Abstract
PURPOSE:
To determine the impact of type 2 diabetes mellitus (T2DM) for men with localized prostate cancer receiving definitive radiation therapy (RT).
PATIENTS AND METHODS:
We perform a retrospective review of 3,217 patients, from 1998-2013, subdivided into 5 subgroups: (I) no T2DM; (II) T2DM on oral antihyperglycemic that contains metformin, no insulin; (III) T2DM on non-metformin oral agent alone, no insulin; (IV) T2DM on any insulin; (V) T2DM not on medication. Outcome measures were overall survival (OS), freedom from biochemical failure (FFBF), freedom from distant metastasis (FFDM), cancer specific survival (CSS), and toxicities. Kaplan-Meier analysis, log rank tests, Fine and Gray competing risk regression (to adjust for patient and lifestyle factors), Cox models, and subdistribution hazard ratios (sHRs) were used.
RESULTS:
Of the 3,176 patients, 38% were low-, 41% intermediate-, and 21% high-risk. The group I-V distribution was 81%, 8%, 5%, 3%, 4%. The median dose was 78 Gy, and the median follow-up time was 50 months (range, 1-190). Group V had increased mortality (sHR 2.1, 95% confidence interval [CI] 0.66 - 1.54), BF (sHR 2.14, 0.88 - 1.83), and CSM (sHR 3.87, 1.31 - 11). Acute toxicities were higher in group IV vs group I (GU: 38% vs. 26%, p = 0.01; GI: 21% vs. 5%, p = 0.01). Late toxicities were higher in groups IV and V vs. group I (12-14% vs. 2-6%, p < 0.01).
CONCLUSIONS:
Men with T2DM not on medication and men with T2DM on insulin have worse outcomes and toxicities compared to other patients.
Keywords: antihyperglycemic agents, diabetes mellitus, insulin, metformin, prostate cancer, radiation therapy
GRAPHICAL ABSTRACT
MICROABSTRACT
We evaluated the impact of type 2 diabetes, and medications used in its management, on prostate cancer patients receiving radiation therapy. Men who were on insulin and those not on any medication had increased risk of death and toxicity than those without diabetes.
INTRODUCTION
Prostate cancer is the second most prevalent solid tumor diagnosed in men of the United States and Western Europe.1 The etiology and biological mechanisms for the development of prostate cancer are complex.2 A consensus statement from the American Cancer Society and the American Diabetes Association emphasized a link between type 2 diabetes mellitus (T2DM) and prostate cancer.3 This association is believed to be rooted on both biological evidence of insulin and insulin-like growth factors (IGFs) potentiating cancer cell growth and cell cycle progression 4-7 and the clinical findings of increased all-cause mortality among diabetic patients as compared to their nondiabetic counterparts.8, 9
Among prostate cancer patients, hyperinsulinemia is associated with increased cancer-specific mortality.10 Moreover, studies suggest that metformin use is associated with improved rates of overall survival (OS), freedom from biochemical failure (FFBF), freedom from distant metastasis (FFDM), cancer specific survival (CSS), and the transformation of prostate cancer from androgen-sensitive to castrate-resistant disease.11, 12 However, the type of antihyperglycemic medication (e.g. metformin, insulin) best used for these patients is unknown.
We evaluated the impact of T2DM, oral antihyperglycemics (subdivided into those containing metformin or not), and insulin, on the outcomes and toxicities among men undergoing definitive radiation therapy (RT) for localized prostate cancer. We hypothesized that men without T2DM would have the best outcomes and toxicities compared to other diabetic patients (specifically those on insulin or those not on medication).
MATERIALS AND METHODS
Study design
After institutional review board approval, we reviewed our prospectively collected institutional database of men undergoing RT for localized prostate adenocarcinoma, clinical Stage T1-4, N0/X, M0. Men were staged using National Comprehensive Cancer Network (NCCN) criteria.13, 14
Patient evaluation details are listed in Supplement Materials and Methods. Using our drug database, we were able to parse out the medications in combination pills (e.g. Actoplus MET®: metformin and pioglitazone) to create diabetes groups (Supplementary Table 1). Men were subdivided into five subgroups, depending on use of T2DM medication: (I) no T2DM; (2) T2DM on oral antihyperglycemic that contains metformin, but not on insulin; (3) T2DM on non-metformin oral antihyperglycemic alone (e.g. glyburide; sitagliptin; pioglitazone), but not on insulin; (4) T2DM on any insulin, with or without oral antihyperglycemic; (5) T2DM not on medication. We created this distinction to parse out patients on metformin, who are hypothesized to have improved outcomes to those not on metformin;11, 15, 16 and to separate men who have an advanced stage of T2DM requiring insulin, which is typically started only after oral antihyperglycemics have failed17, 18 and is associated with increased cancer-related death.10 The techniques used for three dimensional conformal RT (3D-CRT) and intensity modulated RT (IMRT) have been previously reported19, 20 and are further described in the Supplementary Materials and Methods.
Outcome measures and statistical analysis
Patients were followed with clinical exam (including rectal exam) every six months for the first year; then, yearly with PSAs drawn every 6 months. For FFBF, time to event was determined from date of initial RT to date of biochemical event (either date of nadir + 2 PSA, in ng/mL 21, or date that salvage hormones were started), or to date of last PSA measurement recorded in the database for those censored. For FFDM, CSS, and OS, censoring was determined as time from date of start of RT to either date of event or status date. The time component is from start of RT.
