Abstract
Intravitreal injections (IVI) of anti–vascular endothelial growth factor (anti–VEGF) agents have become the most prevalent intraocular procedure as they represent the major therapeutic modality for prevalent retinal conditions such as age-related macular degeneration (AMD) and diabetic retinopathy. Effective therapy requires adherence to a schedule of iterative IVI as well as routine clinic appointments. The ongoing coronavirus disease 2019 (COVID-19) pandemic has resulted in the reduction of attendance at scheduled clinic visits and IVI. In this study, we attempted to analyze the effect of COVID-19 on compliance with anti–VEGF therapy. A total of 636 eyes received injections during a 4-week period of the COVID-19 outbreak in the Retina Clinic. The number of clinic visits for IVI during 1 month from March 15 to April 14 of 2020 was compared to a similar time period in each of the last 4 years. The study demonstrates a decrease in clinic visits for IVI when compared with the same 4-week interval in the four previous years. Based on the trend of the previous 4 years, 10.2% of the year’s total was expected for this time period. Using this model, the 636 reported number of injections for the March–April 2020 period was ~ 5%. This represents a decrease of ~ 50% of the expected IVI for this time period. The COVID-19 outbreak in Israel severely impacted compliance with anti–VEGF treatments.
Keywords: Intravitreal injections, Coronavirus, Pandemic, Compliance
Introduction
Intravitreal injections (IVI) are a prevalent intraocular procedure in the ophthalmology clinic and have been described to treat various retinal pathologies [1]. Individuals often receive serial injections and require these treatments on a regular basis [2]. Compliance with the treatment prescription is crucial [3–8]. Even slight deviations may be associated with decreased vision [9]. The coronavirus (COVID-19) outbreak in Israel resulted in a curtailment of ophthalmologic clinic appointments. This began in mid-March 2020 by order of the Ministry of Health of the State of Israel in order for hospitals and healthcare facilities to accommodate the onslaught of COVID-19 patients. To assess the risk of potential visual loss, we compared IVI clinic attendance during the pandemic to clinic attendance in previous years.
Methods
Data Collection
Initially, the number of intravitreal injections performed at the Shaare Zedek Medical Center retina clinic during 4 weeks of the COVID-19 pandemic in March 15 to April 14, 2020, was analyzed. Injections with anti–vascular endothelial growth factor (anti–VEGF) agents bevacizumab (Avastin), ranibizumab (Lucentis), and aflibercept (Eylea) were analyzed jointly and individually. These results were compared to the same time period in the previous 4 years, March 15 to April 14 of 2016, 2017, 2018, and 2019. The Shaare Zedek Medical Center Institutional Ethics Committee approved the study and did not require an individual informed consent from the subjects. All indications for anti–VEGF were included, regardless of diagnosis, duration of treatment, or previous compliance to the injection schedule. Individual injections were tabulated, and patients who received treatment for both eyes were counted twice.
Statistical Analysis
All data collected in this study were analyzed using SPSS software (SPSS 24.0, SPSS Science, Chicago, IL, USA). Linear regression was performed for the present analysis.
Results
A total of 636 injections were performed during the period of the COVID-19 outbreak in Israel, from March 15 to April 14, 2020 (Table 1). Of these, there were 364 injections of bevacizumab, 75 injections of ranibizumab, and 197 injections of aflibercept. These injections were compared to the injections performed in March 15 to April 14 of the four previous years during which time the number of IVI in the retina clinic steadily increased. The gradual increase of IVI during these 4 weeks is consistent with the annual increase in IVI in our center over the last several years. In 2015, 6880 injections were performed, 8143 in 2016, 8547 in 2017, 10,570 in 2018, and 12,349 in 2019. When comparing March 15 to April 14, 2020, to the same period in 2019, we see an overall drop of 36% for all IVI: 44% for bevacizumab, 17% for ranibizumab, and 22% for aflibercept. For this period of time, we have precise figures and the March–April proportion is approximately 10.2% of the year’s total. Using a linear regression approach for analyzing the data, the findings showed a very clear and significant trend (adjusted R2 = .552, p < .001). Based on this model, it was expected that the injections would increase to 12,672 in 2020. As a proportion of what is expected for the entire year, the 636 reported number of injections for the March–April 2020 period was about 5%, less than half the actual proportion observed in previous years.
