Abstract
In people with young onset Parkinson’s disease (YOPD), onset of symptoms is between 21 and 40 years of age. The distinction between YOPD and late-onset Parkinson’s disease is supported by genetic differences (a genetic etiology is more common in people with YOPD) and clinical differences (e.g., dystonia and levodopa-induced dyskinesias are more common inYOPD). Moreover, people with YOPD tend to have different family and societal engagements compared to those with late-onset PD. These unique features have implications for clinical management, and call for a tailored multidisplinary approach involving shared-decision making.
Keywords: Parkinsons’s disease, young-onset, quality of life, work, caregiver, genetics, dystonia
INTRODUCTION
The prevalence of Parkinson’s disease (PD) rises sharply with age, reaching 2.6% in people aged 85 to 89 years [1, 2]. In the Western world, the mean age of onset of PD is in the early-to-mid 60s [3], but in 3–5% of cases symptoms start decades earlier, before the age of 40 [4, 5]. In Japan, higher percentages of early onset PD have been reported (up to 10–14%) [4], possibly due to a higher genetic suspectability. Early-onset PD can be further subdivided into the rare juvenile parkinsonism and young-onset PD (YOPD) [6, 7]. Disease onset of juvenile parkinsonism is below 21 years, while the age of onset of YOPD lies between 21 and 40 years, although some studies use 50 years of age as the cut off [8]. The distinction between juvenile parkinsonism and YOPD is supported by clinical, pathological and genetical differences [6]. Genotypical and phenotypical differences have also identified between YOPD and late-onset PD. Moreover, people with YOPD tend to have other roles in society compared to those with late-onset PD [9]. These differences make YOPD a unique group, which requires a personalized multidisciplinary approach to management, assessing and subsequently targeting the specific needs of people with YOPD (see Box 1). In this viewpoint we highlight unique features of YOPD, and its implications for daily clinical practice.
GENETICS IN YOPD AND ITS IMPLICATIONS FOR MANAGEMENT
The genetic background of PD is gradually being revealed and consists of the spectrum from common variants that have small contributions to an increased vulnerability, to true monogenic forms [10]. Some of the genes that previously received a PARK locus symbol are in fact unconfirmed, are risk alleles, or— if mutated— give rise to a more complex phenotype. A new nomenclature of genetic movement disorders, including PD, was recently proposed and has tried to deal with these complexities [11]. Here, we focus on the confirmed genes that can be considered monogenic forms of PD. These mainly include the dominant genes SNCA, LRRK2, GBA, and VPS35, and the recessive genes Parkin, PINK1, DJ1. The common picture from the literature is that PD patients with a mutation in one of these genes present at an earlier age, particularly for the recessive genes and SNCA [12]. So, vice versa, if a PD patient presents at a young age, the option of a genetic etiology is often considered. While next generation sequencing platforms have simplified screening the relevant genes, we have to critically address the question: what is the actual benefit of genetic testing in YOPD?
In practice, the yield of genetic testing is relatively limited, with the exception of selective testing in certain ethnic populations (e.g., LRRK2 mutations in Ashkenazi Jewish PD patients), and often significantly lower than published in papers that screened cohorts soon after a new gene was discovered. Knowledge of the a priori chance of an underlying gene mutation is crucial for pre-test counseling sessions. Also, there are some important issues that make genetic counseling a very challenging matter in PD, particularly in terms of risk predictions for family members and their offspring. These issues include 1) the incomplete penetrance of some variants, e.g., in LRRK2; 2) the ongoing controversy of whether single heterozygous mutations in the recessive PD genes impose an increased risk to develop PD; and 3) the difficulties to follow-up and interpret variants of unknown significance or previously unreported variants in one of the genes. The advantage of an identified PD gene mutation in the diagnostic process of YOPD is limited to those with a very early onset (especially a juvenile onset) [7] and those with complex or atypical phenotypes, as this will end any further diagnostic odysseys related to the long and exotic differential of young-onset parkinsonism.
In terms of prognostic value, the identification of a mutation might allow some predictions on the further evolution of the disease. However, even within one genotype, there is a large clinical variability. Also, differences in relevant disease milestones are more likely related to the younger age at onset, rather than to the gene involved. For example, one study of YOPD patients with versus without Parkin mutations found that these two groups were clinically indistinguishable [13].