We used Kaplan-Meier methods to generate survival curves for OS, FFBF, FFDM, and CSS, and compared groups II-V vs group I using log-rank tests. To adjust for patient and lifestyle factors, we used competing risk regression models (variables in models are listed in Supplementary Materials and Methods). For FFBF and FFDM, subdistribution hazard ratios (sHRs) were estimated using Fine and Gray competing risk regression 22. We evaluated genitourinary (GU) and gastrointestinal (GI) toxicities using the Radiation Therapy Oncology Group (RTOG) definitions (Supplementary Table 2). We used competing risk regression to estimate sHRs for late toxicities (occurring >3 months after RT). Competing risk regression analyses and survival plots were done using Stata version 12; additional analyses were performed with SAS 9.2, and a p-value <0.05 was considered significant.
RESULTS
Patient characteristics are listed in Table 1. From 1998 to 2013, 3,217 men were treated with RT, with a median dose of 78 Gy (range, 76 – 80). The median follow-up was 4.9 years (range 1 – 190 months). Of these men, 40% were low-, 37% intermediate-, and 20% high-risk, based on NCCN criteria. Of the 3,217 men, 80.9% were in group I, 7.8% in group II, 4.6% in group III, 2.8% in group IV, and 3.9% in group V. There was no statistically significant difference in distribution of the patients among risk groups; or among Gleason score groups, PSA groups, or T-stage groups. Men in groups II – V were more likely to have hypertension and heart disease than those in group I (p < 0.0001). The average age among the groups was similar, 67 years. Men in group V were more frequently treated with 3D-CRT than with IMRT, compared to other groups (p < 0.0001) because most of these men were treated before 2002, when our institution acquired IMRT, which was controlled for on multivariate analysis.
Table 1.
Patient characteristics.
| Diabetes groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | I: No T2DM | II: Metformin | III: Non- metformin oral antihyperglyce mic |
IV: Any insulin | V: T2DM, no meds |
Chi-square p-value |
|||||||
| N = 3,217 |
100% | N = 2,603 |
80.9% | N = 251 | 7.8% | N = 148 | 4.6% | N = 89 | 2.8% | N = 126 | 3.9% | ||
| NCCN risk group | 0.43 | ||||||||||||
| Low | 1295 | 40.3 | 1067 | 41 | 94 | 38 | 56 | 38 | 30 | 34 | 48 | 38 | |
| Intermediate | 1192 | 37.1 | 964 | 37 | 97 | 39 | 55 | 37 | 35 | 39 | 41 | 33 | |
| High | 652 | 20.3 | 515 | 20 | 54 | 22 | 33 | 22 | 20 | 23 | 30 | 24 | |
| Unknown | 78 | 2.4 | 57 | 2 | 6 | 2 | 4 | 3 | 4 | 5 | 7 | 6 | |
| Gleason score | 0.083 | ||||||||||||
| 6 | 1706 | 53 | 1411 | 54 | 115 | 46 | 75 | 51 | 38 | 43 | 67 | 53 | |
| 7 | 1075 | 33.4 | 854 | 33 | 93 | 37 | 54 | 37 | 37 | 42 | 37 | 29 | |
| 8-10 | 436 | 13.6 | 338 | 13 | 43 | 17 | 19 | 13 | 14 | 16 | 22 | 18 | |
| PSA (ng/mL) | 0.59 | ||||||||||||
| <10 | 2472 | 76.8 | 1990 | 77 | 204 | 81 | 110 | 74 | 68 | 76 | 100 | 79 | |
| 10-20 | 523 | 16.3 | 427 | 16 | 38 | 15 | 26 | 18 | 15 | 17 | 17 | 14 | |
| >20 | 222 | 6.9 | 186 | 7 | 9 | 4 | 12 | 8 | 6 | 7 | 9 | 7 | |
| Mean (SD) | 9.7 | (17.3) | 8.4 | (9.8) | 9.1 | (10) | 8.9 | (10) | 11 | (20) | |||
