Abstract
Background:
Oculopharyngeal muscular dystrophy (OPMD) is a late onset progressive neuromuscular disorder. Although dysphagia is a pivotal sign in OPMD it is still not completely understood.
Objective:
The aim of this study was to systematically investigate oropharyngeal functioning in a large OPMD population.
Methods:
Forty-eight genetically confirmed OPMD patients completed questionnaires, performed clinical tests on swallowing, chewing, speaking, tongue strength and bite force, and underwent videofluoroscopy of swallowing. Descriptive statistics was used for all outcomes and logistic regression to investigate predictors of abnormal swallowing.
Results:
Eighty-two percent reported difficulties with swallowing, 27% with chewing and 67% with speaking. Patients performed significantly worse on all oropharyngeal tests compared to age-matched controls except for bite force. Also asymptomatic carriers performed worse than controls: on chewing time, swallowing speed and articulation rate. During videofluoroscopy, all patients (except one asymptomatic) had abnormal residue and 19% aspirated. Independent predictors of abnormal residue were reduced swallowing capacity for thin liquids (OR 10 mL = 0.93; 20 mL = 0.95) and reduced tongue strength for thick liquids (OR 10 mL = 0.95); 20 mL = 0.90). Aspiration of thin liquids was predicted by disease duration (OR = 1.11) and post-swallow residue with 20 mL (OR = 4.03).
Conclusion:
Next to pharyngeal dysphagia, chewing and speaking are also frequently affected in OPMD patients, even in asymptomatic carriers. Residue after swallowing is a very early sign, while aspiration is a later sign in OPMD. For clinical follow-up monitoring of subjective complaints, swallowing capacity and tongue strength seems relevant.
Keywords: Oculopharyngeal muscular dystrophy, dysphagia, dysarthria, neuromuscular diseases, cohort studies
INTRODUCTION
Oculopharyngeal muscular dystrophy (OPMD) is a progressive, usually autosomal dominantly inherited, muscle disease, starting around the 5th decade of life and caused by an extended repeat mutation in the polyadenylate binding protein nuclear 1 (PABPN1) [1, 2]. The name of the disease refers to the most prominent and early features being ptosis and dysphagia, but the upper and lower extremities can also be affected [3]. The typical swallowing complaint is solid food getting stuck in the throat [4]. Primary functional impairments with swallow inefficiency (i.e. pharyngeal residue) and aspiration of food has been identified [5, 6], which may result in life threatening complications such as choking, aspiration pneumonia or malnutrition [7].
Recent reports suggest that swallowing problems in OPMD are not limited to pharyngeal weakness, but that tongue strength and oral bolus control may also be reduced [8]. Even speech has been reported to change in OPMD, ranging from palatal weakness causing a nasal voice [1], to articulation problems and decreased speech rate [9]. However, because of the rareness of the disease, these studies include small patient populations (5 up to 22 patients), focusing on only one or two aspects of oropharyngeal functioning. Hence, a comprehensive understanding of oropharyngeal functioning in a large and genetically confirmed cohort of OPMD patients is lacking.
The aim of this study is to examine subjective and objective deterioration of swallowing, chewing and speaking because of OPMD, with an extensive set of questions, functional testing and videofluoroscopic imaging, to find directions for clinical management.
METHODS
Participants
OPMD patients
Sixty-three OPMD patients were invited by their treating physicians or from the CRAMP (Computer Registry of All Myopathies and Polyneuropathies) database that contains a Dutch cohort of neuromuscular patients [10]. Our center is the national referral center for OPMD. OPMD patients older than 18 years were included, but irrespective of disease duration. Exclusion criteria were tube feeding and possible causes of dysphagia other than OPMD.
Family members who had not been genetically tested yet were invited as well to find asymptomatic gene carriers. They could choose whether they wanted to know their DNA result or not. If they wanted to know the outcome, this was provide by a neurologist, including extensive counselling. Most of them had a symptomatic family member and were already familiar with the consequences of the disease. Seven family members were tested and they all wanted to know their DNA result. Thirteen participants declined to participate because of practical reasons (such as travel distance); two family members were tested negative for OPMD and were therefore excluded, resulting in 48 genetically confirmed OPMD patients (25 women) who agreed to be enrolled in the study. From these patients we collected age, body mass index (BMI), gene mutation and their score on the EAT-10, a screening tool to identify dysphagia risk [11] and patients reported their age and initial symptoms at onset.
The study protocol [12] was approved by the regional medical ethics committee (nr. NL54606.091.15) and all participants gave written informed consent. All patients were examined by one researcher (RK).
Healthy controls
Normal values of the maximum performance tests used in this study had been collected in previous studies from a large healthy Dutch population aged 18 to 92 years and this allowed for age-matched comparisons [13– 17].
Assessments
To investigate oropharyngeal functions extensively in three domains (swallowing, chewing and speaking), we combined the following approaches:
-
1.
Questionnaire and interview to identify subjective complaints;
-
2.
Maximum performance tests for swallowing, chewing and speaking;
-
3.
Videofluoroscopy of swallowing.
1. Subjective complaints
Subjective complaints were captured by using a questionnaire and an interview.
