Abstract
Activation of the substance P (SP)/neurokinin 1 receptor (NK1R) axis triggers biliary damage/senescence and liver fibrosis in bile duct ligated and Mdr2−/− (alias Abcb4−/−) mice through enhanced transforming growth factor-β1 (TGF-β1) biliary secretion. Recent evidence indicates a role for miR-31 (MIR31) in TGF-β1–induced liver fibrosis. We aimed to define the role of the SP/NK1R/TGF-β1/miR-31 axis in regulating biliary proliferation and liver fibrosis during cholestasis. Thus, we generated a novel model with double knockout of Mdr2−/− and NK1R−/ (alias Tacr1−/−) to further address the role of the SP/NK1R axis during chronic cholestasis. In vivo studies were performed in the following 12-week–old male mice: (i) NK1R−/−; (ii) Mdr2−/−; and (iii) NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) and their corresponding wild-type controls. Liver tissues and cholangiocytes were collected, and liver damage, changes in biliary mass/senescence, and inflammation as well as liver fibrosis were evaluated by both immunohistochemistry in liver sections and real-time PCR. miR-31 expression was measured by real-time PCR in isolated cholangiocytes. Decreased ductular reaction, liver fibrosis, biliary senescence, and biliary inflammation were observed in NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice. Elevated expression of miR-31 was observed in Mdr2−/− mice, which was reduced in NK1R−/−/Mdr2−/− mice. Targeting the SP/NK1R and/or miR-31 may be a potential approach in treating human cholangiopathies, including primary sclerosing cholangitis.
In addition to modifying bile of canalicular origin by a series of coordinated reabsorptive and secretory events,1 cholangiocytes are the target cells of several chronic cholestatic liver diseases (ie, cholangiopathies), such as primary sclerosing cholangitis (PSC), primary biliary cholangitis, biliary atresia, and cholangiocarcinoma.2, 3, 4, 5 PSC is a liver disease (more frequent in middle-aged men)4 that targets extrahepatic and/or intrahepatic bile ducts and is characterized by biliary strictures and activation of hepatic stellate cells (HSCs), causing excessive collagen deposition.2,3,6,7 PSC phenotypes can also progress to severe disorders, such as cholangiocarcinoma and pancreatic and colorectal cancer.4 There is growing evidence that during the progression of cholangiopathies, cholangiocytes undergo cellular senescence, thereby secreting several cytokines (ie, senescence-associated secretory phenotypes), including transforming growth factor-β1 (TGF-β1), IL-6, IL-8, C-C motif chemokine ligand 2, and plasminogen activator inhibitor-1, which trigger activation of HSCs by paracrine mechanisms.8, 9, 10, 11
Several studies have demonstrated that during the progression of cholestatic liver diseases, cholangiocytes acquire neuroendocrine phenotypes and secrete and respond to several neuropeptides, including α-calcitonin gene related peptide (α-CGRP) and substance P (SP).9,12 Both α-CGRP and SP are secreted by neuronal and nonneuronal cells, including macrophages, eosinophils, and cholangiocytes.9,12, 13, 14, 15 There is also growing information regarding the role of neurotransmitters and sensory neuropeptides in the regulation of biliary damage and liver fibrosis.9,12 For example, the α1-adrenergic receptor agonist, phenylephrine, stimulates biliary proliferation via Ca2+-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1.16 In addition, parasympathetic innervation has been shown to modulate biliary proliferation and apoptosis in the bile duct ligated (BDL) rat model.17 Regarding sensory innervation, knockout of α-CGRP (Calca) improves biliary damage and liver fibrosis by reduced biliary senescence, but enhances HSC senescence.12 Furthermore, SP has been shown to stimulate biliary mass/senescence as well as liver inflammation and fibrosis by binding to tachykinin neurokinin 1 receptor (NK1R) expressed by both cholangiocytes and HSCs,18, 19, 20 whereas knockout of NK1R in cholestatic BDL mice, and treatment of multidrug-resistance protein 2 knockout (Mdr2−/−, alias Abcb4−/−) mice with an NK1R antagonist (L-733,060), reduces biliary senescence and liver fibrosis.9
Several rodent models of cholangiopathies [eg, extrahepatic bile duct obstruction, BDL, dominant-negative TGF-β receptor II (primary biliary cholangitis), and Mdr2−/− (PSC)] have been developed and used to define the molecular mechanisms and test new therapies for the management of biliary damage and liver fibrosis in cholestatic liver diseases.9,10,12,21, 22, 23 Mdr2−/− mice are deficient in canalicular phospholipid flippase24 and develop PSC phenotypes as a result of an increase in bile concentration and the absence of phospholipids from bile.23 On the basis of this information, the development and phenotypic characterization of Mdr2−/− mice lacking NK1R (NK1R−/−/Mdr2−/−, alias Tacr1−/−/Abcb4−/−) would be an important tool to evaluate the role of sensory innervation in the regulation of PSC phenotypes, such as changes in biliary mass/senescence, activation of HSCs and liver fibrosis, as well as TGF-β1/miR-31 signaling, which is important in biliary senescence and activation of HSCs.10,25
Materials and Methods
Materials
Reagents were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO), unless otherwise indicated. The iScript cDNA Synthesis Kit and iTaq Universal SYBR Green Supermix were purchased from Bio-Rad Laboratories (Hercules, CA). The mouse and human PCR primers were purchased from Qiagen (Germantown, MD) (Table 1). The rabbit monoclonal antibody against cytokeratin-19 (CK-19; ab52625), rabbit polyclonal antibody hepatocyte nuclear factor 4α (ab78356), rabbit polyclonal antibody to collagen I (ab34710), the rabbit polyclonal antibody against anti-CDKN2A/p16INK4a (ab108349), and rabbit monoclonal antibody against NK1R (ab183713) were purchased from Abcam (Cambridge, MA). The anti-desmin antibody (Y66; Alexa Fluor 488) was purchased from Abcam. The rabbit polyclonal antibody against NK1R was purchased from Thermo Fisher Scientific Company (Mountain View, CA). The rat monoclonal antibody against CK-19 was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA). The rabbit monoclonal antibody against F4/80 (marker of macrophages)21 and the rabbit polyclonal antibody against tata-binding protein were purchased from Cell Signaling (Danvers, MA). The total RNA isolation and TGF-β1 enzyme-linked immunosorbent assay kits were obtained from Thermo Fisher Scientific Company. The SP Enzyme Immunoassay kits were purchased from Phoenix Pharmaceuticals (Burlingame, CA). Research was performed in the Indiana Center for Liver Research at the Richard L. Roudebush VA Medical Center and Indiana University.