Table 1.
Number of intravitreal injections during 4 weeks of the COVID-19 pandemic as compared to the same month in previous years
| March 15 to April 14 | Bevacizumab | Ranibizumab | Aflibercept | Total |
|---|---|---|---|---|
| 2016 | 614 | 130 | 40 | 784 |
| 2017 | 531 | 87 | 115 | 733 |
| 2018 | 523 | 57 | 152 | 732 |
| 2019 | 654 | 90 | 251 | 995 |
| 2020 | 364 | 75 | 197 | 636 |
As compared to the same period in 2019, there is a decrease for all intravitreally injected anti–VEGF compounds. Throughout the 5 years presented, bevacizumab reached a low point during the COVID-19 crisis
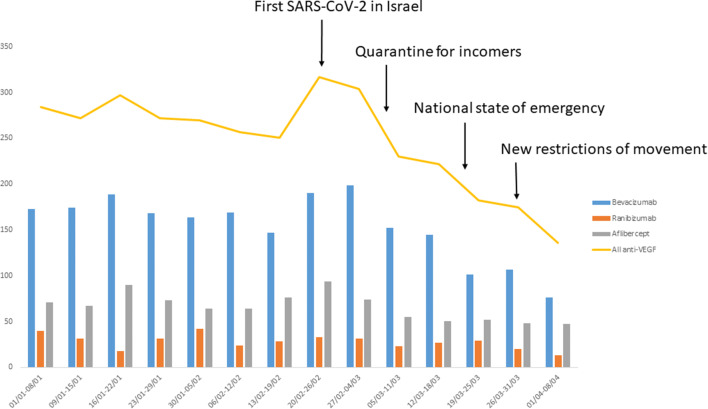
Figure 1 represents the downward trend of IVI over the time associated with the COVID-19 outbreak milestones in Israel. From the week of February 20, 2020, to the week of April 1, 2020, there was a 58% decrease of total IVI: 60% for bevacizumab, 61% for ranibizumab, and 50% for aflibercept.
Fig. 1.
As governmental measures became more stringent, the number of delivered injections dropped
Discussion
This analysis demonstrates a marked decrease in IVI of anti–VEGF agents delivered during a 4-week period of the COVID-19 outbreak. The decrease in IVI correlated with the advancing coronavirus outbreak in Israel, namely increasing numbers of individuals testing positive for the virus, hospitalizations, and deaths. The outbreak resulted in an increase in governmental restriction on local and foreign travel and quarantine of Jerusalem neighborhoods. Hospital services including elective surgeries and clinics were curtailed. Although IVI were considered essential clinic visits and this service was not curtailed, this study indicates that many individuals requiring IVI chose not to attend previously scheduled appointments.
Compliance in individuals obtaining IVI has been previously described to vary based on the treated condition [10, 11]. The risk for mortality from COVID-19 rises substantially in individuals older than 65 years of age [12], those living in skilled nursing facilities [13], and individuals with co-morbidities such as diabetes [14] and obesity [15]. As the two major indications for iterative IVI are neovascular AMD and diabetic ocular complications [16–18], these individuals are precisely those at highest risk for COVID-19-associated mortality. Furthermore, since viral transmission has been documented in asymptomatic individuals, it is understandable that an ophthalmologic patient may be fearful regarding exposure to coronavirus at a crowded outpatient hospital [19–21]. A study from Portugal describes a similar 33% decrease (from 304 to 204) in IVI injections from January to April 2020 due to the COVID-19 pandemic [22].
As ophthalmologic specialists, we are necessarily concentrating our efforts on the ophthalmologic care of our patients and are concerned about resultant potential vision loss from missing IVI [23]. However, in this circumstance, it is our duty to balance the desire to treat their ophthalmic disease while protecting them from being harmed from inadvertent viral transmission [23–32]. Several ways may be considered to improve delivery of retinal care [33]. One option is to provide home injections with appropriate protection of the healthcare workers [34]. A second option may be to concentrate IVI in skilled nursing facilities and day care centers. A third option, albeit a more distant goal, is accelerating the development of long-acting anti–VEGF therapeutics which would reduce the frequency of clinic visits.
Limitations include the relatively brief period and the single-center nature of the study.