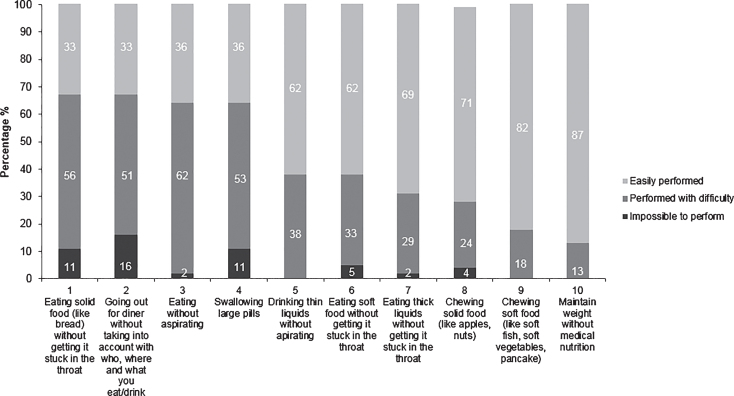
Because there is no dysphagia questionnaire specifically for OPMD we identified 10 characteristics of normal chewing and swallowing (Fig. 2). Patients were asked to choose one of three response options: (0) ‘impossible to perform’, (1) ‘performed with difficulty’, (2) ‘easily performed’. The questionnaire was sent online to all patients and 45 (of 48) patients completed the questionnaire. In addition, all patients were interviewed about other possible subjective complaints including speaking because of OPMD. The interview was semi-structured, as its purpose was to globally investigate any subjective complaints, next to the questionnaire about chewing, swallowing and speaking complaints.
Fig.2.
Results of the questionnaire on swallowing and chewing. Answers on the questionnaire were classified into ‘easily performed’, ‘performed with difficulty’ and ‘impossible to perform’.
2. Maximum performance tests
Seven clinical tasks to assess maximum performance and strength related to swallowing, chewing, and speaking were performed.
Swallowing
-
•
Maximum swallowing speed (MSS) in mL/s was measured by recording the time participants needed to drink 150 ml room temperature tap water as fast as possible [18].
-
•
Maximum swallowing volume (MSV) is the maximum amount of water (mL) a participant is able to swallow in one swallow [15].
-
•
Maximal isometric tongue pressure (MITP) was measured using the Iowa Oral Performance Instrument (IOPI Medical LLC, Model 2.3). Participants were instructed to push the bulb on the tongue against the roof of the mouth as hard as possible [19]. The highest pressure of three trials was used in the analysis.
Chewing
-
•
Maximum chewing time (MCT) was measured using the Test of Masticating and Swallowing Solids (TOMASS) by recording the time the participant needs to eat a 5×5 cm cracker as fast as possible [14].
-
•
Maximum bite force (MBF) was measured using the Bite Force Gauge (Vrije Universiteit, Amsterdam). Participants were asked to bite as hard as possible on the biting element between their upper and lower front teeth. The highest pressure of three trials was used for analysis.
Speaking
-
•
Maximum phonation time (MPT) measures how long a participant can produce an /a/ after one inhale, in seconds [20]. The best of three trials was used in the analysis.
-
•
Maximum repetition rate (MRR) is the number of syllables per second during the first 5 seconds, while the participant is instructed to produce the monosyllabic sequences /pa/, /ta/ and /ka/ and the trisyllabic sequence /pataka/ as fast as possible [21]. The best of three trials was used in the analysis.
All maximum performance tests were compared with norm values from 82 to 130 age-matched healthy controls that were collected in a previous study by our department [13– 16].
3. Videofluoroscopy of swallowing
Videofluoroscopy of swallowing (VFS) was performed at 30 frames per second and recorded with the Digital Swallowing Workstation (DSW, Swallowing Signals Lab, model 7120) to quantify swallowing efficiency and safety. Thin liquid was made by using thin liquid contrast fluid. Thick liquid was made by using thin liquid contrast fluid and one spoon of the gum type thickener. For a solid bolus we took a toast cracker of 2.5 cm×2.5 cm (Albert Heijn Basic™) pasted with a teaspoon of thick liquid contrast fluid. Then we tested it with the flow test of the International Dysphagia Diet Standardization Initiative (IDDSI) [22]: thin liquid correspond with IDDSI 0, thick liquid with IDDSI 3 and solid with IDDSI 7.
Each participant was asked to swallow 10 mL and 20 mL of thin liquid that was recorded in lateral direction. This was followed by 10 mL and 20 mL thick liquid and solid food.
To quantify the swallow efficiency we used the Normalized Residue Ratio Scale (NRRS, abnormal values: NRRSv≥0.09, NRRSp≥0.20) [23] to assess the post swallow residue in the valleculae and pyriform sinus. The NRRS residue ratio of the valleculae and the pyriform sinus is calculated by the following formula:
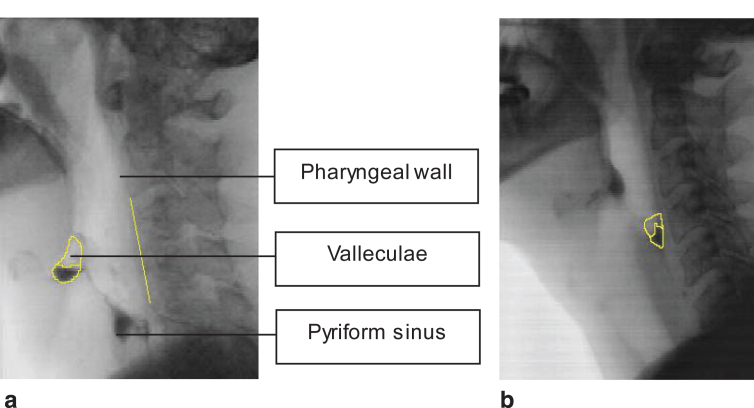
The frame of the post swallow residue was set after the participants swallowed the bolus in one or two swallows. In case of more than two swallows, the frame of post swallow ‘rest’ residue was set after the second swallow. ImageJ (National Institutes of Health, LOCI, University of Wisconsin) was used to draw the residue in the valleculae (Fig. 1a) and pyriform sinus (Fig. 1b). The videofluoroscopic images were independently analyzed by two trained research assistants.
Fig.1.
a. Post swallow residue in the valleculae and the scalar reference line from C2 to C4. b. Post swallow residue in the pyriform sinus.