Table 1.
List of PCR and miRNA Primers
| Gene | Species | Reference sequence | Manufacturer |
|---|---|---|---|
| Acta2 (α-SMA) | Mouse | NM_007392∗ | Qiagen |
| Actb | Mouse | NM_007393∗ | Qiagen |
| Cdkn1a (p21) | Mouse | NM_007669∗ | Qiagen |
| Cdkn2a (p16) | Mouse | NM_009877∗ | Qiagen |
| Col1α1 (Col1a1) | Mouse | NM_007742∗ | Qiagen |
| IL-1β (Il1b) | Mouse | NM_008361∗ | Qiagen |
| Il-6 (Il6) | Mouse | NM_031168∗ | Qiagen |
| TAC-1 (Tac1) | Mouse | NM_009311∗ | Qiagen |
| TGF-β1 (Tgfb1) | Mouse | NM_011577∗ | Qiagen |
| TNF-α (Tnf) | Mouse | NM_013893∗ | Qiagen |
| Mme | Mouse | NM_008604∗ | Qiagen |
| ACTB | Human | NM_001101∗ | Qiagen |
| MME | Human | NM_000902∗ | Qiagen |
| RNAU6 | Mouse and human | NR_004394† | Thermo Fisher Scientific (Mountain View, CA) |
| miR31 | Mouse | MI0000579† | Thermo Fisher Scientific |
| miR31 | Human | MI0000089† | Thermo Fisher Scientific |
National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov).
miRbase (http://www.mirbase.org).
Animal Models
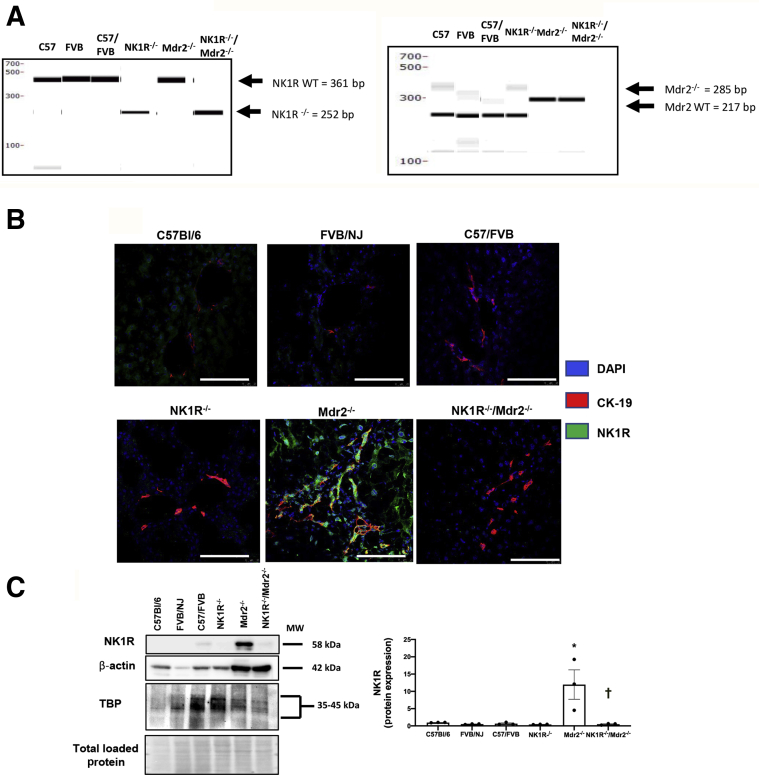
Animal experiments were performed in male mice according to protocols approved by the Baylor Scott & White and Indiana University School of Medicine Institutional Animal Care and Use Committees. C57/BL6 wild-type (WT) mice (25 to 30 g; 12 weeks of age; control for NK1R−/− mice) were purchased from Charles River Laboratories (Wilmington, MA). FVB/NJ WT mice (25 to 30 g; 12 weeks of age; control for Mdr2−/− mice) were purchased from Jackson Laboratories (Bar Harbor, ME); Mdr2−/− mice (25 to 30 g; 12 weeks of age) are available in our breeding colony. C57/FVB/NJ WT mice (control for NK1R−/−/Mdr2−/− mice) were generated by breeding C57/BL6 mice with FVB/NJ mice for several generations. The original breeding pair for the NK1R−/− mouse (25 to 30 g; 12 weeks of age) model was a gift from Dr. Norma Gerard (Harvard Medical School, Boston, MA) to Dr. Donald DiPette (University of South Carolina School of Medicine, Columbia, SC); the breeding pair of mice was used in our previous studies.9,18 These mice are knockout of the NK1R gene (ie, Tacr1−/−, which we refer to as NK1R−/− as described9,18). With our established NK1R−/− and Mdr2−/− mouse colonies in our animal facility, the two strains (having different backgrounds) were bred together for several generations, after which the homozygous double-knockout (NK1R−/−/Mdr2−/−double-knockout) mice were obtained. Charles River Laboratories confirmed the genotype of each NK1R−/−/Mdr2−/− mouse through PCR amplification of DNA extracted from tail snips. The NK1R−/− mice were identified with the following primers: i) NK1Rrev (5′-ACAGCTGTCATGGAGTAGATAC-3′); ii) NK1Rfwd (5′-CTGTGGACTCTGATCTCTTCC-3′); and iii) NeoF2 (5′-ATCGCCTTCTTGACGAGTTC-3′). Migration positions were observed at 406 and 252 bp for WT and NK1R−/−, respectively. Mdr2−/− animals were identified with the following primers: i) 12,834 (mutant reverse: 5′-GCTACTTCCATTTGTCACGTCC-3′); ii) 26,728 (forward, common: 5′-TGGGAAGAGTGGAGAAATCG-3′); and iii) 26,729 (WT reverse: 5′-TGAAGACATCGGTGTTCAGAG-3′), with migration positions observed at 217 and 285 bp for WT and Mdr2−/−, respectively (Figure 1A).