| T-stage | 0.35 | ||||||||||||
| T1-T2a | 2455 | 76.3 | 1990 | 77 | 195 | 78 | 119 | 80 | 67 | 75 | 84 | 67 | |
| T2b-T2c | 438 | 13.6 | 360 | 14 | 30 | 12 | 16 | 11 | 9 | 10 | 23 | 18 | |
| T3-T4 | 188 | 5.8 | 149 | 6 | 15 | 6 | 7 | 5 | 8 | 9 | 9 | 7 | |
| TX | 136 | 4.2 | 104 | 4 | 11 | 4 | 6 | 4 | 5 | 6 | 10 | 8 | |
| Initial ADT use | 833 | 25.9 | 643 | 25 | 80 | 32 | 46 | 31 | 27 | 30 | 37 | 29 | 0.034 |
| Hypertension | 1763 | 54.8 | 1337 | 51 | 173 | 69 | 108 | 73 | 67 | 75 | 78 | 62 | <0.0001 |
| Heart Disease | 727 | 22.6 | 541 | 21 | 67 | 27 | 52 | 35 | 23 | 26 | 44 | 35 | <0.0001 |
| Time of medication start | |||||||||||||
| Pre RT | 155 | 62 | 116 | 78 | 47 | 53 | |||||||
| Post RT | 96 | 38 | 32 | 22 | 42 | 47 | |||||||
| Age at initiation | 0.059 | ||||||||||||
| 36-55 | 246 | 7.6 | 204 | 8 | 19 | 8 | 9 | 6 | 6 | 7 | 8 | 6 | |
| 56-65 | 992 | 30.8 | 793 | 31 | 91 | 36 | 45 | 30 | 35 | 39 | 28 | 22 | |
| 66-75 | 1518 | 47.2 | 1224 | 47 | 116 | 46 | 69 | 47 | 33 | 37 | 76 | 60 | |
| 76-89 | 461 | 14.3 | 382 | 15 | 25 | 10 | 25 | 17 | 15 | 17 | 14 | 11 | |
| Mean (SD) | 67 | (7.8) | 67 | (7.7) | 66 | (7.1) | 68 | (8.0) | 67 | (8.3) | 68 | (7.4) | |
| RT technique | <0.0001 | ||||||||||||
| 3D-CRT | 902 | 28 | 758 | 29 | 38 | 15 | 31 | 21 | 10 | 11 | 65 | 52 | |
| IMRT | 2315 | 72 | 1845 | 71 | 213 | 85 | 117 | 79 | 79 | 89 | 61 | 48 | |
| Follow-up in months: mean (SD) | 58 (34) | 63 | (35) | 62 | (37) | 63 | (33) | 51 | (33) | 49 | (30) | 0.36 | |
| min | 1.1 | 1.1 | 4.7 | 5.5 | 5.8 | 1.7 | |||||||
| max | 212.1 | 197.5 | 212.1 | 162.8 | 167.7 | 167.9 | |||||||
Abbreviations: 3D-CRT: 3D conformal radiation therapy; ADT: androgen deprivation therapy; IMRT: intensity modulated radiation therapy; NCCN: National Comprehensive Cancer Network; PSA: prostate specific antigen; RT: radiation therapy; T2DM: type 2 diabetes mellitus;
Notes: All staging information (e.g. risk group, PSA, T-stage, GS) is pre-RT. Comorbidities (e.g. hypertension) were typically present pre-RT; some patients were diagnosed with these conditions during or post-RT, but detailed information on exact date of diagnosis is unavailable. There was no statistically significant difference in the distribution of RT doses (three levels: 76 Gy, 78 Gy, 79 – 80 Gy) among the patient subgroups.
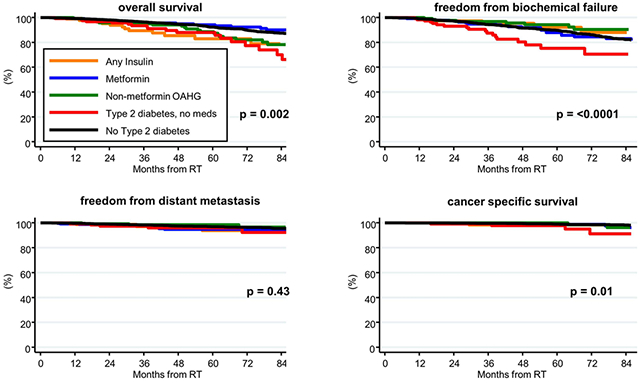
Patient outcomes are shown in Table 2 and Figure 1. The 5-year OS rates for low-, intermediate-, and high-risk men were 94%, 91% (p = 0.01), and 88% (p < 0.0001), respectively (Table 1, upper portion). The 5-year OS rates for men in groups III, IV, and V were significantly worse compared to men in group I: 92% for group I (reference), 94% for group II (p = 0.97), 89% for group III (p = 0.03), 83% for group IV (p = 0.01), and 88% for group IV (p = 0.002), as shown in Table 1, middle portion and Figure 1, upper left panel. After adjusting for competing risk factors (Table 2, lower portion), men in groups IV and V were twice as likely to experience non-cancer related death as those in group I. Men in group II (i.e. those taking metformin) had no difference in OS compared to men in group I.
Table 2.
Patient outcomes, stratified by T2DM group.