The Penetration – Aspiration Scale (PAS) was used to evaluate swallowing safety [24]. This scale ranges from 1 to 8 : 1 = Material does not enter the airway, 2 = Material enters the airway, remains above the vocal folds, and is ejected from the airway, 3 = Material enters the airway, remains above the vocal folds, and is not ejected from the airway, 4 = Material enters the airway, contacts the vocal folds, and is ejected from the airway, 5 = Material enters the airway, contact the vocal folds, and is not ejected from the airway, 6 = Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway, 7 = Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort, 8 = Material enters the airway, passes below the vocal folds, and no effort is made to eject. Score 1 and 2 is defined as safe swallowing and 3 to 8 as unsafe swallowing [5, 6].
Statistical analyses
IBM SPSS Statistics (version 25) was used to conduct all statistical analyses and for all estimates and p-values of <0.05 were considered statistically significant. Subjective complaints were analyzed by calculating percentages.
The results of the maximum performance tests were compared between OPMD patients and age-matched healthy controls using independent t-tests and between asymptomatic OPMD patients and age-matched controls using the Mann-Whitney U test.
Descriptives (median, min – max) and frequencies (numbers and percentages) were calculated for the NRRS scores and PAS scores. Interrater reliability of the NRRS and PAS between the two trained research assistants was tested by calculating the intraclass correlation coefficient (ICC), two-way random for absolute agreement, single measure (ICC2,1) [25].
To test the hypothesis that a longer repeat of the PABPN1 mutation predicts increased clinical severity, repeat length was correlated with the maximum performance tests by Spearman rho correlation.
Post hoc analyses: To further analyze differences within the OPMD group, the cohort was dichotomized for every domain (chewing, swallowing, speaking) into patients with and patients without subjective complaints according to the questionnaire (chewing and swallowing) and the semi-structured interview (speaking). Age and disease duration were compared between the three dichotomized groups and performances on maximum performance tests were compared per domain (MCT and MBF for chewing, MMS, MSV, MITP for swallowing and MPT, MRR for speaking). Mann-Whitney U test was used to estimate any differences.
To try to identify predicting factors a predictive model for either aspiration or pharyngeal residue, we used multivariate analyses. As the first step we correlated all clinical features (patient variables and clinical scores) for each volume and consistency with abnormal vallecular residue (NRRSv≥0.09), pyriform sinus residue (NRRSp≥0.20) and aspiration (PAS≥3). Only the variables that correlated significantly were kept as potentially predictive or explanatory factors. In the second step we constructed models using logistic regression with the presence of post-swallow residue and aspiration for each volume and consistency as dependent variables and the corresponding potentially predictive factors as their independent variables.
Data availability statement
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Patients
Table 1 shows the patient characteristics. None of these patients had been treated with a cricopharyngeal myotomy or dilatation, except one patient in 2001 without a positive result and none of the eligible participants had to be excluded because of tube feeding or possible other causes of dysphagia.
Table 1.
Characteristics of 48 OPMD patients
| N | 48 |
| Men/women (%) | 23 (48) / 25 (52) |
| Age in years (SD) | 61 (8.6) |
| Disease duration in years (SD)* | 10.6 (9.4) |
| Body Mass Index (SD) | 24.7 (3.6) |
| Initial symptom (number of patients)** | |
| Ptosis | 20 |
| Dysphagia | 20 |
| Weakness extremities | 5 |
| Diplopia | 1 |
| None | 4 |
| Unknown | 1 |
| PABPN1 mutation (number of patients (%)) | |
| GCN11/11 | 2 (4) |
| GCN10/12 | 3 (6) |
| GCN10/13 | 6 (12) |
| GCN10/14 | 8 (16) |
| GCN10/15 | 2 (4) |
| GCN10/16 | 27 (56) |
| Eat-10*** (% ≥3) | 86,7% |
*Onset of initial complaints. **Two patients had both dysphagia and ptosis as initial symptom, one patient had leg weakness and dysphagia as initial symptom. ***EAT-10 swallow screening tool with ten questions (score 0– 4, total score 0– 40), with ≥3 representing possible problems in swallowing efficiency and safety [11, 42].
Subjective complaints
Eighty-seven percent of the OPMD patients reported difficulties on chewing and/or swallowing (Fig. 2). During the interview, sixty-seven percent of the OPMD patients complained about speech problems. Six patients (13%) did not report subjective complaints on chewing and swallowing. These included the four asymptomatic gene carriers and two patients with isolated ptosis or muscle weakness of the legs.
Swallowing
Thirty-seven patients (82%) reported swallowing problems (Fig. 2: items 1, 3, 4, 5, 6, 7). These patients had on average 8 years longer disease duration compared to patients without swallowing complaints (12.0 y vs. 4.0 y; p = 0.002). The other patient characteristics (as shown in Table 1) did not differ significantly.
Chewing
Thirteen patients (27%) reported chewing problems on the questionnaire (Fig. 2: items 8 and 9) of which two were unable to chew solid food at all. Patients with chewing complaints were 5.9 years older than those without chewing complaints (65.2 y vs. 59.3 y; p = 0.02). The other patient characteristics (as shown in Table 1) did not differ significantly.
Speaking
Thirty-two patients (67%) reported nasal speech, changes in articulation and reduction of intelligibility and loudness of their speech. Patients with speech complaints were 9.2 years older (64.0 y vs. 54.8 y; p < 0.01) and had OPMD 9.5 years longer (13.5 y vs. 4.0 y; p < 0.01), compared to patients without speech complaints. The other patient characteristics (as shown in Table 1) did not differ significantly.
Maximum performance tests
Table 2 gives the results of all the maximum performance tests.
Table 2.
