Abstract
Over 80% of females experience nipple discharge during their life. Differently from lactational (milk production) and physiological (white, green, or yellow), which are usually bilateral and involving multiple ducts, pathologic nipple discharge (PND) is a spontaneous commonly single-duct and unilateral, clear, serous, or bloody secretion. Mostly caused by intraductal papilloma(s) or ductal ectasia, in 5-33% of cases is due to an underlying malignancy. After clinical history and physical examination, mammography is the first step after 39, but its sensitivity is low (7–26%). Ultrasound shows higher sensitivity (63–100%). Nipple discharge cytology is limited by a false negative rate over 50%. Galactography is an invasive technique that may cause discomfort and pain; it can be performed only when the duct discharge is demonstrated at the time of the study, with incomplete/failed examination rate up to 15% and a difficult differentiation between malignant and benign lesions. Ductoscopy, performed under local anesthesia in outpatients, provides a direct visualization of intraductal lesions, allowing for directed excision and facilitating a targeted surgery. Its sensitivity reaches 94%; however, it is available in only few centers and most clinicians are unfamiliar with its use. PND has recently emerged as a new indication for contrast-enhanced breast MRI, showing sensitivity superior to galactography, with an overall sensitivity up to 96%, also allowing tailored surgery. Surgery no longer can be considered the standard approach to PND. We propose a state-of-the art flowchart for the management of nipple discharge, including ductoscopy and breast MRI as best options.
Introduction
Nipple discharge is a relatively frequent event in females, being the third most common breast symptoms prompting medical care, after breast pain and breast palpable mass. Over 80% of females will develop an episode of nipple discharge during their fertile life,1 which can be categorized as lactional, physiological, and pathological according to the clinical history and the characteristics of the discharge.
Lactional nipple discharge is considered as a normal milk production. It is expected during pregnancy and lactation and may persist for up to one year post-partum or after cessation of breastfeeding. When a milky nipple discharge occurs in females without recent history of pregnancy or lactation it is called galactorrhea and commonly involves bilateral multiple duct being the result of an inappropriate increase in prolactin release, usually supported by a prolactinoma, a prolactin-producing benign tumor of pituitary gland.2
Physiological nipple discharge is a benign entity, usually bilateral and white, green, or yellow in color. It involves multiple ducts and is associated with nipple squeezing. Some causes of physiological nipple discharge are hypothyroidism and medication side-effects.3
Pathologic nipple discharge (PND) is defined as a clear, serous, or bloody secretion (not green or milky), spontaneous, discharging from a single duct and unilateral. It is frequently caused by a benign lesion, such as intraductal papilloma(s) (35–56% of cases) or ductal ectasia (6–59%), but an underlying malignancy can be present in a percentage of cases reported to be variable from 5 to 33%.3–12
According to literature,4,5,11,13–16 the first diagnostic work-up of females with nipple discharge includes clinical history and physical examination. Although conventional imaging, including mammography and ultrasonography are usually performed, these investigations are not always able to exclude an underlying malignancy. More in-depth investigations are proposed such as galactography, ductoscopy, and MRI.
Because to differentiate between a benign from a malignant etiology of a PND based on clinical and diagnostic assessment is not easy, surgical excision has been considered the main way for getting both definitive diagnosis and eliminating the symptom.17
In this article, we try to define a state of the art of management of patients with nipple discharge.
CLINICAL HISTORY AND PHYSICAL EXAMINATION
Clinical history plays an important role for evaluating the probability of malignancy. BRCA1/2 mutations, history of ipsilateral cancer, and age over 50 years are predicting factors for malignancy in the presence of PND.18 In a study including 318 patients with nipple discharge (any fluid from the nipple, spontaneous discharge or observed during breast examination), Seltzer19 has reported a higher incidence of breast cancer equal to 9% in females over 50 (95 patients and 9 cancers) while the incidence was of only 1.3% in younger patients (223 patients and 3 cancers). Previous breast biopsy with diagnosis of atypia is also considered a predictor of malignancy.20
Physical examination has the aim of distinguishing between benign and pathological discharge and of verifying the presence of palpable mass or other associated findings. It usually includes a complete breast evaluation with inspection and palpation, followed by a focused inspection of the nipple area using a magnifying lamp. The physical examination is essential to investigate the color of discharge, the number of ducts involved, the frequency of discharge (persistent or intermittent), and if it is unilateral or bilateral. A spontaneous single-pore bloody and clear discharge is suspect for pathological discharge.
Mammography
Mammography represents the first conventional imaging technique to investigate nipple discharge, at least after 39 years old. For patients with PND, aged between 30 and 40 years old with high-familiarity risk, mammography could be appropriated in order to exclude the presence of microcalcification, as well as for females younger than 30 of age when initial ultrasound shows suspicious findings.9 The protocol includes the standard craniocaudal and mediolateral oblique views.
Mammography findings that are suspect to be associated to an occult malignancy can range from microcalcifications, masses, focal density asymmetry, architectural distortion or ductal ectasia, or otherwise no abnormality can be identified. Mammography has low sensitivity and limited accuracy in the detection of retroareolar lesions that are often small, intraductal, and without calcifications.5 Ductal ectasia may occur as a general increase in density of the retroareolar region and in order to better visualize the area spot compression views could be performed.
In order to improve spatial resolution, magnification mammography can be performed to identify microcalcifications and to distinguish between benign or malignant duct disease.
Microcalcifications with branching or linear pattern, variable density, or distributed in a segmental way, are all highly suspicious of malignancy, whereas round or rod-like calcifications suggest for benign disease.
Bahl et al11 studied 252 patients with at least one pathological feature of nipple discharge (unilateral, clear or bloody, or spontaneous discharge) who underwent surgical excision or a 2-year follow-up. Of 20 cancers diagnosed, only 3 were revealed by mammography, with a 15% (3/20) sensitivity. In other studies,5,21,22 the sensitivity of mammography ranged from 7 to 26%.
