Abstract
Background
Both triglyceride glucose-body mass index (TyG-BMI) and non-alcoholic fatty liver disease (NAFLD) are linked to insulin resistance (IR). Prospective studies linking TyG-BMI to NAFLD have been limited by short follow-up. This study investigated the longitudinal association between TyG-BMI and NAFLD occurrence in the non-obese Chinese individuals.
Methods
This study determined TyG-BMI at baseline and the incidence of NAFLD at follow-up and performed a post hoc analysis of a prospective cohort study that involved assessing the risk of NAFLD in non-obese Chinese residents from January 2010 to December 2014. The incidence of NAFLD during the 5-year follow-up was identified as the endpoint. Cox proportional hazards regression analysis was used to evaluate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the incidence of NAFLD. Receiver operating characteristic (ROC) curve analysis was conducted to estimate the predictive power of TyG-BMI and its components for NAFLD. Subgroup analysis was performed to better understand other factors that may affect the association between TyG-BMI and NAFLD to identify potential special populations.
Results
During the follow-up period, 841 (8.61%) of 9767 non-obese subjects who met the screening criteria were diagnosed with NAFLD. After confounding factors were fully adjusted for, the HR of NAFLD was 3.09 (95% CI 2.63–3.63) per standard deviation (SD) increase in TyG-BMI. Furthermore, TyG-BMI had a strong predictive value (area under ROC = 0.85; 95% CI 0.84–0.86) for the incidence of NAFLD, with a specificity of 0.73 and sensitivity of 0.82. Additionally, in the male population, each SD increase in TyG-BMI was linked to an increased risk of NAFLD (HR = 2.85, 95% CI 2.30–3.53), but the risk was higher in the female population (HR = 3.58, 95% CI 2.80–4.60). Gender and TyG-BMI interacted significantly with NAFLD incidence (P < 0.0001).
Conclusion
In the normolipidaemic and non-obese subset of the Chinese population, an increase in TyG-BMI is related to an increased incidence of NAFLD. TyG-BMI may have clinical significance in identifying groups at high risk of NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-020-01409-1.
Keywords: Insulin resistance, Triglyceride, Fasting plasma glucose, Non-alcoholic fatty liver disease, Body mass index, Triglyceride glucose body mass index, Secondary analysis, Association
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a common form of chronic liver disease [1], and is closely related to type 2 diabetes and metabolic syndrome, with a prevalence between 18 and 45% [2, 3]. Regardless of whether there is underlying cirrhosis, NAFLD is considered to be the cause of hepatocellular carcinoma [4]. Therefore, early identification patients with a high risk of NAFLD is of great significance. NAFLD is more common among obese individuals than among nonobese individuals. Nevertheless, NAFLD has a prevalence rate of 3 to 30% in the non-obese population [5]. Several studies have shown that there is no significant difference in inflammation or fibrosis between non-obese NAFLD and obese NAFLD [6]. Dyslipidaemia is a well-known risk factor for NAFLD [7]. However, few studies have focused on the incidence of NAFLD in individuals with normal blood lipids [8]. A study by Sun et al. showed that increased normal low-density lipoprotein cholesterol (LDL-C) levels are associated with an elevated incidence of NAFLD [9]. Therefore, the risk of NAFLD merits attention even in people with normal blood lipids.
Although the underlying mechanism of NAFLD is unclear, insulin resistance (IR) is related to NAFLD development [10]. IR is considered to play an essential role in NAFLD pathogenesis in non-obese patients, regardless of the presence or absence of metabolic syndrome [11, 12]. The triglyceride and glucose (TyG) index, which combines fasting triglyceride (TG) and fasting plasma glucose (FPG), has been proposed as an effective substitute for IR [13]. Recently, triglyceride glucose body mass index (TyG-BMI), which combines TG, FPG, and obesity status, has been deemed more reliable than TyG for the identification of IR. The roles of all the parameters determining IR have been fully verified [14]. A cross-sectional study showed that TyG-BMI is linked to NAFLD [15]. However, it is not known whether the progression of time affects the association between TyG-BMI and NAFLD, and a subgroup analysis of gender has not been conducted, but previous studies have shown that gender affects the relationship [14]. Therefore, in this study, we attempted to collect 5-year longitudinal follow-up data from non-obese individuals with normal lipid profiles to investigate the association between TyG-BMI and NAFLD and explore whether gender affects the association between TyG-BMI and NAFLD.
Methods
Data source
The original data analysed were obtained from http://Datadryad.org, a public database that allows other investigators to reanalyse the data published by previous researchers. In keeping with the terms of service, this research cites data packets shared by Sun et al. [9, 16].
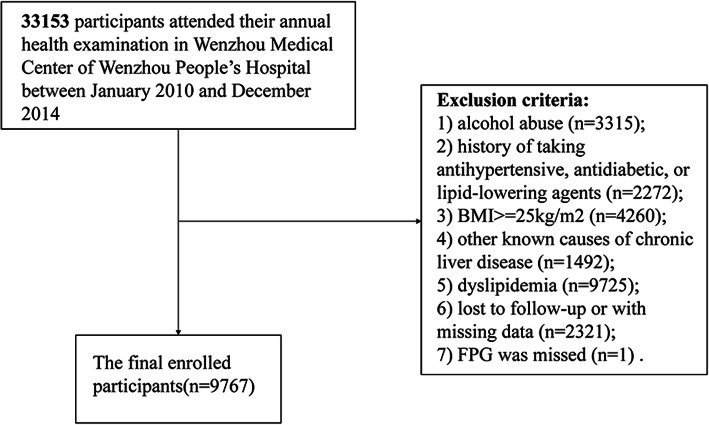
A post hoc analysis was conducted based on previous research that was a cohort study of 33,153 individuals enrolled at Wenzhou People’s Hospital from January 2010 to December 2014. All patients underwent a 5-year follow-up. The follow-up endpoint was the incidence of NAFLD. Subjects were assessed annually during the follow-up period. Subjects with the following conditions were excluded: (1) excessive drinking (> 70 g/week for females or 140 g/week for males) (n = 3315); (2) consumption of lipid-lowering, antidiabetic, or antihypertensive agents (n = 2272); (3) BMI ≥25 (n = 4260) [17]; (4) chronic liver disease caused by other factors (n = 1492); (5) dyslipidaemia [high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L, LDL-C > 3.12 mmol/L, total cholesterol (TC) > 5.2 mmol/L, triglyceride (TG) > 1.7 mmol/L] (n = 9725); (6) loss to follow-up or missing information (n = 2321); and (7) missing FPG (n = 1) (shown in Fig. 1).
