016;116(11):958–966. [DOI] [PubMed] [Google Scholar]
- 43. Abdel-Wahab N, Talathi S, Lopez-Olivo M, Suarez-Almazor M. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27(4):572–583. [DOI] [PubMed] [Google Scholar]
- 44. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Hepatitis C virus infection and risk of venous thromboembolism: a systematic review and meta-analysis. Ann Hepatol. 2017;16(4):514–520. [DOI] [PubMed] [Google Scholar]
- 45. Galloula A, Rossi A, Gautier V, Minozzi C, Messas E, Mirault T. Portal vein thrombosis associated with an acute cytomegalovirus infection. J Mal Vasc. 2014;39(3):224–230. [DOI] [PubMed] [Google Scholar]
- 46. Khan IH, Savarimuthu S, Leung MST, Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg. 2020;72(3):799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russell L, Holst LB, Kjeldsen L, Stensballe J, Perner A. Risks of bleeding and thrombosis in intensive care unit patients with haematological malignancies. Ann Intensive Care. 2017;7(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10(4):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goeijenbier M, Van Wissen M, Van De Weg C, et al. Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075–1079. [DOI] [PubMed] [Google Scholar]
- 51. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ng K, Wu A, Cheng V, et al. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad Med J. 2005;81(956):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. 2004;251(10):1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6(10):491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levi M. Coagulation Disorders in Intensive Care Patients. Perioperative Hemostasis Springer; 2015:377–389. [Google Scholar]
- 57. Hager K, Setzer J, Vogl T, Voit J, Platt D. Blood coagulation factors in the elderly. Arch Gerontol Geriatr. 1989;9(3):277–282. [DOI] [PubMed] [Google Scholar]
- 58. Vaziri N, Gonzales E, Wang J, Said S. Blood coagulation, fibrinolytic, and inhibitory proteins in end-stage renal disease: effect of hemodialysis. Am J Kidney Dis. 1994;23(6):828–835. [DOI] [PubMed] [Google Scholar]
- 59. Lorentsen E, Eika C, Godal H. Coagulation studies in chronically bedridden patients. Acta Med Scand. 1974;195(1-6):79–80. [DOI] [PubMed] [Google Scholar]