Mean (SD) and percentages of normal values of maximum performance tests in OPMD patients compared to age-matched Dutch controls
| Mean | OPMD: % of normal | P | ||
| OPMD (SD) | Controls (SD) | value (95% CI) | ||
| Swallowing | ||||
| Maximum swallowing speed (mL/s) | 9.4 (7.2) | 25.8 (9.7) | 39 (27;51) | p < 0.01 |
| Maximum swallowing volume (mL) | 27.3 (19.2) | 54.6 (19.9) | 50 (38;62) | p < 0.01 |
| Maximum isometric tongue pressure anterior (kPa) | 31.0 (14.0) | 52.4 (13.6) | 59 (50;68) | p < 0.01 |
| Chewing | ||||
| Test of Masticating and Swallowing Solids chewing time (s) | 69.8 (40.6) | 31.9 (12.9) | 219 (188;249)* | p < 0.01 |
| Maximum bite force (kg) | 14.6 (7.4) | 17.0 (8.3) | 86 (70;102) | 0.09 |
| Speaking | ||||
| Maximum phonation time (s) | 15.5 (8.1) | 22.0 (9.9) | 70 (56;85) | p < 0.01 |
| Maximum repetition rate /PA/ (syl/s) | 5.6 (0.7) | 6.7 (0.6) | 84 (81;88) | p < 0.01 |
| Maximum repetition rate /TA/ (syl/s) | 5.4 (0.8) | 6.5 (0.8) | 83 (78;86) | p < 0.01 |
| Maximum repetition rate /KA/ (syl/s) | 5.0 (0.9) | 6.0 (0.8) | 83 (78;87) | p < 0.01 |
| Maximum repetition rate /PATAKA/ (syl/s) | 4.0 (0.7) | 6.8 (1.0) | 59 (54;63) | p < 0.01 |
*all variables are lower in OPMD patients indicating worse performance; but chewing time becomes abnormal when duration is longer than normal (here more than twice as long: 219%).
Swallowing
Three patients were not able to perform the maximum swallowing speed task, because their dysphagia was too severe, therefore their scores were set at 0 mL/s. OPMD patients scored significantly lower than age-matched Dutch controls on swallowing speed (MSS) and volume (MSV) and on maximum isometric tongue pressure (Table 2). Patients with subjective swallowing complaints according to the questionnaire (n = 37), presented a lower MSS (14.5 ml/s vs. 9.0 ml/s; p = 0.03) and lower MSV (38.8 ml vs 24.7 ml; p = 0.03), compared to patients without swallowing complaints. Tongue strength did not differ between these subgroups.
Chewing
Maximum chewing time was twice as long in OPMD patients compared to age-matched Dutch controls (70 vs. 32 seconds; p = 0.00), but maximum bite force did not differ significantly (Table 2). Patients with subjective chewing complaints (n = 13) did not perform differently on swallowing and speech tasks, but showed a lower bite force, compared to patients without chewing complaints (17.0 kg vs. 8.4 kg; p < 0.01).
Speaking
The maximum phonation time and maximum repetition rates were significantly lower in OPMD patients compared to age-matched Dutch controls (Table 2). OPMD patients with subjective speech complaints (n = 32) presented equal scores on all measures except on maximum repetition rate of /PA/, which was lower compared to patients without speech complaints (5.9 syl/s vs. 5.5 syl/s; p = 0.03).
Maximum performance tests in asymptomatic carriers
Asymptomatic carriers scored significantly lower than age-matched controls on maximum swallowing speed, chewing time and maximum articulation rate (Table 3), but not on maximum bite force, maximum tongue strength or maximum swallowing volume (data not shown).
Table 3.
Mean values of asymptomatic OPMD carriers (n = 4) compared to age-matched Dutch controls
| Mean | |||
| Asymptomatic* (SD) | Controls (SD) | P | |
| Swallowing | |||
| Maximum swallowing speed (mL/s) | 18.9 (2.0) | 28.6 (9.8) | 0.02 |
| Chewing | |||
| Test of Masticating and Swallowing Solids chewing time (s) | 43.8 (7.0) | 29.2 (10.2) | 0.01 |
| Speaking | |||
| Maximum repetition rate /PA/ (syl/s) | 6.0 (0.1) | 6.8 (0.6) | p < 0.01 |
| Maximum repetition rate /TA/ (syl/s) | 5.3 (0.4) | 6.8 (0.8) | p < 0.01 |
| Maximum repetition rate /PATAKA/ (syl/s) | 4.8 (0.4) | 6.8 (0.9) | p < 0.01 |
P-values of the non-parametric test (Mann-Whitney U test). *N = 4.
Videofluoroscopy
Forty-five patients underwent a videofluoroscopic swallowing study (VFSS) of which one patient could not swallow thick liquid 20 ml and three patients were not able to swallow solid food. Three patients refused VFSS because of the following reasons: it was physically too stressful, because of intestinal problems and refusal of X-ray. Interrater reliability between the two trained research assistants for all the consistencies of the NRRS valleculae and pyriform sinus was good to excellent (ICC = 0.91, CI 95% 0.72– 0.97 and ICC = 0.78, CI 95% 0.31– 0.94). The PAS scale showed a perfect agreement between the two research assistants (ICC = 1).
Swallowing efficiency
Overall all the patients had abnormal post-swallow residue at any point, in the valleculae (≥ 0.09) or pyriform sinus (≥ 0.20), except one asymptomatic gene carrier who had no residue at all, but frequencies differed across consistencies and place (Table 4).
Table 4.
Median, minimum and maximum of the NRRS, abnormal residues of the valleculae and pyriform sinus and the scores of the PAS of each consistency and volume
| NRRS valleculae | NRRS pyriform sinus | PAS | ||||||||||||
| Median | Min – Max | ≥0.09 | Median | Min – Max | ≥0.20 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Thin liquid 10 mL | 0.11 | 0.00– 0.87 | 24 (53) | 0.27 | 0.00– 2.20 | 26 (58) | 37 (82) | – | – | 1 (2) | 1 (2) | – | – | 6 (13) |
| Thin liquid 20 mL | 0.21 | 0.00– 0.88 | 32 (71) | 0.42 | 0.00– 2.98 | 31 (69) | 36 (80) | – | 1 (2) | 1 (2) | 1 (2) | – | – | 6 (13) |
| Thick liquid 10 mL | 0.24 | 0.00– 1.77 | 31 (69) | 0.21 | 0.00– 2.00 | 23 (53) | 45 (100) | – | – | – | – | – | – | – |
| Thick liquid 20 mL | 0.31 | 0.00– 1.53 | 36 (82) | 0.21 | 0.00– 2.85 | 23 (52) | 44 (100) | – | – | – | – | – | – | – |
| Solid | 0.16 | 0.00– 1.18 | 27 (64) | 0.02 | 0.00– 0.81 | 11 (26) | 42 (100) | – | – | – | – | – | – | – |
Min: minimum, Max: maximum, N: number of patients, ≥0.09: cut off score of abnormal vallecular residue, ≥0.20: cut off score of abnormal residue in the pyriform sinus.
