Abstract
In parallel with the increasingly widespread availability of high performance imaging platforms and recent progresses in pathobiological characterisation and treatment of gastrointestinal malignancies, imaging biomarkers have become a major research topic due to their potential to provide additional quantitative information to conventional imaging modalities that can improve accuracy at staging and follow-up, predict outcome, and guide treatment planning in an individualised manner. The aim of this review is to briefly examine the status of current knowledge about imaging biomarkers in the field of upper gastrointestinal cancers, highlighting their potential applications and future perspectives in patient management from diagnosis onwards.
introduction
The term “biomarker” refers to a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or biological responses to therapeutic interventions.1–3 Biomarkers can be derived directly from human biological samples (including blood or other biological fluids, tissues or cells) or biosignals detected by diagnostic imaging techniques (such as multidetector CT, MRI, ultrasound or PET), providing anatomical and/or functional parameters that correlate with histopathological or molecular data.4–7 Imaging biomarkers can be either quantitative (e.g. lesion diameter or volume, CT density, MR signal intensity, radiomics features, and any other biomarker whose magnitude can be expressed as a quantity value) or qualitative (e.g. pathological grading systems that can be expressed as ordinal rather than continuous quantitative data, such as clinical TNM staging, BI-RADS, LI-RADS, PI-RADS, C-RADS, and so on).8,9 Compared to “real” biological samples, imaging biomarkers have the advantage of being noninvasive (or minimally invasive) and easily obtainable, making them attractive for repeated testing and follow-up. In addition, while conventional biopsy captures only a fraction of tumour cells that may not be representative of the entire tumour, imaging biomarkers can capture the whole tumour heterogeneity, thus playing a potentially critical role into guiding biopsy or whenever biopsy is unobtainable.10–12
Due to the incremental diagnostic and prognostic value that imaging biomarkers can provide over conventional modalities (which, however, remain indispensable for the routine diagnostic workup of cancer patients), a great interest has arisen in the scientific and medical community in searching for biomarkers and building biobanks that may lead to more effective, patient-specific treatment. In this perspective, extensive research has recently been made in the field of radiomics, a discipline dealing with high-throughput extraction and analysis of quantitative features from diagnostic images that cannot usually be derived from visual assessment (including shape/size-, histogram- or filter-based texture analysis) and form the quantitative imaging expression of pathological processes that are also expressed by other “omics”, such as genomics, transcriptomics, metabolomics and proteomics.5,8
In this paper we will give a brief, yet hopefully explanatory overview of the current potential role of imaging biomarkers in the management of upper gastrointestinal cancers, with oropharyngeal, oesophageal, and gastric cancers as paradigm diseases.
oropharyngeal cancer
Head and neck cancer is the seventh most frequent cancer and the ninth most frequent cause of cancer-related death, with a worldwide incidence of 550,000 cases and 380,000 deaths annually, of which 90% are squamous cell carcinomas (SCC) in the adult population.13,14 Most oropharyngeal cancers are diagnosed at a locally advanced stage, usually requiring multimodality treatment (including surgery, chemotherapy and/or radiation therapy), and the rate of locoregional recurrence is as high as 25–50% depending on the lesion site, making recurrence risk the greatest hurdle in improving survival rate. Moreover, tumour biology (with particular reference to human papillomavirus [HPV] positivity) plays a pivotal role in defining the prognosis and optimal therapeutic strategy for patients with head and neck SCC, with HPV+oropharyngeal SCC being associated with a better overall survival. Therefore, a comprehensive assessment of disease extension (including primary tumour site and locoregional lymph nodes) at baseline and after treatment is mandatory, also in consideration that oropharyngeal and other head and neck SCC recurrences tend to occur at a locoregional level within the same volume of treatment.15–17
Although conventional multidetector CT and MR imaging performed with state-of-the-art equipment is the mainstay for diagnosis, staging and follow-up of patients with oropharyngeal SCC, morphologic imaging can be limited in detecting subtle disease or differentiating disease recurrence from benign post-treatment changes (e.g. after surgery and/or radiation therapy) that may obscure viable tumour tissue or mimic disease, and usually cannot provide per se any insight about prognosis, likelihood of early and/or sustained treatment response, or eligibility to patient-tailored target therapies.14,18–20 On the other hand, both standard CT and MRI data and those derived from more complex implementations of cross-sectional imaging can be joined to build specific radiomic signatures that can lead to improved patient stratification and potentially more personalised treatment8,21 (Table 1). In this latter setting, a recent large multicentre study by Leijenaar et al combining 902 radiomic features from 778 patients with oropharyngeal SCC showed that CT-based radiomics can predict HPV+ (p16) status from multivariable models obtained from standard pre-treatment CT images. More specifically, a model was developed on all training data (Mall) and on an artefact-free subset of training data (Mno art) to assess the impact of CT artefacts in predicting HPV (p16), and a consistent and significant split was observed between survival curves with HPV status determined by p16 [p = 0.007, hazard ratio (HR) 0.46], Mall (p = 0.036, HR 0.55) and Mno art (p = 0.027, HR 0.49), along with an area under the receiver operator curve ranging between 0.70 and 0.80 for both Mall and Mno art.22 In addition, evidence is growing that a radiomic approach can provide useful prognostic quantitative information in patients undergoing chemo-radiation therapy (CRT), such as primary tumour volume (which correlates with treatment outcome better than T or N stages),23 CT texture analysis (which can predict local failure after CRT),24 and 18F-FDG PET texture as a predictor of post-CRT patient survival in addition to clinical variables.25
Table 1. .