Figure 1.
A: PCR genotyping demonstrates the deletion of NK1R (Tacr1) and Mdr2 (Abcb4) in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice. B: Immunofluorescence in liver sections demonstrates neurokinin 1 receptor (NK1R) immunoreactivity in cholangiocytes from wild-type (WT) and Mdr2−/− mice, immunoreactivity that was absent in both NK1R−/− and NK1R−/−/Mdr2−/− mice. C: By immunoblots, there is low expression of NK1R in cholangiocytes from WT mice, which increased in cholangiocytes from Mdr2−/− mice; no expression for NK1R is observed in either NK1R−/− or NK1R−/−/Mdr2−/− mice. The immunoblot was performed in cumulative preparations of isolated cholangiocytes from six mice. ∗P < 0.05 versus WT; †P < 0.05 versus Mdr2−/− mice. Scale bars = 100 μm (B). Original magnification, ×40 (B). CK-19, cytokeratin-19; MW, molecular weight; TBP, tata-binding protein.
All mice were maintained in a temperature-controlled environment (20°C to 22°C) with 12:12-hour light/dark cycles and fed standard chow with access to drinking water ad libitum. In summary, all experiments were performed in the following groups: NK1R−/−, Mdr2−/−, and NK1R−/−/Mdr2−/− and their corresponding WT animals. Before each experimental procedure, animals were injected with euthasol (50 to 100 mg/kg body weight). Because there was no difference in intrahepatic bile duct mass (IBDM), liver fibrosis, and biliary senescence (see section below) between the different WT mice used (C57/BL6, FVB/NJ WT, and C57/FVB WT mice), some of the experiments were performed only in C57/BL6 or C57/FVB/NJ mice. Serum, total liver samples, cholangiocytes, and cholangiocyte supernatant (after incubation at 37°C for 4 hours)26 were collected from the selected groups of animals; samples were stored at −80°C until used.
Isolated Cholangiocytes and Human Samples
Cholangiocytes were purified by immunoaffinity separation26,27 using a monoclonal antibody IgG2a (a gift from Dr. R. Faris, Brown University, Providence, RI). Cell viability was evaluated by trypan blue exclusion. Liver tissue from healthy patients (n = 4) was bought from Sekisui XenoTech Company (Kansas City, KS). In addition, liver tissues from healthy (n = 6) and PSC patients (n = 7) were obtained from S.L. under a protocol approved by the Indiana University–Purdue University Indianapolis Institutional Review Board. The unidentified coded human liver specimens for mRNA analysis were obtained through the Liver Tissue Procurement and Distribution System (Minneapolis, MN).28 Liver specimens from PSC patients were obtained from the explant during liver transplantation. Control liver samples were from patients with no known history of chronic liver diseases and collected during abdominal surgeries for various causes. Written informed consent was received from participants before inclusion in the study. Information on human healthy control and PSC patients is shown (Table 2).
Table 2.
Characteristics of PSC Patients
| Groups | Patient | Sample | Sex | Ethnicity | Cirrhosis | Therapy |
|---|---|---|---|---|---|---|
| Control | H1255 | RNA from total liver | Female | African American | No | No |
| H1293 | RNA from total liver | Female | White | No | No | |
| H1296 | RNA from total liver | Male | White | No | No | |
| H1299 | RNA from total liver | Female | White | No | No | |
| 1 | RNA and protein from total liver | NA | NA | NA | NA | |
| 2 | RNA and protein from total liver | NA | NA | NA | NA | |
| 3 | RNA and protein from total liver | NA | NA | NA | NA | |
| 4 | RNA and protein from total liver | NA | NA | NA | NA | |
| 5 | RNA and protein from total liver | NA | NA | NA | NA | |
| 6 | RNA and protein from total liver | NA | NA | NA | NA | |
| 1 | RNA and protein from total liver | Male | White | No | Untreated | |
| 2 | RNA and protein from total liver | NA | NA | No | Untreated | |
| PSC | 3 | RNA and protein from total liver | Male | White | No | Untreated |
| 4 | RNA and protein from total liver | Male | White | No | Untreated | |
| 5 | RNA and protein from total liver | Female | White | No | Untreated | |
| 6 | RNA and protein from total liver | Male | White | No | Untreated | |
| 7 | RNA and protein from total liver | Male | White | No | Untreated |
NA, not applicable; PSC, primary sclerosing cholangitis.
Measurement of NK1R Immunoreactivity in Liver Sections and NK1R Protein Expression in Isolated Cholangiocytes
Measurement of TAC1 and MME mRNA Expression in Total Liver and Isolated Cholangiocytes and Human Total Liver Samples and SP Serum Levels
By immunofluorescence, the immunoreactivity of NK1R costained with CK-19 (biliary marker),27 glial fibrillary acidic protein (marker of HSCs),29,30 or hepatocyte nuclear factor 4α (hepatocyte marker)31 in frozen liver sections (6 to 8 μm thick) was measured. Following staining, sections were incubated with the respective secondary antibody (Cy2 anti-rabbit and Cy3 anti-rat; Jackson Immunochemicals, West Grove, PA). Following incubation, the slides were washed in 1× phosphate-buffered saline and coverslipped with antifade gold containing DAPI as a counterstain (Molecular Probes, Eugene, OR). Negative controls were included; images from the selected sections were obtained using Leica TCS SP5 X system (Leica Microsystems Inc., Buffalo Grove, IL).
