Abstract
Diffusion-weighted imaging (DWI) of the breast is a MRI sequence that shows several advantages when compared to the dynamic contrast-enhanced sequence: it does not need intravenous contrast, it is relatively quick and easy to implement (artifacts notwithstanding). In this review, the current applications of DWI for lesion characterization and prognosis as well as for response evaluation are analyzed from the point of view of the necessary steps to become a useful surrogate of underlying biological processes (tissue architecture and cellularity): from the proof of concept, to the proof of mechanism, the proof of principle and finally the proof of effectiveness. Future applications of DWI in screening, DWI modeling and radiomics are also discussed.
In this era of precision medicine, the traditional role of the radiologist providing high quality (albeit qualitative) images needs to evolve further in order to help clinicians in their decision-making and contribute to the radiology value chain. Integrating quantitative imaging biomarkers into clinical practice allows us to measure the underlying pathophysiological mechanisms non-invasively, revealing properties relevant to detection, diagnosis, prognosis or response to therapy. The data sets produced by the imaging modalities can be extracted and analyzed and thus act as valid and reproducible surrogates of the intrinsic biological processes.1
It is therefore important for radiologists to become acquainted with the process of implementation, validation, and standardization of imaging biomarkers that are considered in vivo (or, more accurately in silico) virtual biopsies2
In this review, diffusion-weighted imaging (DWI) of the breast will be analyzed from the perspective of the development of an imaging biomarker and its potential future role in the field of breast imaging.
The development and validation of diffusion-MRI as an imaging biomarker
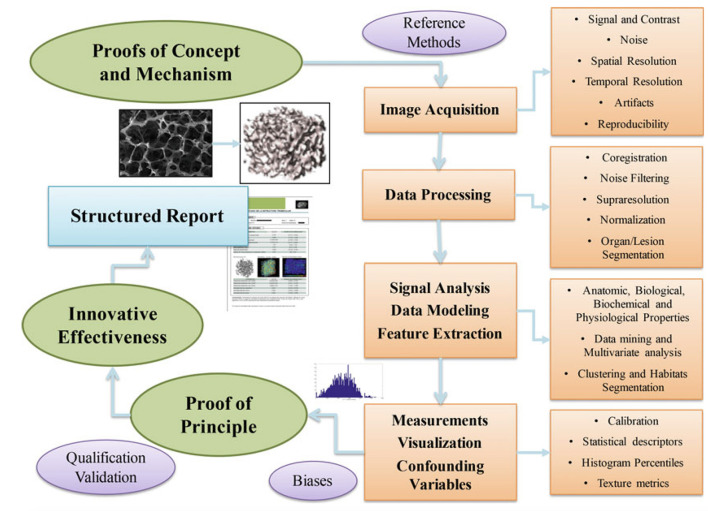
The stepwise development of imaging biomarkers, provides an orderly framework within which to discuss the technique of DWI, as well as its clinical applications and its validation as an imaging biomarker (Figure 1).
Figure 1.
The stepwise development of imaging biomarkers. From Introduction to the Stepwise Development of Imaging Biomarkers (Chapter 2) in Imaging Biomarkers. Martí-Bonmatí L, Alberich-Bayarri A, editors. Springer; 2016. With permission of the author.
The proof of concept in DWI
The first phase is the proof of concept, whereby the underlying pathophysiological mechanism to be evaluated by DWI is demonstrated. Diffusion is the process of random motion of water molecules in a free medium. In vivo, water mobility or diffusivity is limited by intracellular and extracellular compartments, as well as tissue cellularity. Although the motion of water molecules is said to be “restricted” in tissues with a high cellular density (e.g. tumor tissue), in fact, there are three principal physical modes of diffusion: free, hindered and restricted diffusion.3–5 Free diffusion describes the random or Brownian motion of water molecules due to thermal agitation in the absence of any obstacles that follows a Gaussian distribution. Hindered diffusion refers to the delay of passage as they navigate around cellular obstacles, as in the extracellular space. Restricted diffusion is a term classically used to describe the trapping of water molecules within an enclosed compartment (i.e., the cell plasma membrane). The degree of water diffusion in tissue is inversely correlated with tissue cellularity and the integrity of cell membranes.
The proof of mechanism in DWI
The proof of mechanism refers to the relationship between the extracted imaging biomarker and the relevant disease. In order for the biomarker to become a surrogate of the underlying biological process, it has to undergo a process of technical and biological validation.
DWI is used to visualize the degree of water molecule diffusion at in vivo MR imaging. DWI was initially applied in the clinical setting in the mid 1990s for the diagnosis of acute stroke5 and in 1997, Englander et al6 investigated the possibility of applying DWI to the human breast.
Image acquisition
The first step in the proof of mechanism of an imaging biomarker is the image acquisition, whereby data quality has to be checked regarding signal and contrast-to-noise ratios, spatial and temporal resolution, artifacts and reproducibility.
Description of the EPI sequence
In clinical imaging, the sequence used is a T 2 weighted spin-echo sequence with two motion-probing gradients next to the 180° refocusing pulse.7 where segmented or single shot echoplanar readout techniques (EPI) are used to accelerate data acquisition. The faster a molecule diffuses, the greater the attenuation after applying the diffusion gradients and the weaker the corresponding pixel signal intensity at DWI. Thus, regions with restricted diffusion (i.e. higher density of cells) will show a higher signal than regions with fast water mobility. Typical DWI acquisitions have a diffusion time in the range of 30–50 ms, corresponding to an approximate displacement of 14–17 µm for unrestricted free water at body temperature. The degree of diffusion weighting, described by the b-value and determined by the strength and timing of the applied diffusion gradients (in s/mm2), is the primary factor that affects the sensitivity of the diffusion sequence to water motion. In human tissues, the diffusion process is not free and is influenced by a combination of mechanisms (diffusion, microstructural restrictions, microcirculation) that contribute to the final signal attenuation. In vivo signal decay is considered mono-exponential within intermediate b-value ranges, but due to the fact that the diffusion environment is highly complex, the concept of a single diffusion coefficient is arguable and as such is reported as an “apparent” diffusion coefficient or ADC.
At higher b -values, the signal decay fits the multiexponential model in vivo. At lower b -values (b < 150 s/mm2), the ADC is influenced by tissue microperfusion and can lead to overestimations of the ADC. Thus, the choice of the optimal b -values will influence the conspicuity of the lesions in the DWI images but will also modify the ADC value. Pereira et al8 analyzed the ADC values of breast cancers at b -values ranging from 0 to 1000 s/mm2 and found that the ADC value calculated for a combination of b -values of 0 and 750 s/mm2 showed a slightly better sensitivity and specificity than did the ADC value calculated for other b-value combinations. Current recommendations propose two b -values of 50 and 800 s/mm2.9 Pereira et al8 also analyzed the ADC values of benign and malignant breast tumors with various combinations of b -values (0, 250, 500, 750, and 1000 s/mm2) and found that it is not necessary to use multiple b -values because the sensitivity of ADC with two b -values is equivalent to that with multiple b -values and the specificity of b -values 0 and 750 is higher than that of multiple b -values.
The ADC describes the average area occupied by a water molecule per unit time (mm2/s) and as a reference point, the ADC value of free water molecules at 37°C is 3.0 × 10−3 mm2/s. In general, determination of ADC is performed by acquiring images at two b -values and fitting the corresponding signal intensities into a monoexponential model. ADC values can be influenced by which b -values are applied, a fact that highlights the need for consistent and standardized protocols. At higher b -values, ADC decreases in normal breast tissue, fibrocystic changes and breast cancer.7,9
The observed ADC value depends on many factors: fluid viscosity, membrane permeability, water transport mechanisms and the microstructure of the compartment containing diffusing water. Thus, ADC value can provide a specific information on the proof of concept (tissue microstructure, cellularity). For DWI acquired with two or more different b -values, ADC can be calculated for each voxel in the image and presented as a parametric map. Malignant lesions will appear brighter on DWIs and darker on ADC maps compared with normal fibroglandular tissue (Figures 2 and 3). ADC is indeed the surrogate biomarker of diffusion that has to undergo all the necessary technical and biological validation steps in order to become a clinically useful imaging biomarker.