Ultrasound
Ultrasound offers a better performance than mammography for detecting intraductal lesions.3 Ductal ectasia, defined by a duct caliber greater than 3 mm, is one of the most common findings well seen on ultrasound and it appears as dilated retroareolar ducts containing anechoid fluid or hypoecoic debris. It is often caused by intraductal papilloma appearing as a hypoechoic nodule with a central vascular pedicle on color Doppler. Doppler ultrasound is helpful in differentiating intraductal viscous secretion versu s intraductal nodule with vascular sign. Irregular duct margins, wall thickening or hypoechoic intraductal mass with acoustic shadowing are ultrasound malignant features.
In a study5 evaluating 38 patients with nipple discharge (32 of them with PND) with mammography and ultrasound, the overall sensitivity for malignant and high-risk lesions (papillomas and atypical intraductal hyperplasia) were 26% for mammography and 63% for ultrasound; specificity was 94% and 84%, respectively. In another study,23 the sensitivity of ultrasound for cancer was 100% (8/8) and the specificity was 73% (102/140). When compared to mammography, ultrasound improves the evaluation of the retroareolar region and ductal system that can be obscured in patients with dense breasts.24
In a study by Park et al15 the detection rate of malignant lesions occult on mammography and ultrasound-detected was reported to be 8 of 53 females with PND examined (15%). Yoon et al25 have also reported that adding ultrasound to mammography in the pre-operative setting of PND led to the detection of malignancies in 26% of patients (ultrasound detected 5 breast cancers in addition to the 19 breast cancers found by mammography).
The role of ultrasound elastography is disputable in predicting malignancy in patients with PND. Guo et al16 have evaluated the diagnostic accuracy of elastography in patients with PND, affirming that it is a useful tool for predicting malignancy, with sensitivity for malignancy of 90% and that it could be used as a helpful test before more invasive examination (such as ductoscopy or duct excision). However, it is only a preliminary study and further studies are needed to verify the diagnostic performance of elastography.
NIPPLE DISCHARGE CYTOLOGY
Nipple discharge cytology is performed by squeezing the nipple with a gentle compression of the areola area and spreading the secretion onto a glass slide. After smearing, the slides are immediately fixed by spray fixation or by immersion in 95% ethyl alcohol and then stained with the Papanicolaou stain.3
It is a simple and fast examination, easy to perform and painless, but strongly limited by a low sensitivity for cancer, with a false negative rate over 50%.3,5,26–29 Moreover, it can be technically impossible when discharge is not present on the moment of the examination. According to the American College of Radiology, this examination has not proven to be effective in differentiating benign from malignant lesions.9 Therefore, discharge cytology is not routinely recommended.10 Nipple discharge smears are classified as abnormal if they contained papillary, atypical, suspicious, or malignant cells; malignant nipple discharge cytology (C4-C5 categories) is correlated with more high-specificity values.30
Galactography
For a long time, galactography (also called ductography, or ductogalactography) has been considered the gold standard to assess PND before surgery. It is performed by cannulating the discharging duct with a blunt dedicated cannula, through which non-ionic iodined contrast agent is injected. Usually a volume of 0.5–1.0 ml of iodined contrast material is administered. After contrast injection, the breast is compressed and standard mammogram is obtained (craniocaudal and mediolateral oblique projections). At the end of examination, the contrast material is aspirated with the same syringe that was used to inject it and gentle manual pressure is exerted on the breast to discharge it. All the procedure usually takes about 15 min31 but longer times are sometimes required. Contraindications to galactography include severe allergy to iodinated contrast material and a history of prior nipple surgery that would completely disconnect the nipple pores from the underlying ducts. The resulting ductograms are classified as normal or showing ductal ectasia (i.e. duct diameter over 2 mm), filling interruption, filling stops, and ductal distortions.32
Nowadays, galactography has lost some of its clinical relevance due to many disadvantages of the investigation and the availability of other options. As reported by Scheurlen et al33 in a recent systematic review, “galactography can no longer be considered as a mandatory standard in modern multimodal imaging of the breast”. Indeed, it is an invasive technique and may cause discomfort and pain.9 Galactography can also be performed only when the duct discharge is demonstrated at the time of the study. Therefore it can be technically impossible, especially in patients with intermittent discharge or nipple retraction.10,34 Duct rupture can happen if too much contrast material or pressure is used during injection. The incomplete or failed galactography rate has been reported as high as 15%.6
Galactography allows to visualize and localize intraductal lesions, but usually it does not allow to differentiate between benign and malignant pathology.1,4,34
Ductoscopy
Differently from galactography, ductoscopy provides a direct visualization of intraductal lesions, allowing for directed excision and facilitating a targeted surgery. The benefit is being able to avoid extensive surgery resection with healthy breast tissue conservation.35
The offending duct is identified through gentle pressure on the areola and noticing the source of discharge and the ductal orifice is dilated with a suitable probe. The micro-endoscope (0.9 mm in size) is inserted through the duct and a saline solution is injected through the working channel (0.2 mm in size) of the endoscope to obtain a clear visualization of the intraductal space. The injection of saline solution permits to do a ductal lavage and the lavage effluent can be also collected for cytological examination. The major duct and all the branches are inspected to the maximum depth possible according to the ductal size (median depth 5–6 cm).35 Limitation is related to the length and to the outer diameter of the scope, in that the ductoscope cannot reach the far and minute ductal branches with the risk of not seeing lesions in these ductal branches.36
It can be performed under local anesthesia in outpatients increasing patient tolerability and decreasing risk and possible complications.37 In the case of a dilated nipple orifice, the cannulation can be easier. According to the literature, there are contradictory views about the correlation between intraductal visual observations and histopathological diagnosis. Despite some researchers stating a significant correlation, and therefore intraductal visual observations seem to be sufficient to characterize lesion, others affirm that visual inspection alone could not be sufficient to differentiate between benign and malignant lesions.37,38 Therefore only when no intraductal abnormalities are found, ductoscopy is considered negative. Anyway, ductoscopy has to be considered as an invasive technique and, like for galactography, inverted nipples, narrow or unidentifiable ducts, obstructing lesions behind the nipple, and pain during the procedure represent relevant substantial obstacles to perform it.37
In a recent systematic review and meta-analysis Waaijer et al39 assessed the diagnostic accuracy of ductoscopy in patients with PND. Including 12 studies with a total of 1,994 patients, for which any intraductal lesion visualized by ductoscopy was classified as a positive finding and normal ducts were classified as negative, ductoscopy detected about 94% of all underlying malignancies. Results were compared with histopathology outcomes or follow-up for a total of 151 malignancies [invasive breast cancer and ductal carcinoma in situ (DCIS)]. Specificity, defined as the proportion of females with negative ductoscopy among all females with benign outcomes, was low, equal to only 47%. The authors reported that owing to the high sensitivity of ductoscopy and low incidence of malignancy in PND, this method has a 98–100% negative predictive value (NPV). Thus, a negative ductoscopy could make surgery unnecessary, avoiding risk and complications.