The expression of NK1R in isolated cholangiocytes from selected groups of animals was measured by immunoblots. The loading controls used were β-actin and tata-binding protein (Cell Signaling number 8515) (by immunoblots) and total protein loading that was performed by Ponceau S staining because of the dynamic changes of loading control in mouse model of liver fibrosis.32 The bands were analyzed by the BioRad ChemiDoc Imaging System (BioRad Laboratories, Hercules, CA); the NK1R quantification was performed as ratio of total protein load by ImageJ version 1.52n (NIH, Bethesda, MD; https://imagej.nih.gov/ij). This study also measured: i) the mRNA expression of tachykinin precursor 1 (Tac1; gene encoded for SP) in total liver from C57/FVB/NJ (WT), Mdr2−/−, and NK1R−/−/Mdr2−/− mice; ii) membrane metalloendopeptidase (MME; the enzymes regulating the synthesis and degradation of SP)33 in cholangiocytes from FVB/NJ (WT) and Mdr2−/− mice as well as total liver samples from control and late-stage PSC patients by real-time PCR, respectively; and iii) SP serum levels by Enzyme Immunoassay kits.9
Evaluation of Liver Histology and IBDM
The histology of liver and other organs (heart, lung, stomach, pancreas, small and large intestine, spleen, and kidney) was evaluated by hematoxylin and eosin staining in paraffin-embedded liver sections (4 to 5 μm thick); slides were evaluated by a Leica DM 4500B in a blinded manner by a board-certified pathologist (A.S.).
IBDM was determined by quantitative immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick) for CK-19. IBDM was quantified as follows: (area of CK-19–positive bile ducts/total area) × 100; for each experimental group, eight stained slides from four different animals for each group were scanned by a digital scanner (Aperio Scanscope CS System; Aperio Digital Pathology, Leica Biosystems, Milan, Italy) and processed by ImageScope version 12.3.3 (Leica Biosystems).
Evaluation of Liver Inflammation and Fibrosis
Liver inflammation was evaluated by quantitative immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick) for F4/80 (marker of macrophages)34; and real-time PCR for IL-1β and tumor necrosis factor-α in total liver samples. Liver fibrosis was assessed by Sirius red staining in paraffin-embedded liver sections (4 to 5 μm thick); 10 random fields (n = 3) were examined by Visiopharm (Westminister, CO); collagen deposition was measured as follows: (area of red-positive collagen fibers/total area) × 100. Liver sections were scanned with Leica Aperio AT2 Scanner; images were taken with Aperio ImageScope version 12.4.0.5043.
The extent of liver fibrosis was also assessed by costaining CK-19 and collagen 1 in frozen tissue liver (6 to 8 μm thick) as well as measurement of hydroxyproline levels in total liver samples using the Hydroxyproline Assay Kit (MAK008; Sigma-Aldrich). The expression of specific fibrosis genes (Acta2 and Col1a1) were measured by real-time PCR in total liver samples from the selected groups of animals.
Evaluation of Cellular Senescence
Cellular senescence was measured in frozen liver sections (10 μm thick) by staining for SA-β-galactosidase by a commercially available kit (MilliporeSigma, Billerica, MA). Observations were made with the Olympus BX40 microscope (Tokyo, Japan). To further demonstrate biliary senescence, immunofluorescence was performed for the senescence marker p16 (costained with CK-19) in frozen liver sections (6 to 8 μm thick); 10 fields were analyzed from three different liver samples from three different animals from the selected groups of animals. Observations were made with the Leica TCS SP5 X system (Leica Microsystems Inc.). Cellular senescence was also measured by real-time PCR for Cdkn2a (p16) and Cdkn1a (p21) in total liver samples from the selected groups of animals.
Measurement of TGF-β1 Levels and miR-31 Expression
Because Ingenuity Pathway Analysis software version 01-16 (Qiagen, Redwood City, CA) suggested a link between SP/NK1R axis and TGF-β1/miR-31 signaling pathways, experiments aimed to demonstrate that the effects of the SP/NK1R on liver fibrosis is mediated by activation of the TGF-β1/miR-31 axis were performed. The rationale for these experiments is also based on the finding that SP activation of biliary senescence and liver fibrosis is mediated by enhanced expression/secretion of cholangiocyte TGF-β1.9 First, TGF-β1 levels were measured in both serum and cholangiocyte supernatant by commercially available enzyme-linked immunosorbent assay kits.9 The expression levels of miR-31 (expressed as ratio to the housekeeping, RNAU6) was evaluated by real-time PCR in isolated cholangiocytes from the selected groups of animals and total liver samples from late-stage PSC and healthy control patients.
Statistical Analysis
The GraphPad Prism software version 8.3.1 (GraphPad Software, San Diego, CA) was used to perform the statistical analyses. Data are expressed as the means ± SEM. Differences between groups were analyzed by the unpaired t-test when two groups were analyzed or by analysis of variance when more than two groups were analyzed. P < 0.05 was considered significant.