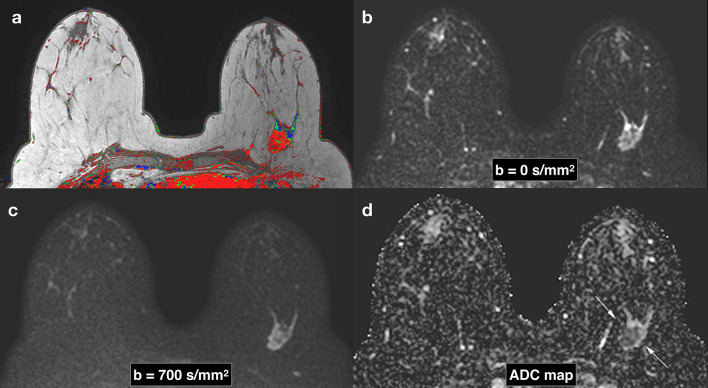
Figure 2.
Breast images obtained with DWI in a patient with a Triple Negative subtype breast cancer in the left breast. Corresponding slices from DCE postcontrast image (a), DWI at b = 0 s/mm2 (b), DWI at b = 700 s/mm2. (c), ADC map (d). Invasive tumors show reduced diffusivity on DW imaging, appearing hyperintense on b = 700 s/mm2 image (c) and hypointense on the ADC map (arrows) (d). ADC, apparent diffusion coefficient; DCE, dynamiccontrast-enhanced; DWI, diffusion-weighted imaging.
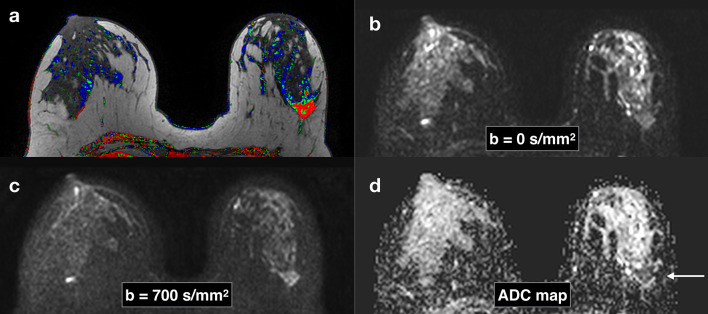
Figure 3.
Breast images obtained with DWI in a patient with a HER-2 subtype breast cancer in the left breast. Corresponding slices from DCE post-contrast image (a), DWI at b = 0 s/mm2 (b), DWI at b = 700 s/mm2 . (c), ADC map (d). Invasive tumors (arrows) show reduced diffusivity on DW imaging, appearing hyperintense on b = 700 s/mm2 image (c) and hypointense on the ADC map (arrow) (d). ADC, apparent diffusion coefficient; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging.
EPI-related technical issues
Conventional EPI-based DWI sequences provide fast motion-freezing imaging but DWI acquisition protocols must be optimized to reduce artifacts and achieve adequate signal-to-noise ratio (SNR).10
Common EPI-related artifacts must be avoided during image acquisition in order to evade ADC quantification imprecisions.
The magnetic susceptibility artifact, due to magnetic field inhomogeneities, can cause severe image distortions at the interface of materials with different magnetic susceptibilities such as the air/tissue or adipose/glandular tissue interfaces. Good patient positioning (avoiding skin folds) and improved shimming to reduce magnetic field inhomogeneities can avoid this artifact.
Motion artifacts can cause misregistration, leading to false ADC values. Parallel imaging shortens acquisition times and minimizes these artifacts, ultimately leading to decreased image distortion (but also reduces SNR).
Chemical shift artifacts can be overcome by adequate fat suppression and good-quality shimming.
Ghosting artifacts are caused by phase mismatch during the EPI readout introduced by eddy currents or inadequate shimming, causing a mirror image that is shifted in the phase direction by half the field of view10,11
Eddy currents are caused by strong gradients (especially evident in diffusion tensor imaging or DTI) and also originate image distortions, causing inaccuracies in ADC calculation.
Low spatial resolution is a limitation of DWI that can be overcome by a higher SNR, afforded by higher magnetic field strength MR units.
Image analysis
The second phase in the proof of mechanism of DWI as an imaging biomarker is image analysis.
Once the source image quality is improved by implementation of noise reduction techniques, avoidance of artifacts and improvement of spatial resolution, signal analysis and feature extraction will yield the calculated tissue properties obtained from each voxel. In DWI, the final image with different ADC values calculated for each voxel is the parametric ADC map. ADC maps have poor anatomic detail and should be analyzed in conjunction with other MR images such as DWI, T 2 weighted or dynamic contrast-enhanced (DCE) images in a multiparametric way.12 Data analysis in this phase includes post-processing of the images before ADC calculation, measurement of the ADC through region of interest (ROI) techniques and identification of confounding variables.
Post-processing of the images before ADC calculation with image registration software tools is applied to reduce spatial misalignment or pixel shifts due to patient motion, susceptibility or eddy current based distortions, using the b = 0 s/mm2 as a reference.
ADC measurements of lesions differ depending the ROI techniques, especially in large or non-mass lesions. Small ROI placed over the most hypointense area of the b = 700 s/mm2 or b = 800 s/mm2 image may provide more accurate measures in heterogeneous lesions while placing the ROI over the whole lesion may provide more reproducible results.13–15 Semiautomated ROI selection methods may allow for more consistent results16 and lately some authors claim histogram analysis17–19 might be an even more accurate way of assessing intra tumoral heterogeneity.
The T2 shine-through effect is a confounding variable that has to be taken into account when interpreting DW images. The standard diffusion image generates a T 2 weighted reference image obtained without diffusion gradients and a DWI that reflects the water mobility but is also a T 2 weighted image. In tissues with long relaxation times, the strong T2 signal maybe mistaken for restricted diffusion, a phenomenon known as the T2 shine-through effect.11,12
Biomarker validations. The proof of principle in DWI
To be clinically useful, a biomarker needs to undergo validation (performance of the test) and qualification (clinical approval) in order to ensure that the obtained measurements have a precise relationship with the underlying biological reality.2 Several validations including technical, biological and clinical have to be conducted before a biomarker is introduced in clinical research. These initial pilot tests performed on a small group of subjects are known as the proof of principle, to verify that the concept and related methods have the potential of being used before embarking on large-scale multicenter projects and clinical trials. Accuracy, repeatability and reproducibility studies of ADC20–23 evaluate the different factors that may affect ADC’s precision. Precise measurement of ADC is important since the dynamic range of the biomarker is quite small, from approximately 0.5 × 10−3 mm2/s in densely packed cells to 3.0 × 10−3 mm2/s in fluid-filled cysts.24
Technical validations of DWI are performed using different measurements related to image acquisition, processing, analysis and ADC measurements These measurements are done with phantoms and in healthy volunteers or in patients24,25
Biological validation shows the link to tumor biology and is the correlation between preclinical disease models or human studies and reference methods such as histopathology, immunohistochemistry or genomics. In these studies, the influence of epidemiological data (sex, age, physiological status) and genuine biological variations has to be studied.2
1. Correlation with histopathology. It has been proven that ADC values correlates well with the degree of cellular density in breast cancers26–29 as well as with the extravascular extracellular fraction in preclinical models30
2. Influence of hormonal status.
Menstrual cycle. Although initial studies posited increased ADC values during the second half of the menstrual cycle,7,31 these variations were non-statistically significant and subsequent studies confirmed no influence of the menstrual cycle32–35 or background parenchymal enhancement36–39 on ADC values.
