Abstract
Objective:
To investigate differences in the degree of enhancement on contrast-enhanced mammography (CEM) between patients with invasive lobular (ILC) and infiltrating ductal carcinoma (IDC) not otherwise specified.
Methods and materials:
Between 2010 and 2017, all patients diagnosed with ILC and who underwent CEM were included for this dual center study. Twenty-two patients with IDC, matched by size, were identified for comparison. Three independent readers, blinded for histopathology results, re-evaluated all CEM exams to determine degree of lesion enhancement according to a previously defined scoring scale ranging from minimal to strong enhancement. Interobserver agreement among the three readers was calculated by quadratic weighted κ coefficient.
Results:
44 patients were included: 22 patients with ILC and 22 patients with IDC. There were no significant differences in age, mean tumor size, tumor grade or receptor status between the two subgroups. Degree of lesion enhancement on CEM was more often considered weak in case of ILC compared to IDC according to two out of three readers (31.8% vs 4.5 %, p = 0.045 and 22.7 vs 4.5 %, p = 0.185). All other lesions showed moderate or strong enhancement. Interobserver agreement between the three independent readers was good (κ = 0.72).
Conclusion:
In patients with ILC, degree of lesion enhancement on CEM appears to be more often weak than in infiltrating ductal carcinoma not otherwise specified. Radiologists should be aware that weakly enhancing lesions may in fact be malignant and particularly invasive lobular cancers.
Advances in knowledge:
Three independent readers evaluated 44 CEM cases with ILC or IDC. Degree of lesion enhancement seems more often weak in case of ILC. Radiologists should be aware of ILC in case of weak CEM enhancement.
Introduction
Invasive lobular carcinoma (ILC) is the second most common histologic type of breast cancer, after infiltrating ductal carcinoma not otherwise specified (IDC), occurring in approximately 10%–15% of all breast cancer patients.1 Sensitivity of mammography is limited for ILC (56%–84%), due to its growth as single files of cells often lacking calcifications.2 Subtle focal asymmetries or architectural distortion may be the only signs of cancer, which are not infrequently missed.3,4 Other imaging techniques such as breast MRI or contrast-enhanced mammography [i.e. CEM, synonyms contrast-enhanced spectral mammography (CESM) or contrast-enhanced dual-energy mammography (CEDM)] are more sensitive for the detection of mammographically occult tumors due to direct visualization of neovascularity as a tumor-specific feature.5–8
Previous studies have demonstrated an at least equal performance of CEM when compared to breast MRI for breast cancer detection.9–12 Decreased enhancement in patients with ILC have been observed on MRI.13,14 Lewin et al demonstrated that malignant lesions were more likely to be intensely enhancing than benign lesions on CEM.15 Kamal et al evaluated 109 malignant lesions on CEM and demonstrated strong correlation between morphologic and enhancement characteristic descriptors and diagnosis of cancer indicating intense enhancement as a characteristic feature of malignancy.16 Nevertheless, it is critical to be aware that even weakly enhancing lesions may be malignant. In light of the distinctly different growth patterns of ILC with decreased mass formation and its different enhancement patterns on MRI,13 we hypothesized that CEM enhancement might be more often weak in patients with ILC than in patients with IDC. Therefore, the aim of the current study was to investigate differences in the degree of enhancement on contrast mammography in patients with ILC compared to those with IDC.
methods and materials
Patient cohort
Due to the retrospective design of this HPAA compliant study, the necessity of informed consent from study subjects was waived and approved by the local Institutional Review Boards. Breast cancer patients diagnosed with ILC who underwent CEM as part of the diagnostic work-up between 2010 and 2017 were eligible for this study. 22 patients with IDC who underwent CEM served as a comparison, matched by size. Patient age, type of surgery and histopathology were collected.
Contrast-enhanced mammography
In Maastricht University Medical Center + (Maastricht UMC+), CEM exams were performed on a Senographe Essential unit equipped with the Senobright* CEM upgrade (GE Healthcare, Chalfont St Giles, UK) using iopromide as contrast agent (Ultravist® 300, Bayer Healthcare, Berlin, Germany) at a dose of 1.5 mL/kg body weight injected with a flow rate of rate of 3 ml s−1. 2 min after injection of contrast agent, mammograms were acquired in random order.
In Memorial Sloan-Kettering Cancer Center (MSKCC), CEM exams were performed on a Senobright unit (GE Healthcare, Buc, France) using iohexol as contrast agent (Omnipaque ® 350, GE Healthcare, Shanghai, China) with the same injection parameters. Images were acquired approximately 2.5 min after injection of contrast agent. Image acquisition was random depending on the individual technologist’s standard protocol.