Results
Measurement of NK1R Immunoreactivity in Liver Sections and Protein Expression in Isolated Cholangiocytes
Measurement of TAC1 and MME mRNA Expression in Mouse and Human Total Liver and Isolated Cholangiocytes and Human Total Liver Samples and SP Serum Levels
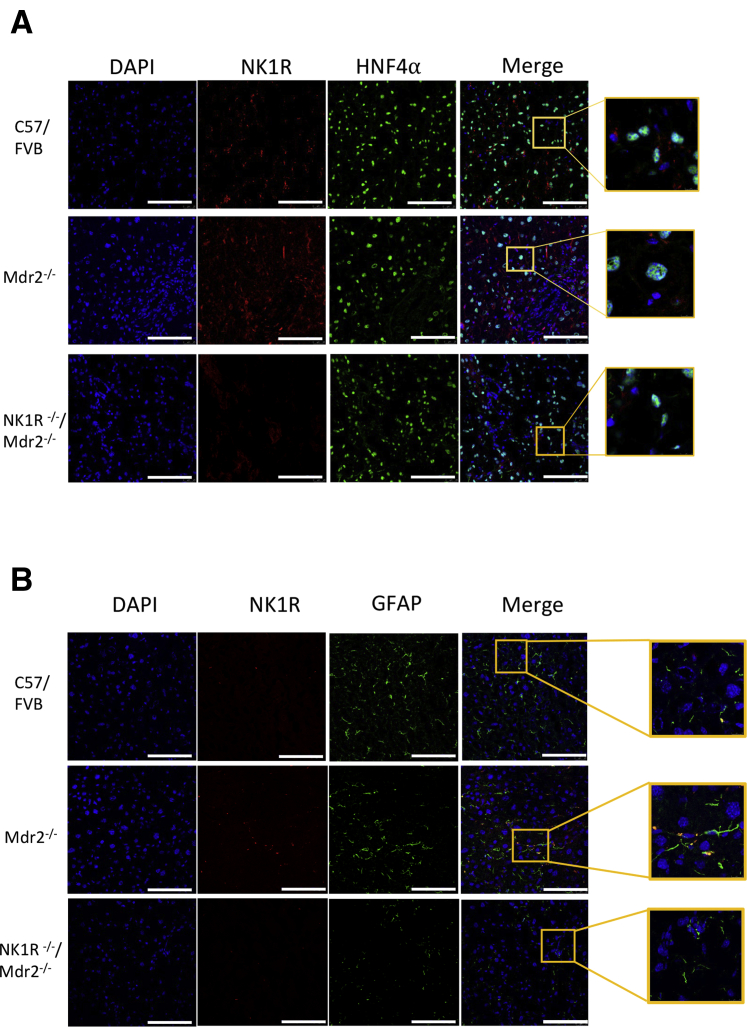
All of the animal groups were subjected to PCR genotyping to evaluate the deletion of NK1R and Mdr2 in NK1R−/−/Mdr2−/− mice (Figure 1A). The presence/absence of NK1R in bile ducts was confirmed by immunofluorescence for NK1R in liver sections costained with CK-19; NK1R biliary immunoreactivity was absent in NK1R−/− and NK1R−/−/Mdr2−/− mice (Figure 1B). By immunoblots, there was low expression of NK1R in cholangiocytes from WT mice, which increased in cholangiocytes from Mdr2−/− mice; no expression for NK1R was observed in either NK1R−/− or NK1R−/−/Mdr2−/− mice (Figure 1C). NK1R immunoreactivity was detected in HSCs9 but not hepatocytes from WT and Mdr2−/− mice, and was absent in both HSCs and hepatocytes from NK1R−/−/Mdr2−/− mice (Figure 2).
Figure 2.
A and B: Neurokinin 1 receptor (NK1R) immunoreactivity is also present in hepatic stellate cells [HSCs; costained with glial fibrillary acidic protein (GFAP)] but not hepatocytes [costained with hepatocyte nuclear factor 4α (HNF4α)] from wild-type and Mdr2−/−(Abcb4−/−) mice, but absent in both HSCs and hepatocytes from NK1R−/−(Tacr1−/−) mice and NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice. Enlarged areas (10× enlargement for hepatocytes and 7× enlargement for HSCs) show an example of NK1R expression in hepatocytes and HSCs. Scale bars = 100 μm (A and B). Original magnification, ×40 (A and B).
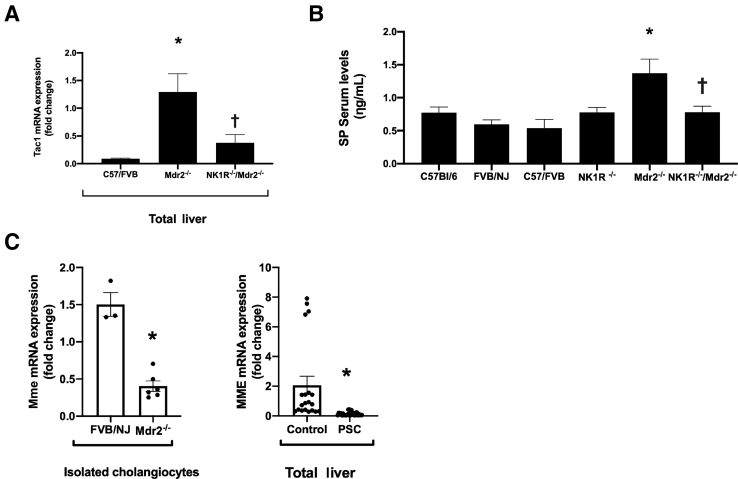
There was increased Tac1 mRNA expression and SP serum levels in Mdr2−/− mice compared with the corresponding WT mice, which was reversed in NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice (Figure 3, A and B); and decreased mRNA expression of MME in Mdr2−/− mice and late-stage PSC liver samples compared with control samples (Figure 3C). These findings further support a previous study, which demonstrated enhanced TAC1 mRNA expression in total liver samples from late-stage PSC patients compared with healthy control samples.9
Figure 3.
There is increased Tac1 mRNA expression (in total liver samples) and substance P (SP) serum levels in Mdr2−/−(Abcb4−/−) mice compared with wild-type mice, which is reversed in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice compared with Mdr2−/− mice (A and B); and decreased mRNA expression of MME in cholangiocytes from Mdr2−/− mice and total liver samples from late-stage primary sclerosing cholangitis (PSC) patients compared with control samples (C). A: Data related to Tac1 mRNA expression are of three PCRs from three different total liver mouse samples. B: Data related to SP serum levels are performed in triplicate from three mice. C: Data are performed in triplicate from cumulative preparations of cholangiocytes from six mice. Data are performed in quadruplicate in total liver from control and PSC patients. Data are expressed as means ± SEM (A–C). n = 5 controls (C); n = 7 PSC patients (C). ∗P < 0.05 versus FVB/NJ mice and control; †P< 0.05 versus Mdr2−/− mice.