Waaijer and coworkers38 also investigated the therapeutic effect of ductoscopy in patients with PND without suspected malignancy on routine diagnostic evaluation. 53 abnormalities in 82 patients were visualized on ductoscopy (n = 29 were polypoid lesions) and 27 lesions were removed in 34 attempted ductoscopic extractions. In 56 of 82 patients (68%) surgery was avoided.
High sensitivity value of ductoscopy was confirmed by Yilmaz et al.40 They compared MRI and ductoscopy, reporting that these techniques have not statistical superiority over each other in the diagnosis of intraductal lesion in patients with PND (sensitivity was 94% for ductoscopy, 90% for MRI).
Despite these good results, ductoscopy remains still unpopular, it is available in only a few centers and most clinicians are unfamiliar with its use. Furthermore, it is difficult to source the intraductal needle biopsy devices that are not yet commercially available in Europe and in USA, with unresolved issues regarding cost and reimbursement.39,41,42
MRI
According to the European recommendations,43 nipple discharge has recently emerged as a new indication for breast MRI. In fact, it is an effective alternative to galactography, non-invasive although it requires the i.v. injection of a gadolinium-based contrast material, with an overall sensitivity for breast cancer ranging from 90 to 99%.9,43,44
Contrast-enhanced MRI allows detecting a “mass enhancement” or “non-mass enhancement” lesions. The main MRI finding in patients with PND is “non-mass enhancement”: a segmental and linear “non-mass enhancement” is more often associated with malignancy. Intraductal papilloma appears as a well-circumscribed mass with homogeneous enhancement. Other times ductal or segmental enhancement can be seen in both papillomas and papillomatosis. Dilated ducts appear as signal hyperintensity on T 2 weighted imaging or as an area of high-signal intensity in pre-contrast T 1 weighted sequences, if endoluminal high protein or hemorrhagic content is present.
Contrast-enhancement MRI allows imaging of both breasts with a good visualization of retroareolar regions. It outlines enhancing pathology in good detail, thereby providing physiological information in addition to the morphological detail provided by mammography and ultrasound.45 It also permits to detect multifocal or multicentric disease or to find an occult contralateral lesion.46 This MRI ability can play a crucial role when a PND involves more than one duct or it is unclear which duct is involved (e.g. intermittent suspicious discharge).
These advantages, makes MRI a better tool compared to the other diagnostic techniques despite higher cost.
Lorenzon et al5 reported a cancer sensitivity of contrast-enhanced MRI of 100% on a small series (5 out of 5 malignancies and all associated with PND). Three of these cancers were missed at conventional imaging (mammography and ultrasound) and detected only with MRI. Other studies compared MRI against galactography. Morrogh et al6 studied 306 patients with PND. They reported a positive predictive value of 56% and a NPV of 87% for contrast-enhanced MRI, while the same data were 13% and 63% for galactography respectively, concluding that galactography cannot exclude malignancy. Manganaro et al34 evaluated retrospectively 53 patients with unilateral bloody or serous-bloody nipple discharge. They found a significant difference in sensitivity between contrast-enhanced MRI (98%) and galactography (49%) to identify ductal pathologies (papilloma, papillomatosis, DCIS, papillary carcinoma) and they also found a significant association between DCIS and segmental enhancement on MRI.
Berger et al7 demonstrated the accuracy of MRI versu s galactography in a systematic review and meta-analysis, including 10 articles for a total of 921 patients. MRI showed significantly higher sensitivity (92%) and specificity (76%) for abnormal findings than galactography (69% and 39%, respectively). When considering only breast cancers, MRI sensitivity was 92%, specificity 97%. These results confirmed on a pooled large case series that galactography is outdated and MRI should be performed in the presence of PND when conventional imaging is negative or inconclusive. Other experiences support the role of MRI in the evaluation of patient with nipple discharge and negative conventional imaging.8,14,47 Many authors highlight the value of targeted ltrasound looking for suspicious finding on MRI.3,5
According to the latest American research, in about of 60% of cases radiologists recommended breast MRI in the setting of pathologic nipple discharge after the negative results of diagnostic breast imaging.48
Bahl et al47 had reported a NPV of MRI for in situ or invasive malignancy of 96% (75/78), from a series of 105 who underwent MRI after a negative or inconclusive mammography for evaluation of nipple discharge.
Furthermore, MRI can help to better identify lesions located in the deepest areas of breast, at a significant distance from the nipple as it is difficult to visualize them with other techniques as ductoscopy or galactography. Compared to galactography in which only one duct can be cannulated, MRI allows evaluating at the same time all the ductal system. Both invasive or in situ cancer and papilloma may affect more than one duct. Sanders et al13 reported that four of eight cancers detected on MRI were located between 3 and 12 cm far from the nipple. Thus, pre-operative MRI should be performed to facilitate optimal treatment planning in patients with nipple discharge.14,46,47 Breast MRI is a useful diagnostic tool in the characterization of BIRADS (Breast Imaging Reporting and Data System) for mammographic microcalcifications to exclude malignancy.49
Concerning the MRI protocols for investigating PND, we should differentiate the indirect MR-galactogram from direct MR-galactography.50,51 The indirect MR-galactogram accentuates the visibility of structures containing fluids using heavily T 2 weighted sequences (as is for MR-cholangiography) and avoiding contrast material injection and ductal cannulation. By acquiring short tau inversion-recovery T 2 weighted sequences, and obtaining maximum intensity projection images, it permits in a non-invasive way, to obtain images similar with those of galactography, with the advantages of receiving great compliance from patients and avoiding radiation exposure. Dilated duct shows hyperintense signal while intraductal abnormalities appear as an hypointense filling defect or filling stop. The spatial resolution is lower than that of galactography, but the size of malignant lesions determining PND (usually at least 2–3 mm) allows this limitation not to be clinically relevant. In addition, it is possible to use specific microscopic coil to improve spatial resolution.52
Direct MR-galactography is performed by cannulating the discharging duct and directly filling it with contrast material. This technique does not save patient from discomfort of cannulation and may fail due to the same issues as conventional galactography.