Only residue in the pyriform sinus, but not in the valleculae and only residue of liquids but not of solids was significantly correlated with any of the patient characteristics and clinical measures (Table 5). Logistic regression identified reduced maximum swallowing volume as the single independent predictor for abnormal residue after swallowing thin liquid (10 mL: OR = 0.93; 95% CI 0.89– 0.98, 20 mL: OR = 0.95; 95% CI 0.91– 0.98) and reduced maximum tongue strength as the single independent predictors for risk of abnormal residue swallowing thick liquids (10 mL: OR = 0.95; 95% CI 0.91– 1.00, 20 mL: OR = 0.90; 95% CI 0.85– 0.96).
Table 5.
Odds ratios of predicting factors of the risk of abnormal residue and aspiration in the pyriform sinus
| Dependent variable | Independent variables* | Predicting factor: OR (95% CI) |
| Swallowing efficiency (abnormal post-swallow residue) | ||
| Thin liquid 10 mL | Higher age, longer disease duration, worse performances on MSS and MSV, weaker tongue, worse score on EAT-10 | maximum swallowing volume: OR = 0.93 (0.89 – 0.98) |
| Thin liquid 20 mL | Higher age, longer disease duration, worse performances on MSS and MSV, weaker tongue | maximum swallowing volume: OR = 0.95 (0.91 – 0.98) |
| Thick liquid 10 mL | Longer disease duration, weaker tongue | maximum isometric tongue pressure: OR = 0.95 (0.91 – 1.00) |
| Thick liquid 20 mL | Lower BMI, longer disease duration, higher age at disease onset, worse performance on the MSS and MSV, weaker tongue pressure and worse score on EAT-10 | maximum isometric tongue pressure: OR = 0.90 (0.85 – 0.96) |
| Swallowing safety (aspiration: PAS≥3) | ||
| Thin liquid 10 and 20 mL | Longer disease duration, lower BMI, worse performance on the MSS and MSV, weaker tongue pressure, more thin liquid residue in the pyriform sinuses swallowing thin liquid 10 mL and 20 mL | residue in the pyriform sinus after swallowing 20 ml thin liquid OR = 4.03 (1.29 – 12.65) |
| disease duration OR = 1.11 (1.01 – 1.23) | ||
*Factors significantly correlating with the independent variable.
Swallowing safety
Unsafe swallowing (PAS≥3) was seen in 19% (n = 10) of the participants during swallowing thin liquid, but no aspiration was observed during or after swallowing thick liquid or solid food (Table 4). Seven patients showed silent aspiration (PAS = 8) while swallowing thin liquid. All patients with unsafe swallowing also showed abnormal residue after swallowing and three patients were not able to perform the MSS and TOMASS because of their severe swallowing problems.
Table 5 lists the variables that correlate with unsafe swallowing. Logistic regression identified residue in the pyriform sinus after swallowing 20 mL thin liquid as single independent predictor for the risk of aspiration (OR = 4.03; CI 95% 1.29– 12.65) and disease duration as single independent predictor for the risk of aspiration (OR = 1.11; CI 95% 1.01– 1.23).
Relation of clinical severity with repeat length
Comparison between repeat length and the maximum performance tests, severity of abnormal residue and aspiration showed no linear relation with any of these variables.
DISCUSSION
The main finding of this study is that OPMD patients performed worse on all but one oropharyngeal test which not only addresses pharyngeal weakness, but also oral weakness, explaining difficulties with swallowing and chewing and speaking. Furthermore, all patients, except one asymptomatic carrier, showed abnormal pharyngeal residue during videofluoroscopy in our large, but heterogenic patient population regarding age and disease duration.
In the following paragraphs, we will discuss the subjective and objective findings for each of the domains swallowing, chewing and speaking separately and will highlight the meaning of the results for the asymptomatic carriers.
Swallowing complaints are reported by 82% of the OPMD patients. The main subjective complaints are ‘solid food getting stuck in the throat’ (67%) and ‘aspirating during eating’ (64%), which is in coherence with a previous questionnaire-based study [4]. Problems with drinking thin liquids are subjectively reported less frequently by 38% of patients confirming that drinking thin liquids without aspirating is difficulty (Fig. 2), while objectively during instrumental examination drinking of liquids resulted in aspiration in 19%. Although this is only half compared to the frequency of subjective complaints, possibly because of more careful drinking during the examination, the majority of these events (70%; 12/17) was silent aspiration. Instrumental observation also showed abnormal pharyngeal residue in 71%, which turned out to be an independent predictor of aspiration. Together this suggests that ‘food getting stuck in the throat’ is indeed a common complaint, but aspiration of liquids should be taken into account as well.