Fig. 1.
Flow chart
Because the data were de-identified, the requirement for informed consent was waived. The ethics committee of Wenzhou People’s Hospital had approved the previous study on which this one was based. Thus, this research did not require separate ethical approval. This study followed the Declaration of Helsinki.
Data collection
As described in previous research, general clinical baseline data were collected by trained nurses through standardized patient-completed forms, and blood indicators were measured based on standard methods using an automated analyser (Abbott AxSYM) [18]. The following data were gathered: age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), weight, height, total bilirubin (TB), direct bilirubin (DBIL), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), globulin (GLB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (ALB), total protein (TP), LDL-C, HDL-C, TG, TC, FPG, creatinine (Cr), blood urea nitrogen (BUN) and uric acid (UA). As introduced in the previous literature, TyG = Ln [TG (mg/dL) × FPG (mg/dL)/2] [19], and TyG-BMI = TyG × BMI [13].
Diagnosis of NAFLD by ultrasound
NAFLD was diagnosed by ultrasound as described by the Chinese Liver Disease Association [18]. In short, NAFLD was defined as a diffusion-enhanced near-field echo in the liver region and gradual decay of the far-field echo in combination with one of the following conditions: the structure of the hepatic lacunae was not clearly displayed; mild to moderate hepatomegaly with peripheral and marginal passivation was present; the blood flow signal was reduced, but the blood flow distribution was normal; or the right liver lobe and diaphragm muscle capsule were unclear or incomplete [20].
Missing data
For the 9767 participants in the analysed dataset, the Additional File Table S1 shows the missing data for each variable. For the missing covariates, multiple multivariate imputations were applied [21]. In order to minimize the bias that may be induced by excluding missing data from data analysis and maximizing statistical power [22], the Multiple Imputation by Chained Equations (MICE) software package was applied to create five estimated data sets with chained equations [21]. Additionally, as presented in Additional File Table S2, a sensitivity analysis was conducted to explore whether the resulting complete data were significantly different from the original data. The results indicated that the complete generated data were not significantly different from the original data. Thus, the multivariate analysis results were based on the original data set, as were all other analyses in this paper.
Statistical analysis
All statistical analyses were conducted with EmpowerStats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the statistical software package R (http://www.R-project.org, The R Foundation). Continuous variables that followed a normal distribution were expressed as the mean ± standard deviation (SD), and those that were not normally distributed were expressed as the median (quartile 1-quartile 3). Categorical variables were expressed as the frequency (percentage). The Mann-Whitney U test and chi-square test were employed as appropriate to evaluate the difference between NAFLD and non-NAFLD individuals. Results were considered statistically significant at a two-tailed P value of < 0.05.
The independent risk factors for NAFLD were determined by establishing univariate and multivariate Cox proportional hazard models. First, univariate analysis was performed to assess all variables, then, all variables that were statistically significant (P < 0.05) or regarded as clinically significant were included in the multivariate analysis. A correlation matrix was used to assess the collinearity of all explanatory variables. Collinearity between variables was tested using the variance inflation factor (VIF) based on a multiple regression model [23]. As shown in Additional File Table S3, the variables with VIF > 5 were considered to exhibit collinearity. Three different models were built: Model 1, with no adjustment for covariates; Model 2, adjusted for sex and age; and Model 3, adjusted for sex, age, ALP, LDL-C, HDL-C, UA, Cr, ALB, AST, ALT, GGT, GLB, FPG, TG, SBP, DBP, and DBIL.
A Cox proportional hazard model was generated for subgroup analysis. In the case of continuous variables, first, according to the clinical cut-off point or dichotomy, these variables were converted to categorical variables and then the interaction tests were performed. The subgroup effect modification test used the interaction terms between the subgroup indicators; then, likelihood ratio tests were carried out.
In order to verify the data analysis results and explore the possibility of nonlinearity, TyG-BMI was converted to categorical variables according to quartiles, and the P value for the trend was calculated. Receiver operating characteristic (ROC) curves were constructed to estimate the ability of TyG-BMI, TG, ALT, TyG, FPG, and BMI to predict NAFLD. In addition, the ratio of TG to HDL-C (TG/HDL-C) is reported to be associated with incident NAFLD [24]; accordingly, the ROC curve of TG/HDL-C was also drawn. The Kaplan-Meier method was applied to draw cumulative hazard curves and compare the cumulative incidence of NAFLD among the hierarchical TyG-BMI quartiles using the log-rank test.
Results
Description of the study groups
Of the 33,153 subjects recruited in the previous study, 9767 met the inclusion criteria for the present post hoc analysis (seen in Fig. 1). The subjects’ average age was 42.5 ± 14.7 (14–90) years, and 48.58% were women. Table 1 lists the baseline characteristics of the subjects. Individuals in the highest TyG-BMI group (Q4) were usually older and had higher BMI, LDL-C, TG, UA, FPG, Cr, TB, ALP, GGT, ALT, AST, GLB, SBP, DBP, TyG, TC, and BUN values than individuals in the lowest TyG-BMI group (Q1). In contrast, the ALB, HDL-C, and DBIL values of the Q3 and Q4 groups were lower than those of Q1. In addition, as TyG-BMI values increased, the incidence of NAFLD gradually increased (Q1: 0.29% vs. Q2: 1.43% vs. Q3: 7.58% vs. Q4: 25.14%).
Table 1.