Evaluation of Liver Histology and IBDM
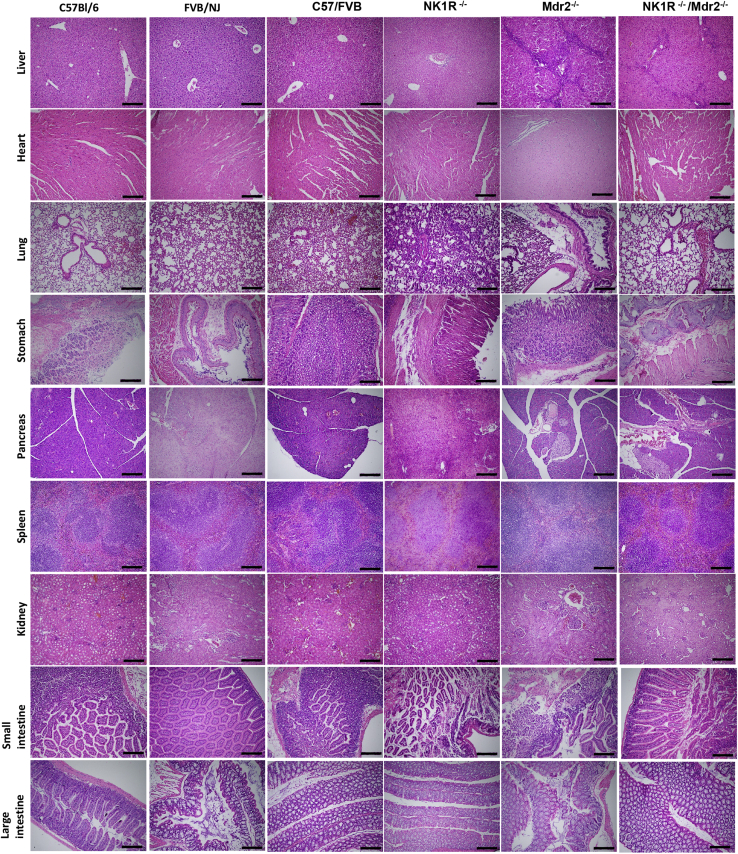
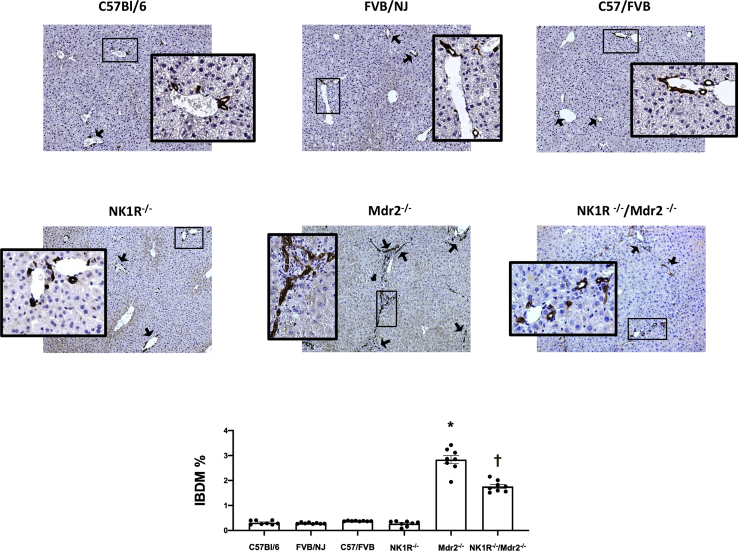
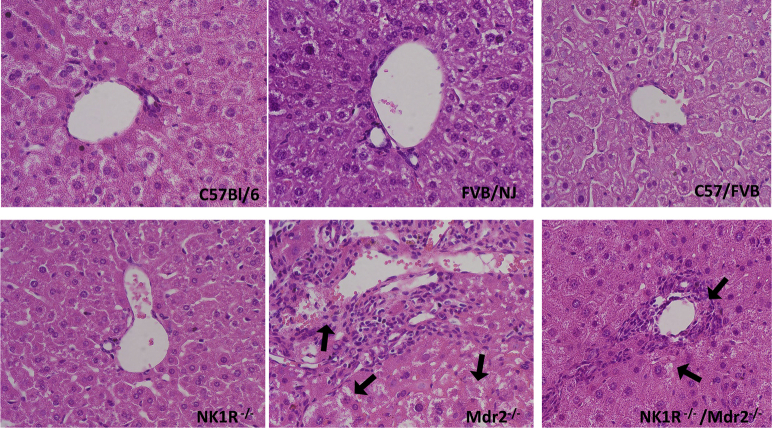
By hematoxylin and eosin staining in the livers of Mdr2−/− mice, portal areas are expanded by an inflammatory infiltrate that appears composed predominantly of lymphocytes with rare eosinophils; there was mild activity at the limiting plate with possible entrapment of few hepatocytes. Lobules show reactive, foamy cytoplasm, but no evidence of collapse, necrosis, ballooning, or steatosis (Figure 4). In the livers of NK1R−/−/Mdr2−/− mice, a mild inflammatory infiltrate composed predominantly of lymphocytes and neutrophils and no significant lobular inflammation or steatosis was observed; at the limiting plate, few necrotic hepatocytes were seen (Figure 4 and Supplemental Figure S1). The architecture of the livers of WT and NK1R−/− mice did not present any abnormality (Figure 4). No significant pathologic changes were observed in other organs (except lung) among all of the animal groups (Figure 4); specifically, in lung tissue from NK1R−/−/Mdr2−/− mice, there was increased septal cellularity composed predominantly of neutrophils with scattered aggregates of lymphocytes and increased intra-alveolar macrophages (Figure 4). There was increased IBDM in Mdr2−/− mice (compared with the corresponding WT mice) that was significantly reduced in NK1R−/−/Mdr2−/−mice (Figure 5); no significant changes in IBDM were observed between control and NK1R−/− mice (Figure 5).