Nicholson et al53 compared contrast-enhanced MRI and indirect MR-galactography against conventional galactography. They found that contrast-enhanced MRI has the highest sensitivity, positive and NPVs. Sensitivity for intraductal pathology was 95% for contrast-enhanced MRI, 52–55% for MR-galactography, 65% for galactography. Although indirect MR-galactography showed interesting results, it is not as sensitive for malignancy as is contrast-enhanced MRI. In that study, all of five cancers were visualized on contrast-enhanced MRI (three as non-mass enhancement and two as mass enhancements) while MR-galactography was negative in three out of five cases (two DCIS and one mucinous carcinoma).
Nevertheless, we must consider the false-negative ratings that could be low-grade DCIS or very small invasive ductal carcinoma due to the lack of enhancement47, as well as the high false positive rate as reflected by the detection of additional incidental lesions afterwards proved negative by biopsy.6
Strobel et al54 reported that MRI assessment has reduced the number of false positive findings at mammography from 152 to 14 after MRI. MRI dismissed three low-grade DCIS (of 25 findings) with a false negative rate of 12%.
Also Manganaro et al34 had only one case of false negative on MRI (a DCIS, not identified on galactography). In Lubina’s study,8 MRI did not diagnose three cases of low-grade DCIS and one invasive carcinoma.
GALACTOGRAPHY COMBINED WITH TOMOSYNTHESIS
Although MRI and ductoscopy represent the innovative techniques in the evaluation of PND, a possible alternative approach could be the association between conventional galactography with digital breast tomosynthesis. Digital breast tomosynthesis, also called three-dimensional mammography, provides sectional images from different projection angles with the advantage to remove the masking created by overlying structures. It allows creating digital three-dimensional images with a better localization of suspicious findings and higher spatial resolution.
According to Cohen,55 the union of these two techniques could be a useful procedure for the workup of PND, especially when MR is not available, with a lower cost. However, up to now, there is only a recent preliminary original research (based only on five patients) that compares ductal sonography with synthetic digital two-dimensional full-field mammograms generated using contrast-enhanced galactography with tomosynthesis, reporting that both techniques were approximately equivalent with respect to the correct diagnosis of suspicious findings and the final histopathological results.56 Obviously, the disadvantages of conventional galactography are maintained, including cannulating the discharging duct, the direct injection of contrast agent, and radiation exposure.
Surgery
For a long time, surgery has been considered the standard approach to PND.17 Operation consists of either total subareolar duct excision (major duct excision) or selective duct excision (microdochectomy) of the single duct affected. The benefit of microdochectomy is the preservation of remaining ductal systems in continuity with the nipple and is usually the best option for young females who wish to breastfeed. In other cases major duct excision is preferred due to a higher detection of occult cancers than that of microdochectomy, resulting in fewer patients requiring repeated duct excision.57 Possible complications of surgery are inability to breastfeed, loss of nipple sensation, and retroareolar necrosis.
Sabel et al18 reported that in 69 patients with PND and normal conventional imaging (ultrasound and mammograms), cancer was found (after surgery or follow-up) in only 1% of cases (1/69), saying that the risk of an occult malignant lesion is relatively low in patients without abnormal findings at clinical examination and imaging tests. Most of these cases are low-grade DCIS with very good prognosis.18 MRI showed high sensitivity and NPV, being an accurate imaging test in the workup of nipple discharge in patients with inconspicuous mammography and ultrasound.8 Therefore, surgery cannot be longer always considered the best option and the potential of imaging should be more extensively explored.
In 2014, Ashfaq et al23 proposed again the treatment algorithm already envisaged by Gray in 2007,58 suggesting a distinction among PND patients between a high-risk group requiring surgery versu s a low-risk group in whom a short-term surveillance is reasonable. Low-risk patients include those without abnormal findings on clinical and radiological assessment that can be monitored every 6 months up to 2 years or until the discharge resolved, with physical and ultrasound examinations every 6 months and yearly mammogram. High-risk patients include those with history or family history of breast cancer and they should undergo duct excision. In addition, duct excision could also be proposed as palliative therapy or in the event of refusal of surveillance.18 By using this approach, Ashfaq et al23 have determined that it is possible to avoid unnecessary operation in 66% of patients with PND, by reserving it only in selective cases. They also documented that among the patients who never underwent operation, 81% had a resolution of their discharge.
A FLOWCHART FOR THE MANAGEMENT OF NIPPLE DISCHARGE
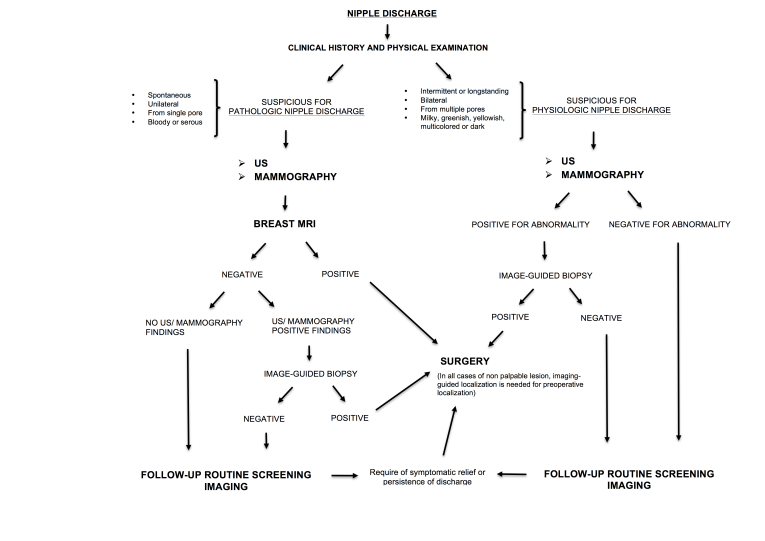
We propose here a flowchart for the management of nipple discharge (Figure 1). Evaluation of clinical history and physical examination are the first approaches to differentiate pathological from physiological discharge. Bloody or serous, spontaneous and unilateral nipple discharge from a single pore should be considered suspicious for breast cancer.