Baseline characteristics of subjects
| TyG-BMI | P value | ||||
|---|---|---|---|---|---|
| Q1 (114.23–157.67) | Q2 (157.68–172.11) | Q3 (172.11–188.07) | Q4 (188.08–230.41) | ||
| N | 2442 | 2441 | 2442 | 2442 | |
| Age, years | 41.28 ± 14.48 | 41.71 ± 14.19 | 43.20 ± 15.01 | 43.66 ± 15.03 | < 0.001 |
| Sex | < 0.001 | ||||
| Women | 1243 (50.90%) | 1264 (51.78%) | 1185 (48.53%) | 1053 (43.12%) | |
| Men | 1199 (49.10%) | 1177 (48.22%) | 1257 (51.47%) | 1389 (56.88%) | |
| BMI, kg/m2 | 18.56 ± 1.10 | 20.31 ± 0.87 | 21.75 ± 0.88 | 23.49 ± 0.89 | < 0.001 |
| ALP, U/L | 64.16 ± 19.48 | 67.33 ± 23.42 | 70.88 ± 22.89 | 75.39 ± 22.12 | < 0.001 |
| GGT, U/L | 17.00 (14.00–21.00) | 17.00 (14.00–22.00) | 20.00 (16.00–26.00) | 24.00 (19.00–35.00) | < 0.001 |
| ALT, U/L | 13.00 (10.00–17.00) | 14.00 (11.00–18.00) | 15.00 (12.00–21.00) | 19.00 (14.00–25.00) | < 0.001 |
| AST, U/L | 19.00 (17.00–22.00) | 20.00 (17.00–23.00) | 21.00 (18.00–25.00) | 22.00 (19.00–26.00) | < 0.001 |
| TB, μmol/L | 12.22 ± 4.98 | 11.89 ± 4.71 | 12.18 ± 5.01 | 12.46 ± 4.97 | 0.012 |
| GLB, g/L | 29.35 ± 3.62 | 29.48 ± 3.73 | 29.53 ± 4.06 | 29.64 ± 4.05 | 0.104 |
| ALB, g/L | 44.48 ± 2.64 | 44.36 ± 2.75 | 44.21 ± 2.83 | 44.30 ± 2.78 | 0.009 |
| TP, g/L | 73.78 ± 3.97 | 73.77 ± 4.09 | 73.69 ± 4.19 | 73.89 ± 4.16 | 0.455 |
| Cr, mmol/L | 70.16 ± 21.11 | 73.21 ± 17.26 | 78.70 ± 26.06 | 86.05 ± 31.73 | < 0.001 |
| UA, μmol/L | 233.34 ± 69.44 | 248.48 ± 72.94 | 270.44 ± 78.98 | 302.16 ± 80.37 | < 0.001 |
| LDL-C, mmol/L | 1.97 ± 0.39 | 2.08 ± 0.40 | 2.16 ± 0.41 | 2.26 ± 0.41 | < 0.001 |
| HDL-C, mmol/L | 1.61 ± 0.32 | 1.55 ± 0.29 | 1.49 ± 0.28 | 1.41 ± 0.26 | < 0.001 |
| TG, mmol/L | 0.76 ± 0.23 | 0.90 ± 0.27 | 1.01 ± 0.28 | 1.20 ± 0.27 | < 0.001 |
| TC, mmol/L | 4.23 ± 0.54 | 4.31 ± 0.53 | 4.37 ± 0.52 | 4.45 ± 0.50 | < 0.001 |
| FPG, mmol/L | 4.85 ± 0.41 | 4.97 ± 0.45 | 5.09 ± 0.59 | 5.38 ± 1.04 | < 0.001 |
| SBP, mmHg | 111.13 ± 13.65 | 114.95 ± 14.27 | 119.68 ± 15.66 | 126.49 ± 16.40 | < 0.001 |
| DBP, mmHg | 67.74 ± 8.64 | 69.64 ± 9.31 | 72.09 ± 9.76 | 75.71 ± 10.22 | < 0.001 |
| Time, days | 1036.08 ± 398.69 | 1012.46 ± 401.00 | 995.29 ± 394.25 | 925.52 ± 399.07 | < 0.001 |
| TyG | 7.94 ± 0.31 | 8.13 ± 0.31 | 8.28 ± 0.30 | 8.50 ± 0.28 | < 0.001 |
| BUN, mmol/L | 4.29 ± 1.36 | 4.42 ± 1.31 | 4.55 ± 1.36 | 4.74 ± 1.43 | < 0.001 |
| DBIL, μmol/L | 2.46 ± 1.21 | 2.41 ± 1.28 | 2.39 ± 1.29 | 2.35 ± 1.17 | 0.136 |
| NAFLD | < 0.001 | ||||
| No | 2435 (99.71%) | 2406 (98.57%) | 2257 (92.42%) | 1828 (74.86%) | |
| Yes | 7 (0.29%) | 35 (1.43%) | 185 (7.58%) | 614 (25.14%) | |
The variables are presented as n (%) or the mean ± SD or median (quartile 1-quartile 3), TyG = Ln [TG (mg/dL) × FPG (mg/dL)/2], TyG-BMI = TyG × BMI
Abbreviations: TyG-BMI triglyceride glucose-body mass index, BMI body mass index, NAFLD non-alcoholic fatty liver disease, TyG triglyceride and glucose index, DBP diastolic blood pressure, SBP systolic blood pressure, DBIL direct bilirubin, TB total bilirubin, GLB globulin, GGT gamma-glutamyl transferase, TP total protein, ALB albumin, UA uric acid, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, Cr creatinine, BUN blood urea nitrogen, FPG fasting plasma glucose, TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
Predictive values of TyG-BMI for the incidence of NAFLD
The ROC curve was plotted to measure the predictive power of TyG-BMI, TyG, BMI, ALT, TG, FPG and TG/HDL-C for NAFLD. Table 2 shows the predicted values of NAFLD. In ascending order, the predictive values of the variables for NAFLD were as follows: FPG [95% confidence interval (CI), 0.6291–0.6680; AUC (area under the curve) = 0.6485], TG (95% CI, 0.6829–0.7179; AUC = 0.7004), ALT (95% CI, 0.6858–0.7222; AUC = 0.7040), TG/HDL-C (95% CI, 0.6944–0.7288; AUC = 0.7116), TyG (95% CI, 0.7096–0.7433; AUC = 0.7264), BMI (95% CI, 0.8109–0.8361; AUC = 0.8235) and TyG-BMI (95% CI, 0.8375–0.8603; AUC = 0.8489). TyG-BMI predicted NAFLD with a specificity of 0.7348 and a sensitivity of 0.8205. The ROC curves of TyG-BMI and its components for predicting NAFLD are shown in Fig. 2. In addition, even when males and females were separated, TyG-BMI’s predictive ability for NAFLD was still better than that of other indicators (Additional File Fig. S1, S2).
Table 2.
AUC of TyG-BMI, TyG, BMI, TG, FPG, ALT, and TG/HDL-C for predicting NAFLD
| Variables | AUC | 95% CI lower bound | 95% CI upper bound | Best threshold | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| TyG-BMI | 0.8489 | 0.8375 | 0.8603 | 183.8263 | 0.7348 | 0.8205 |
| TyG | 0.7264 | 0.7096 | 0.7433 | 8.3219 | 0.6325 | 0.7015 |
| BMI | 0.8235 | 0.8109 | 0.8361 | 22.1444 | 0.7308 | 0.7824 |
| TG | 0.7004 | 0.6829 | 0.7179 | 1.0450 | 0.6524 | 0.6397 |
| FPG | 0.6485 | 0.6291 | 0.6680 | 5.0350 | 0.5810 | 0.6397 |
| ALT | 0.7040 | 0.6858 | 0.7222 | 16.5000 | 0.6045 | 0.7047 |
| TG/HDL-C | 0.7116 | 0.6944 | 0.7288 | 0.6865 | 0.6053 | 0.7146 |
Abbreviations: AUC area under the curve, CI confidence interval
Fig. 2.