| 5-year KM (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCCN risk group | N | % | OS | 95% CI | p-val | FFBF | 95% CI | p-val | FFDM | 95% CI | p-val | CSS | 95% CI | p-val | |
| Low | 1295 | 41.3 | 94.3 | 92.6-95.6 | Ref | 96.4 | 94.7-97.5 | Ref | 99.4 | 98.5-99.7 | Ref | 99.9 | 99.1-100 | Ref | |
| Intermediate | 1192 | 38.0 | 91.0 | 88.8-92.8 | 0.01 | 87.3 | 84.7-89.6 | 0.12 | 96.9 | 95.4-97.9 | < 0.0001 | 99.2 | 98.0-99.6 | 0.12 | |
| High | 652 | 20.8 | 88.4 | 85.2-90.9 | < 0.0001 | 79.1 | 74.8-82.7 | < 0.0001 | 90.8 | 87.8-93.1 | < 0.0001 | 96.8 | 94.7-98.0 | < 0.0001 | |
| T2DM group | N | % | OS | 95% CI | p-val | FFBF | 95% CI | p-val | FFDM | 95% CI | p-val | CSS | 95% CI | p-val | |
| I | No T2DM | 2603 | 81 | 92.3 | 91.0-93.5 | Ref | 89.7 | 88.2-91.1 | Ref | 96.9 | 96.1-97.6 | Ref | 99 | 98.4-99.4 | Ref |
| II | Metformin | 251 | 7.8 | 94.3 | 89.9-96.8 | 0.97 | 87.8 | 81.1-92.3 | 0.48 | 94.8 | 90.5-97.2 | 0.15 | 98.8 | 94.9-99.7 | 0.07 |
| III | Non-metformin oral antihyperglycemic | 148 | 4.6 | 88.7 | 81.0-93.4 | 0.03 | 94.3 | 87.4-97.4 | 0.04* | 98.5 | 94.2-99.6 | 0.43 | 100 | 0.90 | |
| IV | Any insulin | 89 | 2.8 | 82.9 | 70.6-90.4 | 0.01 | 92.1 | 78.8-97.2 | 0.43 | 94 | 80.9-98.2 | 0.64 | 98.4 | 89.1-99.8 | 0.88 |
| V | T2DM, no meds | 126 | 3.9 | 88.0 | 79.0-93.3 | 0.002 | 75.3 | 62.0-84.5 | < 0.0001 | 96 | 89.6-98.5 | 0.28 | 97.8 | 94.5-99.5 | 0.01 |
| sHR | |||||||||||||||
| T2DM group | N | % | OM | 95% CI | p-val | BF | 95% CI | p-val | DM | 95% CI | p-val | CSM | 95% CI | p-val | |
| I | No T2DM | 2603 | 81 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | ||||
| II | Metformin | 251 | 7.8 | 0.99 | 0.65-1.52 | 0.98 | 1.22 | 0.84-1.77 | 0.29 | 1.49 | 0.78-2.85 | 0.22 | 2.13 | 0.90-5.08 | 0.09 |
| III | Non-metformin oral antihyperglycemic | 148 | 4.6 | 1.48 | 0.96-2.28 | 0.07 | 0.54 | 0.27-1.06 | 0.074 | 0.67 | 0.20-2.26 | 0.52 | 1.11 | 0.25-5.01 | 0.89 |
| IV | Any insulin | 89 | 2.8 | 2.06 | 1.17-3.63 | 0.012 | 0.60 | 0.27-1.33 | 0.21 | 1.24 | 0.38-4.04 | 0.73 | 1.20 | 0.17-8.54 | 0.86 |
| V | T2DM, no meds | 126 | 3.9 | 2.01 | 1.24-3.26 | 0.005 | 2.22 | 1.46-3.39 | < 0.001 | 1.94 | 0.76-4.86 | 0.17 | 3.91 | 1.33-11.46 | 0.013 |
Note: All p-values are pair-wise comparisons to reference group (No T2DM). Bold faced font denotes p-values < 0.05. For OM, Cox proportional hazards models were used. For all sHRs, covariates included Gleason score, T-stage, prostate specific antigen group, initial hormone therapy (Y vs N), RT type (2 levels), RT dose (three levels), treatment year, and age at start of treatment. Additionally, for OM, a history of hypertension was included.
Abbreviations: BF or FFBF: biochemical failure or freedom from biochemical failure; CI: confidence interval; CSM or CSS: cause specific survival or cause specific mortality; DM or FFDM: distant metastasis or freedom from distant metastasis; KM: Kaplan-Meier; NCCN: National Comprehensive Cancer Network; OM/OS: overall mortality / overall survival; sHR: sub hazard ratio; T2DM: type 2 diabetes mellitus
Figure 1.
Kaplan-Meier curves of FFBF for all patients (top left), FFDM (top right), CSS (lower left), and OS (lower right). The x-axis on each plot is follow-up time (in months); the y-axis is percent. The number at risk of patients on MHSs (red lines) and those not on MHSs (blue lines) are listed for reference in each plot. MHS use was associated with improved OS in this analysis; but not with a change in FFBF, DM, or CSS.
The 5-year FFBF rates for low-, intermediate-, and high-risk men were 96%, 87% (p = 0.12), and 79% (p < 0.0001), respectively (Table 1, upper portion). The 5-year FFBF rates for men in groups V were significantly worse compared to men in group I: 90% for group I (reference), 88% for group II (p = 0.48), 94% for group III (p = 0.04), 92% for group IV (p = 0.43), and 75% for group IV (p = < 0.0001), as shown in Table 1, middle portion and Figure 1, upper right panel. After adjusting for competing risk factors (Table 2, lower portion), men in group V were twice as likely to experience BF than those in group I. Men in group II (i.e. those taking metformin) had no difference in BF compared to men in group I.
The 5-year FFDM rates for low-, intermediate-, and high-risk men were 99%, 97% (p < 0.0001), and 91% (p < 0.0001), respectively (Table 1, upper portion). The FFDM rates were similar among all groups (Table 1, middle portion; Figure 1, lower left panel). After adjusting for competing risk factors (Table 2, lower portion), the FFDM rates remained similar among all of the groups. Men in group II (i.e. those taking metformin) had no difference in FFDM compared to men in group I.
The 5-year CSS rates for low-, intermediate-, and high-risk men were 100%, 99% (p = 0.12), and 97% (p < 0.0001), respectively (Table 1, upper portion). The CSS rates for group V were significantly worse than those in group I: 98% vs. 99% (p = 0.01); there was no difference in any other group compared to group I (Table 1, middle portion; Figure 1, lower right panel). After adjusting for competing risk factors (Table 2, lower portion), the cancer specific mortality was 3.87 times higher in men in group V that in group I (p = 0.01); it was 2.32 times higher in group II than in group I (borderline significant at p = 0.05).