Summary of the features and main findings of the studies on imaging biomarkers in head and neck cancer (with particular reference to oropharyngeal cancer) cited in the text. Case reports and general reviews articles are not included. References are sorted in the order in which they appear in the text
| Reference | Tumour type and location | Imaging features | Endpoints | Main findings |
| van der Hoorn et al.20 | Head and neck SCC (oral cavity, pharynx, larynx)a | Anatomical MRI, ADC | Diagnostic accuracy for treatment response evaluation |
|
| Leijenaar et al.22 | HPV+ oropharyngeal SCC | Radiomic signatures (Mall, Mno art) for HPV (p16) prediction from standard pre-RT/CRT CT imaging | Diagnostic accuracy, OS |
|
| Strongin et al.23 | Locally advanced SCC (oropharynx, hypopharynx and larynx) | Primary tumour volume (TV) before CRT | PFS, OS |
|
| Kuno et al.24 | Head and neck SCC (oropharynx, larynx, hypopharynx and oral cavity) | CT texture parameters | Local recurrence after CRT |
|
| Feliciani et al.25 | Head and neck SCC (oral cavity, oropharynx, hypopharynx, nasopharynx and larynx) |
Pre-CRT 18F-FDG PET texture features for prediction of treatment failure | Local control rate, PFS, OS |
|
| Holzapfel et al.26 | SCC cervical nodal metastases | ADC | Diagnostic accuracy for differentiating between benign and metastatic lymph nodes |
|
| Jin et al.27 | Nasopharyngeal SCC cervical nodal metastases* | ADC | Diagnostic accuracy for differentiating between benign and metastatic lymph nodes |
|
| Hwang et al.28 | Head and neck SCC (oral cavity, oropharynx, sinonasal cavity, nasopharynx, hypopharynx, external auditory canal) | ADC | Diagnostic accuracy for differentiating tumour recurrence from post-treatment (surgery and/or chemo/radiotherapy) changes |
|
| Ryoo et al.29 | Head and neck SCC (oral cavity, oropharynx, nasopharynx, supraglottic larynx, maxillary sinus) | ADC | Diagnostic accuracy for predicting response to induction chemotherapy |
|
| Preda et al.30 | Head and neck SCC | SUV, ADC | DFS |
|
| Surov et al.31 | Head and neck SCC | SUV, ADC | Tumour proliferation indexes (Ki67, cell count, total nucleic area, average nucleic area) |
|
| Dong et al.34 | Head and neck SCC | DCE-MRI (Ktrans, Ve, iAUC) | Tumour grading |
|
| Chen et al.35 | Head and neck cancer (nasopharynx, oropharynx, tongue base, larynx) | DCE-MRI (Ktrans, Ve, kep) | Differentiation among tumours, metastatic nodes and normal tissue |
|
| Baker et al.36 | Head and neck SCC murine xenografts | R1 and R2* relaxation, functional MRI, relative 18F-FDG uptake | Association with acquired resistance to targeted EGFR therapy within size-matched EGFR TKI-resistant CAL 27 (CALR) and sensitive (CALS) tumour xenografts in vivo |
|
| Zhong et al.37 | Lymph node metastases from head and neck SCC (larynx, tongue, nasopharynx, floor of mouth, nasal cavity, oropharynx, gingiva) | Preoperative DWI and perfusion CT | Diagnostic accuracy for differentiation between metastatic and benign lymph nodes |
|
| Lam et al.40 | Head and neck SCC (primary and lymph node metastases) | DECT image quality | SNR, attenuation difference between tumour and muscles, lesion CNR |
|
| Tawfik et al.43 | Metastatic SCC cervical lymph nodes | DECT-derived iodine content and iodine overlay | Differentiation among normal, inflammatory and metastatic SCC lymph nodes |
|
| Foust et al. 201844 | Oropharyngeal SCC with nodal metastases | DECT-derived iodine content and iodine spectral attenuation slope | Diagnostic accuracy for differentiation between metastatic and nonmetastatic lymph nodes |
|
| Rizzo et al.45 | Metastatic lymph nodes from various primary cancers (lung, gynaecological)* | DECT-derived material decomposition maps and intranodal attenuation (HU) distribution | Differentiation between metastatic and nonmetastatic lymph nodes based on spectral structure |
|
ADC, apparent diffusion coefficient; AUC, area under the ROC curve; CNR, contrast-to-noise ratio; DFS, disease free survival; HR, hazard ratio; NPV, negative predictive value; OS, overall survival; PFS, progression free survival; PPV, positive predictive value; SNR, signal-to-noise ratio; SUV, standardised uptake value.
Meta-analysis article. (*) References 27 and 45 were included due to the general value of their respective findings, although not specific to oropharyngeal cancer.
Among MRI techniques, diffusion-weighted imaging (DWI) is a powerful quantitative biomarker that can identify foci of residual disease as areas of restricted diffusivity of water molecules, due to cancer cells being densely packed with a relatively high nuclear-cytoplasmic ratio compared with non-neoplastic tissues. This allows overcoming potential confounding factors such as diffuse oedema and contrast enhancement as a result of inflammation and/or post-radiation sequelae, or faint or no enhancement in poorly vascularised and/or highly necrotic tumours, resulting in increased sensitivity in detection of primary oropharyngeal SCC.14,20 In a recent large meta-analysis, DWI-based apparent diffusion coefficient (ADC) quantitative assessment outperformed anatomical MRI for detection of primary tumour site with a pooled sensitivity and specificity of 89% and 86% vs 84% and 82%, respectively.20 Lower ADC values have also been found in malignant than in benign lymph nodes sized down to 5–10 mm, and Holzapfel et al were able to correctly classify cervical lymph nodes as benign or malignant using an ADC threshold of 1.02 × 10−3 mm2/s in 94.3% of cases.14,26,27 Furthermore, Hwang et al assessed the performance of standard (b = 1000 s/mm2) and high b-value (2000 s/mm2) DWI in differentiating between recurrent tumour and post-treatment changes, and found that the ratio between ADC2000 and ADC1000 was significantly higher in the former than in the latter (73.5% vs 56.9%, p < 0.001), with a cut-off value of 62.6% leading to a sensitivity, specificity, and diagnostic accuracy of 95.0%, 69.2%, and 84.8%, respectively.28 Of interest, patients with a good response to induction neoadjuvant chemotherapy were found to have lower ADC2000 values along with smaller tumour volume than poor responders, suggesting a potential role of high ADC values in predicting tumour response.29 In terms of risk stratification, it has been reported that the combination of poor prognostic factors such as high 8F-FDG PET-CT-derived SUVmaxT/B and high ADCmin values improves the prognostic role of the two separate parameters (with high ADCmin identifying SCC patients with worse prognosis among those with high SUVmaxT/B).30 Moreover, a significant association was observed between histopathological markers of tumour proliferation and ADC and SUV values calculated at simultaneous 18F-FDG-PET/MRI, with mean ADC being negatively correlated with Ki67 level (r = −0.728, p = 0.011) and total nucleic area (r = −0.691, p = 0.019), and combined SUVmax/ADCmin positively correlated with average nucleic area (r = 0.627, p = 0.039).31