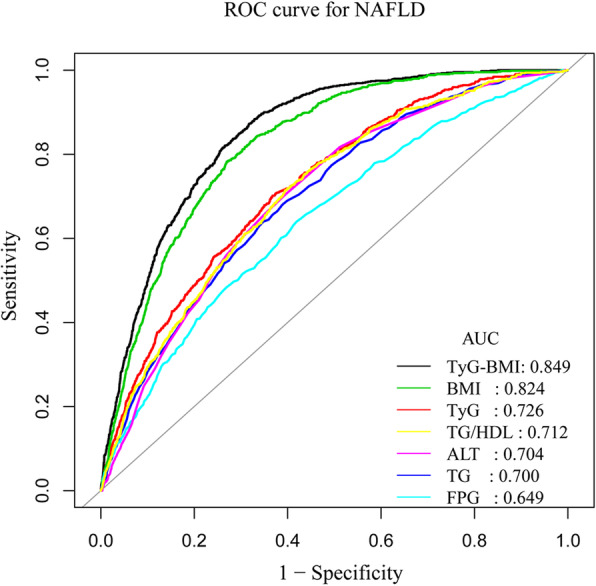
ROC curves for NAFLD
Association between TyG-BMI and NAFLD
The results of the univariate analysis indicated that in the non-obese population, sex, age, DBP, SBP, BMI, LDL-C, HDL-C, TG, TC, FPG, UA, Cr, TB, GLB, ALB, AST, GGT, ALT, ALP, TP, BUN, DBIL, and TyG-BMI were crucial risk factors for NAFLD (Table 3). The effect sizes of the association between TyG-BMI and NAFLD incidence in females, males, and subjects in general are listed in Table 4. Model 1 is a crude model. This model showed that TyG-BMI was positively related to the incidence of NAFLD. In Model 2, for every 1-SD increase in TyG-BMI, the risk of NAFLD increased 4.046-fold (HR = 4.046, 95% CI 3.717–4.405, P < 0.001) after adjusting for sex and age. The fully adjusted HR (95% CI) for the incidence of NAFLD in all subjects was 3.089 (95% CI 2.628–3.631, P < 0.001) for every 1-SD increase in TyG-BMI. The fully adjusted HRs (95% CI) for women and men, respectively, were 3.583 (2.796, 4.593) and 2.849 (2.298, 3.532) .
Table 3.
Univariate Cox regression model showing variables associated with NAFLD risk
| Variables | Hazard ratio | Lower limit of 95% CI | Upper limit of 95% CI | P value |
|---|---|---|---|---|
| Sex (male) | 1.127 | 0.984 | 1.29 | 0.085 |
| Age | 1.008 | 1.003 | 1.012 | 0.001 |
| ALP | 1.01 | 1.008 | 1.012 | < 0.001 |
| GGT | 1.007 | 1.006 | 1.008 | < 0.001 |
| ALT | 1.007 | 1.006 | 1.009 | < 0.001 |
| AST | 1.013 | 1.007 | 1.018 | < 0.001 |
| TP | 1.005 | 0.987 | 1.023 | 0.592 |
| ALB | 0.972 | 0.947 | 0.997 | 0.032 |
| GLB | 1.021 | 1.002 | 1.04 | 0.026 |
| TB | 0.988 | 0.972 | 1.004 | 0.131 |
| Cr | 1.005 | 1.004 | 1.006 | < 0.001 |
| UA | 1.005 | 1.004 | 1.006 | < 0.001 |
| FPG | 1.322 | 1.276 | 1.37 | < 0.001 |
| TC | 1.398 | 1.221 | 1.601 | < 0.001 |
| TG | 8.217 | 6.654 | 10.146 | < 0.001 |
| HDL-C | 0.253 | 0.195 | 0.328 | < 0.001 |
| LDL-C | 3.24 | 2.702 | 3.884 | < 0.001 |
| BMI | 1.929 | 1.844 | 2.019 | < 0.001 |
| SBP | 1.025 | 1.022 | 1.029 | < 0.001 |
| DBP | 1.048 | 1.041 | 1.054 | < 0.001 |
| BUN | 0.989 | 0.941 | 1.039 | 0.651 |
| DBIL | 0.59 | 0.54 | 0.646 | < 0.001 |
| TyG-BMI | 1.071 | 1.067 | 1.076 | < 0.001 |
Table 4.