Early toxicity analysis is displayed in Table 3, upper portion; late toxicity analysis is displayed in Table 3, lower portion and Figure 2. Early RTOG grade 2-4 GU toxicity was significantly higher in group IV vs group I (38% vs. 26%, p = 0.01). Early RTOG grade 2-4 GI toxicity was significantly higher in group IV vs I (12% vs. 5%, p = 0.01). Late RTOG grade 2-4 GU toxicity was significantly higher in group IV (11%, p = 0.001) and group V (12%, p = 0.001) than in group I (2.5%). Similarly, late RTOG grade 2-4 GI toxicity was significantly higher in group IV (14%, p = 0.01) and group V (14%, p = 0.001) than in group I (6%).
Table 3.
Patient toxicity, stratified by T2DM group.
| T2DM group | RTOG toxicity, grade 2-4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early, incidence | GU | GI | |||||||||
| N | % | % | n | p-val | % | n | p-val | ||||
| I | No T2DM | 2603 | 81 | 26 | 688 | Ref | 5 | 146 | Ref | ||
| II | Metformin | 251 | 7.8 | 28 | 76 | 0.19 | 7 | 18 | 0.31 | ||
| III | Non-metformin oral antihyperglycemic | 148 | 4.6 | 21 | 32 | 0.20 | 4 | 6 | 0.42 | ||
| IV | Any insulin | 89 | 2.8 | 38 | 34 | 0.01 | 12 | 11 | 0.01 | ||
| V | T2DM, no meds | 126 | 3.9 | 22 | 28 | 0.29 | 9 | 11 | 0.14 | ||
| Late, KM at 3 years | |||||||||||
| N | % | % | 95% CI | p-val | % | 95% CI | p-val | ||||
| I | No T2DM | 2603 | 81 | 2.5 | 1.9 | 3.3 | Ref | 5.9 | 5.0 | 7.0 | Ref |
| II | Metformin | 251 | 7.8 | 2.1 | 0.8 | 5.6 | 0.25 | 6.1 | 3.5 | 10.5 | 0.42 |
| III | Non-metformin oral antihyperglycemic | 148 | 4.6 | 4.8 | 2.2 | 10.4 | 0.18 | 7.8 | 4.2 | 14.0 | 0.49 |
| IV | Any insulin | 89 | 2.8 | 11.4 | 5.5 | 22.6 | 0.001 | 13.7 | 7.6 | 24.1 | 0.01 |
| V | T2DM, no meds | 126 | 3.9 | 11.9 | 6.3 | 21.9 | 0.001 | 13.9 | 8.4 | 22.5 | 0.001 |
Abbreviations: CI: confidence interval; KM: Kaplan-Meier; RTOG: Radiation Therapy Oncology Group; sHR: sub hazard ratio
Figure 2.
Kaplan-Meier curves of the incidence of late GU toxicities, grade 2-4 (left); and late GI toxicities, grade 2-4 (right), among the T2DM groups.
DISCUSSION
In this study, we analyzed the impact of metformin-containing oral antihyperglycemics, non-metformin oral antihyperglycemics, insulin, and non-medication controlled T2DM on the outcomes and toxicities of men with prostate cancer treated with definitive RT. We found that men with T2DM on insulin and those not on medication are twice as likely to die of non-cancer causes are those without T2DM; moreover, men with non-medication controlled T2DM are twice as likely to experience BF than those without T2DM, and they are almost four times as likely to experience death from prostate cancer than men without T2DM. With respect to toxicity, men on insulin have about a two-fold higher incidence of acute GU and GI toxicity; men on insulin and those with non-medication controlled T2DM have an eight-fold increase in late GU complications, and two-fold increase in late GI complications. Men with T2DM not on medication and men with T2DM on insulin have worse outcomes and toxicities than those without T2DM or those on oral antihyperglycemics. The type of oral antihyperglycemic (i.e. presence or absence of metformin) used for control of T2DM may be minimally important for prostate cancer; rather, the development of hyperinsulinemia should be avoided.
These findings have several implications: (1) Physicians caring for men with T2DM who are receiving RT for prostate cancer should counsel the patients and refer them to appropriate specialists (e.g. endocrinologists) who may help them with T2DM management (including proper diet and exercise). (2) These physicians should also try to select a treatment modality with minimal toxicity impact by T2DM (e.g. avoid brachytherapy with IMRT, as the complication rates are higher for men with T2DM);23, 24 subsequently, physicians should have a lower threshold to suspect toxicity in men with poorly-managed T2DM. (3) Clinical trialists evaluating toxicity as an endpoint should be mindful of patient comorbidites (including T2DM), which may predispose certain patients to worse outcomes and toxicities.25 (4) Men who are having their prostate cancer treated should be mindful of their comorbidities, they should not put these on the “backburner,” but instead continue to see physicians who will manage these conditions appropriately. (5) Further research is necessary to explore the interplay among diabetes, anti-diabetes medications, and cancer.