Figure 1.
Flowchart for managing patients with nipple discharge.
Diagnostic imaging evaluation should be performed in all females with PND and mammography and breast ultrasound are the first examinations to do. Discharge cytology is not routinely recommended9 (and it is not considered in this flowchart), but it may be useful if combined with other finding.
Contrast-enhanced MRI, due to its highest sensibility and NPV should be considered in the management of all patients with PND, in particular in those with negative mammography and ultrasound9 (Figures 2 and 3). MRI provides excellent visualization of dilated ducts and their contents and it outlines enhancing pathology in good detail, adding physiological information to the morphological detail provided by mammography and ultraound.45 When MRI reveals a suspicious lesion that can be the cause of the discharge (confirmed or not confirmed at conventional modalities), image-guided needle biopsy should be performed before sending the patient to surgery. If technically possible, ultrasound-guided biopsy should be preferred.
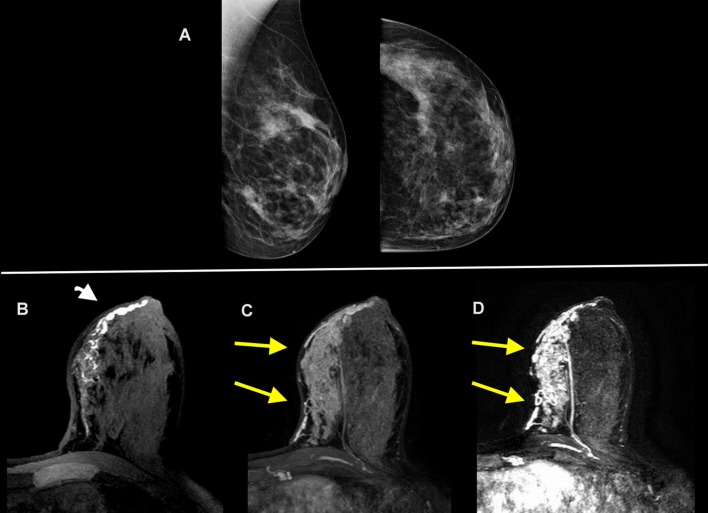
Figure 2.
A 40-year-old female with dense breast and 4 months of pathological discharge from left nipple. Digital mammography (craniocaudal and mediolateral-oblique views) reveals scattered areas of fibroglandular density with no evidence of masses or suspicious calcifications (A). The unenhanced T 1 weighted image in the axial plane shows ductal ectasia in the inner quadrant with endoluminal proteinaceous material (head of arrow) (B). The contrast-enhanced axial T 1 weighted image (C) and T 1 weighted subtracted maximum intensity projection reconstruction (D) revealed an extensive segmental enhancement at the inner quadrants of the left breast (arrows). Findings were confirmed at pathology to be invasive ductal carcinoma of the breast.
Figure 3.
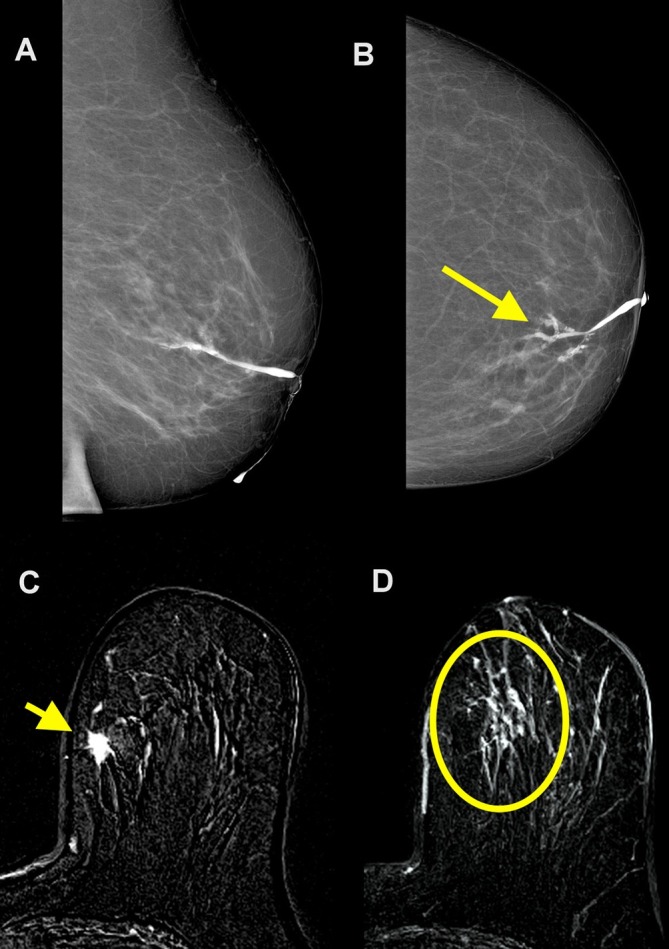
A 75-year-old female with 6 months of pathological discharge from the left nipple. Duct ectasia was diagnosed on ultrasound. No abnormal findings were found on mammography. Cytology of discharge showed amorphous material, red cells, and macrofages without ductal cell. Galactography allowed for a good visualization of the main duct with a filling stop for secondary ducts (arrow) (A, B). Contrast-enhanced subtracted axial MR images show an enhancing spiculated mass at the upper inner quadrant of left breast (arrow in C) associated with a segmental non-mass enhancement (circle in D). At pathology, an invasive ductal carcinoma with extensive intraductal component was revealed.