Association between TyG-BMI and NAFLD risk in different models
| Exposure | Model 1 | P value | Model 2 | P value | Model 3 | P value |
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Female | ||||||
|
TyG-BMI (1-SD increase) |
4.170 (3.692, 4.709) | < 0.001 | 4.166 (3.688, 4.705) | < 0.001 | 3.583 (2.796, 4.593) | < 0.001 |
| TyG-BMI Quartile | ||||||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 3.237 (1.186, 8.836) | 0.022 | 3.237 (1.186, 8.836) | 0.022 | 3.164 (0.669, 14.958) | 0.146 |
| Q3 | 19.206 (7.800, 47.293) | < 0.001 | 19.200 (7.797, 47.279) | < 0.001 | 10.716 (2.566, 44.744) | 0.001 |
| Q4 | 77.980 (32.206, 188.810) | < 0.001 | 77.920 (32.173, 188.713) | < 0.001 | 30.978 (7.427, 129.208) | < 0.001 |
| P for trend | 5.689 (4.762, 6.797) | < 0.001 | 5.687 (4.759, 6.797) | < 0.001 | 3.648 (2.733, 4.870) | < 0.001 |
| Male | ||||||
|
TyG-BMI (1-SD increase) |
3.954 (3.513, 4.451) | < 0.001 | 3.945 (3.504, 4.440) | < 0.001 | 2.849 (2.298, 3.532) | |
| TyG-BMI Quartile | ||||||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 9.985 (2.326, 42.868) | 0.002 | 9.964 (2.321, 42.778) | 0.002 | 8.338 (1.057, 65.749) | 0.044 |
| Q3 | 49.743 (12.264, 201.752) | < 0.001 | 49.323 (12.160, 200.065) | < 0.001 | 24.649 (3.369, 180.346) | 0.002 |
| Q4 | 171.006 (42.585, 686.699) | < 0.001 | 169.762 (42.273, 681.737) | < 0.001 | 56.284 (7.712, 410.750) | < 0.001 |
| P for trend | 5.098 (4.298, 6.047) | < 0.001 | 5.090 (4.291, 6.038) | < 0.001 | 3.098 (2.387, 4.020) | < 0.001 |
| Total | ||||||
|
TyG-BMI (1-SD increase) |
4.053 (3.724, 4.412) | < 0.001 | 4.046 (3.717, 4.405) | < 0.001 | 3.089 (2.628, 3.631) | < 0.001 |
| TyG-BMI Quartile | ||||||
| Q1 | 1 | 1 | 1 | |||
| Q2 | 5.164 (2.294, 11.626) | < 0.001 | 5.161 (2.292, 11.618) | < 0.001 | 4.718 (1.387, 16.040) | 0.013 |
| Q3 | 27.953 (13.142, 59.456) | < 0.001 | 27.832 (13.085, 59.202) | < 0.001 | 15.062 (4.743, 47.835) | < 0.001 |
| Q4 | 104.387 (49.549, 219.914) | < 0.001 | 103.788 (49.261, 218.670) | < 0.001 | 38.242 (12.065, 121.213) | < 0.001 |
| P for trend | 5.387 (4.762, 6.094) | < 0.001 | 5.373 (4.749, 6.079) | < 0.001 | 3.336 (2.750, 4.047) | < 0.001 |
Model 1: unadjusted; Model 2: adjusted for sex and age; Model 3: adjusted for sex, age, ALP, GGT, ALT, AST, ALB, GLB, Cr, UA, FPG, TG, HDL-C, LDL-C, SBP, DBP, and DBIL
In order to explore the nonlinearity of TyG-BMI related to NAFLD events in patients, the continuous variable TyG-BMI was converted to categorical variables according to quartiles. The effect size trends of different TyG-BMI groups were equidistant, consistent with the P value for the trend of TyG-BMI for the occurrence of NAFLD in the patients (P < 0.001).
A sensitivity analysis was performed for imputation of missing covariates, and five imputed datasets were created by using the MICE software package with chained equations. When the same analysis was conducted in the five estimated data sets, the core results of the complete data analysis were stable and consistent with the original data (Additional File Table S2).
Follow-up results
During the follow-up period, 841 (8.61%) of the non-obese subjects in the study were diagnosed with NAFLD. Figure 3 illustrates the significant difference in NAFLD risk between the TyG-BMI quartile groups (log-rank test P < 0.0001). As TyG-BMI increased, the cumulative risk of NALFD gradually increased.
Fig. 3.
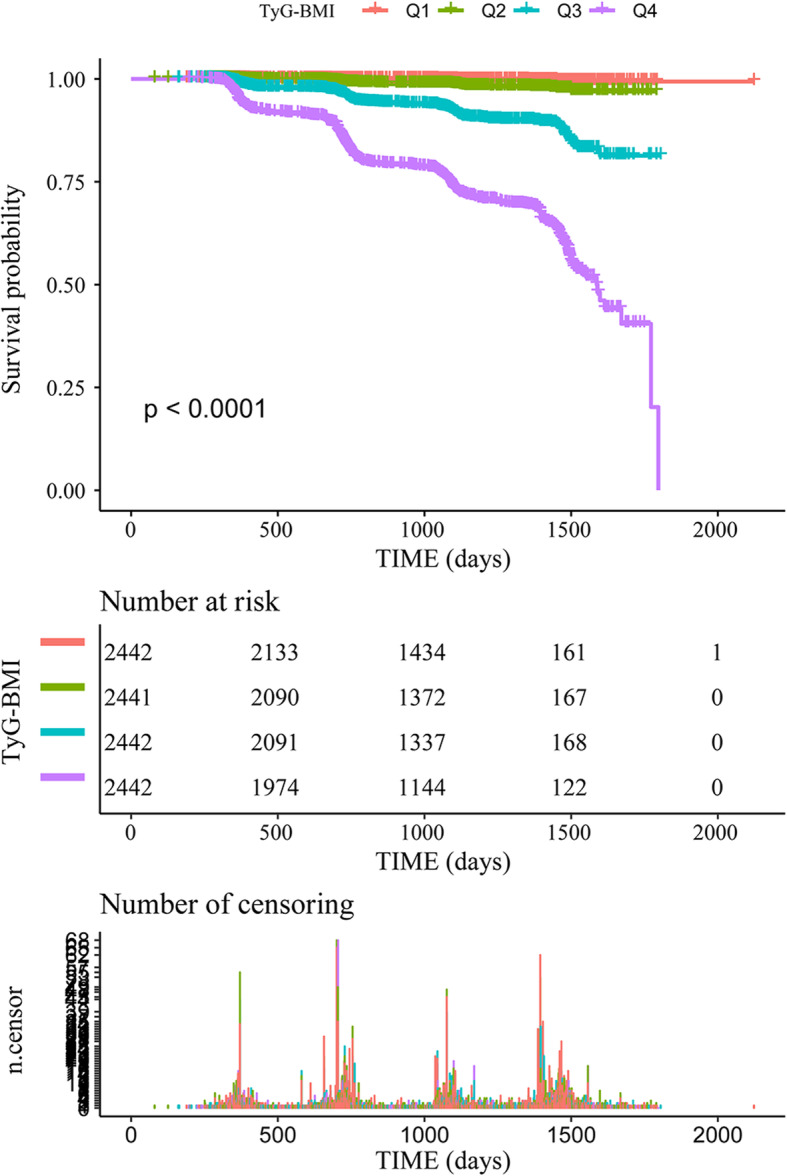
Kaplan-Meier analysis of NAFLD incidence according to TyG-BMI quartiles (P < 0.0001)
Subgroup analysis
In order to better understand other factors that may affect the association between TyG-BMI and NAFLD incidence and to further identify potential special populations, subgroup analysis was performed. The full variables were presented hierarchically based on clinical significance or bisection, and interaction tests were also performed (Additional File Table S4). The relationship between baseline TyG-BMI and NAFLD was weaker for men than for women (HR 2.849 versus HR 3.583, P < 0.05 for the TyG-BMI-gender interaction effect on NAFLD). The interactions of TyG-BMI with sex, ALP, GGT, HDL-C, TG, and BMI were significant. (shown in Fig. 4).
Fig. 4.