Hence, genetic testing should not be considered lightly as a diagnostic test. Importantly, for the majority of people with YOPD, family planning will be affected if a gene mutation is detected [14]. Still it will increasingly be a topic in YOPD consultations. A previous study showed that PD patients have a high level of interest in genetics and genetic testing, but at the same time they seem to lack genetic knowledge and overestimate the risk of a genetic mutation [15]. This clearly indicates that genetic counseling is necessary to make well informed decisions about whether or not genetic testing is desired. Genetic testing should therefore be done at centres that have proven experience in the clinical aspects, counseling dilemma’s, and genetic pitfalls of testing the PD genes. These experiences should be shared to improve clinical practice of testing PD genes.
Until recently, the absence of therapeutic consequences of an identified PD gene mutation was another reason to hold a reserved attitude towards genetic testing in PD. However, gene-specific interventions are now entering clinical trials, such as for GBA and for LRRK2. Many patients are, via internet and social media, aware of these developments and want genetic testing done for this reason. There are also recent indications that gene status might also affect outcome of DBS in PD. Carriers of a GBA mutation were more likely to develop cognitive impairment during the follow-up after DBS [16], while carriers of the G2019S andLRRK2 mutation were suggested to have better DBS outcomes compared to non-carriers [17]. These findings have to be confirmed, but fuel an interesting area of research referred to as ‘surgicogenomics’. These emerging therapeutic implications for specific genotypes will probably be the main driver that will change the attitude towards genetic testing in patients and doctors alike.
CLINICAL ASPECTS of YOPD AND ITS IMPLICATIONS FOR MANAGEMENT
Several phenotypical differences between YOPD and late-onset PD have been identified at group level [18]. Here, we elaborate on those phenotypical differences that significantly impact daily clinical practice.
Dystonia
At early disease stages, dystonia is more common in YOPD than in late-onset PD [19]. Dystonia is a well-known feature of later stages of classic PD, either as part of levodopa-induced dyskinesias or as a disease-related motor feature (e.g., striatal hand and antecollis). However, in drug-naive YOPD, dystonia is a very common, early co-existing and occasionally presenting feature. Especially exercise-induced dystonia in patients aged 21 or older should always raise the suspicion of PD [19]. This is typically a mobile dystonia that can affect all body parts, although foot or leg involvement seems to be the most common. We have seen people with YOPD presenting with writer’s cramp, exercise-induced dystonia of the toes (‘dystonic claudication’), and retrocollis that responded to a sensory trick. While in such cases, the dystonia is clearly an intrinsic part of the phenotype, the response to levodopa does not always parallel that of the hypokinetic-rigid features. Alternative strategies are required in many people with YOPD, including oral medications (e.g., anticholinergics), physiotherapy (e.g., taping to elicit the sensory trick effect) or botulinum toxin injections. In some patients, GPi or STN deep brain stimulation can be considered to treat treatment-refractory dystonia, even if hypokinetic-rigid features are well controlled with levodopa.
Levodopa-induced dyskinesias
Second, in addition to dystonia, levodopa-induced dyskinesias are more common in YOPD than in late-onset PD [20–23]. It is not unravelled yet why levodopa-induced dyskinesias are more common in young patients [24]. Potentially, the development of levodopa-induced dyskinesias reflects a greater capacity to exhibit maladaptive plastic responses [25]. Alternatively, higher synaptic dopamine turnovers have been reported in YOPD patients compared to patients with late-onset PD, resulting in larger swing in dopamine synaptic levels, and possibly contributing to the occurrence of levodopa-induced dyskinesias [26].