Other functional imaging modalities such as dynamic contrast enhancement (DCE)-MRI, CT perfusion imaging and dual energy CT (DECT) can enable a quantitative evaluation of tumour vascularity that can be useful in the pre- and post-treatment setting.14,32 In general, highly vascular SCC tend to have a better treatment response compared to less vascular ones due to their greater sensitivity to radiation and better delivery of chemotherapeutic drugs, yet they may have poorer outcome due to a higher metastatic potential.14,33 In terms of grading, quantitative DCE-MRI parameters such as the blood volume transfer constant (Ktrans), the extracellular volume fraction (Ve), and the initial area under the curve (iAUC) were shown to be higher in poorly than in well-differentiated SCC, with Ktrans having the greatest diagnostic significance at a cut-off value of 0.270 min−1 and an overall diagnostic sensitivity and specificity of 95.0 and 90.9%.34 Chen et al found that DCE-MRI parameters [Ktrans, Ve and the rate constant of contrast transfer (Kep)] allowed to discriminate among tumour, metastatic lymph nodes and normal tissue, with Ktrans and Ve values of normal tissue being significantly lower from those of nodes (p < 0.001) and primary tumours (p < 0.001), whereas Kep values of primary tumours were significantly higher from those of nodes (p = 0.001) and normal tissue (p = 0.002), respectively.35 Moreover, Baker et al reported that DCE-MRI can predict tumour response to target therapies with EGFR-targeted tyrosine kinase inhibitors, in conjunction with 18F-FDG PET.36
Similarly, perfusion CT can quantify the vascularity of primary head-and-neck SCC by applying specific bio-mathematical models of tumour neoangiogenesis (Figure 1). Evidence exists that a) better treatment response can be usually be predicted at perfusion CT before the beginning of therapy in more perfused tumours, and b) early perfusion response to CRT can be assessed before the onset of morphological changes at conventional morphological imaging.37,38 On the other hand, DECT is emerging as an increasingly widespread imaging technique that can join the fast imaging time, high spatial resolution, and excellent morphological detail of multidetector CT with the possibility to collect functional data via material decomposition, allowing to calculate iodine content as a biomarker of tumour vascularity with a potentially lower overall radiation and contrast dose compared with standard single-energy CT imaging39 (Figure 2). DECT can be effective in improving the detection and local staging of primary tumours, with particular reference to N-staging, which is often limited due to small and/or morphologically indeterminate lymph nodes leading to inconclusive findings at conventional anatomical imaging.38,40–42 To this regard, Tawfik et al reported significant differences in DECT-derived iodine content and overlay among normal, inflammatory and metastatic SCC lymph nodes.43 Moreover, DECT showed lower iodine content and spectral attenuation slope in metastatic oropharyngeal SCC than in non-neoplastic cervical lymph nodes, in line with the finding of a different intranodal iodine distribution that reflects pathological structure.44,45
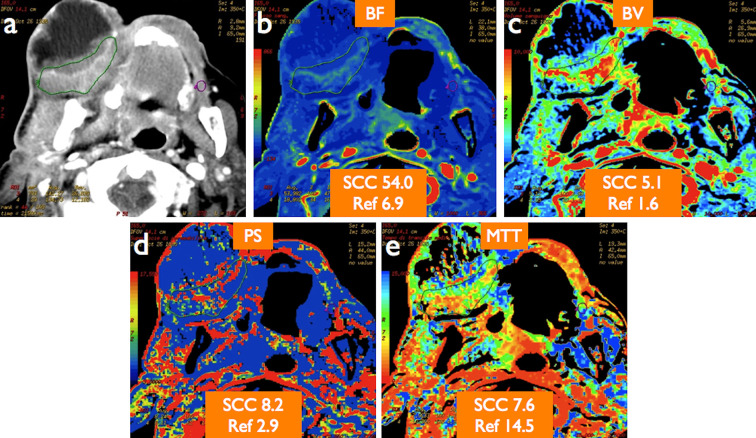
Figure 1.
An 83-year-old male with locally advanced, partially necrotic gingival SCC as displayed on standard morphological CT image (a). Quantitative CT perfusion analysis revealed increased blood flow (BF) (b), blood volume (BV) (c), permeability surface (PS) product (d), and reduced mean transit time (MTT) (e) values in the viable portion of the tumour compared with contralateral normal tissue (Ref) taken as reference. Note lack of perfusion inside the necrotic portion of the tumour as visually depicted on colour-coded perfusion maps.
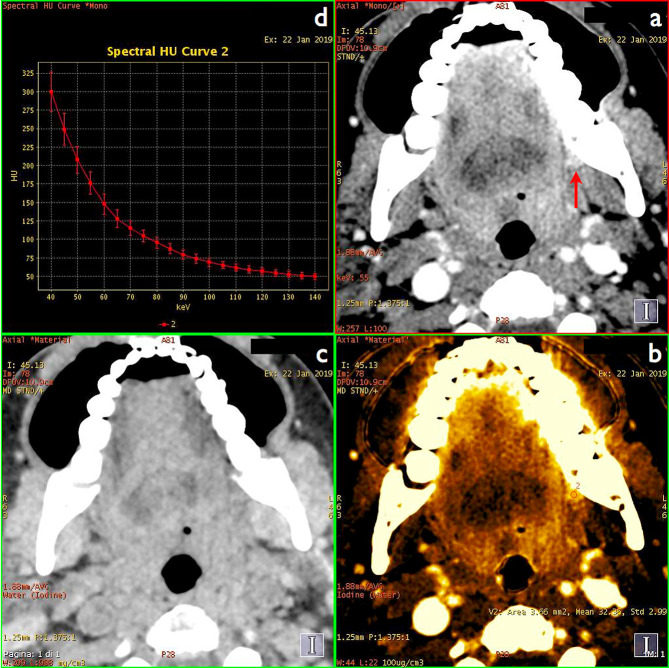
Figure 2.
A 51-year-old female with small keratinising SCC of the left retromolar trigone (arrow on DECT monochromatic 55keV image, (a). Material decomposition iodine/water image (b) improves lesion conspicuity by boosting iodine signal and reveals high lesion vascularisation (measured mean iodine concentration of 32 × 100 µg ml−1), whereas virtual precontrast image (c) shows no abnormal density or beam hardening artefacts at the lesion site. Avid lesion enhancement was confirmed by spectral curve analysis (d). All images had been obtained at the same anatomic level from a single contrast-enhanced DECT acquisition.
Overall, the integration of quantitative image biomarkers in the diagnostic workup of patients with oropharyngeal cancer and other tumours of the uppermost portion of the digestive tract looks promising in an effort to optimise lesion detection and staging, either at primary sites (especially in case of suspected disease recurrence, when diagnosis by conventional imaging is often hampered by gross post-treatment sequelae) and at lymph node levels (possibly overcoming the poor performance of standard morphological imaging in differentiating smaller benign from metastatic lymph nodes and guiding the choice towards more or less aggressive lymph node resection). Moreover, the correlation between imaging biomarkers and the biological features of tumours (related e.g. to lesion aggressiveness) may be of great value to improve patient selection for the best possible treatment in an individualised fashion.