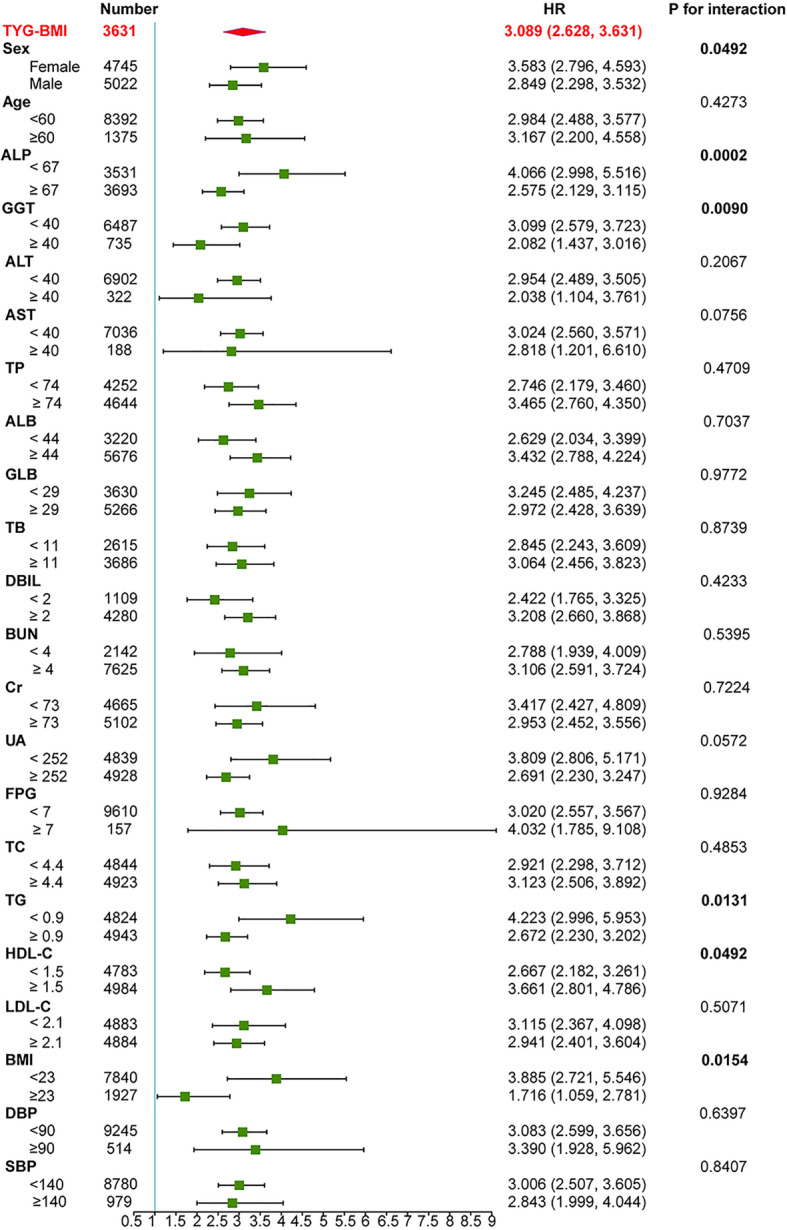
Subgroup analysis of the association between TyG-BMI and NAFLD. The HR (95% CI) was derived from the Cox regression model. (Sex, age, DBP, SBP, LDL-C, HDL-C, TG, UA, Cr, GLB, ALB, AST, ALP, GGT, ALT, FPG, and DBIL were adjusted)
Discussion
In this secondary analysis of a prospective cohort study, the association between TyG-BMI and non-obese NAFLD incidence in Chinese adults with normal lipid levels was explored. The results showed that in normal-weight individuals, TyG-BMI still showed a strong and positive association with NAFLD after adjusting for other covariates (HR = 3.089, 95% CI 2.628–3.631). Furthermore, TyG-BMI had a stronger predictive value than its components for the incidence of NAFLD, with larger AUC values in both genders. Therefore, TyG-BMI may be a valid indicator to predict the occurrence of NAFLD in non-obese Chinese individuals. As the Kaplan-Meier curves showed, the cumulative hazard of NAFLD gradually increased as TyG-BMI increased. Additionally, in the subgroup analysis, interaction effects on NAFLD risk were detected between TyG-BMI and sex (P value for interaction = 0.0492), ALP (P value for interaction = 0.0002), GGT (P value for interaction = 0.0090), TG (P value for interaction = 0.0131), HDL-C (P value for interaction = 0.0492) and BMI (P value for interaction = 0.0154).
Among non-obese people, NAFLD is not uncommon. Due to differences in study subjects’ choices, diagnostic methods, and lifestyles, the reported global prevalence of non-obese NAFLD ranges from 3 to 30% [25]. Kwon et al. [26] reported that among 29,994 subjects who underwent routine medical examinations, the prevalence of non-obese NAFLD was 12.6%. In a study from China, a total of 5562 participants completed a 5-year follow-up, of whom 494 (8.88%) developed non-obese NAFLD [7]. A study conducted in India reported that subjects with BMI < 23 kg/m2 and BMI < 25 kg/m2 had NAFLD prevalence rates of 5.5 and 7.4%, respectively [27]. Interestingly, in the stratified analysis, interactions (P value for interaction = 0.0154) were observed in individuals with BMI < 23 (HR = 3.885, 95% CI 2.721–5.546) and ≥ 23 (HR = 1.716, 95% CI 1.059–2.781). The relationship between baseline TyG-BMI and NAFLD was stronger in individuals with BMI < 23 than in those with higher BMI values. As shown in Additional File Table S4, when height and weight were devided into 3 groups each, an interaction effect of weight tertile and TyG-BMI on NAFLD risk was found (P value for interaction = 0.0154). Thus, the interaction with BMI may come from weight. Previous studies have reported that nicotine can reduce appetite and increase energy expenditure, leading to weight loss, and smoking can increase insulin resistance [28, 29], which may explain the interaction between body weight and TyG-BMI in this study. However, the raw data analysed in this study did not include smoking; therefore, further studies are still needed to verify this result.
Although the mechanism of NAFLD onset is poorly understood, IR is involved in NAFLD development [10]. Some studies have revealed that IR also plays an essential role in NAFLD pathogenesis in non-obese patients [11]. IR identification may help stratify non-obese NAFLD patients and support the development of personalized treatment measures. Nevertheless, the measurement of IR in clinical practice is not easy. The gold standard for testing IR is hyperinsulinaemic-euglycaemic clamp (HEC) [30], but this approach is time consuming and is not suitable for clinical application. Currently, the homeostasis model assessment of IR (HOMA-IR) is a universally accepted alternative indicator for IR. Previous studies have reported an independent association between NAFLD and HOMA-IR [31]. Additionally, other studies have suggested that HOMA-IR diagnostic criteria could be applied to predict NAFLD [32]. However, in many laboratories that perform routine tests, insulin measurement remains challenging, and there are problems with standardization [33]. Therefore, it is necessary to find more accessible and practical laboratory indicators.