Lactation. Breast tissue ADC values in lactating females are significantly reduced probably due to high lipid content and viscosity of the milk,34 but do not influence breast cancer ADC measurements.40
Menopausal status. ADC is significantly lower in postmenopausal compared with premenopausal patients,32,34,35 probably related to changes in water content, microcirculation and adipose content with age. A recent paper by Horvat et al41 does not find any difference in ADC values between benign and malignant lesions in patients stratified by BPE level, amount of fibro-glandular tissue or menopausal status.
3. Intravenous contrast. The influence of contrast administration on DW is controversial, most likely due to the different acquisition parameters in the published papers42–46 A meta-analysis by Dorrius et al47 confirmed that contrast administration does not significantly affect breast lesion ADC values. The EUSOBI consensus statement9 recommends performing DWI before DCE-MRI and consistency in timing across examinations is also advocated.11
Clinical validations to see how well the biomarker works are initially performed as single-center observational studies in small groups of patients or meta-analyses of these studies.
In the realm of DWI, these studies focus mainly on three distinct clinical applications: lesion characterization, the prognostic value of ADC and response evaluation to neoadjuvant treatments with DWI. Screening with DWI has also been explored and will be included in the section on future perspectives of DWI (see below).
1. Lesion characterization with DWI. The most investigated clinical application of DWI is as an adjunct sequence to reduce the false positives encountered in contrast-enhanced MRI and avoid unnecessary biopsies (Figures 4 and 5). Since the first publication by Guo et al27 many studies have shown breast malignancies have significantly lower ADC values than benign lesions. Two meta-analyses48,49 evaluating the diagnostic performance of quantitative breast DW imaging demonstrated overall better specificity than DCE MR imaging. More recently, another meta-analysis of 14 studies further confirmed that DWI can increase the accuracy of DCE-MRI50 with a resulting area under the summary receiver operating characteristics (ROC) curve of 0.94. Variations in DWI acquisition protocols as well as types of lesions (mass vs non-mass) and patient cohorts across studies prevent the issuance of an optimal ADC threshold value to distinguish benign from malignant lesions due to dependence of lesion ADC measures on choice and combination of b -values.51 Dorrius et al showed that despite the influence of choice of b values on ADC measurements, sensitivity and specificity are not significantly affected by choice of b -value.47 Moreover, a meta-analysis of 61 studies by Shi et al52 found that there was no significant difference in diagnostic accuracy for breast cancer between 1.5 and 3.0 T scanners. The EUSOBI consensus statement offers a proposal for an analysis and interpretation scheme based on both qualitative and quantitative ADC value ranges on which future research protocols could be based in order to standardize DWI.9
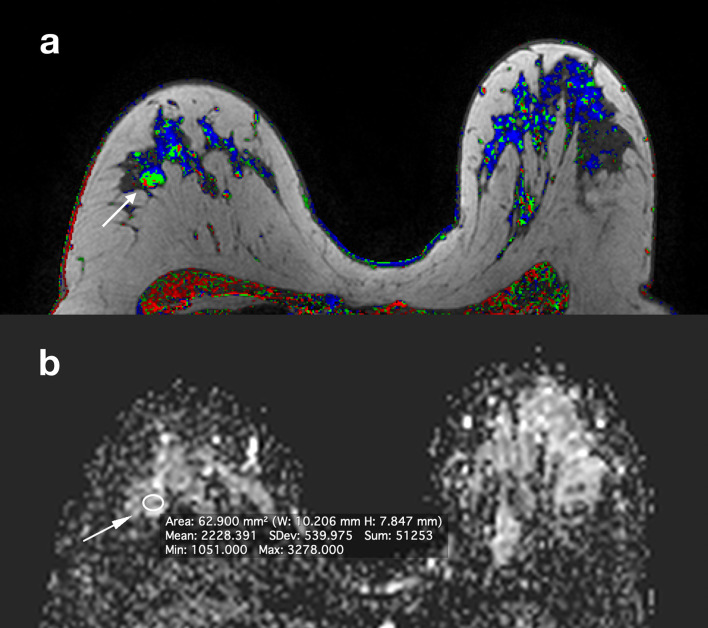
Figure 4.
Same patient in Figure 2. Corresponding slices from DCE postcontrast image (a), ADC map (b). A BI-RADS four lesion (arrow) was identified in the contralateral breast and DWI characterized it as benign (ADC = 2,22×10−3 mm2/s). It was decided not to biopsy. 2 years later, the lesion was stable and 3 years later the patient underwent contralateral prophylactic mastectomy which did not show any malignant lesions. ADC, apparent diffusion coefficient; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging.
Figure 5.
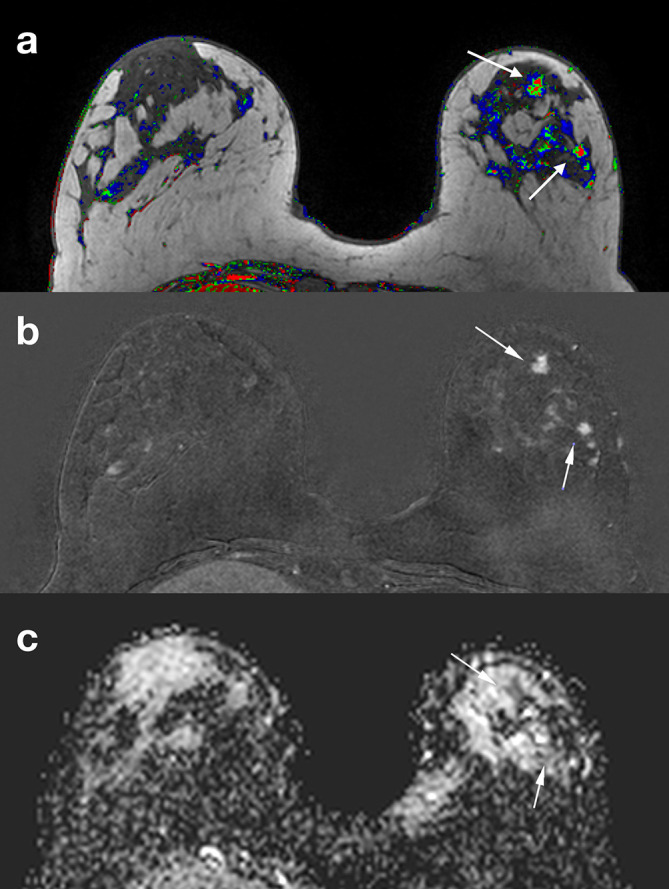
Same patient in Figure 2. Corresponding slices from DCE postcontrast image (a), DCE subtraction image (b) ADC map (c). Two BI-RADS four lesions (arrows) were identified in a different quadrant from the index tumor. ADC characterized them as malignant (ADC = 1,28×10−3 mm2/s). An MR-guided vacuum-assisted biopsy was performed in the largest of the two lesions and an additional multicentric invasive ductal cancer was confirmed. ADC, apparent diffusion coefficient; DCE, dynamiccontrast-enhanced; DWI, diffusion-weighted imaging.