Although there is consensus that levodopa is still the most effective therapy for treating motor symptoms in PD, the relative high risk of developing levodopa-induced dyskinesias in YOPD and the small amount of young onset patients included in trials could explain why there is still reluctance to start with levodopa in some patients and clinicians [27–29]. For instance in the PDMED study [30] only 12% of patients had an age under 60, and in the recently published LEAP study [31] a mere 11% of patients was under 50 years of age (personal communication). In a recent review on the initiation of treatment in PD [32], the authors state that young age is an important factor to consider alternatives to levodopa. Importantly, alternatives for levodopa (e.g., dopamine agonists and MAO-B-inhibitors) are not without side effects either. Hence, starting pharmacological treatment in people with YOPD has to be made in close collaboration with the patient and their caregivers and carefully monitored. There is need for a patient centered approach using shared decision making. In our opinion, the choice of the drug depends on the impact of improving motor disability (better with levodopa compared to dopamine agonist of MAO-B inhibitor) in relation to the risk of motor complications (more risk of motor complications with levodopa compared to dopamine agonists and MAO-B-inhibitors, especially at younger age) and the risk of neuropsychiatric complications (higher risk with dopamine agonists, compared to levodopa and MAO-B-inhibitors [33], especially in male patients with younger age [34]). Later on in the disease course DBS is an important consideration in YOPD patients. The EARLYSTIM study, with a mean age at inclusion of 52 years and mean disease duration of 7 years, suggests that this treatment should be considered early in the disease course of YOPD patients with motor complications [35].
Anxiety and depression
When looking at anxiety, conflicting epidemiological data have been reported. One study using case-series of 79 patients reported that patients with YOPD are more likely to experience anxiety compared to late onset-PD [36], but a disadvantage of the latter study is that patients were dichotomised into young-onset and late-onset PD using a cut-off of 62 years, which is not in accordance with the commonly used definitions of YOPD [6]. In contrast to the latter findings, it has also been reported that anxiety is more common in late-onset PD compared to YOPD [37]. Moreover, others did not find a difference in anxiety rates between YOPD and late-onset PD [38]. When compared to the general population, anxiety is more common in YOPD [9]. Conflicting results are also present when looking at the presence of depression, as higher [22, 39, 40], equal [38], and lower incidences [41] have been reported, but the general consensus is that depression is more common in people with YOPD compared to PD in general [42]. Hence, in YOPD, evaluation of mood is of particular importance, and both pharmacological and non-pharmacological treatment modalities (such as cognitive behavioural therapy) should be made available [43].
CIRCUMSTANCES AND SOCIETAL ENGAGEMENT IN YOPD AND IMPLICATIONS FOR MANAGEMENT
In general, people with YOPD tend to have different family and societal engagements to those with late-onset PD. For example, most people diagnosed with YOPD will have a job, whereas some people with late-onset PD have already retired. Additionaly, it is not unusual that people with YOPD have young children (who need to be educated about the disease), or may want to start a family.
The patient’s perspective
How YOPD impacts on social engagement may differ between patients.
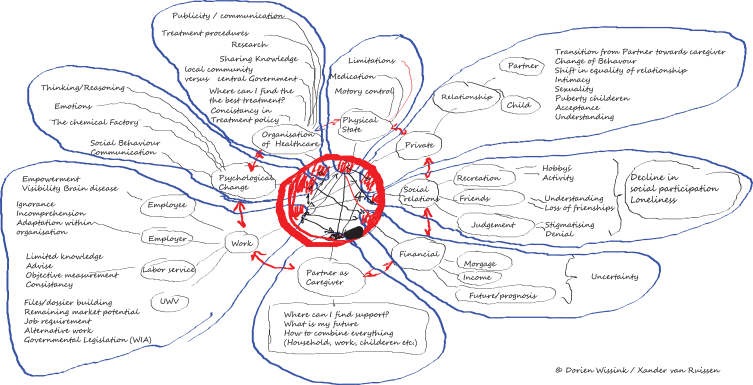
At our center, creating a mind map provides a tool to assess the needs for (young persons) living with Parkinson’s disease, which subsequently helps to organize personalized healthcare using cocreation (see Box 1 and Fig. 1). Common topics include pregnancy, work and relations, on which we elaborate here.
Fig. 1.
Mindmap visualizing the challenges faced by a young onset Parkinson’s disease patient. This mindmap displays the clinical aspects, circumstances, societal engagements and complexity of the young onset Parkinson’s disease patient. It helps to facilitate person-centred care based on the individual needs of the person living with Parkinson’s disease on a young age. This mindmap is created by Xander van Ruissen (YOPD patient and co-author of this paper) and his wife Dorien Wissink.