oesophageal and gastric cancer
Oesophageal cancer is the eighth most common cancer and the sixth most common cause of cancer-related deaths worldwide, accounting for more than 509,000 deaths in 2018.46 While initial staging of oesophago-gastric cancer is usually performed with contrast-enhanced CT, multimodality imaging with endoscopic ultrasound and 18F-FDG PET is useful to define local disease extent and rule out metastatic spread in patients being considered for curative treatment, in an effort to reduce futile surgery.47 MRI is a less routinely used, yet valuable tool that can reveal the detailed anatomic structure of the oesophagus, differentiating among the various wall layers for proper local staging and providing an accurate assessment of lesion length before and after radiation therapy.48,49
Several imaging biomarkers have been identified that can help predict response of oesophageal cancer to CRT (Table 2). In this latter setting, Aoyagi et al reported that ADC values were significantly higher in patients with better survival rates,50 whereas in a recent meta-analysis the variation of ADC before and after treatment and post-treatment ADC were found to have good performance for evaluating CRT response.51 Furthermore, van Rossum et al showed that a treatment-induced relative increase in ADC during the first 2–3 weeks of neoadjuvant CRT for oesophageal cancer was highly predictive of histopathologic response,52 and also AUC changes at DCE-MRI were predictive of complete pathological response to neoadjuvant CRT at a threshold of −24.6% with sensitivity, specificity, positive and negative predictive values of 83%, 88%, 71% and 93%, respectively.53
Table 2.
Summary of the features and main findings of the studies on imaging biomarkers in oesophageal cancer cited in the text. Case reports and general reviews articles are not included. References are sorted in the order in which they appear in the text
| Reference | Tumour type and location | Imaging features | Endpoints | Main findings |
| Ge et al.46 | Oesophageal cancer | Pre- and post-CRT DECT-derived normalised iodine concentration (NIC) and normalised CT (NCT) values | Comparison of pre- and post-CRT NIC and NCT values obtained in the arterial (NIC-A, NCT-A) and portal venous phases (NIC-V, NCT-V) in good and poor responders |
|
| Guo et al.49 | Oesophageal cancer (SCC, adenocarcinoma, small cell carcinoma) | CT, ADC | Diagnostic accuracy before treatment, outcome prediction during and after radiotherapy in CR and PR patients |
|
| Aoyagi et al.50 | Oesophageal SCC | Pre-CRT ADC | Prediction of CRT response or prognosis |
|
| Cheng et al.51 | Oesophageal cancer | ADC [ADC variation before and after CRT (∆ADC), post-ADC] | Prediction of early response to CRT |
|
| van Rossum et al.52 | Oesophageal cancer (adenocarcinoma, SCC) | ADC [ADC variation during treatment (∆ADCduring)] | Prediction of pathologic response to neoadjuvant CRT |
|
| Heethuis et al.53 | Oesophageal cancer (SCC, adenocarcinoma, adenosquamous carcinoma) | DCE-MRI [change of AUC before neoadjuvant CRT (AUCpre), at 2–3 weeks during neoadjuvant CRT (AUCper), and after neoadjuvant CRT before surgery (AUCpost)] | Prediction of pathologic response to neoadjuvant CRT |
|
| Shen et al.54 | Lymph node metastases from oesophageal cancer | CT radiomics features | Prediction of lymph node metastasis status in the preoperative setting |
|
| Hou et al.55 | Oesophageal carcinoma | CT radiomics features | Prediction of CRT response |
|
| Hou et al.56 | Oesophageal SCC | MRI radiomics features (T2w, SPAIR T2w) | Prediction of CRT response |
|
| Tan et al.57 | Lymph node metastases from resectable oesophageal SCC | CT radiomics features | Diagnostic accuracy |
|
CR, complete response; PR, partial response; SD, stable disease.
In parallel, a comparative study of DECT and standardised iodine concentrations in patients with oesophageal cancer before and after CRT showed a significant reduction of tumour normalised iodine concentration on iodine maps in responders, allowing to functionally evaluate treatment response and prognosis in terms of reduced tumour iodine uptake.46 Radiomics approaches have also recently been proposed for preoperative prediction of lymph node metastasis54 and tumour response to CRT in patients with oesophageal cancer,55,56 and of note, it has emerged that radiomics features can outperform size criteria in discriminating lymph node metastasis in resectable oesophageal SCC.57
Gastric cancer is the fourth most common malignancy and the second most common cause of cancer-related death worldwide (738,000 deaths annually), whose prognosis and survival rate are poor in advanced stage disease and for which only surgical resection of the primary tumour can be curative.58,59 In light of this, quantitative imaging represents a promising area for gastric cancer research and management, and in particular, ADC and contrast-enhanced CT-based texture features are noninvasive, quantitative imaging biomarkers that hold promise in the evaluation of tumour aggressiveness, treatment response and prognosis (Table 3).47,60,61 CT texture analysis can predict histopathological characteristics of gastric cancers (such as differentiation degree, Lauren classification and vascular invasion status),62 the expression level of immunohistochemical biomarkers (including E-cadherin, Ki67, and VEGFR2),63 and even occult peritoneal carcinomatosis in patients with advanced disease64 (Figure 3). Radiomic feature extraction is another area of active research that has yielded promising results, especially in terms of prognostic assessment.47,65–67
Table 3. .