2. The prognostic value of ADC . An area of active research is the correlation of ADC with molecular or traditional pathologic prognostic factors, as well as surrogate tumor phenotypes or recurrence scores. Although most of the associations have been non-significant to date, with as many studies finding significant or no significant correlation, there is a trend of concordance between ADC and more aggressive cancer features. This characterization of biological aggressiveness can be of help for selecting adequate treatments. In the most recent published literature, some authors find an inverse significant relationship between ADC and tumor grade,13,19,53,54 while others do not55–58 59 extending the debate that has been taking place since the earliest publications in 2009.On the other hand, most authors agree on the inverse correlation between ADC and the ki67 proliferation index 19,53,60 a fact that could be of weight in considering neoadjuvant treatment, although a recent multicenter analysis in 845 patients did not find any relationship.54 Although the earliest papers found a significant inverse correlation between ER-positive tumors and ADC, the latest publications either find a significant relationship61–65 or not,13,17,19 which is also in line with the fact that ER positive tumors are slow-growing and lower-grade cancers and suggests that studies with larger number of patients and in multiple centers are needed.Most studies dealing with HER-2-positive tumors and ADC have found a positive correlation,66–69 possibly due to a higher degree of angiogenesis overcoming cellularity in this type of breast cancers.As would be expected by the underlying biological cellular density, most recent studies dealing with the difference in ADC values between invasive and in situ tumors have found lower ADC values in invasive cancers.55,70–72 Moreover, some authors can predict invasiveness in pure-DCIS lesions72–74 by detecting the areas with the lowest ADC values or indexes and even use ADC to up-grade to malignancy in high-risk or B3 lesions.75 Attempts to differentiate between low grade and high-grade ductal carcinoma in situ ( DCIS ) are in the center of the debate over the necessity of surgically treating all DCIS and previous as well as current literature does not find ADC a significant biomarker to stratify patients.72,76 Associations between DWI characteristics (ADC and contrast-to-noise ratio) and recurrence scores in ER-positive, HER-2-negative breast cancers have yielded promising results77,78 and can potentially help stratifying patients into avoiding chemotherapy as well as being more cost-effective than the current recurrence scores schemes.
3. Response evaluation to neoadjuvant chemotherapy with DWI. One of the advantages of imaging biomarkers is to be able to non-invasively monitor response to drug treatments in a spatially- and time-resolved manner. The main utility of imaging techniques in this clinical setting is dual: to predict response early in the treatment course in order to spare the patients side-effects of unnecessary chemotherapeutic treatments and to accurately assess the extent of residual disease before surgery in order to plan the most adequate surgical approach. Lately, it has become even more important to predict pathological complete response (pCR) in order to omit surgery79 in patients with the highest pCR rates (tumoral subtypes HER2 and triple negative).
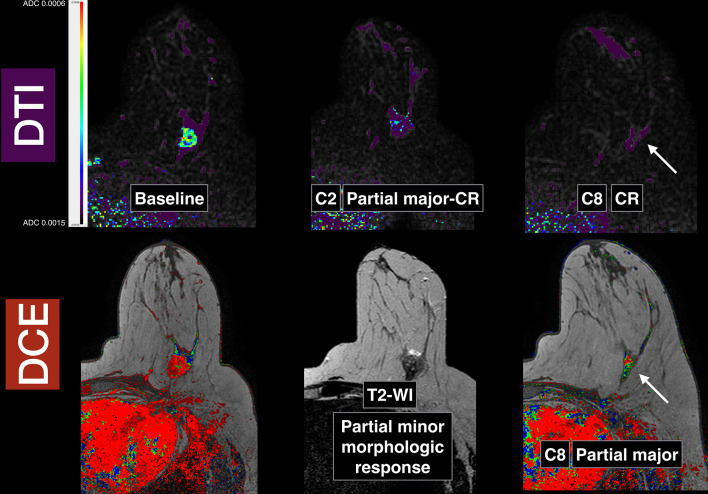
Early response prediction. Cytotoxic effects of chemotherapeutic agents (apoptosis, cell lysis) increase the mobility of water in the tissues, reflected in the increase of ADC value. Early studies with mice80 showed that increased diffusivity could be shown within days of therapy onset in implanted breast cancers. DWI may be able to offer earlier and more precise information on treatment response than DCE-MRI, as macroscopic volume changes are only the downstream manifestations of underlying patho-physiological phenomena (cell swelling and cell death) which occur early after treatment, and DCE can also show enhancement due to inflammatory changes. Several studies in breast cancer patients have proven that ADC can be an early predictor of response, as early as after the first cycle81–85 and earlier than DCE-MRI81,82 (Figure 6). The introduction of histogram analysis19 or voxel-by-voxel functional maps to parametrize response can be of help especially in very heterogeneous tumors85 and might be helpful in overcoming bias related to tumor segmentation techniques. Baseline ADC does not seem to predict response in all tumor subtypes and the usefulness of this parameter pre-treatment is not clear.86–88
Residual disease evaluation. Residual disease has traditionally been evaluated with DCE-MRI and meta-analyses comparing both techniques have shown that DWI adds sensitivity for predicting pCR to the high specificity of DCE-MRI for residual disease.89 Both techniques combined achieve the best diagnostic accuracy.50 A recent meta-analysis focusing solely on DWI90 revealed a pooled sensitivity of 0.89 and a specificity of 0.72 for detecting pCR. The data definitely support the use of DWI in response evaluation, although future trials must incorporate rigorous quality assurance and control and reproducibility measures as well as multicenter designs91 in order to ensure the standardization of ADC as a response biomarker.92
Figure 6.
Breast images obtained in a patient with a Triple Negative subtype breast cancer in the left breast (same patient from Figure 1): baseline pre-neoadjuvant chemotherapy, early after two cycles and pre-surgery after eight cycles. Slices from baseline, early (after two cycles) and pre-surgical (after eight cycles) DTI in the top row. Corresponding slices (bottom row) from baseline post-contrast image, T 2 weighted image after two cycles and post-contrast image after eight cycles, pre-surgery. Note that in the DTI images after two cycles, there is a functional qualitative partial major almost complete response whilst in the corresponding T 2 weighted image, the morphologic response is minor or minimal. When comparing the DTI images after eight cycles with the corresponding DCE image, there is a complete response in the DTI images (arrow, top row) and a residual enhancement in the DCE images (arrow, bottom row) interpreted as partial major response. The final pathology confirmed a complete response that was already predicted by the early DTI exam after two cycles. DCE, dynamic contrast-enhanced; DTI, diffusion tensor imaging.
The proof of effectiveness and clinical qualification in DWI
Through qualification of a biomarker, the link between surrogate and clinical endpoints is established and it becomes a clinical decision-making tool.93 In order to achieve this, prospective large multicenter trials have to be undertaken to validate the wealth of evidence provided by single-center studies. American College of Radiology Imaging Network (ACRIN) multicenter studies underway (ACRIN 6702), or with published results,91 reveal the complexity of standardization at all levels.24,92
Future role of DWI
Screening with DWI
The high intrinsic contrast achieved with DWI without the injection of external agents and the ease of implementation into multiparametric protocols due to the fact that it is an inexpensive and fast sequence has triggered several studies on the role of DWI as an alternative to DCE for breast cancer screening. This research has been undertaken in different populations, mostly in patients with suspicious mammographic and ultrasonographic findings94–103 and due to the issues raised by the breast density legislation in USA, as well as concerns on the long-term use of gadolinium contrast agents, it is a very active area of research with promising preliminary data.
DWI modeling
The monoexponential decay model is the most commonly used in clinical applications, but more sophisticated techniques are being explored in order to extract additional biological information. These techniques require, on the other hand, increased acquisition times (due the wider sampling of b-values and diffusion directions) and complex post-processing tools.
DTI characterizes the diffusivity (rate of water diffusion) and the directionality (anisotropy) of water molecules, providing data on the microarchitecture through anisotropy measures10 It has proven its role in both lesion characterization and response evaluation.104–106 (Figure 5).