Pregnancy
The incidence of pregnancy during PD is unknown, and available knowledge is based on case reports and case-series [44, 45]. In about 50% of women with YOPD, symptoms seem to deteriorate during pregnancy. The risk of deterioration is smaller when dopaminergic medication is continued and when needed adjusted throughout pregnancy. The mechanism underlying a worsening of symptoms during pregnancy is likely multifactorial, including hormonal changes, physiological changes resulting in altered pharmacokinetics, and physical and social stress. The effect of anti-parkinsonian medication during pregnancy has been best documented for levodopa/carbidopa, with no evidence of major fetal abnormalities and a small amount of pregnancy related complications effects [44]. Levodopa appears to be the safest option as first-line treatment in pregnant women with YOPD, although this continues to be an area of further study and the proposed prospective registry in the latest review on this topic seems to be a good start [44]. Data on the effects of other pharmacological agents during pregnancy is limited [45]. Amantadine should be avoided as it has been associated with teratogenicity in both animal and human studies [46]. The effects of DBS seems to be safe although data are based on a small number of cases [47]. Compared to the normal population, there appears to be no differences in deliveries by women with YOPD [45]. Despite some case reports [48], describing succesfull breastfeeding in woman on anti-parkinsonian medication, there is insufficient data on the safety of breastfeeding when using dopaminergic medication, and as a result, it is usually discouraged to breastfeed when using anti-parkinsonian medication.
Box 1:
The perspective of a patient
Being diagnosed with a non-curable chronic disease at the age of 41 turns your world upside down. Especially since we were familiar with the impact of PSP, the disease my father in law suffered from. The diagnosis of YOPD disrupted my family and social life. All the things we took for granted became questionable: ‘will I be able to see my daughter through college?’, ‘what is the impact on her?’, ‘will our marriage last?’, ‘to what extend will my partner be able to care for me?’, ‘what about work and financial issues?’
In search for answers to these fundamental questions, my wife and I found little recognition and even disbelieve, even by close friends and relatives.
In 2014 my neurologist and I teamed up for a fundraising cycling event. During this ride we talked about the impact of my disease on my personal life. This conversation inspired my wife and myself to make a mind map of the areas of concern and how these impacts on our lives (Fig. 1).
Take Home messages
– Young onset Parkinson’s disease arbitrarily is defined as an age at onset between 21 and 40–50 years
– Genetic testing can be considered in young onset Parkinson’s disease but should be done at centers that have proven experience in the clinical aspects, counseling dilemma’s, and genetic pitfalls of testing the genes associated with Parkinson’s disease
– The unique challenges faced with when living with Parinson’s disease at a younger age, require personalized healthcare, targeting the specific needs of the patients
– Our mindmap (Fig. 1) can function as a way to start exploring the needs of young onset Parkinson’s disease patients
Work
Workplace success is another important topic for many people with YOPD. A retrospective study performed in Ireland found that unemployment rates for men with PD were higher compared to the general population, with a standardized ratio of 1.6; interestingly, this discrepancy was not found for women [49]. Retirement age was approximately 4–5 years younger compared to the general population. Importantly, average age of diagnosis in this study was 58 years, so this number is likely higher in the YOPD-group. Indeed, it has been reported that patients diagnosed before the age of 45 years stop working on average 6 to 7 years after diagnosis, although large differences between persons existed, but no differences between sexes [50].
Signs such as dystonia and levodopa-induced dyskinesias can influence the ability to work considerably [51]. However, severity of symptoms is not the only factor that determines how long someone is able to work after the onset of PD [50]. A qualitative study found that workplace success for people with YOPD depends on both internal and external factors [52]. Internal factors involve symptom severity, daily fluctuations in PD symptoms, but also the way in which a patient copes with and adapts to the disease. External factors, on the other hand, involve the presence of supportive employers and colleagues who enable appropriate adjustments to the working environment. Importantly, for external and internal factors to be present in a successful manner, sufficient knowledge on PD needs to be present both to patients, employers and colleagues. In the latter qualitative study, almost all patients reported that better workplace education would have improved their workplace success. Hence, targeted workplace education should be an element of rehabilitation programs for people with YOPD. Job coaching (e.g., by an occupational therapist or an occupational doctor) that targets both these internal and external factors and provides workplace eduction should therefore be available for people with YOPD. The scientific evidence for workplace education in PD remains to be investigated in coming years.