Conclusions
During the 4-week period of the COVID-19 outbreak in Israel, we observed a greater than 50% drop in attendance at the IVI in our Retina Clinic. It became evident that many of our patients were being lost to follow-up. This marked decrease in attendance is understandable taking into consideration that many of these individuals exhibit co-morbidities that place them at increased risk for mortality should they contract COVID-19 infection. It is imperative, however, that we begin to plan for the consequences of this missed therapy.
Abbreviations
- IVI
Intravitreal injections
- Anti–VEGF
Anti–vascular endothelial growth factor
- COVID-19
Coronavirus 19
- AMD
Age-related macular degeneration
Author Contributions
All of the authors contributed substantially to this work, participated in the drafting and writing of the manuscript, and approved the final version prior to submission.
Data Availability
The datasets analyzed during the current study are not publicly available due to the concern for patient safety and confidentiality but are available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
The Shaare Zedek Medical Center Institutional Ethics Committee approved the study and did not require an individual informed consent from the subjects.
Consent for Publication
Not applicable.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parikh R, Pirakitikulr N, Chhablani J, Sakurada Y, Singh RP, Modi YS. A multinational comparison of anti-vascular endothelial growth factor use: the United States, the United Kingdom, and Asia-Pacific, (in eng) Ophthalmol Retina. 2019;3(1):16–26. doi: 10.1016/j.oret.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Haller JA. Current anti-vascular endothelial growth factor dosing regimens: benefits and burden, (in eng) Ophthalmology. 2013;120(5 Suppl):S3–S7. doi: 10.1016/j.ophtha.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 3.Angermann R, et al. Treatment compliance and adherence among patients with diabetic retinopathy and age-related macular degeneration treated by anti-vascular endothelial growth factor under universal health coverage, (in eng) Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2119–2125. doi: 10.1007/s00417-019-04414-y. [DOI] [PubMed] [Google Scholar]
- 4.Sun JK, Wang PW, Taylor S, Haskova Z. Durability of diabetic retinopathy improvement with as-needed ranibizumab: open-label extension of RIDE and RISE studies, (in eng) Ophthalmology. 2019;126(5):712–720. doi: 10.1016/j.ophtha.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, et al. Monthly versus as-needed ranibizumab injections in patients with retinal vein occlusion: the SHORE study, (in eng) Ophthalmology. 2014;121(12):2432–2442. doi: 10.1016/j.ophtha.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Ehlken C, Helms M, Bohringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients, (in eng) Clin Ophthalmol (Auckland, NZ) 2018;12:13–20. doi: 10.2147/opth.s151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regillo CD, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1, (in eng) Am J Ophthalmol. 2008;145(2):239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Ziemssen F, et al. Demographics of patients receiving intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials, (in eng) BMC Ophthalmol. 2017;17(1):7. doi: 10.1186/s12886-017-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massamba N, Dirani A, Knoeri J, Pasquier B, Ingram A, Soubrane G. Evaluating the impact of summer vacation on the visual acuity of AMD patients treated with ranibizumab, (in eng) Eye (London, England) 2015;29(11):1453–1457. doi: 10.1038/eye.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss M, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice, (in eng) Retina (Philadelphia, Pa) 2018;38(12):2293–2300. doi: 10.1097/iae.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 11.Polat O, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration, (in eng) Turk J Ophthalmol. 2017;47(4):205–210. doi: 10.4274/tjo.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan A, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China, (in eng). Jama. 2020. 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed]
- 13.American Geriatrics Society (AGS) Policy Brief: COVID-19 and nursing homes, (in eng). J Am Geriatr Soc. 2020. 10.1111/jgs.16477.