Intravoxel Incoherent Motion (IVIM) explores the microvascularity of the tissues through a bi-exponential signal decay model.5,107 It has likewise proven useful in lesion characterization and response evaluation.108
Non-Gaussian diffusivity (diffusion kurtosis, stretched exponential diffusion) explores the complexity of the tissue microenvironment and its physical barriers to diffusion using higher b-value ranges than DWI (b > 1000 s/m2). Recent studies have explored this technique in lesion characterization.109,110
Radiomics
The main objective of radiomics is the discovery of signatures (imaging biomarkers) which have strong associations with patient’s prognoses or tumor subtype classification.111 Imaging biomarkers have the potential to capture the entire tumoral area, including its heterogeneity in a non-invasive and quick way. In radiomics, a great number of image features (shape, histogram, texture features) are extracted from medical images. Through specific selection methods of stability analysis (repeatability and reproducibility tests) significant features are chosen and their prognostic powers are tested in order to choose a subset of significant features or signatures that will have prognostic information. When these phenotypic signatures are correlated with genotypes, it is called radio-genomics or even panomics (genomics, proteomics, metabolomics) to better stratify patients for more precise therapeutic care in precision medicine. There have been few reports on the use of radiomics with DWI images yielding information that goes beyond the ADC measurements: looking for radiomics classifiers for lesion characterization in BI-RADS four lesions,112 or in BI-RADS 4a and 4b lesions based on kurtosis DWI,113 building multiparametric radiomic feature maps for analysis of textural information and correlation with tissue biology113 or predicting sentinel lymph node metastasis.114
Conclusions
DWI is a technique that provides complementary information to DCE-MRI exams. It has proven its value in single-center studies on lesion characterization and response evaluation, with a less clear role in tumor profiling through prognostic associations. Technical issues due to standard echoplanar imaging are being solved but the technique must be completely standardized and clear interpretation guidelines must be issued in order for DWI to become fully incorporated in breast cancer diagnosis and response evaluation. Potential areas of growth include detection with DWI without contrast agents, investigation of additional biological properties through DWI modeling and analysis of radiomics classifiers in order to better stratify patients and enable a real precision medicine.
Multicenter trials are clearly needed to validate single-center studies and establish DWI as a useful clinical decision-making tool.
REFERENCES
- 1. European Society of Radiology (ESR) ESR statement on the stepwise development of imaging biomarkers. Insights Imaging 2013; 4: 147–52. doi: 10.1007/s13244-013-0220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martí-Bonmatí L. Introduction to the stepwise development of imaging biomarkers : Imaging Biomarkers. 30: The British Institute of Radiology.; 2016. 9–27. [Google Scholar]
- 3. Paran Y, Bendel P, Margalit R, Degani H. Water diffusion in the different microenvironments of breast cancer. NMR Biomed 2004; 17: 170–80 2004. doi: 10.1002/nbm.882 [DOI] [PubMed] [Google Scholar]
- 4. White NS, McDonald C, McDonald CR, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res 2014; 74: 4638–52. doi: 10.1158/0008-5472.CAN-13-3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161: 401–7. doi: 10.1148/radiology.161.2.3763909 [DOI] [PubMed] [Google Scholar]
- 6. Englander SA, Uluğ AM, Brem R, Glickson JD, van Zilj PCM. Diffusion imaging of human breast. NMR in Biomedicine 1997; 10: 348–52. doi: [DOI] [PubMed] [Google Scholar]
- 7. Woodhams R, Ramadan S, Stanwell P, Sakamoto S, Hata H, Ozaki M, et al. Diffusion-weighted imaging of the breast: principles and clinical applications. Radiographics 2011; 31: 1059–84. doi: 10.1148/rg.314105160 [DOI] [PubMed] [Google Scholar]
- 8. Pereira F, Martins G, Figueiredo E, Domingues MNA, Da Fonseca LMB, Gasparetto EL. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different B values. AJR American Journal of Roentgenology 2011; 196: 210–7. [DOI] [PubMed] [Google Scholar]
- 9. Baltzer P, Mann RM, Iima M, Sigmund EE, Clauser P, Gilbert F, et al. Diffusion-weighted imaging of the breast: a consensus and mission statement from the EUSOBI International Breast diffusion- weighted imaging Working Group. European Radiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: clinical applications and emerging techniques. J Magn Reson Imaging 2017; 45: 337–55. doi: 10.1002/jmri.25479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am 2013; 21: 601–24. doi: 10.1016/j.mric.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011; 31: 1773–91. doi: 10.1148/rg.316115515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arponen O, Arponent O, Sudah M, Masarwah A, Taina M, Rautiainen S, Könönen M, et al. Diffusion-weighted imaging in 3.0 Tesla breast MRI: diagnostic performance and tumor characterization using small subregions vs. Whole tumor regions of interest. PLoS One 2015; 10: e0138702–17. doi: 10.1371/journal.pone.0138702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bickel H, Pinker K, Polanec S, Magometschnigg H, Wengert G, Spick C, et al. Diffusion-weighted imaging of breast lesions: Region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol 2017; 27: 1883–92. doi: 10.1007/s00330-016-4564-3 [DOI] [PubMed] [Google Scholar]
- 15. Ahlawat S, Khandheria P, Del Grande F, Morelli J, Subhawong TK, Demehri S, et al. Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: comparison with whole tumor volume measurements. J Magn Reson Imaging 2016; 43: 446–54. doi: 10.1002/jmri.24994 [DOI] [PubMed] [Google Scholar]
- 16. Rahbar H, Kurland BF, Olson ML, Kitsch AE, Scheel JR, Chai X, et al. Diffusion-weighted breast magnetic resonance imaging: a semiautomated Voxel selection technique improves Interreader reproducibility of apparent diffusion coefficient measurements. J Comput Assist Tomogr 2016; 40: 428–35. doi: 10.1097/RCT.0000000000000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi Y, Kim SH, Youn IK, Kang BJ, Park W-chan, Lee A. Rim sign and histogram analysis of apparent diffusion coefficient values on diffusion-weighted MRI in triple-negative breast cancer: comparison with ER-positive subtype. PLoS One 2017; 12: e0177903. doi: 10.1371/journal.pone.0177903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suo S, Zhang K, Cao M, Suo X, Hua J, Geng X, et al. Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging 2016; 43: 894–902. doi: 10.1002/jmri.25043 [DOI] [PubMed] [Google Scholar]
- 19. Kim YJ, Kim SH, Lee AW, Jin M-S, Kang BJ, Song BJ. Histogram analysis of apparent diffusion coefficients after neoadjuvant chemotherapy in breast cancer. Jpn J Radiol 2016; 34: 657–66. doi: 10.1007/s11604-016-0570-2 [DOI] [PubMed] [Google Scholar]
- 20. Spick C, Bickel H, Pinker K, Bernathova M, Kapetas P, Woitek R, et al. Diffusion-weighted MRI of breast lesions: a prospective clinical investigation of the quantitative imaging biomarker characteristics of reproducibility, repeatability, and diagnostic accuracy. NMR Biomed 2016; 29: 1445–53. doi: 10.1002/nbm.3596 [DOI] [PubMed] [Google Scholar]
- 21. Giannotti E, Waugh S, Priba L, Davis Z, Crowe E, Vinnicombe S. Assessment and quantification of sources of variability in breast apparent diffusion coefficient (ADC) measurements at diffusion weighted imaging. Eur J Radiol 2015; 84: 1729–36. doi: 10.1016/j.ejrad.2015.05.032 [DOI] [PubMed] [Google Scholar]