Some studies have suggested that TyG could be used as an alternative index to assess IR [34, 35]. This parameter does not require a measurement of insulin concentration and requires only two simple indicators, namely, FPG and TG [13]. Moreover, TyG has been shown to be related to HOMA-IR and HEC by several studies and could be used to identify IR [36]. A study by Lee et al. [31] concluded that TyG performed better than HOMA-IR in predicting NAFLD. Regarding the role of obesity in IR, Er et al. and Lim et al. [13, 37] indicated that TyG-BMI performed better than TyG in predicting IR. Additionally, a study by Zeng et al. revealed that TyG-BMI performed better than TyG in identifying prehypertension in the non-obese population [14]. Zhang et al. [15] analysed the relationship between TyG-BMI and non-obese NAFLD in a cross-sectional study. They concluded that TyG-BMI was more effective than TyG alone in identifying non-obese NAFLD patients. The results of the present cohort study were consistent with theirs. However, their research has no follow-up data, and it is not known whether the passage of time affects the association between TyG-BMI and NAFLD. In addition, they did not consider the effect of gender on TyG, which was mentioned in previous articles [38]. In the present study, it was clearly observed that TyG-BMI was linked to the incidence of non-obese NAFLD in both genders. In male subjects, the TyG-BMI increase per SD was linked to an increased risk of NAFLD (HR = 2.85, 95% CI 2.30–3.53), but the risk was higher in female subjects (HR = 3.58, 95% CI 2.80–4.60). Gender and TyG-BMI had significant interactions with the occurrence of NAFLD (P < 0.0001). How gender affects the relationship between TyG-BMI and NAFLD is unclear, but it may be related to the effect of gender on glucose, lipid metabolism and IR [39, 40]. In addition, a protective effect of oestrogen against NAFLD has been suggested [41]; oestrogen levels drop in women after menopause, and subjects’ average age was 42.5 ± 14.7 years in this study, which may partly explain the higher HR of women in this study. Nevertheless, the specific mechanism requires further study.
Strength and limitations of this study
This study has several strengths: (1) This was a 5-year longitudinal population-based study. (2) This study strictly adjusted for confounding factors. (3) The target independent variable was converted to a categorical variable for analysis, and both the complete data and the original data were analysed simultaneously to improve the reliability of the results. (4) Effect sizes were calculated in different populations.
Nevertheless, several limitations should be noted: (1) In this study, the diagnosis of NAFLD was based on ultrasonography rather than liver biopsy. The accuracy of NAFLD diagnosis may be reduced. In addition, ultrasonography cannot discriminate between steatosis and steatohepatitis. However, ultrasound examination for the diagnosis of NAFLD has been widely used in epidemiological studies [42]. (2) Some indicators associated with NAFLD and IR, such as waist circumference, waist-to-hip ratio, and HOMA-IR, were not collected in the raw data. (3) This study did not record information about energy intake and nutritional habits, but we indirectly adjusted for other covariates related to dietary habits, such as TC, HDL-C, LDL-C, and ALB. (4) Because the subjects included only non-obese people in China, the conclusion is not generalizable to other populations.
In short, the incidence of NAFLD without obesity is not uncommon in Chinese people. TyG-BMI is positively correlated with the incidence of NAFLD in non-obese people. In addition, the effect size may vary by gender. Thus, non-obese people with higher TyG-BMI need to be particularly concerned even if their blood lipids are at normal levels.
Conclusion
In non-obese Chinese individuals with normal lipid levels, an increase in TyG-BMI is related to an increased incidence of NAFLD. TyG-BMI may have clinical significance in the early identification of groups with a high risk of NAFLD. This index is a simple and low-cost biochemical measurement value that may be used for large-scale NAFLD screening and risk assessment. Perhaps a strategy to prevent NAFLD can be developed based on the TyG-BMI index. Additionally, the findings of this study should be helpful for future research on the establishment of diagnostic or predictive models of incident NAFLD.
Supplementary Information
Additional File 1 Table S1. Description of the missing data.
Additional File 2 Table S2. Comparative analysis of sensitivity before and after imputation.
Additional File 3 Table S3. Result of the collinearity test of each variable.
Additional File 4 Table S4. Subgroup analysis of the association between TyG-BMI and NAFLD risk.
Additional File 5 Fig. S1. ROC curves for NAFLD in males.
Additional File 6 Fig. S2. ROC curves for NAFLD in females.
Acknowledgements
We are grateful to Sun et al. for making their data publicly available.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- TyG-BMI
Triglyceride glucose body mass index
- IR
Insulin resistance
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- HR
Hazard ratio
- CI
Confidence interval
- SD
Standard deviation
- TC
Total cholesterol
- TG
Triglyceride
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- ALB
Albumin
- GLB
Globulin
- GGT
Gamma-glutamyl transferase
- TP
Total protein
- DBIL
Direct bilirubin
- TB
Total bilirubin
- UA
Uric acid
- Cr
Creatinine
- BUN
Blood urea nitrogen
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ALP
Alkaline phosphatase
- BMI
Body mass index
- FPG
Fasting plasma glucose
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- TyG
Triglyceride and glucose index
- TG/HDL-C
Ratio of triglyceride to high-density lipoprotein cholesterol
- VIF
Variance inflation factor
Authors’ contributions
Conceptualization, methodology: Yaling Li, Rui Zheng and Zhiming Huang. Project administration: Yaling Li and Zhiming Huang. Software: Rui Zheng. Visualization: Jie Li. Supervision: Yaling Li and Zhiming Huang. Writing – Original draft preparation: Yaling Li and Rui Zheng. Writing – Reviewing and Editing: Li Wang and Shuyi Feng. All the authors listed have read and approved the manuscript.
Funding
No funding support was provided.
Availability of data and materials
The data are available from the ‘DataDryad’ database (www.datadryad.org).
Ethics approval and consent to participate
Because the data were de-identified, informed consent was waived. The ethics committee of Wenzhou People’s Hospital approved the previous study, Thus, our research did not require ethical approval.
Consent for publication
Not applicable.