Degree of lesion enhancement
Degree of lesion enhancement on recombined CEM images was independently re-evaluated by three readers, who were asked to determine the degree of enhancement of specified lesions according to previously defined criteria by Lewin et al.15 All three readers used a Likert scale for the assessment of degree of enhancement: possible, weak, moderate and strong enhancement. Readers read contrast mammograms, both on craniocaudal and mediolateral oblique views, in a random selection of those with ILC and IDC and were blinded to histopathology.
The first reader (MJ) has 35 years of experience in breast imaging, including 7 years of experience in reading CEM exams. The second reader (DK) has 22 years of experience in breast imaging, including 4 years of experience in CEM. The third reader (KP) has 12 years of experience in breast imaging, including 3 years of experience in CEM.
Statistics
Differences in degree of lesion enhancement on CEM between patients with ILC and IDC were calculated for each reader separately. The highest score of the degree of enhancement on either craniocaudal or mediolateral oblique view for each CEM exam was considered the final score. For each reader, scores were compared between ILC and IDC patients by using (two-sided) χ2 test and if necessary Fisher’s exact test. Interobserver agreement between the three readers, according to the initial scoring scale no to strong enhancement, was calculated by quadratic weighted κ coefficient (κ).17
The remaining categorical data were analyzed by χ2 test, continuous data by Mann–Whitney U test. Statistical analyses were performed by using Statistical Package for the Social Sciences software (v. 24, IBM, Armonk, NY). p-values (two-sided) <0.05 were considered statistically significant.
Results
Patient characteristics
22 patients with ILC and 22 patients with IDC were included in this study: 26 (15 ILC; 11 IDC, represents respectively 59% of the total population) from Maastricht UMC + and 18 (7 ILC; 11 IDC, represents respectively 41% of the total population) from MSKCC. The mean patient age was 61 and 58 years and the mean tumor size was 24 and 25 mm, respectively for patients with ILC and IDC. Patient characteristics are summarized in Table 1. Multifocal disease was significantly more common in patients with ILC (31.8% vs 4.5%, p = 0.046). Breast-conserving surgery was less frequently performed in patients with ILC compared to IDC (54.5% vs 84.2%, p = 0.042).
Table 1.
Patient characteristics
| Invasive lobular carcinoma (n = 22) | Infiltratin ductal carcinoma NOS (n = 22) | p-value | |
| Mean age (years) (range) | 61 (43–75) | 58 (41–79) | 0.372 |
| Site (%) | |||
| Left | 10 (45.5) | 11 (50.0) | 0.763 |
| Right | 12 (54.5) | 11 (50.0) | |
| Multifocal (%) | 7 (31.8) | 1 (4.5) | 0.046 |
| Mean clinical tumor size (mm) (range) | 25 (5–132) | 24 (8–133) | 0.860 |
| Primary surgery (%) | |||
| Breast-conserving surgery | 12 (54.5) | 17 (85.0) | 0.042 |
| Mastectomy | 10 (45.5) | 3 (15.0) | |
| No surgery a | - | 2 a | |
| Positive surgical margins (%) | 1 (4.5) | 1 (5.3) | 1.000 |
| Tumor grade | |||
| 1 | 4 (18.2) | 5 (32.7) | 0.709 |
| 2 | 15 (68.2) | 11 (50.0) | 0.220 |
| 3 | 3 (13.6) | 6 (27.3) | 0.262 |
| Hormonal and receptor status | |||
| ER/PR+, HER2- | 21 (95.5) | 18 (81.8) | 0.345 |
| ER/PR+, HER2+ | 1 (4.5) | - | |
| Triple negative | - | 4 (18.2) |
ER/PR, estrogen/progesterone; Her2, Human Epidermal growth factor Receptor 2; NOS, not otherwise specified.
No surgery performed in two cases, due to distant metastases at presentation
Evaluation of degree of lesion enhancement
According to the first two readers, degree of lesion enhancement was more frequently scored as weak in cases of ILC compared to IDC: 32 vs 5% (p = 0.046) and 23 vs 5% (p = 0.185), while Reader 3 (the least experienced) scored 36 vs 18% (p = 0.310) cases as weak. Enhancement of IDC was considered stronger than with ILC by the first two readers: 50 vs 23% (p = 0.060) and 73 vs 41% (p = 0.033). Interobserver agreement among the three readers was considered good (κ = 0.723 (0.584–0.862) for the two experienced CEM readers; and κ = 0.728 (0.598–0.858) for the expert vs less-experienced CEM reader). Table 2 demonstrates an overview of the results of all three readers. Figure 1 demonstrates an example of two ILC cases one considered to have strong lesion enhancement (Figure 1a) and one scored as weak lesion enhancement (Figure 1b), by all three readers.