Although pharyngeal residue is a known problem in OPMD [5, 6] and a clinical predictor of aspiration generally in dysphagia [26], this is the first time that this phenomenon has been demonstrated in a large cohort and in almost all patients, even in those who do not report this subjectively. Following up on earlier work [5], we used multiple consistencies and larger volumes (10 and 20 mL instead of 5 mL) to study swallowing efficiency and safety during videofluoroscopy. Pharyngeal residue is generally more common after swallowing solid food, because a thicker consistency requires more pharyngeal constriction and hyolaryngeal excursion to clear the pharynx [22]. But our results show that post-swallow residue is common in all consistencies and volumes, which suggests a more complex pathophysiology of pharyngeal weakness in OPMD. When looking for factors to explain this residue we found several features that correlated with residue in the pyriform sinuses, but none of these correlated with residue in the valleculae. This may be due to the size of the residue, which is clearly smaller in the valleculae and therefore less likely to correlate with reduced functional capacity or strength. Our analyses revealed two independent predictors of liquid residue in the pyriform sinus, i.e. reduced maximum swallowing volume and reduced tongue strength. The maximum volume of a large liquid bolus to be swallowed in one swallow, obviously will decrease when oral and pharyngeal strength deteriorate, but further research is needed to understand whether this is characteristic for causing residue in the pyriform sinus in OPMD, other than reduced pharyngeal constriction or cricopharyngeal dysfunction [27]. Similarly, it is indeed plausible that deterioration of oral tongue strength increases the risk of post-swallow residue of thick liquids. This would suggest that tongue strengthening exercises may be a treatment option to maintain muscle function, but how this relates to pharyngeal clearance in OPMD also needs to be studied further. Until then, maximum swallowing volume and maximum tongue strength are simple measures to monitor progression of dysphagia and the risk of pharyngeal residue.
To identify and compare abnormal pharyngeal residue we used the cut-off values proposed by Waito et al. [5] and Molfenter et al. [26], which are based on using small thin liquid boluses like 5 mL or tea spoon amount. By using larger amounts of 10 and 20 mL we might have found more frequent or more severe abnormal residue compared to others [6], but this was not the case. Together with the low frequency of aspiration in this heterogenic population, this suggests that in this population, small volumes are not necessarily safer, neither would they reduce the risk of post-swallow residue. This is coherent with a recent retrospective study showing a rate of aspiration pneumonia as low as 8% [28].
In this study aspiration was much less common than post-swallow residue. In particular aspiration of solids remained absent, despite this being the most common subjective complaint. This may be due to patients paying more attention to careful chewing and swallowing in the laboratory setting, compared to being at home where dining with others is dual tasking, increasing the risk of aspiration of accumulating residue. In addition, we only tested one type of solid food, which may have been too easy to swallow. Aspiration of thin liquids however was present in a fifth, most of them having more severe oropharyngeal disorders and predicted by longer disease duration and pharyngeal residue after swallowing 20 mL of thin liquid. The latter is easy to explain, because the larger the amount of abnormal residue, the greater the risk of aspiration [29]. Longer disease duration increased the risk of aspiration irrespective of the presence of subjective swallowing complaints, which is highly important to be aware of in clinical follow-up. Furthermore, despite the known slow progression of OPMD, it is also relevant for clinicians to monitor the nutritional status and weight of OPMD patients, as those with aspiration had significantly lower BMI, which may reflect malnutrition.
Chewing problems are reported by a quarter of the OPMD patients and chewing time was more than twice as long compared to age-matched controls. This longer chewing time may be explained by patients’ behavior to take their time to chew to ensure that the texture of the bolus is easier to swallow. Although bite force was not significantly reduced on a group level, patients with chewing complaints had a lower bite force than those without chewing complaints. This is relevant because muscle weakness in OPMD is assumed to be mainly present in the pharyngeal muscles. Only one earlier report [4] also identified chewing complaints in OPMD, but without objective measurement. While oromandibular weakness and chewing problems are common in other neuromuscular diseases, e.g. in Duchenne muscular dystrophy or myotonic dystrophy [30, 31], our results now show that biting and chewing problems are also part of the clinical phenotype of OPMD.
Speech complaints are reported by 67% of our patients. Although in neuromuscular diseases the prevalence of dysarthria is high [32], detailed information about dysarthria in OPMD was largely missing. Some studies with various patient populations describe speech and voice changes, such as nasal voice, decreased rate of speech or articulation problems [8, 9, 33– 35]. In our study, performances on all diadochokinetic tasks were significantly slower compared to age-matched Dutch controls. This is in contrast to Neel et al. [8], who showed slower rates only for monosyllabic sequences in OPMD patients compared to controls. This may be explained by differences in disease severity, but Neel et al. [8] did not report detailed patient characteristics to allow for such a comparison. Also the mean maximum phonation time was significantly reduced, suggesting that OPMD patients are indeed at risk of flaccid dysarthria. This implies that examination of speech and voice with a validated dysarthria assessment [36] is clinically useful when speech capacity or intelligibility becomes a problem.
Asymptomatic carriers had already significantly lower scores on several maximum performance tests. They were slower during chewing, swallowing and speaking, but did not show reduced strength on maximum tongue force, bite force or phonation time (which can be interpreted as a form of expiratory strength). Although the asymptomatic carriers form a small subgroup, these slower performances, without reduction of strength yet, could be an early feature of OPMD. Also, during videofluoroscopy, three out of four showed abnormal residue in at least one consistency. Pharyngeal residue may not be clinically relevant at first, but residue after swallowing larger volumes (20 mL) was a four-fold risk for aspiration in this population (OR = 4.03), suggesting problems in the future.
Limitations
This study was not without shortcomings. The interview was semi-structured, as its purpose was to globally investigate any subjective complaints, next to the questionnaire about swallowing complaints. For identifying the speech problems, a structured interview or validated questionnaire may have revealed more detailed information. However, only general speech questionnaires were available, which at the time did not seem valid for this first investigation. Secondly, bite force was measured by a validated tool that measures the maximum bite force with the front teeth, which does not represent full bite force with the molar teeth. This technique might have underestimated bite force, explaining the insignificant difference with controls. Thirdly, in our study we observed a small number of asymptomatic gene carriers, further research about the performance of asymptomatic gene carriers needs to be done to confirm our findings.
Regarding the interrater reliability of the ratings of pharyngeal residue in the pyriform sinus, the ICCs were more than adequate, but with rather wide confidence intervals, because the calculation was based on a small number of observations. Meanwhile for future experiments, the NRRS to assess pharyngeal residue has been revised [37].