Men in groups II-IV (Table 1) did not have more aggressive cancers than those without T2DM, as suggested by the relatively equal distribution of patients among NCCN risk groups, Gleason score groups, PSA groups, or T-stage groups. Our findings are consistent with data from Germany and the United Kingdom, which revealed no evidence of metformin or sulfonylureas having a protective effect among multiple lung cancers,26, 27 with data from Canada that revealed no association between metformin use and prostate cancer aggressiveness,28 and with the patient characteristics from MSKCC.11 Additionally, our findings are consistent with a meta-analysis of studies showing no link between insulin use and incidence of prostate cancer.29 The results do not suggest that T2DM is a protective factor for prostate cancer.30
Men in group V were more likely to be treated with 3D-CRT than with IMRT, most likely because more of the patients in group V were treated from 1998-2001 when IMRT was not implemented at our institution. Although the outcomes with these two technologies are considered to be equivalent, toxicity is typically more frequently observed with 3D-CRT than with IMRT;20, 31, 32 thus, we controlled for this covariate when performing the toxicity analysis. It is hard to fully adjust for the difference in planning technique and reduce it to a single universal coefficient; thus, some of the toxicity may be due to treatment technique. Nonetheless, based on clinical trials32 and data from Memorial Sloan Kettering31 comparing IMRT and 3D-CRT, we would expect the rate of Grade 2+ toxicities to be <15% for 3D-CRT and <6% for IMRT.
On outcomes analysis (Table 2, Figure 1), men in groups III-V had worse OS when compared to group I; the significantly worse OS was present in groups IV and V, after controlling for covariates (Table 2, lower portion). Our findings are consistent with data from the UK, which revealed that T2DM was associated with a 23% increased risk of prostate cancer mortality (HR 1.23, 95 % CI 1.04-1.46) and a 25% increased risk in all-cause mortality (HR 1.25, 95 % CI 1.11-1.40).33 With respect to cancer-related outcomes, patients in groups II-IV did not have worse FFBF, FFDM or CSS, before or after adjustment for covariates (Table 2, middle and lower portions, respectively).
CSS is worse for group V, and this may be because distant metastases are relatively common during the disease course of patients (occurring within 5-10 years of diagnosis), vs. death from prostate cancer, which is relatively uncommon, occurring in <5-10% of patients treated with RT.32 Among all patients treated at our institution, almost all who died of prostate cancer were in group V; thus, the corresponding p-value was low and the confidence intervals were narrow. On the other hand, patients with DMs were scattered among the groups; thus, for group V, the p-value was not as low, and confidence interval was relatively wide. Additionally, it is possible that group V and diabetics in general had more comorbidities and therefore received ADT at a lesser rate (after adjusting for severity of disease) or for a shorter duration; this may also contribute to their apparent increase in cancer-specific mortality and biochemical recurrence rates.
Our findings are consistent with (1) a Saskatchewan Health database study where cancer patients with T2DM exposed to sulfonylureas and exogenous insulin had a significantly worse OS compared with patients exposed to metformin;34 (2) a UK study, where the use of metformin was not associated with a change in OS or CSS;35 and (3) a Mount Sinai study, which revealed no impact of metformin on FFBF, CSS, or OS.36 Our finding suggest that diabetes should be reported among randomized controlled trials of prostate cancer patients because these may affect outcomes and toxicities.25
The Memorial Sloan Kettering Cancer Center (MSKCC) experience11 revealed that metformin may prevent the development of castrate resistant disease. Our results support the hypothesis that insulin a growth factor and promotes tumor progression, as patients who were on oral antihyperglycemics (with or without metformin) had improved outcomes than those on insulin. Thus, oral antihyperglycemics may abrogate the negative impact of advanced T2DM; on the other hand, hyperinsulinemia further fuels cancer progression.
The mechanisms where hyperinsulinemia potentiates prostate cancer cell growth are under investigation and have overlap with those of increased obesity and adiposity.6, 37, 38 For example, hyperinsulinemia causes a decrease in sex hormone-binding globulins, increasing free unbound androgens, which stimulate hormone-response cancers (e.g. breast, prostate).39, 40 Diet-induced hyperinsulinemia accelerates tumor growth in prostate cancer xenograft models,41 purportedly by increasing insulin receptor expression.42 Additionally, insulin and IGF-I potentiate the PI3K/Akt/mTOR signaling cascade, which regulate cell growth, cell cycle progression, and angiogenesis.4-6 Finally, diabetic angiopathy may cause tumoral hypoxia, which may stimulate hypoxia inducible factor 1 alpha (HIF-1α).43
Men on insulin had a 50% to 100% higher incidence of acute GU and GI toxicity, an eight-fold increase in late GU complications, and two-fold increase in late GI complications (Table 3, lower portion). These results are similar to those of the University of Chicago, such patients had a 1.4 relative risk of ≥ grade 2 GU toxicity.44 Hypothetically, the presence of T2DM impairs leukocyte function, decreases phagocytosis, impairs bacterial killing, and impairs chemotaxis, thus decreasing host immunity.45 RT also damages endothelial cells, denuding blood vessels, resulting in diminished blood flow and capillary necrosis.46
Our study has limitations. First, it is retrospective; thus, we may infer association but not causation. Second, we do not have exact start and stop times of medications; and, since 34% of our patients took medications before initiation of RT, we may have some immortal time bias, which was suggested47 to be present in the MSKCC study.11 We do not report analyses for immortal time bias because (1) similar to the MSKCC analysis and subsequent comments of Spratt and colleagues,48 only 5% of our patients took medications after initiation of RT; in comparison, the time on any event (i.e. FFBF, FFDM, CSS) is relatively long (i.e. > 10 years from BF to cancer-related mortality) in prostate cancer patients;49 and (2) men who were on any medication did not have improved outcomes. Additionally, we did not evaluate outcomes and toxicities among other fractionation schedules (e.g. hypofractionation,50 stereotactic body RT51) or treatment modalities (e.g. brachytherapy,52 brachytherapy boost 23), though we hypothesize that outcomes and toxicities of those patients would similarly be affected. Next, we do not have medication dose information. Finally, we do not have information regarding blood glucose concentrations or Hgb A1c values (which have been shown to be prognostic for pancreatic cancer53); these would have allowed a more robust statistical analysis.