Relationships
YOPD may present an challenge to relationships; in a study with 75 patients with YOPD (<50 years) and 66 patients with late-onset PD, marital discord scores were significantly worse in YOPD compared to those with late-onset PD. In addition, marital satisfaction scores were average in the YOPD group, and very satisfactory in the late-onset group, but these differences reached no significance [39], A control group was not included in the latter study, so it not clear whether the findings reflect a difference between generations or a difference between late-onset and YOPD. In our experience, YOPD affects relationships especially in the presence of difficulties accepting the diagnosis and when non-motor symptoms emerge. The presence of these non-motor symptoms and coping strategies should therefore be monitored, and when needed targeted with a multidisciplinary intervention.
CONCLUSION
YOPD is an unique subgroup among patients with PD. At the level of clinical management, this calls for shared decision making, the possibility of genetic counselling, and appropriate multidisciplinairy treatment options. At the level of scientific research, the paucity of trials that only include people with YOPD calls for studies or powered subgroup analysis focused on treatment effects in YOPD. Such evidence is urgently needed as input during shared-decision processes between doctors and patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 29, 1583–1590. [DOI] [PubMed] [Google Scholar]
- [2]. Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386, 896–912. [DOI] [PubMed] [Google Scholar]
- [3]. Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363, 1783–1793. [DOI] [PubMed] [Google Scholar]
- [4]. Golbe LI (1991) Young-onset Parkinson’s disease: A clinical review. Neurology 41, 168–173. [DOI] [PubMed] [Google Scholar]
- [5]. Quinn N, Critchley P, Marsden CD (1987) Young onset Parkinson’s disease. Mov Disord 2, 73–91. [DOI] [PubMed] [Google Scholar]
- [6]. Schrag A, Schott JM (2006) Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol 5, 355–363. [DOI] [PubMed] [Google Scholar]
- [7]. Morales-Briceno H, Mohammad SS, Post B, Fois AF, Dale RC, Tchan M, Fung VSC (2020) Clinical and neuroimaging phenotypes of genetic parkinsonism from infancy to adolescence. Brain 143, 751–770. [DOI] [PubMed] [Google Scholar]
- [8]. Butterfield PG, Valanis BG, Spencer PS, Lindeman CA, Nutt JG (1993) Environmental antects of young-onset Parkinson’s disease. Neurology 43, 1150–1158. [DOI] [PubMed] [Google Scholar]
- [9]. Mehanna R, Jankovic J (2019) Young-onset Parkinson’s disease: Its unique features and their impact on quality of life. Parkinsonism Relat Disord 65, 39–48. [DOI] [PubMed] [Google Scholar]
- [10]. Blauwendraat C, Nalls MA, Singleton AB (2020) The genetic architecture of Parkinson’s disease. Lancet Neurol 19, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, Mercimek-Mahmutoglu S, Ebrahimi-Fakhari D, Warner TT, Durr A, Assmann B, Lohmann K, Kostic V, Klein C (2016) Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov Disord 31, 436–457. [DOI] [PubMed] [Google Scholar]
- [12]. Trinh J, Zeldenrust FMJ, Huang J, Kasten M, Schaake S, Petkovic S, Madoev H, Grunewald A, Almuammar S, Konig IR, Lill CM, Lohmann K, Klein C, Marras C (2018) Genotype-phenotype relations for the Parkinson’s disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord 33, 1857–1870. [DOI] [PubMed] [Google Scholar]
- [13]. Lohmann E, Thobois S, Lesage S, Broussolle E, du Montcel ST, Ribeiro MJ, Remy P, Pelissolo A, Dubois B, Mallet L, Pollak P, Agid Y, Brice A, French Parkinson’s Disease Genetics Study Group (2009) A multidisciplinary study of patients with early-onset PD with and without parkin mutations. Neurology 72, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Jacobs H, Latza U, Vieregge A, Vieregge P (2001) Attitudes of young patients with Parkinson’s disease towards possible presymptomatic and prenatal genetic testing. Genet Couns 12, 55–67. [PubMed] [Google Scholar]