Another limitation of this study is that we did not measure pharyngeal constriction or cricopharyngeal pressures. However, we carefully measured the severity of aspiration and post-swallow residue of multiple consistencies revealing a pattern that is different from what patients report. High resolution impedance manometry (HRIM) combined with videofluoroscopy is the next step to further explain the pathophysiology of dysphagia in OPMD.
Finally, despite the extended set of measurements, in retrospect some clinical tests were missing. For example regarding measurements of speech and voice, this study suggests that spirometry may be relevant to measure whether vital force capacity (VFC) is also reduced in OPMD. Moreover, respiratory measures could also be significant in relation to dysphagia and coughing, in particular in the light of respiratory diseases being an important cause of death in OPMD [28].
For use in future clinical trials longitudinal data is needed to determine whether the measurements used in this study are also sensitive to change. Additionally, as tongue strength is reduced in OPMD patients, it may be a target for therapy. Currently, there are no tongue strength training studies in neuromuscular diseases, but it has been demonstrated that tongue strength training improves swallowing function in stroke patients and patients with sarcopenic dysphagia [38– 40]. Masticatory training using chewing gum is another treatment option, which has been shown to improve mastication in Duchenne muscular dystrophy patients [41].
To conclude, not only swallowing, but also chewing and speaking are frequently impaired in OPMD patients, even in asymptomatic carriers. Abnormal pharyngeal residue in OPMD patients is an early sign and can be predicted by a decreased swallowing capacity and decreased tongue strength. Aspiration of solids is frequently reported subjectively, while aspiration of liquids mainly seems to occur in advanced OPMD. Based on these findings, for clinical follow-up of swallowing, chewing, and speaking we propose monitoring of subjective complaints and measuring of swallowing capacity and tongue strength.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
FINANCIAL DISCLOSURE
This project is funded by AFM Téléthon (project number 17110).
ACKNOWLEDGMENTS
We thank Lisa Stokman and Dianthe Hermans, speech-language therapists for their support in analyzing the videofluoroscopic images.
REFERENCES
- [1]. Brais B. Oculopharyngeal muscular dystrophy: A late-onset polyalanine disease. Cytogenetic and genome research. 2003;100(1-4):252–60. [DOI] [PubMed] [Google Scholar]
- [2]. Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18(2):164–7. [DOI] [PubMed] [Google Scholar]
- [3]. van der Sluijs BM, Raz V, Lammens M, van den Heuvel LP, Voermans NC, van Engelen BG. Intranuclear Aggregates Precede Clinical Onset in Oculopharyngeal Muscular Dystrophy. J Neuromuscul Dis. 2016;3(1):101–9. [DOI] [PubMed] [Google Scholar]
- [4]. Youssof S, Romero-Clark C, Warner T, Plowman E. Dysphagia-related quality of life in oculopharyngeal muscular dystrophy: Psychometric properties of the SWAL-QOL instrument. Muscle & nerve. 2017;56(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Waito AA, Steele CM, Peladeau-Pigeon M, Genge A, Argov Z. A Preliminary Videofluoroscopic Investigation of Swallowing Physiology and Function in Individuals with Oculopharyngeal Muscular Dystrophy (OPMD). Dysphagia, 2018. [DOI] [PubMed] [Google Scholar]
- [6]. Tabor LC, Plowman EK, Romero-Clark C, Youssof S. Oropharyngeal dysphagia profiles in individuals with oculopharyngeal muscular dystrophy. Neurogastroenterol Motil. 2018;30(4):e13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Abu-Baker A, Rouleau GA. Oculopharyngeal muscular dystrophy: Recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim Biophys Acta. 2017;1772(2): 173–85. [DOI] [PubMed] [Google Scholar]
- [8]. Neel AT, Palmer PM, Sprouls G, Morrison L. Muscle weakness and speech in oculopharyngeal muscular dystrophy. Journal of speech, language, and hearing research: JSLHR. 2015;58(1):1–12. [DOI] [PubMed] [Google Scholar]
- [9]. Young EC, Durant-Jones L. Gradual onset of dysphagia: A study of patients with oculopharyngeal muscular dystrophy. Dysphagia. 1997;12(4):196–201. [DOI] [PubMed] [Google Scholar]
- [10]. van Engelen BG, van Veenendaal H, van Doorn PA, Faber CG, van der Hoeven JH, Janssen NG, et al. The Dutch neuromuscular database CRAMP (Computer Registry of All Myopathies and Polyneuropathies): development and preliminary data. Neuromuscular disorders: NMD. 2007;17(1):33–7. [DOI] [PubMed] [Google Scholar]
- [11]. Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. [DOI] [PubMed] [Google Scholar]
- [12]. Kroon H, Kalf J, de Swart B, Horlings C, van Engelen B. Characteristics and natural history of oculopharyngeal muscular dystrophy (OPMD): The study protocol of ‘OPMD Forte’. Neuromuscular Disord. 2016;26, S139–S40. [Google Scholar]
- [13]. Knuijt S, Kalf J, Van Engelen B, Geurts A, de Swart B. Reference values of maximum performance tests of speech production. Int J Speech Lang Pathol. 2019;21(1):56–64. [DOI] [PubMed] [Google Scholar]
- [14]. Huckabee ML, McIntosh T, Fuller L, Curry M, Thomas P, Walshe M, et al. The Test of Masticating and Swallowing Solids (TOMASS): Reliability, validity and international normative data. Int J Lang Commun Disord. 2018;53(1):144–56. [DOI] [PubMed] [Google Scholar]