Competing interests
No conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaling Li and Rui Zheng contributed equally to this work.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Khashab MA, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr Gastroenterol Rep. 2008;10:73–80. doi: 10.1007/s11894-008-0012-0. [DOI] [PubMed] [Google Scholar]
- 3.Xia C, Li R, Zhang S, Gong L, Ren W, Wang Z, Li Q. Lipid accumulation product is a powerful index for recognizing insulin resistance in non-diabetic individuals. Eur J Clin Nutr. 2012;66:1035–1038. doi: 10.1038/ejcn.2012.83. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Kim JH, Kim YJ, Yoo HJ, Kim HY, Seo JA, Kim NH, Choi KM, Baik SH, Choi DS, Kim SG. Clinical and metabolic factors associated with development and regression of nonalcoholic fatty liver disease in nonobese subjects. Liver Int. 2014;34:604–611. doi: 10.1111/liv.12454. [DOI] [PubMed] [Google Scholar]
- 6.Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, Shu SS, Chim AM, Chan HL, Wong VW. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai study. Am J Gastroenterol. 2013;108:1299–1304. doi: 10.1038/ajg.2013.104. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Qin H, Qiu S, Chen G, Chen Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: a retrospective cohort research. Lipids Health Dis. 2019;18:162. doi: 10.1186/s12944-019-1104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun DQ, Wu SJ, Liu WY, Wang LR, Chen YR, Zhang DC, Braddock M, Shi KQ, Song D, Zheng MH. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6:e013781. doi: 10.1136/bmjopen-2016-013781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado M, Cortez-Pinto H. Non-alcoholic fatty liver disease and insulin resistance. Eur J Gastroenterol Hepatol. 2005;17:823–826. doi: 10.1097/00042737-200508000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Sinn DH, Gwak GY, Park HN, Kim JE, Min YW, Kim KM, Kim YJ, Choi MS, Lee JH, Koh KC, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107:561–567. doi: 10.1038/ajg.2011.400. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Rhee PL, Lee JK, Lee KT, Kim JJ, Koh KC, Paik SW, Rhee JC, Choi KW. Role of hyperinsulinemia and glucose intolerance in the pathogenesis of nonalcoholic fatty liver in patients with normal body weight. Korean J Intern Med. 1998;13:12–14. [PubMed] [Google Scholar]
- 13.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: a cross-sectional study of Chinese adults. J Clin Hypertens (Greenwich) 2020;22:1025–1032. doi: 10.1111/jch.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Du T, Li M, Jia J, Lu H, Lin X, Yu X. Triglyceride glucose-body mass index is effective in identifying nonalcoholic fatty liver disease in nonobese subjects. Medicine (Baltimore) 2017;96:e7041. doi: 10.1097/MD.0000000000007041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun DQ, Wu SJ, Liu WY, Wang LR, Chen YR, Zhang DC, Braddock M, Shi KQ, Song D, Zheng MH. Data from: association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. Dryad Digital Repository. 2016. 10.5061/dryad.1n6c4. [DOI] [PMC free article] [PubMed]
- 17.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Sun DQ, Liu WY, Wu SJ, Zhu GQ, Braddock M, Zhang DC, Shi KQ, Song D, Zheng MH. Increased levels of low-density lipoprotein cholesterol within the normal range as a risk factor for nonalcoholic fatty liver disease. Oncotarget. 2016;7:5728–5737. doi: 10.18632/oncotarget.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 20.Zeng MD, Li YM, Chen CW, Lu LG, Fan JG, Wang BY, Mao YM. Chinese National Consensus Workshop on nonalcoholic fatty liver D. guidelines for the diagnosis and treatment of alcoholic liver disease. J Dig Dis. 2008;9:113–116. doi: 10.1111/j.1751-2980.2008.00332.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernhardt PW. Model validation and influence diagnostics for regression models with missing covariates. Stat Med. 2018;37:1325–1342. doi: 10.1002/sim.7584. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of Coffee Consumption with Total and Cause-Specific Mortality among Nonwhite Populations. Ann Intern Med. 2017;167:228–235. doi: 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wax Y. Collinearity diagnosis for a relative risk regression analysis: an application to assessment of diet-cancer relationship in epidemiological studies. Stat Med. 1992;11:1273–1287. doi: 10.1002/sim.4780111003. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda Y, Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, Ohbora A, Kato T, Kojima T, Fukui M. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 2016;36:713–720. doi: 10.1111/liv.12977. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 27.Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, Dhibar T, Bhattacharya B, Bhattacharya D, Manna B, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 28.Yu XY, Song P, Zou MH. Obesity paradox and smoking gun: a mystery of statistical confounding? Circ Res. 2018;122:1642–1644. doi: 10.1161/CIRCRESAHA.118.312897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 31.Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, Nam JS, Ahn CW, Park JS. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul) 2019;34:179–186. doi: 10.3803/EnM.2019.34.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isokuortti E, Zhou Y, Peltonen M, Bugianesi E, Clement K, Bonnefont-Rousselot D, Lacorte JM, Gastaldelli A, Schuppan D, Schattenberg JM, et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017;60:1873–1882. doi: 10.1007/s00125-017-4340-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, Steffes MW, Insulin standardization work G. toward standardization of insulin immunoassays. Clin Chem 2009; 55:1011–1018. [DOI] [PubMed]
- 34.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the Euglycemic-Hyperinsulinemic clamp. J Clin Endocrinol Metabol. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 37.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and nutrition examination survey. PLoS One. 2019;14:e212963. [DOI] [PMC free article] [PubMed]
- 38.Kitae A, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. The triglyceride and glucose index is a predictor of incident nonalcoholic fatty liver disease: a population-based cohort study. Can J Gastroenterol Hepatol. 2019;2019:5121574. doi: 10.1155/2019/5121574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Giosia P, Giorgini P, Stamerra CA, Petrarca M, Ferri C, Sahebkar A. Gender differences in epidemiology, pathophysiology, and treatment of hypertension. Curr Atheroscler Rep. 2018;20:13. [DOI] [PubMed]
- 41.Gutierrez-Grobe Y, Ponciano-Rodriguez G, Ramos MH, Uribe M, Mendez-Sanchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 42.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1 Table S1. Description of the missing data.
Additional File 2 Table S2. Comparative analysis of sensitivity before and after imputation.
Additional File 3 Table S3. Result of the collinearity test of each variable.
Additional File 4 Table S4. Subgroup analysis of the association between TyG-BMI and NAFLD risk.
Additional File 5 Fig. S1. ROC curves for NAFLD in males.
Additional File 6 Fig. S2. ROC curves for NAFLD in females.
Data Availability Statement
The data are available from the ‘DataDryad’ database (www.datadryad.org).