- [15]. Falcone DC, Wood EM, Xie SX, Siderowf A, Van Deerlin VM (2011) Genetic testing and Parkinson disease: Assessment of patient knowledge, attitudes, and interest. J Genet Couns 20, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Lythe V, Athauda D, Foley J, Mencacci NE, Jahanshahi M, Cipolotti L, Hyam J, Zrinzo L, Hariz M, Hardy J, Limousin P, Foltynie T (2017) GBA-associated Parkinson’s disease: Progression in a deep brain stimulation cohort. J Parkinsons Dis 7, 635–644. [DOI] [PubMed] [Google Scholar]
- [17]. Sayad M, Zouambia M, Chaouch M, Ferrat F, Nebbal M, Bendini M, Lesage S, Brice A, Errahmani MB, Asselah B (2016) Greater improvement in LRRK2 G2019S patients undergoing subthalamic nucleus deep brain stimulation compared to non-mutation carriers. BMC Neurosci 17, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. (1990) Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40, 1529–1534. [DOI] [PubMed] [Google Scholar]
- [19]. Bozi M, Bhatia KP (2003) Paroxysmal exercise-induced dystonia as a presenting feature of young-onset Parkinson’s disease. Mov Disord 18, 1545–1547. [DOI] [PubMed] [Google Scholar]
- [20]. Kostic V, Przedborski S, Flaster E, Sternic N (1991) Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson’s disease. Neurology 41, 202–205. [DOI] [PubMed] [Google Scholar]
- [21]. Wickremaratchi MM, Knipe MDW, Sastry BSD, Morgan E, Jones A, Salmon R, Weiser R, Moran M, Davies D, Ebenezer L, Raha S, Robertson NP, Butler CC, Ben-Shlomo Y, Morris HR (2011) The motor phenotype of Parkinson’s disease in relation to age at onset. Mov Disord 26, 457–463. [DOI] [PubMed] [Google Scholar]
- [22]. Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC (2014) Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord 20, 530–534. [DOI] [PubMed] [Google Scholar]
- [23]. Turcano P, Mielke MM, Bower JH, Parisi JE, Cutsforth-Gregory JK, Ahlskog JE, Savica R (2018) Levodopa-induced dyskinesia in Parkinson disease: A population-based cohort study.e2238-e. Neurology 91, 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, Bezard E, Picconi B, Calabresi P, Lang AE (2018) Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann Neurol 84, 797–811. [DOI] [PubMed] [Google Scholar]
- [25]. Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F; Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28, 1064–1071. [DOI] [PubMed] [Google Scholar]
- [26]. Sossi V, de la Fuente-Fernandez R, Schulzer M, Adams J, Stoessl J (2006) Age-related differences in levodopa dynamics in Parkinson’s: Implications for motor complications. Brain 129, 1050–1058. [DOI] [PubMed] [Google Scholar]
- [27]. Fox SH, Katzenschlager R, Lim S, Barton B, de Bie RM, Seppi K, Coelho M, Sampaio C (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33, 1992–1992. [DOI] [PubMed] [Google Scholar]
- [28]. Espay AJ, Lang AE (2017) Common myths in the use of levodopa in Parkinson disease when clinical trials misinform clinical practice. JAMA Neurol 74, 633–634. [DOI] [PubMed] [Google Scholar]
- [29]. Vlaar A, Hovestadt A, van Laar T, Bloem BR (2011) The treatment of early Parkinson’s disease: Levodopa rehabilitated. Pract Neurol 11, 145–152. [DOI] [PubMed] [Google Scholar]
- [30]. Group PDMC, Gray R, Ives N, Rick C, Patel S, Gray A, Jenkinson C, McIntosh E, Wheatley K, Williams A, Clarke CE (2014) Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): A large, open-label, pragmatic randomised trial. Lancet 384, 1196–1205. [DOI] [PubMed] [Google Scholar]
- [31]. Verschuur CVM, Suwijn SR, Boel JA, Post B, Bloem BR, van Hilten JJ, van Laar T, Tissingh G, Munts AG, Deuschl G, Lang AE, Dijkgraaf MGW, de Haan RJ, de Bie RMA, LEAP Study Group (2019) Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med 380, 315–324. [DOI] [PubMed] [Google Scholar]
- [32]. de Bie RMA, Clarke CE, Espay AJ, Fox SH, Lang AE (2020) Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet Neurol 19, 452–461. [DOI] [PubMed] [Google Scholar]
- [33]. Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, Dietrichs E, Fabbrini G, Friedman A, Kanovsky P, Kostic V, Nieuwboer A, Odin P, Poewe W, Rascol O, Sampaio C, Schupbach M, Tolosa E, Trenkwalder C, Schapira A, Berardelli A, Oertel WH (2013) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20, 5–15. [DOI] [PubMed] [Google Scholar]