- 22. Clauser P, Marcon M, Maieron M, Zuiani C, Bazzocchi M, Baltzer PAT. Is there a systematic bias of apparent diffusion coefficient (ADC) measurements of the breast if measured on different workstations? An inter- and intra-reader agreement study. Eur Radiol 2016; 26: 2291–6. doi: 10.1007/s00330-015-4051-2 [DOI] [PubMed] [Google Scholar]
- 23. Belli G, Busoni S, Ciccarone A, Coniglio A, Esposito M, Giannelli M, et al. Quality assurance multicenter comparison of different Mr scanners for quantitative diffusion-weighted imaging. J Magn Reson Imaging 2016; 43: 213–9. doi: 10.1002/jmri.24956 [DOI] [PubMed] [Google Scholar]
- 24. deSouza NM, Winfield JM, Waterton JC, Weller A, Papoutsaki M-V, Doran SJ, et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: current considerations and future perspectives. Eur Radiol 2018; 28: 1118–31. doi: 10.1007/s00330-017-4972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Padhani AR, Liu G, Mu-Koh D, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009; 11: 102–25. doi: 10.1593/neo.81328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maier CF, Paran Y, Bendel P, Rutt BK, Degani H. Quantitative diffusion imaging in implanted human breast tumors. Magn Reson Med 1997; 37: 576–81. doi: 10.1002/mrm.1910370417 [DOI] [PubMed] [Google Scholar]
- 27. Guo Y, Cai Y-Q, Cai Z-L, Gao Y-G, An N-Y, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: 172–8. doi: 10.1002/jmri.10140 [DOI] [PubMed] [Google Scholar]
- 28. Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med 2008; 26: 222–6. doi: 10.1007/s11604-007-0218-3 [DOI] [PubMed] [Google Scholar]
- 29. Matsubayashi RN, Fujii T, Yasumori K, Muranaka T, Momosaki S. Apparent diffusion coefficient in invasive ductal breast carcinoma: correlation with detailed histologic features and the enhancement ratio on dynamic contrast-enhanced MR images. J Oncol 2010; 2010: 1–602 09 20102010; Article ID. doi: 10.1155/2010/821048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnes SL, Sorace AG, Loveless ME, Whisenant JG, Yankeelov TE. Correlation of tumor characteristics derived from DCE-MRI and DW-MRI with histology in murine models of breast cancer. NMR Biomed 2015; 28: 1345–56. doi: 10.1002/nbm.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Partridge SC, McKinnon GC, Henry RG, Hylton NM. Menstrual cycle variation of apparent diffusion coefficients measured in the normal breast using MRI. J Magn Reson Imaging 2001; 14: 433–8. doi: 10.1002/jmri.1204 [DOI] [PubMed] [Google Scholar]
- 32. O'Flynn EAM, Morgan VA, Giles SL, deSouza NM. Diffusion weighted imaging of the normal breast: reproducibility of apparent diffusion coefficient measurements and variation with menstrual cycle and menopausal status. Eur Radiol 2012; 22: 1512–8. doi: 10.1007/s00330-012-2399-0 [DOI] [PubMed] [Google Scholar]
- 33. Kim JY, Suh HB, Kang HJ, Shin JK, Choo KS, Nam KJ, et al. Apparent diffusion coefficient of breast cancer and normal fibroglandular tissue in diffusion-weighted imaging: the effects of menstrual cycle and menopausal status. Breast Cancer Res Treat 2016; 157: 31–40. doi: 10.1007/s10549-016-3793-0 [DOI] [PubMed] [Google Scholar]
- 34. Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H. Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology 2014; 271: 672–80. doi: 10.1148/radiol.14132084 [DOI] [PubMed] [Google Scholar]
- 35. Shin S, Ko ES, Kim RB, Han B-K, Nam SJ, Shin JH, et al. Effect of menstrual cycle and menopausal status on apparent diffusion coefficient values and detectability of invasive ductal carcinoma on diffusion-weighted MRI. Breast Cancer Res Treat 2015; 149: 751–9. doi: 10.1007/s10549-015-3278-6 [DOI] [PubMed] [Google Scholar]
- 36. McDonald ES, Schopp JG, Peacock S, DeMartini WB, DeMartini WD, Rahbar H, Lehman CD, et al. Diffusion-weighted MRI: association between patient characteristics and apparent diffusion coefficients of normal breast fibroglandular tissue at 3 T. AJR Am J Roentgenol 2014; 202: W496–W502. doi: 10.2214/AJR.13.11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iacconi C, Thakur SB, Dershaw DD, Brooks J, Fry CW, Morris EA. Impact of fibroglandular tissue and background parenchymal enhancement on diffusion weighted imaging of breast lesions. Eur J Radiol 2014; 83: 2137–43. doi: 10.1016/j.ejrad.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 38. Cho GY, Moy L, Kim SG, Klautau Leite AP, Baete SH, Babb JS, et al. Comparison of contrast enhancement and diffusion-weighted magnetic resonance imaging in healthy and cancerous breast tissue. Eur J Radiol 2015; 84: 1888–93. doi: 10.1016/j.ejrad.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 39. Plaza MJ, Morris EA, Thakur SB. Diffusion tensor imaging in the normal breast: influences of fibroglandular tissue composition and background parenchymal enhancement. Clin Imaging 2016; 40: 506–11. doi: 10.1016/j.clinimag.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sah RG, Agarwal K, Sharma U, Parshad R, Seenu V, Jagannathan NR. Characterization of malignant breast tissue of breast cancer patients and the normal breast tissue of healthy lactating women volunteers using diffusion MRI and in vivo (1) H MR spectroscopy. J Magn Reson Imaging 2013; 42: 169–74. [DOI] [PubMed] [Google Scholar]
- 41. Horvat JV, Durando M, Milans S, Patil S, Massler J, Gibbons G, et al. Apparent diffusion coefficient mapping using diffusion-weighted MRI: impact of background parenchymal enhancement, amount of fibroglandular tissue and menopausal status on breast cancer diagnosis. European Radiology 2018; 25: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuen S, Yamada K, Goto M, Nishida K, Takahata A, Nishimura T. Microperfusion-induced elevation of ADC is suppressed after contrast in breast carcinoma. J Magn Reson Imaging 2009; 29: 1080–4. doi: 10.1002/jmri.21743 [DOI] [PubMed] [Google Scholar]
- 43. Ramadan S, Mulkern RV. Comment on ADC reductions in postcontrast breast tumors. J. Magn. Reson. Imaging 2010; 31: 262–4. doi: 10.1002/jmri.21972 [DOI] [PubMed] [Google Scholar]
- 44. Janka R, Hammon M, Geppert C, Nothhelfer A, Uder M, Wenkel E. Diffusion-weighted MR imaging of benign and malignant breast lesions before and after contrast enhancement. Rofo 2014; 186: 130–5. doi: 10.1055/s-0033-1350298 [DOI] [PubMed] [Google Scholar]
- 45. Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Effect of gadolinium injection on diffusion-weighted imaging with background body signal suppression (DWIBS) imaging of breast lesions. Magn Reson Imaging 2014; 32: 1242–6. doi: 10.1016/j.mri.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 46. Nguyen VT, Rahbar H, Olson ML, Liu C-L, Lehman CD, Partridge SC. Diffusion-weighted imaging: effects of intravascular contrast agents on apparent diffusion coefficient measures of breast malignancies at 3 tesla. J Magn Reson Imaging 2015; 42: 788–800. doi: 10.1002/jmri.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of B value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 2014; 24: 2835–47. doi: 10.1007/s00330-014-3338-z [DOI] [PubMed] [Google Scholar]
- 48. Chen X, Li W-ling, Zhang Y-li, Wu Q, Guo Y-min, Bai Z-lan. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer 2010; 10: 693. doi: 10.1186/1471-2407-10-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsushima Y, Takahashi-Taketomi A, Endo K, resonance M. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 2009; 30: 249–55. doi: 10.1002/jmri.21854 [DOI] [PubMed] [Google Scholar]
- 50. Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol 2016; 57: 651–60. doi: 10.1177/0284185115597265 [DOI] [PubMed] [Google Scholar]
- 51. Peters NHGM, Vincken KL, van den Bosch MAAJ, Luijten PR, Mali WPTM, Bartels LW. Quantitative diffusion weighted imaging for differentiation of benign and malignant breast lesions: the influence of the choice of b-values. J Magn Reson Imaging 2010; 31: 1100–5. doi: 10.1002/jmri.22152 [DOI] [PubMed] [Google Scholar]