CONCLUSION
Men with T2DM not on medication and men with T2DM on insulin have worse prostate cancer outcomes and toxicities than those without T2DM or those on oral antihyperglycemics. The type of oral antihyperglycemic (i.e. presence or absence of metformin) used for control of T2DM may be minimally important for prostate cancer; rather, the development of hyperinsulinemia should be avoided.
Supplementary Material
CLINICAL PRACTICE POINTS.
• What is already known about this subject?
Type II diabetes mellitus (T2DM) is hypothesized to potentiate cancer cell growth and increase all-cause mortality among prostate cancer patients. This association is believed to be rooted on both biological evidence of insulin and insulin-like growth factors potentiating cancer cell growth and cell cycle progression and the clinical findings of increased all-cause mortality among diabetic patients as compared to their nondiabetic counterparts
• What are the new findings?
For men receiving radiation therapy for prostate cancer, those on insulin and those not on any medication have increased risk of death and toxicity than those without diabetes. The type of oral medication (i.e. whether or not it contains metformin) is not as important as avoiding hyperinsulinemia (i.e. either from non-management of the disease or the use of insulin).
• How might it impact on clinical practice in the foreseeable future?
Clinicians may use this information to optimally manage diabetes among prostate cancer patients. Additionally, since men not on medication and those on insulin have the worst outcomes and toxicities among all patients, they would be able to enroll these patients on proper clinical trials -- i.e. trials that focus on lifestyle management rather than more aggressive fractionation schemes which may have worse toxicities.
Acknowledgments
Funding sources: This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This publication was supported in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Varian Medical Systems, Inc. No funding agency participated in the design, implementation, analysis, or interpretation of data.
Footnotes
Conflicts of Interest Notification: We have no conflicts of interests.
Approval/disclosures: All authors have read and approved the manuscript. This manuscript is not under consideration at any other journal. We have no financial disclosures. We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this paper. No text, text boxes, figures, or tables in this article have been previously published or owned by another party. This study was approved by the IRB, protocol number IRB 03-835. This study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study. This is a retrospective analysis, and this article does not contain any studies with human subjects performed by any of the authors.
Contributor Information
Nicholas G Zaorsky, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Talha Shaikh, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Karen Ruth, Biostatistics and Bioinformatics Facility, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Pankaj Sharda, Department of Endocrinology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Shelly B Hayes, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Mark L Sobczak, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Mark A Hallman, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Marc C Smaldone, Department of Surgical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
David YT Chen, Department of Surgical Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
Eric M Horwitz, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA..
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA. Cancer J. Clin 2012;62:220–241. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri CE, Bangma CH, Bjartell A, et al. The mutational landscape of prostate cancer. Eur. Urol 2013;64:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song K, Shankar E, Yang J, Bane KL, Wahdan-Alaswad R, Danielpour D. Critical role of a survivin/TGF-beta/mTORC1 axis in IGF-I-mediated growth of prostate epithelial cells. PLoS ONE. 2013;8:e61896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal RR, Ryan CJ, Chan JM. Insulin-like growth factor pathway: a link between androgen deprivation therapy (ADT), insulin resistance, and disease progression in patients with prostate cancer? Urologic oncology. 2013;31:522–530. [DOI] [PubMed] [Google Scholar]
- 6.Palmer JD, Soule BP, Simone BA, Zaorsky NG, Jin L, Simone NL. MicroRNA expression altered by diet: can food be medicinal? Ageing Res. Rev 2014;17:16–24. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Ma Y, Sinyuk M, et al. Insulin-mediated signaling promotes proliferation and survival of glioblastoma through Akt activation. Neuro Oncol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Beer JC, Liebenberg L. Does cancer risk increase with HbA1c, independent of diabetes? Br. J. Cancer 2014;110:2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol 2013;63:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margel D, Urbach DR, Lipscombe LL, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J. Clin. Oncol 2013;31:3069–3075. [DOI] [PubMed] [Google Scholar]
- 13.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 1.2014. J. Natl. Compr. Canc. Netw 2013;11:1471–1479. [DOI] [PubMed] [Google Scholar]
- 14.Zaorsky NG, Li T, Devarajan K, Horwitz EM, Buyyounouski MK. Assessment of the American Joint Committee on Cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the National Comprehensive Cancer Network risk-stratification method. Cancer. 2012;118:5535–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann. Oncol 2011;22:2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int. J. Radiat. Oncol. Biol. Phys 2006;64:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaorsky NG, Harrison AS, Trabulsi EJ, et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol. 2013;10:565–579. [DOI] [PubMed] [Google Scholar]