- 14.Stoian AP, Banerjee Y, Rizvi AA, Rizzo M. Diabetes and the COVID-19 pandemic: how insights from recent experience might guide future management, (in eng). Metab Syndr Relat Disord. 2020. 10.1089/met.2020.0037. [DOI] [PMC free article] [PubMed]
- 15.Simonnet A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation, (in eng). Obesity (Silver Spring, Md). 2020. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed]
- 16.Wecker T, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV, (in eng) Br J Ophthalmol. 2017;101(3):353–359. doi: 10.1136/bjophthalmol-2016-308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham B, et al. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis, (in eng) BMJ Open. 2019;9(5):e022031. doi: 10.1136/bmjopen-2018-022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain P, Sheth J, Anantharaman G, Gopalakrishnan M. Real-world evidence of safety profile of intravitreal bevacizumab (Avastin) in an Indian scenario, (in eng) Indian J Ophthalmol. 2017;65(7):596–602. doi: 10.4103/ijo.IJO_992_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima CKT, et al. The emotional impact of coronavirus 2019-nCoV (new coronavirus disease), (in eng) Psychiatry Res. 2020;287:112915. doi: 10.1016/j.psychres.2020.112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China, (in eng). Int J Environ Res Public Health. 2020;17(5). 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed]
- 21.The L. COVID-19: protecting health-care workers, (in eng) Lancet (London, England) 2020;395(10228):922. doi: 10.1016/s0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos A, Oliveira N, Martins J, Arruda H, Sousa J. The paradigm shift of ophthalmology in the COVID-19 era. Clin Ophthalmol. 2020;14:2625–2630. doi: 10.2147/OPTH.S267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minocha A, Sim SY, Than J, Vakros G. Survey of ophthalmology practitioners in a&E on current COVID-19 guidance at three major UK eye hospitals, (in eng). Eye (London, England). 2020. 10.1038/s41433-020-0857-5. [DOI] [PMC free article] [PubMed]
- 24.Olivia Li J-P, et al. Preparedness among ophthalmologists: during and beyond the COVID-19 pandemic. Ophthalmology. 2020;127(5):569–572. doi: 10.1016/j.ophtha.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrelli E, et al. Taking the right measures to control COVID-19 in ophthalmology: the experience of a tertiary eye care referral center in Italy, (in eng). Eye (London, England). 2020. 10.1038/s41433-020-0880-6. [DOI] [PMC free article] [PubMed]
- 26.Du H, Zhang M, Zhang H, Sun X. Practical experience on emergency ophthalmic surgery during the prevalence of COVID-19, (in eng). Graefes Arch Clin Exp Ophthalmol. 2020. 10.1007/s00417-020-04692-x. [DOI] [PMC free article] [PubMed]
- 27.Lim LW, et al. Sustainable practice of ophthalmology during COVID-19: challenges and solutions, (in eng). Graefes Arch Clin Exp Ophthalmol. 2020. 10.1007/s00417-020-04682-z. [DOI] [PMC free article] [PubMed]
- 28.Ma X, Lin J, Fang S. Precautions in ophthalmic practice in a hospital with the risk of COVID-19: experience from China, (in eng). Acta Ophthalmol. 2020. 10.1111/aos.14436. [DOI] [PMC free article] [PubMed]
- 29.Petrovski BE, et al. Reorganize and survive-a recommendation for healthcare services affected by COVID-19-the ophthalmology experience, (in eng). Eye (London, England). 2020. 10.1038/s41433-020-0871-7. [DOI] [PMC free article] [PubMed]
- 30.Romano MR, et al. Facing COVID-19 in ophthalmology department, (in eng). Curr Eye Res. 2020. 10.1080/02713683.2020.1752737. [DOI] [PubMed]
- 31.Sadhu S, et al. COVID-19: limiting the risks for eye care professionals, (in eng). Ocul Immunol Inflamm. 2020:1–7. 10.1080/09273948.2020.1755442. [DOI] [PubMed]
- 32.Li KZ, Yong VKY, Lee LKM, Chin CF, Yip LWL. When ophthalmologists step up to the COVID-19 frontlines, (in eng). Eye (London, England). 2020. 10.1038/s41433-020-0918-9. [DOI] [PMC free article] [PubMed]
- 33.Shih CK, Chan JCH, Lai JSM. Maintenance of ophthalmic specialist out-patient service during the COVID-19 outbreak: the University of Hong Kong experience, (in eng). Eye (London, England). 2020. 10.1038/s41433-020-0887-z. [DOI] [PMC free article] [PubMed]
- 34.Starr MR, Barkmeier AJ, Engman SJ, Kitzmann A, Bakri SJ. Telemedicine in the management of exudative age-related macular degeneration within an integrated health care system. Am J Ophthalmol. 2019;208:206–210. doi: 10.1016/j.ajo.2019.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to the concern for patient safety and confidentiality but are available from the corresponding author upon reasonable request.