In all cases of non-palpable lesions, an image-guided localization is needed for pre-operative localization. Based on the low risk of underlying malignancy, patients with negative imaging (which includes MRI) can be (re)directed to routine screening follow-up.5,14,47 When, despite a negative MRI, a suspect lesion is found on conventional imaging, ultrasound- or mammography-guided biopsy is recommended. Malignancy cases sent to surgery and patients with benign lesions should be followed-up with routine screening examinations.
If nipple discharge is persistent or recurrent after a 2-year monitoring or for patient choice for symptomatic relief, duct excision may be considered.
In the presence of physiological nipple discharge, image-guided biopsy is recommended just in case of abnormalities at conventional imaging; otherwise routine follow-up should be done.9 MRI is not appropriate in cases of physiological discharge and negative conventional imaging, but can be proposed in the case of malignancy to evaluate the extension of disease.
In conclusion, clinical history and physical examination, mammography, and ultrasound remain important steps in the management of patient with nipple discharge, despite their low diagnostic accuracy. Discharge cytology has a low diagnostic value, and is not routinely recommended. Galactography might not be the best option due to its disadvantages and the increasing availability of MRI and ductoscopy. Unlike in the past years, surgery should no longer be considered the standard of care in patients with PND. Performing an MRI is recommended in those patients.
Contributor Information
Giovanna Panzironi, Email: giovannapanzironi@gmail.com.
Federica Pediconi, Email: federica.pediconi@uniroma1.it.
Francesco Sardanelli, Email: francesco.sardanelli@grupposandonato.it.
REFERENCES
- 1. Morrogh M, Park A, Elkin EB, King TA. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg 2010; 200: 73–80. doi: 10.1016/j.amjsurg.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 2. Huang W, Molitch ME. Evaluation and management of galactorrhea. Am Fam Physician 2012; 85: 1073–80. [PubMed] [Google Scholar]
- 3. Lippa N, Hurtevent-Labrot G, Ferron S, Boisserie-Lacroix M. Nipple discharge: the role of imaging. Diagn Interv Imaging 2015; 96: 1017–32. doi: 10.1016/j.diii.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 4. van Gelder L, Bisschops RH, Menke-Pluymers MB, Westenend PJ, Plaisier PW. Magnetic resonance imaging in patients with unilateral bloody nipple discharge; useful when conventional diagnostics are negative? World J Surg 2015; 39: 184–6. doi: 10.1007/s00268-014-2701-1 [DOI] [PubMed] [Google Scholar]
- 5. Lorenzon M, Zuiani C, Linda A, Londero V, Girometti R, Bazzocchi M. Magnetic resonance imaging in patients with nipple discharge: should we recommend it? Eur Radiol 2011; 21: 899–907. doi: 10.1007/s00330-010-2009-y [DOI] [PubMed] [Google Scholar]
- 6. Morrogh M, Morris EA, Liberman L, Borgen PI, King TA. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol 2007; 14: 3369–77. doi: 10.1245/s10434-007-9530-5 [DOI] [PubMed] [Google Scholar]
- 7. Berger N, Luparia A, Di Leo G, Carbonaro LA, Trimboli RM, Ambrogi F, et al. Diagnostic performance of MRI vs Galactography in women with pathologic nipple discharge: A systematic review and meta-analysis. AJR Am J Roentgenol 2017; 209: 465–71. doi: 10.2214/AJR.16.16682 [DOI] [PubMed] [Google Scholar]
- 8. Lubina N, Schedelbeck U, Roth A, Weng AM, Geissinger E, Hönig A, et al. 3.0 Tesla breast magnetic resonance imaging in patients with nipple discharge when mammography and ultrasound fail. Eur Radiol 2015; 25: 1285–93. doi: 10.1007/s00330-014-3521-2 [DOI] [PubMed] [Google Scholar]
- 9. Expert Panel on Breast Imaging, Lee SJ, Trikha S, Moy L, Baron P, diFlorio RM. ACR appropriateness criteria(®) evaluation of nipple discharge®) evaluation of nipple discharge. J Am Coll Radiol 2017; 14: S138–S153. [DOI] [PubMed] [Google Scholar]
- 10. Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med 2015; 128: 353–60. doi: 10.1016/j.amjmed.2014.09.031 [DOI] [PubMed] [Google Scholar]
- 11. Bahl M, Baker JA, Greenup RA, Ghate SV. Diagnostic value of ultrasound in female patients with nipple discharge. AJR Am J Roentgenol 2015; 205: 203–8. doi: 10.2214/AJR.14.13354 [DOI] [PubMed] [Google Scholar]
- 12. Patel BK, Ferraro C, Kosiorek HE, Loving VA, D’Orsi C, Newell M, et al. Nipple discharge: imaging variability among U.S. radiologists. AJR Am J Roentgenol 2018; 211: 920–5. doi: 10.2214/AJR.18.19622 [DOI] [PubMed] [Google Scholar]
- 13. Sanders LM, Daigle M. The rightful role of MRI after negative conventional imaging in the management of bloody nipple discharge. Breast J 2016; 22: 209–12. doi: 10.1111/tbj.12551 [DOI] [PubMed] [Google Scholar]
- 14. Bahl M, Baker JA, Greenup RA, Ghate SV. Evaluation of pathologic nipple discharge: What is the added diagnostic value of MRI? Ann Surg Oncol 2015; 22(Suppl 3): 435–41. doi: 10.1245/s10434-015-4792-9 [DOI] [PubMed] [Google Scholar]