Summary of the features and main findings of the studies on imaging biomarkers in gastric cancer cited in the text. Case reports and general reviews articles are not included. References are sorted in the order in which they appear in the text
| Reference | Tumour type and location | Imaging features | Endpoints | Main findings |
| Giganti et al.58 | Resectable gastric cancer (adenocarcinoma, signet-ring cell carcinoma) | ADC | Association between ADC and clinicopathological factors (i.e. pathologic T and N stages, tumour location, surgical approach, histologic subtype) |
|
| Liu et al.61 | Gastric cancer (tubular or papillary adenocarcinoma, poorly cohesive adenocarcinoma, signet-ring cell carcinoma) | CT texture parameters in the arterial (AP) and portal venous phase (PVP) | Diagnostic accuracy and correlation between preoperative CT texture parameters and pathological stage |
|
| Liu et al.62 | Gastric cancer (tubular adenocarcinoma, papillary adenocarcinoma, poorly cohesive adenocarcinoma, signet-ring cell carcinoma, mucinous carcinoma, mixed types) | CT texture parameters | Prediction of histopathological characteristics |
|
| Liu et al.63 | Gastric cancer | CT texture parameters | Correlation between CT texture parameters and immunohistochemical markers (E-cadherin, Ki67, VEGFR2 and EGFR) |
|
| Kim et al.64 | Advanced gastric cancer | CT texture parameters | Prediction of occult peritoneal carcinomatosis (PC) detected at surgery |
|
| Li et al.65 | Gastric cancer following curative resection | CT radiomics features | Prognosis prediction |
|
| Li et al.66 | Locally advanced gastric cancer | CT radiomics signature | Outcome prediction of neoadjuvant chemotherapy |
|
| Jiang et al.67 | Gastric adenocarcinoma | CT radiomics signature derived from PVP images | Prediction of survival and response to chemotherapy |
|
| Caivano et al.68 | Gastric adenocarcinoma | Preoperative ADC | Diagnostic accuracy compared to conventional MRI with pathology as gold standard |
|
| Joo et al.69 | Gastric cancer | MRI with and without DWI, CT | Diagnostic accuracy for preoperative staging with pathology as gold standard |
|
| He et al.70 | Gastric cancer | ADC | Correlation between ADC and HER2 expression |
|
| Zongqiong et al.71 | Gastric cancer (tubular adenocarcinoma, signet-ring cell carcinoma, mucinous adenocarcinoma) | Perfusion CT | Tumour grading |
|
| Zhang et al.72 | Gastric adenocarcinoma | Perfusion CT | Correlation with histological grading, serosal and lymphatic involvement, distant metastasis, pTNM stage, and microvessel density (MVD) assessed by CD34 immunohistochemical staining |
|
| Liang et al.73 | Gastric adenocarcinoma | DECT [normalised iodine concentration (NIC) on AP and PVP images] | Correlation with histologic grading, serosal and lymphatic involvement, distant metastasis, pTNM stage and MVD |
|
| Meng et al.74 | Gastric mucosal cancer | DECT (IC and NIC on AP and PVP images) | Diagnostic accuracy for differentiating gastric cancer from normal gastric mucosa (NGM) and gastric inflammation (GI) |
|
| Pan et al.75 | Gastric cancer (adenocarcinoma, signet-ring cell carcinoma, mucinous adenocarcinoma) | DECT (keV images, conventional kVp images, NIC) | Diagnostic accuracy with pathology as gold standard |
|
| Cheng et al.76 | Early and advanced gastric adenocarcinoma | DECT [IC, NIC, curve slope (λHU) in the PVP and delayed phase (DP)] | Association with Ki-67 protein expression as a marker of cell proliferation |
|
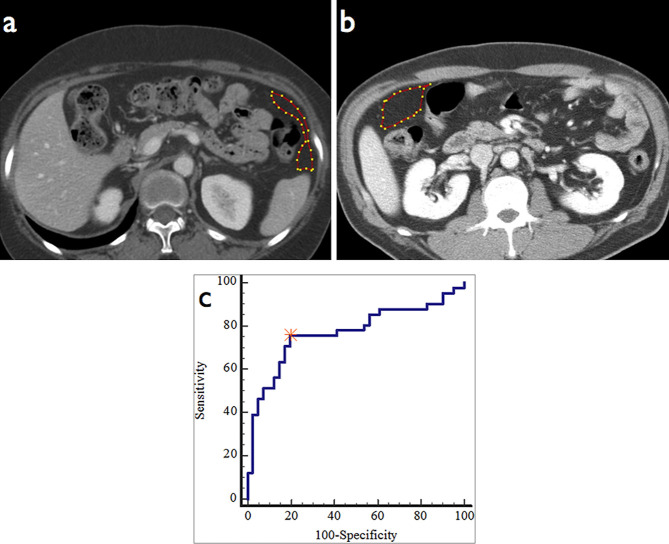
Figure 3.
A 49-year-old male with T3N2 advanced gastric cancer without peritoneal seeding showed entropy of 7.05 within the region of interest (a). A 59-year-old female with T3N2 advanced gastric cancer with occult seeding (b) showed entropy of 7.70, higher than the cut-off value (>7.141) obtained from receiver operating characteristic (ROC) curve analysis (c). Reproduced and adapted from.64
Among quantitative imaging biomarkers, DWI has shown higher accuracy than conventional MRI for T- and N-staging of gastric adenocarcinoma (80% vs 73% and 93% vs 80%, respectively),68 and the addition of 3T-MR DWI provides significantly higher sensitivity over conventional MRI or contrast-enhanced CT for the assessment of lymph node metastasis.69 In a multivariate analysis of 99 patients with gastric cancer (of whom 71 underwent surgery and 28 neoadjuvant chemotherapy, respectively), ADC values of 1.5 × 10−3 mm2/s or lower were associated with a negative prognosis along with pathologic T and N stages, suggesting an important role of DWI in outcome prediction.58 Overall, such findings seem to reflect a relationship between quantitative imaging biomarkers and different molecular tumour fingerprints that may be exploited to tailor treatment, and to this latter regard it is worth mentioning, for instance, that ADC values were found to correlate with HER2 status.70
Perfusion CT and especially DECT can also give a relevant contribution to local staging and prognostic assessment of gastric cancer. Zongqiong et al showed a correlation between perfusion CT parameters (BF, BV and PS values) and the degree of differentiation of gastric adenocarcinoma,71 whereas Zhang et al reported a significant difference in the PS values between patients with or without lymphatic involvement (p = 0.038), different histological grades (p = 0.04) and TNM staging (p = 0.026).72 On the other hand, in Liang et al’s study DECT-derived normalised iodine content on arterial and portal venous phase acquisitions was higher in poorly differentiated gastric adenocarcinomas than in moderately differentiated tumours, along with a significant difference in portal venous phase normalised iodine content between tumours with high and low microvessel density, as determined by CD34 staining.73 Quantitative analysis of DECT imaging parameters for the gastric mucosa can be useful to differentiate malignant from benign gastric mucosal lesions, with iodine concentration and normalised iodine concentration in gastric cancer being significantly different from those in normal and inflammatory mucosa, showing a sensitivity and specificity of about 90% in differentiating gastric cancer from normal mucosa on portal venous phase images.74 More generally, DECT with monochromatic imaging and quantitative iodine concentration measurements has higher diagnostic accuracy than conventional single-energy CT for T and N staging of gastric cancer (81.2% and 80% vs 73.9% and 75%, respectively), with iodine concentration indexes differing significantly between differentiated and undifferentiated gastric carcinoma, and between metastatic and non-metastatic lymph nodes.75 The diagnostic potential of DECT may be pushed even further to a molecular level, with quantitative parameters (such as iodine concentration, normalised iodine concentration, and curve slope) showing a significant positive correlation with Ki-67 antigen expression as a marker of tumour proliferation and allowing to differentiate between early and advanced gastric cancer.76
Based on the above considerations, the evidence collected so far shows that imaging biomarkers can provide a significant added value to conventional imaging for improving T and N staging of oesophageal and gastric cancers and predicting early response to neoadjuvant CRT and overall prognosis. This might play a pivotal role into identifying the best treatment strategy for patients on a case-by-case basis [e.g. surgery with or without CRT, or neoadjuvant CRT? which degree of surgical radicality? which expected lesion aggressiveness (including conditions with a dramatic impact on treatment choice and prognosis, such as peritoneal dissemination)? any and which potential targets for specific therapies?], possibly leading to better patient outcomes, higher quality of life, and more effective use of healthcare resources.