Table 2.
Degree of lesion enhancement on CEM, respectively in case of invasive lobular carcinoma vs infiltrating ductal carcinoma not otherwise specified
| Invasive lobular carcinoma (n = 22) | Infiltratin ductal carcinoma NOS (n = 22) | p-value | |
| Reader 1 | |||
| Weak (%) | 7 (31.8) | 1 (4.5) | 0.046 |
| Moderate (%) | 10 (45.5) | 10 (45.5) | 1.000 |
| Strong (%) | 5 (22.7) | 11 (50.0) | 0.060 |
| Reader 2 | |||
| Weak (%) | 5 (22.7) | 1 (4.5) | 0.185 |
| Moderate (%) | 8 (36.4) | 5 (22.7) | 0.322 |
| Strong (%) | 9 (40.9) | 16 (72.8) | 0.033 |
| Reader 3 | |||
| Weak (%) | 8 (36.4) | 4 (18.1) | 0.310 |
| Moderate (%) | 6 (27.2) | 10 (45.5) | 0.210 |
| Strong (%) | 8 (36.4) | 8 (36.4) | 1.000 |
CEM, contrast-enhanced mammography; NOS, not otherwise specified.
Figure 1.
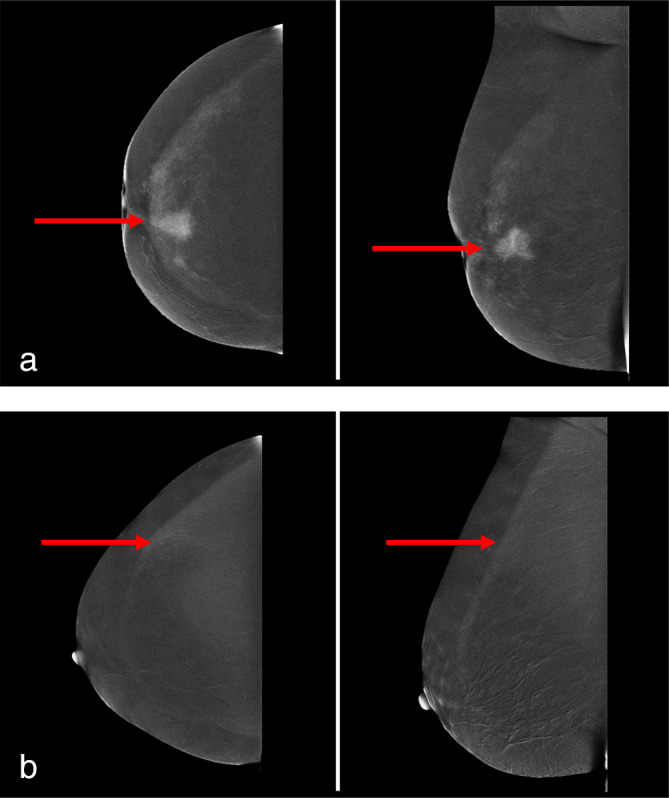
(a) CEM images of a female patient with a 25 mm large invasive lobular carcinoma in her left breast, respectively on CC (left image) and MLO (right image). The red arrows demonstrate the suspicious lesion, which was considered strong enhancement by all three readers. (b) CEM images of a female patient with a 10 mm large invasive lobular carcinoma in her left breast, respectively on CC (left image) and MLO (right image). The red arrows demonstrate the suspicious lesion, which was considered weak enhancement by all three readers.
Discussion
CEM has the ability to detect breast cancers by visualizing enhancing neovascularity in a fashion similar to breast MRI.8,18 Several studies have demonstrated superior results of CEM when compared to full-field digital mammography for population-based breast cancer detection including screening, problem solving and work-up of symptomatic patients and patients with abnormal screening exams.11,19,20 Studies have suggested an at least equal performance of CEM when compared to breast MRI for the detection of malignant lesions.9,20 Assessment of tumor size on CEM is comparable to breast MRI, without reported cases of relevant size discrepancies (i.e. >1 cm) between both imaging modalities.11,12
Since this is a relatively new technique, there is currently no standard lexicon for interpreting CEM as there is for other breast imaging modalities such as the BI-RADS system for mammography, ultrasound and MRI. Proposed language for CEM includes the use of a BI-RADS type mammography lexicon for interpretation of the low energy images combined with language used in the BI-RADS interpretation of MRI excluding kinetics. While it has been reported that tumor enhancement may be more often weak in patients with ILC on MRI, to our knowledge, this is the first study investigating differences in degree of tumor enhancement on CEM between patients diagnosed with ILC compared to those with IDC. As has been reported with MRI, we demonstrated that enhancement trended to be more often weak in a small cohort of patients with ILC compared with IDC. The degree of lesion enhancement can be considered an important subject during interpretation of CEM, since radiologists should be aware of the possibility of weak enhancing lesions being ILC rather than benign.