- 15. Park CJ, Kim EK, Moon HJ, Yoon JH, Kim MJ. Reliability of breast ultrasound BI-RADS final assessment in mammographically negative patients with nipple discharge and radiologic predictors of malignancy. J Breast Cancer 2016; 19: 308–15. doi: 10.4048/jbc.2016.19.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo X, Liu Y, Li W. Diagnostic accuracy of shear wave elastography for prediction of breast malignancy in patients with pathological nipple discharge. BMJ Open 2016; 6: e008848. doi: 10.1136/bmjopen-2015-008848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons R, Adamovich T, Brennan M, Christos P, Schultz M, Eisen C, et al. Nonsurgical evaluation of pathologic nipple discharge. Ann Surg Oncol 2003; 10: 113–6. doi: 10.1245/ASO.2003.03.089 [DOI] [PubMed] [Google Scholar]
- 18. Sabel MS, Helvie MA, Breslin T, Curry A, Diehl KM, Cimmino VM, et al. Is duct excision still necessary for all cases of suspicious nipple discharge? Breast J 2012; 18: 157–62. doi: 10.1111/j.1524-4741.2011.01207.x [DOI] [PubMed] [Google Scholar]
- 19. Seltzer MH. Breast complaints, biopsies, and cancer correlated with age in 10,000 consecutive new surgical referrals. Breast J 2004; 10: 111–7. doi: 10.1111/j.1075-122X.2004.21284.x [DOI] [PubMed] [Google Scholar]
- 20. Dupont SC, Boughey JC, Jimenez RE, Hoskin TL, Hieken TJ. Frequency of diagnosis of cancer or high-risk lesion at operation for pathologic nipple discharge. Surgery 2015; 158: 988–95. doi: 10.1016/j.surg.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 21. Vargas HI, Vargas MP, Eldrageely K, Gonzalez KD, Khalkhali I. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg 2006; 72: 124–8. [PubMed] [Google Scholar]
- 22. Adepoju LJ, Chun J, El-Tamer M, Ditkoff BA, Schnabel F, Joseph KA. The value of clinical characteristics and breast-imaging studies in predicting a histopathologic diagnosis of cancer or high-risk lesion in patients with spontaneous nipple discharge. Am J Surg 2005; 190: 644–6. doi: 10.1016/j.amjsurg.2005.06.032 [DOI] [PubMed] [Google Scholar]
- 23. Ashfaq A, Senior D, Pockaj BA, Wasif N, Pizzitola VJ, Giurescu ME, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208: 222–7. doi: 10.1016/j.amjsurg.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 24. Yoon JH, Yoon H, Kim EK, Moon HJ, Park YV, Kim MJ. Ultrasonographic evaluation of women with pathologic nipple discharge. Ultrasonography 2017; 36: 310–20. doi: 10.14366/usg.17013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon H, Yoon JH, Kim EK, Moon HJ, Park BW, Kim MJ. Adding ultrasound to the evaluation of patients with pathologic nipple discharge to diagnose additional breast cancers: Preliminary data. Ultrasound Med Biol 2015; 41: 2099–107. doi: 10.1016/j.ultrasmedbio.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 26. Abdalla S, Savag L, Masannat Y, Pinder SE, Fentiman IS, Hamed H. Pathological nipple discharge. The Open Access Journal of Science and Technology 2014; 2. doi: 10.11131/2014/101037 [DOI] [Google Scholar]
- 27. Simmons R, Thevarajah S, Brennan MB, Christos P, Osborne M. Methylene blue dye as an alternative to isosulfan blue dye for sentinel lymph node localization. Ann Surg Oncol 2003; 10: 242–7. doi: 10.1245/ASO.2003.04.021 [DOI] [PubMed] [Google Scholar]
- 28. Das DK, Al-Ayadhy B, Ajrawi MT, Shaheen AA, Sheikh ZA, Malik M, et al. Cytodiagnosis of nipple discharge: a study of 602 samples from 484 cases. Diagn Cytopathol 2001; 25: 25–37. doi: 10.1002/dc.1098 [DOI] [PubMed] [Google Scholar]
- 29. Cabioglu N, Hunt KK, Singletary SE, Stephens TW, Marcy S, Meric F, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg 2003; 196: 354–64. doi: 10.1016/S1072-7515(02)01606-X [DOI] [PubMed] [Google Scholar]
- 30. Castellano I, Metovic J, Balmativola D, Annaratone L, Rangel N, Vissio E, et al. The impact of malignant nipple discharge cytology (NDc) in surgical management of breast cancer patients. PLoS One 2017; 12: e0182073. doi: 10.1371/journal.pone.0182073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berná JD, Guirao J, Garcia V. A coaxial technique for performing galactography. AJR Am J Roentgenol 1989; 153: 273–4. doi: 10.2214/ajr.153.2.273 [DOI] [PubMed] [Google Scholar]
- 32. Slawson SH, Johnson BA. Ductography: how to and what if? Radiographics 2001; 21: 133–50. doi: 10.1148/radiographics.21.1.g01ja15133 [DOI] [PubMed] [Google Scholar]
- 33. Scheurlen K, Schnitzer A, Krammer J, Kaiser C, Schönberg SO, Wasser K. Value of galactography for the diagnostic work-up of pathological nipple discharge in multimodal breast diagnostics. Part 2: a systematic review of the literature. Radiologe 2014; 54: 160–6. doi: 10.1007/s00117-013-2573-7 [DOI] [PubMed] [Google Scholar]
- 34. Manganaro L, D'Ambrosio I, Gigli S, Di Pastena F, Giraldi G, Tardioli S, et al. Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: a comparison with galactography. Biomed Res Int 2015; 2015: 806368–9. doi: 10.1155/2015/806368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher CS, Margenthaler JA. A look into the ductoscope: its role in pathologic nipple discharge. Ann Surg Oncol 2011; 18: 3187–91. doi: 10.1245/s10434-011-1962-2 [DOI] [PubMed] [Google Scholar]
- 36. Denewer A, El-Etribi K, Nada N, El-Metwally M. The role and limitations of mammary ductoscope in management of pathologic nipple discharge. Breast J 2008; 14: 442–9. doi: 10.1111/j.1524-4741.2008.00620.x [DOI] [PubMed] [Google Scholar]