Figure 4.
Representative hematoxylin and eosin staining of sections from liver and selected organs from C57/Bl6, FVB/NJ, C57/FVB/NJ, NK1R−/− (Tacr1−/−), Mdr2−/− (Abcb4)−/−, and NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice. Data analyzed from three different animals for each group. Scale bars = 100 μm. Original magnification, ×10.
Figure 5.
There is increased intrahepatic bile duct mass (IBDM) in Mdr2−/− (Abcb4−/−) mice [compared with the corresponding wild-type (WT) mice] that is significantly reduced in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice; no significant changes in IBDM are observed between WT and NK1R−/− (Tacr1−/−) mice. Black arrows indicate bile ducts. Boxed areas are shown at higher magnification in the insets. Data are of eight slides analyzed from four different animals for each group. Data are expressed as means ± SEM. ∗P < 0.05 versus FVB/NJ mice; †P < 0.05 versus Mdr2−/− mice. Original magnification: ×10 (main images); ×40 (insets).
Evaluation of Liver Inflammation and Fibrosis
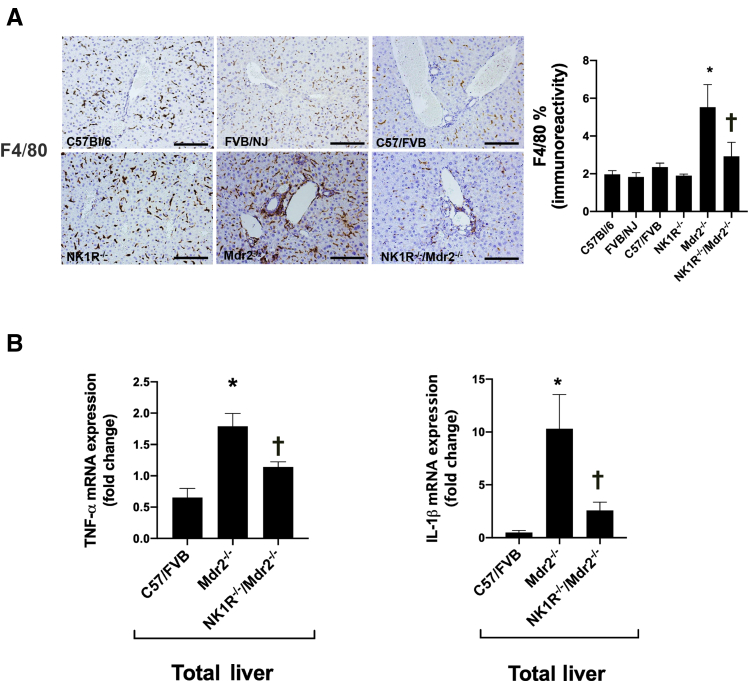
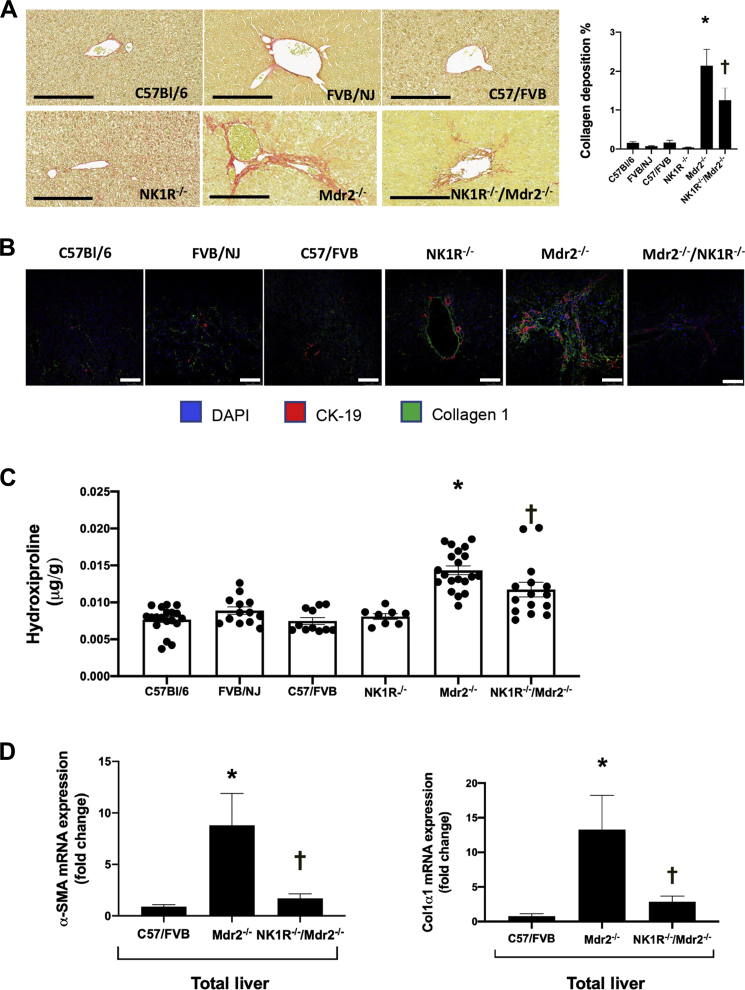
In WT animal groups, macrophages were randomly spread throughout liver tissue; however, a greater number of macrophages was observed in the periportal area of Mdr2−/− mice, which was significantly reduced in NK1R−/−/Mdr2−/− mice (Figure 6A). Also, the mRNA expression of IL-1β (Il1b) and tumor necrosis factor-α was higher in Mdr2−/− compared with WT mice, but significantly decreased in NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice (Figure 6B). The increased collagen deposition observed in Mdr2−/− mice (compared with WT mice) was significantly reduced in NK1R−/−/Mdr2−/− mice; no difference was seen among the corresponding WT mice and NK1R−/− mice (Figure 7A). These data were confirmed by immunofluorescence for collagen1a in liver sections (costained with CK-19) as well as by changes in hydroxyproline levels in total liver samples (Figure 7, B and C). In total liver samples from Mdr2−/− mice, there was increased mRNA expression of Acta2 and Col1a1 compared with WT mice, which was significantly reduced in NK1R−/−/Mdr2−/− mice (Figure 7D).