Conclusions
Imaging biomarkers represent an exciting area of active research that can currently be considered a new frontier of oncologic imaging, owing to their potential to provide a substantial amount of additional information to guide all management steps of cancer patients (from lesion detection and staging to treatment planning, follow-up and prognostic assessment). In particular, the diagnostic workup of upper gastrointestinal cancers should significantly benefit from a combined and thorough analysis of available imaging biomarkers, due to the heterogeneous biological features of such diseases that usually require a complex diagnostic and therapeutic approach. It is likely that in the near future, a growing number of imaging biomarkers and radiomics features will reach the status of validated parameters to be routinely assessed along with established diagnostic imaging criteria, possibly favouring treatment individualisation and improving overall patient outcome.
Contributor Information
Michela Gabelloni, Email: mgabelloni@sirm.org.
Lorenzo Faggioni, Email: lfaggioni@sirm.org.
Emanuele Neri, Email: emanuele.neri@med.unipi.it.
REFERENCES
- 1. Califf RM. Biomarker definitions and their applications. Exp Biol Med 2018; 243: 213–21. doi: 10.1177/1535370217750088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FDA-NIH Biomarker Working Group Best (biomarkers, endpoints. and other Tools) Resource 2018;Available from. [PubMed] [Google Scholar]
- 3. Deigner H. P, Kohl M. Precision medicine: tools and quantitative approaches. London, UK: The British Institute of Radiology.; 2018. . [Google Scholar]
- 4. European Society of Radiology (ESR). white paper on imaging biomarkers. Insights Imaging 2010; 1: 42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neri E, Regge D. Imaging biobanks in oncology: European perspective. Future Oncol 2017; 13: 433–41. doi: 10.2217/fon-2016-0239 [DOI] [PubMed] [Google Scholar]
- 7. Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2018; 2: 36. doi: 10.1186/s41747-018-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neri E, Del Re M, Paiar F, Erba P, Cocuzza P, Regge D, et al. Radiomics and liquid biopsy in oncology: the holons of systems medicine. Insights Imaging 2018; 9: 915–24. doi: 10.1007/s13244-018-0657-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017; 14: 169–86. doi: 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansen RW, van Amstel P, Martens RM, Kooi IE, Wesseling P, de Langen AJ, et al. Non-invasive tumor genotyping using radiogenomic biomarkers, a systematic review and oncology-wide pathway analysis. Oncotarget 2018; 9: 20134–55. doi: 10.18632/oncotarget.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sala E, Mema E, Himoto Y, Veeraraghavan H, Brenton JD, Snyder A, et al. Unravelling tumour heterogeneity using next-generation imaging: radiomics, radiogenomics, and habitat imaging. Clinical Radiology 2017; 72: 3–10. doi: 10.1016/j.crad.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global Burden of Disease Cancer Collaboration Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of Disease Study. JAMA Oncol 2017; 3: 524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nooij RP, Hof JJ, van Laar PJ, van der Hoorn A. Functional MRI for treatment evaluation in patients with head and neck squamous cell carcinoma: a review of the literature from a radiologist perspective. Curr Radiol Rep 2018; 6: 2. doi: 10.1007/s40134-018-0262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talebi Ghane E, Baghestani AR, Zayeri F, Talimkhani I, Masoudi S. Survival and recurrence rate in patients with head and neck cancer and associated prognostic factors. Int J Cancer Manag 2018; 11: e12794. [Google Scholar]
- 16. Windon MJ, D'Souza G, Fakhry C. Treatment preferences in human papillomavirus-associated oropharyngeal cancer. Future Oncol 2018; 14: 2521–30. doi: 10.2217/fon-2018-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oksuz DC, Prestwich RJ, Carey B, Wilson S, Senocak MS, Choudhury A, et al. Recurrence patterns of locally advanced head and neck squamous cell carcinoma after 3D conformal (chemo)-radiotherapy. Radiat Oncol 2011; 6: 54. doi: 10.1186/1748-717X-6-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito N, Nadgir RN, Nakahira M, Takahashi M, Uchino A, Kimura F, et al. Posttreatment CT and MR imaging in head and neck cancer: what the radiologist needs to know. Radiographics 2012; 32: 1261–82. doi: 10.1148/rg.325115160 [DOI] [PubMed] [Google Scholar]
- 19. Lewis-Jones H, Colley S, Gibson D. Imaging in head and neck cancer: United Kingdom National multidisciplinary guidelines. J Laryngol Otol 2016; 130(S2): S28–S31. doi: 10.1017/S0022215116000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Hoorn A, van Laar PJ, Holtman GA, Westerlaan HE. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with head and neck tumors, a systematic review and meta-analysis. PLoS One 2017; 12: e0177986: e0177986. doi: 10.1371/journal.pone.0177986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elhalawani H, Lin TA, Volpe S, Mohamed ASR, White AL, Zafereo J, et al. Machine learning applications in head and neck radiation oncology: lessons from open-source radiomics challenges. Front Oncol 2018; 8: 294. doi: 10.3389/fonc.2018.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leijenaar RTH, Bogowicz M, Jochems A, Hoebers FJP, Wesseling FWR, Huang SH, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol 2018; 5: 20170498. doi: 10.1259/bjr.20170498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 1823–30. doi: 10.1016/j.ijrobp.2010.10.053 [DOI] [PubMed] [Google Scholar]
- 24. Kuno H, Qureshi MM, Chapman MN, Li B, Andreu-Arasa VC, Onoue K, et al. CT texture analysis potentially predicts local failure in head and neck squamous cell carcinoma treated with chemoradiotherapy. AJNR Am J Neuroradiol 2017; 38: 2334–40. doi: 10.3174/ajnr.A5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feliciani G, Fioroni F, Grassi E, Bertolini M, Rosca A, Timon G, et al. Radiomic Profiling of Head and Neck Cancer: 18 F-FDG PET Texture Analysis as Predictor of Patient Survival. Contrast Media Mol Imaging 2018; 2018: 1–8. doi: 10.1155/2018/3574310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol 2009; 72: 381–7. doi: 10.1016/j.ejrad.2008.09.034 [DOI] [PubMed] [Google Scholar]