- 37. Sarica O, Ozturk E, Demirkurek HC, Uluc F, ductoscopy Cof. Comparison of ductoscopy, galactography, and imaging modalities for the evaluation of intraductal lesions: a critical review. Breast Care 2013; 8: 348–54. doi: 10.1159/000355833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waaijer L, van Diest PJ, Verkooijen HM, Dijkstra NE, van der Pol CC, Borel Rinkes IH, et al. Interventional ductoscopy in patients with pathological nipple discharge. Br J Surg 2015; 102: 1639–48. doi: 10.1002/bjs.9950 [DOI] [PubMed] [Google Scholar]
- 39. Waaijer L, Simons JM, Borel Rinkes IH, van Diest PJ, Verkooijen HM, Witkamp AJ. Systematic review and meta-analysis of the diagnostic accuracy of ductoscopy in patients with pathological nipple discharge. Br J Surg 2016; 103: 632–43. doi: 10.1002/bjs.10125 [DOI] [PubMed] [Google Scholar]
- 40. Yılmaz R, Bender Ö, Çelik Yabul F, Dursun M, Tunacı M, Acunas G. Diagnosis of nipple discharge: value of magnetic resonance imaging and iltrasonography in comparison with ductoscopy. Balkan Med J 2017; 34: 119–26. doi: 10.4274/balkanmedj.2016.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dooley WC. Breast ductoscopy and the evolution of the intra-ductal approach to breast cancer. Breast J 2009; 15(Suppl 1): S90–S94. doi: 10.1111/j.1524-4741.2009.00799.x [DOI] [PubMed] [Google Scholar]
- 42. Kapenhas-Valdes E, Feldman SM, Boolbol SK. The role of mammary ductoscopy in breast cancer: a review of the literature. Ann Surg Oncol 2008; 15: 3350–60. doi: 10.1245/s10434-008-9911-4 [DOI] [PubMed] [Google Scholar]
- 43. Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, et al. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 2015; 25: 3669–78. doi: 10.1007/s00330-015-3807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bennani-Baiti B, Bennani-Baiti N, Baltzer PA. Diagnostic performance of breast magnetic resonance imaging in non-Calcified equivocal breast findings: results from a systematic review and Meta-Analysis. PLoS One 2016; 11: e0160346. doi: 10.1371/journal.pone.0160346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ballesio L, Maggi C, Savelli S, Angeletti M, De Felice C, Meggiorini ML, et al. Role of breast magnetic resonance imaging (MRI) in patients with unilateral nipple discharge: preliminary study. Radiol Med 2008; 113: 249–64. doi: 10.1007/s11547-008-0245-x [DOI] [PubMed] [Google Scholar]
- 46. Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008; 26: 3248–58. doi: 10.1200/JCO.2007.15.2108 [DOI] [PubMed] [Google Scholar]
- 47. Bahl M, Gadd MA, Lehman CD. JOURNAL CLUB: Diagnostic utility of MRI after negative or Inconclusive mammography for the evaluation of pathologic nipple discharge. AJR Am J Roentgenol 2017; 209: 1404–10. doi: 10.2214/AJR.17.18139 [DOI] [PubMed] [Google Scholar]
- 48. Patel BK, Ferraro C, Kosiorek HE, Loving VA, D'Orsi C, Newell M, et al. Nipple discharge: imaging variability among U.S. radiologists. AJR Am J Roentgenol 2018; 211: 920–5. doi: 10.2214/AJR.18.19622 [DOI] [PubMed] [Google Scholar]
- 49. Bennani-Baiti B, Baltzer PA. MR Imaging for diagnosis of malignancy in mammographic microcalcifications: A systematic review and Meta-Analysis. Radiology 2017; 283: 692–701. doi: 10.1148/radiol.2016161106 [DOI] [PubMed] [Google Scholar]
- 50. Jara H, Barish MA, Yucel EK, Melhem ER, Hussain S, Ferrucci JT. MR hydrography: theory and practice of static fluid imaging. AJR Am J Roentgenol 1998; 170: 873–82. doi: 10.2214/ajr.170.4.9530026 [DOI] [PubMed] [Google Scholar]
- 51. Hirose M, Nobusawa H, Gokan T. MR ductography: comparison with conventional ductography as a diagnostic method in patients with nipple discharge. Radiographics 2007; 27(Suppl 1): S183–S196. doi: 10.1148/rg.27si075501 [DOI] [PubMed] [Google Scholar]
- 52. Bhattarai N, Kanemaki Y, Kurihara Y, Nakajima Y, Fukuda M, Maeda I. Intraductal papilloma: features on MR ductography using a microscopic coil. AJR Am J Roentgenol 2006; 186: 44–7. doi: 10.2214/AJR.04.1600 [DOI] [PubMed] [Google Scholar]
- 53. Nicholson BT, Harvey JA, Patrie JT, Mugler JP. 3D-MR Ductography and contrast-enhanced MR mammography in patients with suspicious nipple discharge; a feasibility study. Breast J 2015; 21: 352–62. doi: 10.1111/tbj.12417 [DOI] [PubMed] [Google Scholar]
- 54. Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 lesions detected with screening mammography and screening US: utility of MR imaging. Radiology 2015; 274: 343–51. doi: 10.1148/radiol.14140645 [DOI] [PubMed] [Google Scholar]
- 55. Cohen Y. Conventional ductography combined with digital breast tomosynthesis for imaging of pathologic nipple discharge. AJR Am J Roentgenol 2016; 206: W44. doi: 10.2214/AJR.15.15454 [DOI] [PubMed] [Google Scholar]
- 56. Schulz-Wendtland R, Preuss C, Fasching PA, Loehberg CR, Lux MP, Emons J, et al. Galactography with Tomosynthesis Technique (Galactomosynthesis) - Renaissance of a Method? Geburtshilfe Frauenheilkd 2018; 78: 493–8. doi: 10.1055/a-0594-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sharma R, Dietz J, Wright H, Crowe J, DiNunzio A, Woletz J, et al. Comparative analysis of minimally invasive microductectomy vs major duct excision in patients with pathologic nipple discharge. Surgery 2005; 138: 591–7. doi: 10.1016/j.surg.2005.07.015 [DOI] [PubMed] [Google Scholar]
- 58. Gray RJ, Pockaj BA, Karstaedt PJ. Navigating murky waters: a modern treatment algorithm for nipple discharge. Am J Surg 2007; 194: 850–5. doi: 10.1016/j.amjsurg.2007.08.027 [DOI] [PubMed] [Google Scholar]