- 52. Shi R-Y, Yao Q-Y, Wu L-M, Xu J-R, J-R X. Breast lesions: diagnosis using diffusion weighted imaging at 1.5T and 3.0T-Systematic review and meta-analysis. Clin Breast Cancer 2018; 18: e305–20. doi: 10.1016/j.clbc.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 53. Molinari C, Clauser P, Girometti R, Linda A, Cimino E, Puglisi F, et al. MR mammography using diffusion-weighted imaging in evaluating breast cancer: a correlation with proliferation index. Radiol Med 2015; 120: 911–8. doi: 10.1007/s11547-015-0527-z [DOI] [PubMed] [Google Scholar]
- 54. Shin JK, Kim JY. Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 2017; 45: 94–102. doi: 10.1002/jmri.25348 [DOI] [PubMed] [Google Scholar]
- 55. Bickel H, Pinker-Domenig K, Bogner W, Spick C, Bagó-Horváth Z, Weber M, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 2015; 50: 95–100. doi: 10.1097/RLI.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 56. Kızıldağ Yırgın İnci, Arslan G, Öztürk E, Yırgın H, Taşdemir N, Gemici AA, et al. Diffusion weighted MR imaging of breast and correlation of prognostic factors in breast cancer. Balkan Med J 2016; 33: 301–7. doi: 10.5152/balkanmedj.2016.140555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Durando M, Gennaro L, Cho GY, Giri DD, Gnanasigamani MM, Patil S, et al. Quantitative apparent diffusion coefficient measurement obtained by 3.0Tesla MRI as a potential noninvasive marker of tumor aggressiveness in breast cancer. Eur J Radiol 2016; 85: 1651–8. doi: 10.1016/j.ejrad.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Surov A, Clauser P, Chang Y-W, Li L, Martincich L, Partridge SC, et al. Can diffusion-weighted imaging predict tumor grade and expression of Ki-67 in breast cancer? A multicenter analysis. Breast Cancer Research 2018; 20: 252. doi: 10.1186/s13058-018-0991-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kitajima K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol 2016; 85: 943–9. doi: 10.1016/j.ejrad.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 60. Mori N, Ota H, Mugikura S, Takasawa C, Ishida T, Watanabe G, et al. Luminal-type breast cancer: correlation of apparent diffusion coefficients with the Ki-67 labeling index. Radiology 2015; 274: 66–73. doi: 10.1148/radiol.14140283 [DOI] [PubMed] [Google Scholar]
- 61. Cho GY, Moy L, Kim SG, Baete SH, Moccaldi M, Babb JS, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol 2016; 26: 2547–58. doi: 10.1007/s00330-015-4087-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meng L, Ma P. Apparent diffusion coefficient value measurements with diffusion magnetic resonance imaging correlated with the expression levels of estrogen and progesterone receptor in breast cancer: a meta-analysis. J Cancer Res Ther 2016; 12: 36–42. doi: 10.4103/0973-1482.150418 [DOI] [PubMed] [Google Scholar]
- 63. Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol 2012; 22: 1519–28. doi: 10.1007/s00330-012-2403-8 [DOI] [PubMed] [Google Scholar]
- 64. Akın Y, Uğurlu M Ümit, Kaya H, Arıbal E. Diagnostic value of diffusion-weighted imaging and apparent diffusion coefficient values in the differentiation of breast lesions, Histpathologic subgroups and Correlatıon with Prognostıc factors using 3.0 Tesla Mr. J Breast Health 2016; 12: 123–32. doi: 10.5152/tjbh.2016.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guvenc I, Akay S, Ince S, Yildiz R, Kilbas Z, Oysul FG, et al. Apparent diffusion coefficient value in invasive ductal carcinoma at 3.0 Tesla: is it correlated with prognostic factors? Br J Radiol 2016; 89: 20150614. doi: 10.1259/bjr.20150614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med 2014; 55: 736–42. doi: 10.2967/jnumed.113.129395 [DOI] [PubMed] [Google Scholar]
- 67. Park SH, Choi H-Y, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging 2015; 41: 175–82. doi: 10.1002/jmri.24519 [DOI] [PubMed] [Google Scholar]
- 68. Lee HS, Kim SH, Kang BJ, Baek JE, Song BJ. Perfusion parameters in dynamic contrast-enhanced MRI and apparent diffusion coefficient value in diffusion-weighted MRI:: association with prognostic factors in breast cancer. Acad Radiol 2016; 23: 446–56. doi: 10.1016/j.acra.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 69. Belli P, Costantini M, Bufi E, Giardina GG, Rinaldi P, Franceschini G, et al. Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radiol Med 2015; 120: 268–76. doi: 10.1007/s11547-014-0442-8 [DOI] [PubMed] [Google Scholar]
- 70. Shao G, Fan L, Zhang J, Dai G, Xie T. Association of DW/DCE-MRI features with prognostic factors in breast cancer. Int J Biol Markers 2017; 32: 118–25. doi: 10.5301/jbm.5000230 [DOI] [PubMed] [Google Scholar]
- 71. Hussein H, Chung C, Moshonov H, Miller N, Kulkarni SR, Scaranelo AM. Evaluation of apparent diffusion coefficient to predict grade, microinvasion, and invasion in ductal carcinoma in situ of the breast. Acad Radiol 2015; 22: 1483–8. doi: 10.1016/j.acra.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 72. Mori N, Ota H, Mugikura S, Takasawa C, Tominaga J, Ishida T, et al. Detection of invasive components in cases of breast ductal carcinoma in situ on biopsy by using apparent diffusion coefficient Mr parameters. Eur Radiol 2013; 23: 2705–12. doi: 10.1007/s00330-013-2902-2 [DOI] [PubMed] [Google Scholar]
- 73. Yoon H-J, Kim Y, Kim BS. Intratumoral metabolic heterogeneity predicts invasive components in breast ductal carcinoma in situ. Eur Radiol 2015; 25: 3648–58. doi: 10.1007/s00330-015-3761-9 [DOI] [PubMed] [Google Scholar]
- 74. Cheeney S, Rahbar H, Dontchos BN, Javid SH, Rendi MH, Partridge SC. Apparent diffusion coefficient values may help predict which MRI-detected high-risk breast lesions will upgrade at surgical excision. J Magn Reson Imaging 2017; 46: 1028–36. doi: 10.1002/jmri.25656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rahbar H, Partridge SC, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 2012; 263: 374–82. doi: 10.1148/radiol.12111368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thakur SB, Durando M, Milans S, Cho GY, Gennaro L, Sutton EJ, et al. Apparent diffusion coefficient in estrogen receptor-positive and lymph node-negative invasive breast cancers at 3.0T DW-MRI: a potential predictor for an Oncotype DX test recurrence score. J Magn Reson Imaging 2018; 47: 401–9. doi: 10.1002/jmri.25796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Amornsiripanitch N, Nguyen VT, Rahbar H, Hippe DS, Gadi VK, Rendi MH, et al. Diffusion-weighted MRI characteristics associated with prognostic pathological factors and recurrence risk in invasive ER+/HER2- breast cancers. J Magn Reson Imaging 2018; 48: 226–36. doi: 10.1002/jmri.25909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuerer HM, Peeters M-J, Rea DW, Basik M, Santos JL, Heil J. Nonoperative management for invasive breast cancer after neoadjuvant systemic therapy: conceptual basis and fundamental international feasibility clinical trials. Ann Surg Oncol 2017; 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 79. Galons J-P, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ. Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia 1999; 1: 113–7. doi: 10.1038/sj.neo.7900009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 2006; 24: 843–7. doi: 10.1016/j.mri.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 81. Sharma U, Danishad KKA, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 2009; 22: 104–13. doi: 10.1002/nbm.1245 [DOI] [PubMed] [Google Scholar]
- 82. Li SP, Padhani AR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging 2012; 35: 745–63. doi: 10.1002/jmri.22838 [DOI] [PubMed] [Google Scholar]