- 27. Jin GQ, Yang J, Liu LD, Su DK, Wang DP, Zhao SF, et al. The diagnostic value of 1.5-T diffusion-weighted MR imaging in detecting 5 to 10 mm metastatic cervical lymph nodes of nasopharyngeal carcinoma. Medicine 2016; 95: e4286: e4286. doi: 10.1097/MD.0000000000004286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwang I, Choi SH, Kim Y-J, Kim KG, Lee AL, Yun TJ, et al. Differentiation of recurrent tumor and posttreatment changes in head and neck squamous cell carcinoma: application of high b-value diffusion-weighted imaging. AJNR Am J Neuroradiol 2013; 34: 2343–8. doi: 10.3174/ajnr.A3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryoo I, Kim J-H, Choi SH, Sohn C-H, Kim SC. Squamous Cell Carcinoma of the Head and Neck: Comparison of Diffusion-weighted MRI at b-values of 1,000 and 2,000 s/mm(2) to Predict Response to Induction Chemotherapy. Magn Reson Med Sci 2015; 14: 337–45. doi: 10.2463/mrms.2015-0003 [DOI] [PubMed] [Google Scholar]
- 30. Preda L, Conte G, Bonello L, Giannitto C, Travaini LL, Raimondi S, et al. Combining standardized uptake value of FDG-PET and apparent diffusion coefficient of DW-MRI improves risk stratification in head and neck squamous cell carcinoma. Eur Radiol 2016; 26: 4432–41. doi: 10.1007/s00330-016-4284-8 [DOI] [PubMed] [Google Scholar]
- 31. Surov A, Stumpp P, Meyer HJ, Gawlitza M, Höhn A-K, Boehm A, et al. Simultaneous (18)F-FDG-PET/MRI: Associations between diffusion, glucose metabolism and histopathological parameters in patients with head and neck squamous cell carcinoma. Oral Oncol 2016; 58: 14–20. doi: 10.1016/j.oraloncology.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 32. El Beltagi AH, Elsotouhy AH, Own AM, Abdelfattah W, Nair K, Vattoth S. Functional magnetic resonance imaging of head and neck cancer: performance and potential. Neuroradiol J 2018; 6: 1971400918808546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jansen JFA, Parra C, Lu Y, Shukla-Dave A. Evaluation of head and neck tumors with functional MR imaging. Magn Reson Imaging Clin N Am 2016; 24: 123–33. doi: 10.1016/j.mric.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong Ji X, Yan S, Xia S, Guo Y, Shen W. Quantitative parameters correlated well with differentiation of squamous cell carcinoma at head and neck: a study of dynamic contrast-enhanced MRI. Acta Radiol 2018; 20: 284185118809543. [DOI] [PubMed] [Google Scholar]
- 35. Chen L, Ye Y, Chen H, Chen S, Jiang J, Dan G, et al. Dynamic contrast-enhanced magnetic resonance imaging for differentiating between primary tumor, metastatic node and normal tissue in head and neck cancer. Curr Med Imaging Rev 2018; 14: 416–21. doi: 10.2174/1573405614666171205105236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker LCJ, Sikka A, Price JM, Boult JKR, Lepicard EY, Box G, et al. Evaluating imaging biomarkers of acquired resistance to targeted EGFR therapy in xenograft models of human head and neck squamous cell carcinoma. Front Oncol 2018; 8: 271. doi: 10.3389/fonc.2018.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faggioni L, Neri E, Bartolozzi C. CT perfusion of head and neck tumors: how we do it. AJR Am J Roentgenol 2010; 194: 62–9. doi: 10.2214/AJR.09.3187 [DOI] [PubMed] [Google Scholar]
- 38. Zhong J, Lu Z, Xu L, Dong L, Qiao H, Hua R, et al. The diagnostic value of cervical lymph node metastasis in head and neck squamous carcinoma by using diffusion-weighted magnetic resonance imaging and computed tomography perfusion. Biomed Res Int 2014; 2014: 1–7. doi: 10.1155/2014/260859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parakh A, Macri F, Sahani D. Dual-energy computed tomography: dose reduction, series reduction, and contrast load reduction in dual-energy computed tomography. Radiol Clin North Am 2018; 56: 601–24. doi: 10.1016/j.rcl.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 40. Forghani R, De Man B, Gupta R. Dual-energy computed tomography: physical principles, approaches to scanning, usage, and implementation: Part 1. Neuroimaging Clin N Am 2017; 27: 371–84. doi: 10.1016/j.nic.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 41. Pérez-Lara A, Forghani R. Spectral computed tomography: technique and applications for head and neck cancer. Magn Reson Imaging Clin N Am 2018; 26: 1–17. doi: 10.1016/j.mric.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 42. Lam S, Gupta R, Levental M, Yu E, Curtin HD, Forghani R. Optimal virtual monochromatic images for evaluation of normal tissues and head and neck cancer using dual-energy CT. AJNR Am J Neuroradiol 2015; 36: 1518–24. doi: 10.3174/ajnr.A4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol 2014; 24: 574–80. doi: 10.1007/s00330-013-3035-3 [DOI] [PubMed] [Google Scholar]
- 44. Foust AM, Ali RM, Nguyen XV, Agrawal A, Prevedello LM, Bourekas EC, et al. Dual-energy CT-derived iodine content and spectral attenuation analysis of metastatic versus nonmetastatic lymph nodes in squamous cell carcinoma of the oropharynx. Tomography 2018; 4: 66–71. doi: 10.18383/j.tom.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rizzo S, Radice D, Femia M, De Marco P, Origgi D, Preda L, et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol 2018; 28: 760–9. doi: 10.1007/s00330-017-5015-5 [DOI] [PubMed] [Google Scholar]
- 46. Ge X, Yu J, Wang Z, Xu Y, Pan C, Jiang L, et al. Comparative study of dual energy CT iodine imaging and standardized concentrations before and after chemoradiotherapy for esophageal cancer. BMC Cancer 2018; 18: 1120. doi: 10.1186/s12885-018-5058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sah B-R, Owczarczyk K, Siddique M, Cook GJR, Goh V. Radiomics in esophageal and gastric cancer. Abdom Radiol 2018; 24. doi: 10.1007/s00261-018-1724-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tomizawa M, Shinozaki F, Ozaki A, Baba A, Fukamizu Y, Matsunaga F, et al. Diffusion-weighted imaging and diffusion-weighted whole-body imaging with background body signal suppression for characterizing esophageal cancer: a case report. Int Med Case Rep J 2013; 6: 95–8. doi: 10.2147/IMCRJ.S41823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo L, Zhang L, Zhao J. CT scan and magnetic resonance diffusion-weighted imaging in the diagnosis and treatment of esophageal cancer. Oncol Lett 2018; 16: 7117–22. doi: 10.3892/ol.2018.9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aoyagi T, Shuto K, Okazumi S, Shimada H, Kazama T, Matsubara H. Apparent diffusion coefficient values measured by diffusion-weighted imaging predict chemoradiotherapeutic effect for advanced esophageal cancer. Dig Surg 2011; 28: 252–7. doi: 10.1159/000328770 [DOI] [PubMed] [Google Scholar]