Luczyńska et al, observed that the likelihood of malignancy increased with increasing intensity of enhancement. In their study of 193 patients, medium to strong enhancement was more frequent observed in malignant lesions as opposed to benign (70%–90% vs 11%–26%), suggesting that weakly enhancing lesions were more frequently benign.21 In our study, weak enhancement was observed in approximately one-third of all ILC cases. Consequently, it is critical to realize that all enhancing lesions are potentially malignant.
Regarding differences in enhancement on CEM between ILC and IDC, previous studies only included few cases with ILC, preventing any further analysis by breast cancer histology.15,22 Kamal et al described intense enhancement as an indicator of malignancy in mass lesions, since more intense enhancement was more often observed in malignant versus benign mass lesions (82% vs 18%).16 Despite these initial results in their small cohort of patients, they did not investigate differences in enhancement on CEM between patients diagnosed with ILC compared to those with IDC. Our results are therefore of added value, since we studied whether the actual difference in degree of CEM enhancement exists between cases with ILC and IDC.
Despite our good interobserver agreement, visual assessment of lesion enhancement remains subjective and the classification currently used is one of many that have been published before. Perhaps, the introduction of enhancement quantification tools might improve the differentiation between benign and malignant lesions on CEM. In a recent study, Hwang et al investigated quantitative assessment of CEM enhancement, proving that the degree of lesion enhancement can be automatically assessed.23 This opens to the door to a more objective analysis of CEM enhancement, which might be used to further improve lesion classification. Further work in this regard will likely require the ability to include textural characterization of the low energy images obtained below the K-edge of iodine somehow blinding the evaluation to the contrast to get a more accurate depiction of the actual enhancement qualities
Our study had several limitations. Even when combining the cases of two breast cancer institutes, the sample size was small, limiting the power of the study. This is caused by the low prevalence of ILC as breast cancer subtype. Consequently, some of our results might not show statistical significance. Furthermore, there were small differences in CEM imaging protocols between the two institutes, such as the timing of the first image acquisition, the concentration of the contrast agent used and the order in which the images were obtained. In fact Jochelson et al have demonstrated that the order in which each view was acquired did not affect lesion detectability20 and therefore this is unlikely to cause a significant discrepancy in our results..
In conclusion, degree of enhancement in ILC on CEM appears to be more often weak than in IDC. Consequently, radiologists should be aware that weakly enhancing lesions may in fact be malignant and particularly invasive lobular cancers.
Footnotes
Acknowledgment: T.J.A. van Nijnatten received a travel grant from René Vogels Stichting. All other authors declare that they received no funding in the study design, collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to submit the manuscript for publication.
Ethical approval: Due to the retrospective design of this HPAA compliant study, the necessity of informed consent from study subjects was waived and approved by the local Institutional Review Boards.
Contributor Information
Thiemo JA van Nijnatten, Email: thiemovn@gmail.com.
Maxine S Jochelson, Email: jochelsm@mskcc.org.
Katja Pinker, Email: pinkerdk@mskcc.org.
Delia M Keating, Email: keatingd@mskcc.org.
Janice S Sung, Email: sungj@mskcc.org.
Monica Morrow, Email: morrowm@mskcc.org.
Marjolein L Smidt, Email: m.smidt@mumc.nl.
Marc BI Lobbes, Email: marc.lobbes@mumc.nl.