- [34]. Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE (2010) Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Arch Neurol 67, 589–595. [DOI] [PubMed] [Google Scholar]
- [35]. Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, EARLYSTIM Study Group (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368, 610–622. [DOI] [PubMed] [Google Scholar]
- [36]. Dissanayaka NNW, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, Marsh R, Mellick GD (2010) Anxiety disorders in Parkinson’s disease: Prevalence and risk factors. Mov Disord 25, 838–845. [DOI] [PubMed] [Google Scholar]
- [37]. Kagi G, Klein C, Wood NW, Schneider SA, Pramstaller PP, Tadic V, Quinn NP, van de Warrenburg BPC, Bhatia KP (2010) Nonmotor symptoms in parkin gene-related parkinsonism. Mov Disord 25, 1279–1284. [DOI] [PubMed] [Google Scholar]
- [38]. Kummer A, Cardoso F, Teixeira AL (2009) Frequency of psychiatric disorders in young-onset Parkinson’s disease does not differ from typical-onset Parkinson’s disease. Parkinsonism Relat Disord 15, 153–155. [DOI] [PubMed] [Google Scholar]
- [39]. Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M (2003) Young- versus older-onset Parkinson’s disease: Impact of disease and psychosocial consequences. Mov Disord 18, 1250–1256. [DOI] [PubMed] [Google Scholar]
- [40]. Knipe MD, Wickremaratchi MM, Wyatt-Haines E, Morris HR, Ben-Shlomo Y (2011) Quality of life in young- compared with late-onset Parkinson’s disease. Mov Disord 26, 2011–2018. [DOI] [PubMed] [Google Scholar]
- [41]. Spica V, Pekmezovic T, Svetel M, Kostic VS (2013) Prevalence of non-motor symptoms in young-onset versus late-onset Parkinson’s disease. J Neurol 260, 131–137. [DOI] [PubMed] [Google Scholar]
- [42]. Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ (2018) Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol 17, 559–568. [DOI] [PubMed] [Google Scholar]
- [43]. Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, Weintraub D, Sampaio C, the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34, 180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Young C, Phillips R, Ebenezer L, Zutt R, Peall KJ (2020) Management of Parkinson’s disease during pregnancy: Literature review and multidisciplinary input. Mov Disord Clin Pract 7, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Seier M, Hiller A (2017) Parkinson’s disease and pregnancy: An updated review. Parkinsonism Relat Disord 40, 11–17. [DOI] [PubMed] [Google Scholar]
- [46]. Nora JJ, Nora AH, Way GL (1975) Letter: Cardiovascular maldevelopment associated with maternal exposure to amantadine. Lancet 2, 607. [DOI] [PubMed] [Google Scholar]
- [47]. Scelzo E, Mehrkens JH, Botzel K, Krack P, Mendes A, Chabardes S, Polosan M, Seigneuret E, Moro E, Fraix V (2015) Deep brain stimulation during pregnancy and delivery: Experience from a series of “DBS Babies”. Front Neurol 6, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Thulin PC, Woodward WR, Carter JH, Nutt JG (1998) Levodopa in human breast milk: Clinical implications. Neurology 50, 1920–1921. [DOI] [PubMed] [Google Scholar]
- [49]. Murphy R, Tubridy N, Kevelighan H, O’Riordan S (2013) Parkinson’s disease: How is employment affected?. Ir J Med Sci 182, 415–419. [DOI] [PubMed] [Google Scholar]
- [50]. Schrag A, Banks P (2006) Time of loss of employment in Parkinson’s disease. Mov Disord 21, 1839–1843. [DOI] [PubMed] [Google Scholar]
- [51]. Banks P, Lawrence M (2006) The disability discrimination act, a necessary, but not sufficient safeguard for people with progressive conditions in the workplace? The experiences of younger people with Parkinson’s disease. Disabil Rehabil 28, 13–24. [DOI] [PubMed] [Google Scholar]
- [52]. Mullin RL, Chaudhuri KR, Andrews TC, Martin A, Gay S, White CM (2018) A study investigating the experience of working for people with Parkinson’s and the factors that influence workplace success. Disabil Rehabil 40, 2032–2039. [DOI] [PubMed] [Google Scholar]