- 83. Iwasa H, Kubota K, Hamada N, Nogami M, Nishioka A. Early prediction of response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and gray-scale ultrasonography. Oncol Rep 2014; 31: 1555–60. doi: 10.3892/or.2014.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Galbán CJ, Hoff BA, Chenevert TL, Ross BD. Diffusion MRI in early cancer therapeutic response assessment. NMR Biomed 2017; 30: e3458. doi: 10.1002/nbm.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fujimoto H, Kazama T, Nagashima T, Sakakibara M, Suzuki TH, Okubo Y, et al. Diffusion-weighted imaging reflects pathological therapeutic response and relapse in breast cancer. Breast Cancer 2014; 21: 724–31. doi: 10.1007/s12282-013-0449-3 [DOI] [PubMed] [Google Scholar]
- 86. Liu S, Ren R, Chen Z, Wang Y, Fan T, Li C, et al. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J Magn Reson Imaging 2015; 42: 779–87. doi: 10.1002/jmri.24843 [DOI] [PubMed] [Google Scholar]
- 87. Bufi E, Belli P, Costantini M, Cipriani A, Di Matteo M, Bonatesta A, et al. Role of the apparent diffusion coefficient in the prediction of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin Breast Cancer 2015; 15: 370–80. doi: 10.1016/j.clbc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 88. Gu Y-L, Pan S-M, Ren J, Yang Z-X, Jiang G-Q. Role of magnetic resonance imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer 2017; 17: 245–55. doi: 10.1016/j.clbc.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 89. Gao W, Guo N, Dong T. Diffusion-weighted imaging in monitoring the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis. World J Surg Oncol 2018; 16: 145. doi: 10.1186/s12957-018-1438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Partridge SC, Zhang Z, Newitt DC, Gibbs JE, Chenevert TL, Rosen MA, et al. Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: the ACRIN 6698 multicenter trial. Radiology 2018; 289: 618–27. doi: 10.1148/radiol.2018180273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. deSouza NM. Diffusion-weighted MRI in multicenter trials of breast cancer: a useful measure of tumor response? Radiology 2018; 289: 628–9. doi: 10.1148/radiol.2018181717 [DOI] [PubMed] [Google Scholar]
- 92. O'Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017; 14: 169–86. doi: 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuroki-Suzuki S, Kuroki Y, Nasu K, Nawano S, Moriyama N, Okazaki M. Detecting breast cancer with non-contrast MR imaging: combining diffusion-weighted and stir imaging. Magn Reson Med Sci 2007; 6: 21–7. doi: 10.2463/mrms.6.21 [DOI] [PubMed] [Google Scholar]
- 94. Baltzer PAT, Benndorf M, Dietzel M, Gajda M, Camara O, Kaiser WA. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol 2010; 20: 1101–10. doi: 10.1007/s00330-009-1654-5 [DOI] [PubMed] [Google Scholar]
- 95. Partridge SC, Demartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging 2010; 31: 562–70. doi: 10.1002/jmri.22078 [DOI] [PubMed] [Google Scholar]
- 96. Yabuuchi H, Matsuo Y, Sunami S, Kamitani T, Kawanami S, Setoguchi T, et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol 2011; 21: 11–17. doi: 10.1007/s00330-010-1890-8 [DOI] [PubMed] [Google Scholar]
- 97. Kazama T, Kuroki Y, Kikuchi M, Sato Y, Nagashima T, Miyazawa Y, et al. Diffusion-weighted MRI as an adjunct to mammography in women under 50 years of age: an initial study. J Magn Reson Imaging 2012; 36: 139–44. doi: 10.1002/jmri.23626 [DOI] [PubMed] [Google Scholar]
- 98. Wu L-M, Chen J, Hu J, Gu H-Y, Xu J-R, Hua J, L-M W, J-R X, Hua J. Diffusion-weighted magnetic resonance imaging combined with T2-weighted images in the detection of small breast cancer: a single-center multi-observer study. Acta Radiol 2014; 55: 24–31. doi: 10.1177/0284185113492458 [DOI] [PubMed] [Google Scholar]
- 99. Trimboli RM, Verardi N, Cartia F, Carbonaro LA, Sardanelli F. Breast cancer detection using double reading of unenhanced MRI including T1-weighted, T2-weighted stir, and diffusion-weighted imaging: a proof of concept study. AJR Am J Roentgenol 2014; 203: 674–81. doi: 10.2214/AJR.13.11816 [DOI] [PubMed] [Google Scholar]
- 100. Bickelhaupt S, Tesdorff J, Laun FB, Kuder TA, Lederer W, Teiner S, et al. Independent value of image fusion in unenhanced breast MRI using diffusion-weighted and morphological T2-weighted images for lesion characterization in patients with recently detected BI-RADS 4/5 X-ray mammography findings. Eur Radiol 2017; 27: 1–8. doi: 10.1007/s00330-016-4400-9 [DOI] [PubMed] [Google Scholar]
- 101. Baltzer PAT, Bickel H, Spick C, Wengert G, Woitek R, Kapetas P, et al. Potential of noncontrast magnetic resonance imaging with diffusion-weighted imaging in characterization of breast lesions: Intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 2018; 53: 229–35. doi: 10.1097/RLI.0000000000000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pinker K, Moy L, Sutton EJ, Mann RM, Weber M, Thakur SB, et al. Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone parameter: comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Invest Radiol 2018; 53: 587–95. doi: 10.1097/RLI.0000000000000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Eyal E, Shapiro-Feinberg M, Furman-Haran E, Grobgeld D, Golan T, Itzchak Y, et al. Parametric diffusion tensor imaging of the breast. Invest Radiol 2012; 47: 284–91. doi: 10.1097/RLI.0b013e3182438e5d [DOI] [PubMed] [Google Scholar]
- 104. Furman-Haran E, Nissan N, Ricart-Selma V, Martinez-Rubio C, Degani H, Camps-Herrero J. Quantitative evaluation of breast cancer response to neoadjuvant chemotherapy by diffusion tensor imaging: initial results. J Magn Reson Imaging 2018; 47: 1080–90. doi: 10.1002/jmri.25855 [DOI] [PubMed] [Google Scholar]
- 105. Baltzer PAT, Schäfer A, Dietzel M, Grässel D, Gajda M, Camara O, et al. Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol 2011; 21: 1–10. doi: 10.1007/s00330-010-1901-9 [DOI] [PubMed] [Google Scholar]
- 106. Iima M, Le Bihan D. Clinical Intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 2016; 278: 13–32. doi: 10.1148/radiol.2015150244 [DOI] [PubMed] [Google Scholar]
- 107. Kim Y, Kim SH, Lee HW, Song BJ, Kang BJ, Lee A, et al. Intravoxel incoherent motion diffusion-weighted MRI for predicting response to neoadjuvant chemotherapy in breast cancer. Magn Reson Imaging 2018; 48: 27–33. doi: 10.1016/j.mri.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 108. Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, et al. Breast cancer: diffusion Kurtosis Mr Imaging-Diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 2015; 277: 46–55. doi: 10.1148/radiol.15141625 [DOI] [PubMed] [Google Scholar]
- 109. Iima M, Yano K, Kataoka M, Umehana M, Murata K, Kanao S, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol 2015; 50: 205–11. doi: 10.1097/RLI.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 110. Arimura H, Soufi M, Ninomiya K, Kamezawa H, Yamada M. Potentials of radiomics for cancer diagnosis and treatment in comparison with computer-aided diagnosis. Radiol Phys Technol 2018; 11: 365–74. doi: 10.1007/s12194-018-0486-x [DOI] [PubMed] [Google Scholar]
- 111. Bickelhaupt S, Paech D, Kickingereder P, Steudle F, Lederer W, Daniel H, et al. Prediction of malignancy by a radiomic signature from contrast agent-free diffusion MRI in suspicious breast lesions found on screening mammography. J Magn Reson Imaging 2017; 46: 604–16. doi: 10.1002/jmri.25606 [DOI] [PubMed] [Google Scholar]
- 112. Bickelhaupt S, Jaeger PF, Laun FB, Lederer W, Daniel H, Kuder TA, et al. Radiomics based on adapted diffusion Kurtosis imaging helps to clarify most mammographic findings suspicious for cancer. Radiology 2018; 287: 761–70. doi: 10.1148/radiol.2017170273 [DOI] [PubMed] [Google Scholar]
- 113. Parekh VS, Jacobs MA. Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI. NPJ Breast Cancer 2017; 3: 43. doi: 10.1038/s41523-017-0045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dong Y, Feng Q, Yang W, Lu Z, Deng C, Zhang L, et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. Eur Radiol 2018; 28: 87–10. doi: 10.1007/s00330-017-5005-7 [DOI] [PubMed] [Google Scholar]