REFERENCES
- 1. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003; 289: 1421–4. [DOI] [PubMed] [Google Scholar]
- 2. Jayaram G, Swain M, Chew MT, Yip CH. Cytologic appearances in invasive lobular carcinoma of the breast. A study of 21 cases. Acta Cytol 2000; 44: 169–74. doi: 10.1159/000326356 [DOI] [PubMed] [Google Scholar]
- 3. Mariscotti G, Durando M, Houssami N, Zuiani C, Martincich L, Londero V, et al. Digital breast tomosynthesis as an adjunct to digital mammography for detecting and characterising invasive lobular cancers: a multi-reader study. Clin Radiol 2016; 71: 889–95. doi: 10.1016/j.crad.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 4. Oliveira TMG, Elias J, Melo AF, Teixeira SR, Filho SC, Gonçalves LM, et al. Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights Imaging 2014; 5: 183–94. doi: 10.1007/s13244-014-0324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Houssami N, Turner RM, Morrow M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat 2017; 165: 273–83. doi: 10.1007/s10549-017-4324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bansal GJ, Santosh D, Davies EL. Selective magnetic resonance imaging (MRI) in invasive lobular breast cancer based on mammographic density: does it lead to an appropriate change in surgical treatment? Br J Radiol 2016; 89: 20150679. doi: 10.1259/bjr.20150679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blum KS, Rubbert C, Mathys B, Antoch G, Mohrmann S, Obenauer S. Use of contrast-enhanced spectral mammography for intramammary cancer staging: preliminary results. Acad Radiol 2014; 21: 1363–9. doi: 10.1016/j.acra.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 8. Diekmann F, Bick U. Tomosynthesis and contrast-enhanced digital mammography: recent advances in digital mammography. Eur Radiol 2007; 17: 3086–92. doi: 10.1007/s00330-007-0715-x [DOI] [PubMed] [Google Scholar]
- 9. Li L, Roth R, Germaine P, Ren S, Lee M, Hunter K, et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging 2017; 98: 113–23. doi: 10.1016/j.diii.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 10. Jochelson MS, Pinker K, Dershaw DD, Hughes M, Gibbons GF, Rahbar K, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol 2017; 97: 37–43. doi: 10.1016/j.ejrad.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 11. Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014; 24: 256–64. doi: 10.1007/s00330-013-3007-7 [DOI] [PubMed] [Google Scholar]
- 12. Lobbes MBI, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer 2015; 6: 144–50. doi: 10.7150/jca.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mann RM, Veltman J, Huisman H, Boetes C. Comparison of enhancement characteristics between invasive lobular carcinoma and invasive ductal carcinoma. J Magn Reson Imaging 2011; 34: 293–300. doi: 10.1002/jmri.22632 [DOI] [PubMed] [Google Scholar]
- 14. Dietzel M, Baltzer PA, Vag T, Gröschel T, Gajda M, Camara O, et al. Magnetic resonance mammography of invasive lobular versus ductal carcinoma: systematic comparison of 811 patients reveals high diagnostic accuracy irrespective of typing. J Comput Assist Tomogr 2010; 34: 587–95. doi: 10.1097/RCT.0b013e3181db9f0e [DOI] [PubMed] [Google Scholar]
- 15. Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 2003; 229: 261–8. doi: 10.1148/radiol.2291021276 [DOI] [PubMed] [Google Scholar]
- 16. Mohamed Kamal R, Hussien Helal M, Wessam R, Mahmoud Mansour S, Godda I, Alieldin N. Contrast-enhanced spectral mammography: impact of the qualitative morphology descriptors on the diagnosis of breast lesions. Eur J Radiol 2015; 84: 1049–55. doi: 10.1016/j.ejrad.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 17. Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968; 70: 213–20. doi: 10.1037/h0026256 [DOI] [PubMed] [Google Scholar]
- 18. Knogler T, Homolka P, Hoernig M, Leithner R, Langs G, Waitzbauer M, et al. Application of BI-RADS descriptors in contrast-enhanced dual-energy mammography: comparison with MRI. Breast Care 2017; 12: 212–6. doi: 10.1159/000478899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lobbes MBI, Lalji U, Houwers J, Nijssen EC, Nelemans PJ, van Roozendaal L, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014; 24: 1668–76. doi: 10.1007/s00330-014-3154-5 [DOI] [PubMed] [Google Scholar]
- 20. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266: 743–51. doi: 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Łuczyńska E, Niemiec J, Hendrick E, Heinze S, Jaszczyński J, Jakubowicz J, et al. Degree of enhancement on contrast enhanced spectral mammography (CESM) and lesion type on mammography (Mg): comparison based on histological results. Med Sci Monit 2016; 22: 3886–93. doi: 10.12659/MSM.900371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011; 21: 565–74. doi: 10.1007/s00330-010-1944-y [DOI] [PubMed] [Google Scholar]
- 23. Hwang Y-S, Cheung Y-C, Lin Y-Y, Hsu H-L, Tsai H-Y. Susceptibility of iodine concentration map of dual-energy contrast-enhanced digital mammography for quantitative and tumor enhancement assessment. Acta Radiol 2018; 59: 893–901. doi: 10.1177/0284185117740760 [DOI] [PubMed] [Google Scholar]