- [15]. Paepens M, Stepman G, DeHeyder E, Beeckman A, Kalf J. Maximum swallowing volume: Comparison between Flemish and Dutch normal values. Dysphagia. 2020;35, 155–6. [Google Scholar]
- [16]. Kroon R, Kalf J. Dutch normal values of tongue strength and bite force. Dysphagia. 2020;35, 186–7. [Google Scholar]
- [17]. Stepman G, Paepens M, DeHeyder E, Beeckman A, Kalf J. Maximum swallowing speed: Comparison between Flemish and Duthc normal values. Dysphagia. 2020;35, 154–5. [Google Scholar]
- [18]. Nathadwarawala KM, Nicklin J, Wiles CM. A timed test of swallowing capacity for neurological patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(9):822–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: Relation to highly skilled movements. Journal of Speech and Hearing Research. 1992;35(6):1239–45. [DOI] [PubMed] [Google Scholar]
- [20]. Speyer R, Bogaardt HC, Passos VL, Roodenburg NP, Zumach A, Heijnen MA, et al. Maximum phonation time: Variability and reliability. J Voice. 2010;24(3):281–4. [DOI] [PubMed] [Google Scholar]
- [21]. Gadesmann M, Miller N. Reliability of speech diadochokinetic test measurement. Int J Lang Commun Disord. 2008;43(1):41–54. [DOI] [PubMed] [Google Scholar]
- [22]. Cichero JA, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia. 2017;32(2):293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Pearson WG Jr, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: The normalized residue ratio scale. Dysphagia. 2013;28(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. [DOI] [PubMed] [Google Scholar]
- [25]. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Molfenter SM, Steele CM. The relationship between residue and aspiration on the subsequent swallow: An application of the normalized residue ratio scale. Dysphagia. 2013;28(4):494–500. [DOI] [PubMed] [Google Scholar]
- [27]. Castell JA, Castell DO, Duranceau CA, Topart P. Manometric characteristics of the pharynx, upper esophageal sphincter, esophagus, and lower esophageal sphincter in patients with oculopharyngeal muscular dystrophy. Dysphagia. 1995;10(1):22–6. [DOI] [PubMed] [Google Scholar]
- [28]. Brisson JD, Gagnon C, Brais B, Cote I, Mathieu J. A study of impairments in oculopharyngeal muscular dystrophy. Muscle & Nerve 2020. [DOI] [PubMed] [Google Scholar]
- [29]. Shapira-Galitz Y, Shoffel-Havakuk H, Halperin D, Lahav Y. Correlation Between Pharyngeal Residue and Aspiration in Fiber-Optic Endoscopic Evaluation of Swallowing: An Observational Study. Arch Phys Med Rehabil. 2019;100(3):488–94. [DOI] [PubMed] [Google Scholar]
- [30]. van den Engel-Hoek L, de Groot IJ, Sie LT, van Bruggen HW, de Groot SA, Erasmus CE, et al. Dystrophic changes in masticatory muscles related chewing problems and malocclusions in Duchenne muscular dystrophy. Neuromuscular disorders: NMD. 2016;26(6):354–60. [DOI] [PubMed] [Google Scholar]
- [31]. Baptista H, Lopes Cardoso I. Steinert syndrome and repercussions in dental medicine. Arch Oral Biol. 2017;75, 37–47. [DOI] [PubMed] [Google Scholar]
- [32]. Knuijt S, Kalf JG, de Swart BJM, Drost G, Hendricks HT, Geurts ACH, et al. Dysarthria and dysphagia are highly prevalent among various types of neuromuscular diseases. Disabil Rehabil. 2014;36(15):1285–9. [DOI] [PubMed] [Google Scholar]
- [33]. Bouchard JP, Brais B, Brunet D, Gould PV, Rouleau GA. Recent studies on oculopharyngeal muscular dystrophy in Quebec. Neuromuscular disorders: NMD.S. 1997;7(Suppl 1):22–9. [DOI] [PubMed] [Google Scholar]
- [34]. Becher MW, Morrison L, Davis LE, Maki WC, King MK, Bicknell JM, et al. Oculopharyngeal muscular dystrophy in Hispanic New Mexicans. JAMA. 2001;286(19):2437–40. [DOI] [PubMed] [Google Scholar]
- [35]. Duranceau AC, Beauchamp G, Jamieson GG, Barbeau A. Oropharyngeal dysphagia and oculopharyngeal muscular dystrophy. Surg Clin North Am. 1983;63(4):825–32. [DOI] [PubMed] [Google Scholar]
- [36]. Knuijt S, Kalf JG, van Engelen BGM, de Swart BJM, Geurts ACH. The Radboud Dysarthria Assessment: Development and Clinimetric Evaluation. Folia phoniatrica et logopaedica : Official organ of the International Association of Logopedics and Phoniatrics. 2017;69(4):143–53. [DOI] [PubMed] [Google Scholar]
- [37]. Steele CM, Peladeau-Pigeon M, Barbon CAE, Guida BT, Namasivayam-MacDonald AM, Nascimento WV, et al. Reference Values for Healthy Swallowing Across the Range From Thin to Extremely Thick Liquids. Journal of speech, language, and hearing research: JSLHR. 2019;62(5):1338–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Kim HD, Choi JB, Yoo SJ, Chang MY, Lee SW, Park JS. Tongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. J Oral Rehabil. 2017;44(1):59–64. [DOI] [PubMed] [Google Scholar]
- [39]. Namiki C, Hara K, Tohara H, Kobayashi K, Chantaramanee A, Nakagawa K, et al. Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin Interv Aging. 2019;14, 601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Park JS, Kim HJ, Oh DH. Effect of tongue strength training using the Iowa Oral Performance Instrument in stroke patients with dysphagia. J Phys Ther Sci. 2015;27(12):3631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. van Bruggen HW, van den Engel-Hoek L, Steenks MH, van der Bilt A, Bronkhorst EM, Creugers NHJ, et al. Fighting Against Disuse of the Masticatory System in Duchenne Muscular Dystrophy: A Pilot Study Using Chewing Gum. J Child Neurol. 2015;30(12):1625–32. [DOI] [PubMed] [Google Scholar]
- [42]. Steele CM, Grace-Martin K. Reflections on Clinical and Statistical Use of the Penetration-Aspiration Scale. Dysphagia. 2017;32(5):601–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.