- 21.Abramowitz MC, Li TN, Buyyounouski MK, et al. The phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. 2008;112:55–60. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Zaorsky NG, Doyle LA, Yamoah K, et al. High dose rate brachytherapy boost for prostate cancer: A systematic review. Cancer Treat. Rev 2014;40:414–425. [DOI] [PubMed] [Google Scholar]
- 24.Zaorsky NG, Shaikh T, Murphy CT, et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat. Rev 2016;48:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaorsky NG, Egleston BL, Horwitz EM, et al. The Missing Pieces in Reporting of Randomized Controlled Trials of External Beam Radiation Therapy Dose Escalation for Prostate Cancer. Am. J. Clin. Oncol 2016. [DOI] [PubMed] [Google Scholar]
- 26.Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf. 2015;24:865–874. [DOI] [PubMed] [Google Scholar]
- 27.Tsilidis KK, Capothanassi D, Allen NE, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37:2522–2532. [DOI] [PubMed] [Google Scholar]
- 28.Margel D, Urbach D, Lipscombe LL, et al. Association between metformin use and risk of prostate cancer and its grade. J. Natl. Cancer Inst 2013;105:1123–1131. [DOI] [PubMed] [Google Scholar]
- 29.Chen YB, Chen Q, Wang Z, Zhou J. Insulin therapy and risk of prostate cancer: a systematic review and meta-analysis of observational studies. PLoS ONE. 2013;8:e81594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastmanesh R, Hejazi J, Marotta F, Hara N. Type 2 diabetes: a protective factor for prostate cancer? An overview of proposed mechanisms. Clin. Genitourin. Cancer 2014;12:143–148. [DOI] [PubMed] [Google Scholar]
- 31.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 2008;70:1124–1129. [DOI] [PubMed] [Google Scholar]
- 32.Zaorsky NG, Keith SW, Shaikh T, et al. Impact of Radiation Therapy Dose Escalation on Prostate Cancer Outcomes and Toxicities. Am. J. Clin. Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 2014;25:329–338. [DOI] [PubMed] [Google Scholar]
- 34.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29:1990–1991. [DOI] [PubMed] [Google Scholar]
- 35.Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol. Biomarkers Prev 2014;23:2111–2118. [DOI] [PubMed] [Google Scholar]
- 36.Taira AV, Merrick GS, Galbreath RW, Morris M, Butler WM, Adamovich E. Metformin is not associated with improved biochemical free survival or cause-specific survival in men with prostate cancer treated with permanent interstitial brachytherapy. Journal of contemporary brachytherapy. 2014;6:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LS, Murphy CT, Ruth K, et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2015;121:3010–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur. Urol 2013;63:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalving M, Gietema JA, Lefrandt JD, et al. Metformin: taking away the candy for cancer? Eur. J. Cancer. 2010;46:2369–2380. [DOI] [PubMed] [Google Scholar]
- 40.Zaorsky NG, Trabulsi EJ, Lin J, Den RB. Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin. Oncol 2013;40:308–321. [DOI] [PubMed] [Google Scholar]
- 41.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J. Natl. Cancer Inst 2007;99:1793–1800. [DOI] [PubMed] [Google Scholar]
- 42.Cox ME, Gleave ME, Zakikhani M, et al. Insulin receptor expression by human prostate cancers. Prostate. 2009;69:33–40. [DOI] [PubMed] [Google Scholar]
- 43.Fraga A, Ribeiro R, Principe P, Lopes C, Medeiros R. Hypoxia and Prostate Cancer Aggressiveness: A Tale With Many Endings. Clin. Genitourin. Cancer 2015;13:295–301. [DOI] [PubMed] [Google Scholar]
- 44.Kalakota K, Liauw SL. Toxicity after external beam radiotherapy for prostate cancer: an analysis of late morbidity in men with diabetes mellitus. Urology. 2013;81:1196–1201. [DOI] [PubMed] [Google Scholar]
- 45.Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit. Care Med 2005;33:1624–1633. [DOI] [PubMed] [Google Scholar]
- 46.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. [DOI] [PubMed] [Google Scholar]
- 47.Bensimon L, Suissa S, Azoulay L. Re: Spratt Daniel E., Zhang Chi, Zumsteg Zachary S., Pei Xin, Zhang Zhigang, Zelefsky Michael J.. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63:709–16. Eur. Urol. 2013;64:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spratt DE, Zhang Z, Zelefsky MJ. Reply to Bensimon Leah, Suissa Samyand Azoulay’s Laurent letter to the editor re: Spratt Daniel E., Zhang Chi, Zumsteg Zachary S., Pei Xin, Zhang Zhigang, Zelefsky Michael J.. metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63:709–16. Eur. Urol. 2013;64:e29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur. Urol 2015;67:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaorsky NG, Ohri N, Showalter TN, Dicker AP, Den RB. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treat. Rev 2013;39:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaorsky NG, Studenski MT, Dicker AP, Gomella L, Den RB. Stereotactic body radiation therapy for prostate cancer: is the technology ready to be the standard of care? Cancer Treat. Rev 2013;39:212–218. [DOI] [PubMed] [Google Scholar]
- 52.Zaorsky NG, Doyle LA, Hurwitz MD, Dicker AP, Den RB. Do theoretical potential and advanced technology justify the use of high-dose rate brachytherapy as monotherapy for prostate cancer? Expert Rev. Anticancer Ther 2014;14:39–50. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Garcia Rodriguez LA, Malgerud L, et al. New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br. J. Cancer 2015;113:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