- 51. Cheng B, Yu J. Predictive value of diffusion-weighted MR imaging in early response to chemoradiotherapy of esophageal cancer: a meta-analysis. Dis Esophagus 2018;. [DOI] [PubMed] [Google Scholar]
- 52. van Rossum PSN, van Lier ALHMW, van Vulpen M, Reerink O, Lagendijk JJW, Lin SH, et al. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015; 115: 163–70. doi: 10.1016/j.radonc.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 53. Heethuis SE, van Rossum PSN, Lips IM, Goense L, Voncken FE, Reerink O, et al. Dynamic contrast-enhanced MRI for treatment response assessment in patients with oesophageal cancer receiving neoadjuvant chemoradiotherapy. Radiother Oncol 2016; 120: 128–35. doi: 10.1016/j.radonc.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 54. Shen C, Liu Z, Wang Z, Guo J, Zhang H, Wang Y, et al. Building CT radiomics based nomogram for preoperative esophageal cancer patients lymph node metastasis prediction. Transl Oncol 2018; 11: 815–24. doi: 10.1016/j.tranon.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hou Z, Ren W, Li S, Liu J, Sun Y, Yan J, Wan S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget 2017; 8: 104444–54. doi: 10.18632/oncotarget.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hou Z, Li S, Ren W, Liu J, Yan J, Wan S. Radiomic analysis in T2W and SPAIR T2W MRI: predict treatment response to chemoradiotherapy in esophageal squamous cell carcinoma. J Thorac Dis 2018; 10: 2256–67. doi: 10.21037/jtd.2018.03.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tan X, Ma Z, Yan L, Ye W, Liu Z, Liang C. Radiomics nomogram outperforms size criteria in discriminating lymph node metastasis in resectable esophageal squamous cell carcinoma. Eur Radiol 2019; 29: 392–400. doi: 10.1007/s00330-018-5581-1 [DOI] [PubMed] [Google Scholar]
- 58. Giganti F, Orsenigo E, Esposito A, Chiari D, Salerno A, Ambrosi A, et al. Prognostic role of diffusion-weighted MR imaging for resectable gastric cancer. Radiology 2015; 276: 444–52. doi: 10.1148/radiol.15141900 [DOI] [PubMed] [Google Scholar]
- 59. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018; 10: 239–48. doi: 10.2147/CMAR.S149619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giganti F, Tang L, Baba H. Gastric cancer and imaging biomarkers: Part 1 – a critical review of DW-MRI and CE-MDCT findings. Eur Radiol 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu S, Shi H, Ji C, Zheng H, Pan X, Guan W, et al. Preoperative CT texture analysis of gastric cancer: correlations with postoperative TNM staging. Clinical Radiology 2018; 73: 756.e1–756.e9 e9. doi: 10.1016/j.crad.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 62. Liu S, Liu S, Ji C, Zheng H, Pan X, Zhang Y, et al. Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol 2017; 27: 4951–9. doi: 10.1007/s00330-017-4881-1 [DOI] [PubMed] [Google Scholar]
- 63. Liu S, Shi H, Ji C, Guan W, Chen L, Sun Y, et al. CT textural analysis of gastric cancer: correlations with immunohistochemical biomarkers. Sci Rep 2018; 8: 11844. doi: 10.1038/s41598-018-30352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim HY, Kim YH, Yun G, Chang W, Lee YJ, Kim B. Could texture features from preoperative CT image be used for predicting occult peritoneal carcinomatosis in patients with advanced gastric cancer? PLoS One 2018; 13: e0194755: e0194755. doi: 10.1371/journal.pone.0194755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li W, Zhang L, Tian C, Song H, Fang M, Hu C, et al. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur Radiol 2018; 1. doi: 10.1007/s00330-018-5861-9 [DOI] [PubMed] [Google Scholar]
- 66. Li Z, Zhang D, Dai Y, Dong J, Wu L, Li Y, et al. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: a pilot study. Chin J Cancer Res 2018; 30: 406–14. doi: 10.21147/j.issn.1000-9604.2018.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang Y, Chen C, Xie J, Wang W, Zha X, Lv W, et al. Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine 2018; 36: 171–82. doi: 10.1016/j.ebiom.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Caivano R, Rabasco P, Lotumolo A, D' Antuono F, Zandolino A, Villonio A, et al. Gastric cancer: the role of diffusion weighted imaging in the preoperative staging. Cancer Invest 2014; 32: 184–90. doi: 10.3109/07357907.2014.896014 [DOI] [PubMed] [Google Scholar]
- 69. Joo I, Lee JM, Kim JH, Shin C-I, Han JK, Choi BI. Prospective comparison of 3T MRI with diffusion-weighted imaging and MDCT for the preoperative TNM staging of gastric cancer. J Magn Reson Imaging 2015; 41: 814–21. doi: 10.1002/jmri.24586 [DOI] [PubMed] [Google Scholar]
- 70. He J, Shi H, Zhou Z, Chen J, Guan W, Wang H, et al. Correlation between apparent diffusion coefficients and HER2 status in gastric cancers: pilot study. BMC Cancer 2015; 15: 749. doi: 10.1186/s12885-015-1726-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zongqiong S, Xiaohong L, Wei C, Jiangfeng Z, Yuxi G, Zhihui X, et al. CT perfusion imaging of the stomach: a quantitative analysis according to different degrees of adenocarcinoma cell differentiation. Clin Imaging 2016; 40: 558–62. doi: 10.1016/j.clinimag.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 72. Zhang H, Pan Z, Du L, Yan C, Ding B, Song Q, et al. Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Korean J Radiol 2008; 9: 119–27. doi: 10.3348/kjr.2008.9.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liang P, Ren X-C, Gao J-B, Chen K-S, Xu X, Ren XC. Iodine concentration in spectral CT: assessment of prognostic determinants in patients with gastric adenocarcinoma. AJR Am J Roentgenol 2017; 209: 1033–8. doi: 10.2214/AJR.16.16895 [DOI] [PubMed] [Google Scholar]
- 74. Meng X, Ni C, Shen Y, Hu X, Chen X, Li Z, et al. Differentiating malignant from benign gastric mucosal lesions with quantitative analysis in dual energy spectral computed tomography: initial experience. Medicine 2017; 96: e5878: e5878. doi: 10.1097/MD.0000000000005878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pan Z, Pang L, Ding B, Yan C, Zhang H, Du L, et al. Gastric cancer staging with dual energy spectral CT imaging. PLoS One 2013; 8: e53651: e53651. doi: 10.1371/journal.pone.0053651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cheng S-M, Ling W, Zhu J, Xu J-R, Wu L-M, Gong H-X. Dual energy spectral CT imaging in the assessment of gastric cancer and cell proliferation: a preliminary study. Sci Rep 2018; 8: 17619. doi: 10.1038/s41598-018-35712-w [DOI] [PMC free article] [PubMed] [Google Scholar]