Figure 6.
A: There are greater number of macrophages in the periportal area of Mdr2−/− (Abcb4−/−) mice [compared with wild-type (WT) mice] that are significantly reduced in NK1R−/−/Mdr2−/−(Tacr1−/−/Abcb4−/−) mice. B: The mRNA expression of IL-1β (Il1b) and tumor necrosis factor-α (TNF-α) (Tnf) are higher in Mdr2−/− mice compared with WT mice, but significantly decreased in NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice. Data are of three PCRs (performed in triplicate) from three different total liver samples. Data are expressed as means ± SEM (A and B). ∗P < 0.05 versus FVB/NJ mice; †P< 0.05 versus Mdr2−/− mice. Scale bars = 100 μm (A). Original magnification, ×20 (A).
Figure 7.
A: The increased collagen deposition observed in Mdr2−/− (Abcb4−/−) mice is significantly reduced in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice; no difference is seen among the corresponding wild-type (WT) mice and NK1R−/− mice. B and C: By immunofluorescence for collagen 1 in liver sections [costained with cytokeratin-19 (CK-19)] and measurement of hydroxyproline levels in total liver samples, increased fibrosis is observed in Mdr2−/− mice, which is significantly reduced in NK1R−/−/Mdr2−/− mice. D: There is increased mRNA expression of Acta2 (α-SMA) and Col1a1 (Col1α1) in total liver samples from Mdr2−/− mice compared with WT mice, which is reduced significantly in NK1R−/−/Mdr2−/− mice. Data are of three evaluations from four different total liver samples (C); data are of three PCRs (performed in triplicate) from three different total liver samples (D). Data are expressed as means ± SEM (A, C, and D). ∗P < 0.05 versus FVB/NJ mice; †P< 0.05 versus Mdr2−/− mice. Scale bars = 100 μm (A and B). Original magnification: ×20 (A); ×60 (B). α-SMA, α-smooth muscle actin.
Knockout of NK1R Decreases Biliary Senescence in Mdr2−/− Mice
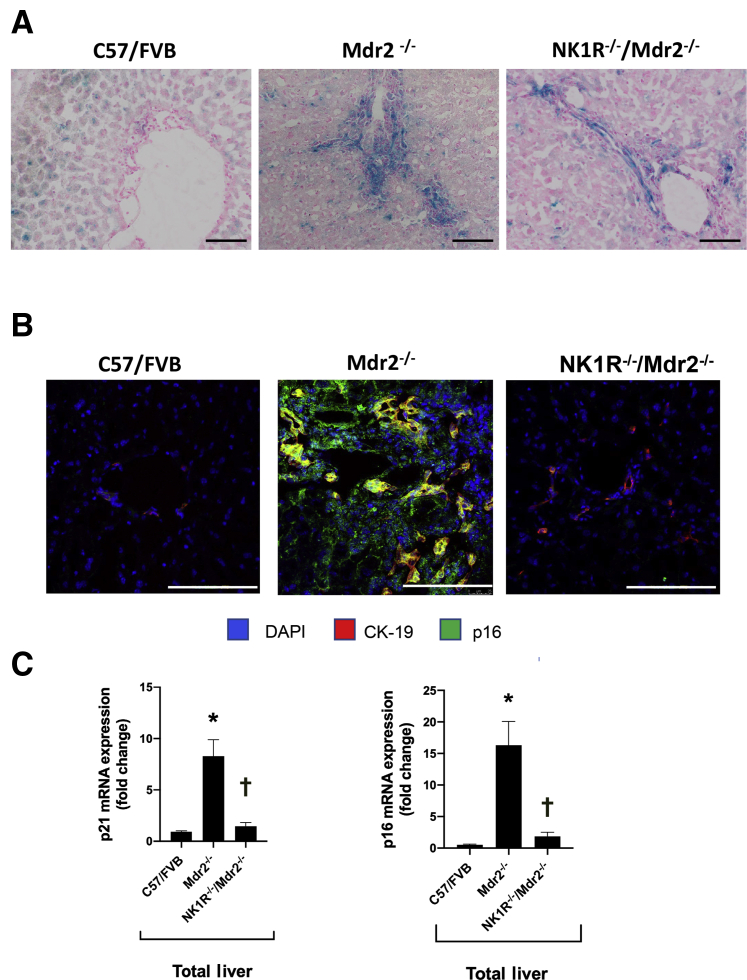
Previously, no significant differences in biliary senescence in the respective WT mice and NK1R−/− mice were noted.9 Parallel to a previous study,9 an enhanced biliary senescence was demonstrated in Mdr2−/− mice, which was reduced in NK1R−/−/Mdr2−/− mice, as evidenced by SA-β-galactosidase staining (Figure 8A) and immunofluorescence for p16 (Figure 8B) (costained for CK-19) in liver sections. An increased mRNA expression of Cdkn2a (p16) and Cdkn1a (p21) was demonstrated in total liver samples from Mdr2−/− mice compared with the corresponding WT mice; and decreased mRNA expression of Cdkn2a (p16) and Cdkn1a (p21) was demonstrated in total liver samples from NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice (Figure 8C).
Figure 8.
A and B: By both SA-β-galactosidase staining and immunofluorescence for p16 in liver sections, there is enhanced biliary senescence in Mdr2−/− (Abcb4−/−) mice that is reduced in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice. C: There is increased mRNA expression of Cdkn2a (p16) and Cdkn1a (p21) in total liver from Mdr2−/− mice compared with the corresponding wild-type mice, which decreased in cholangiocytes from NK1R−/−/Mdr2−/− compared with Mdr2−/− mice. Data are performed in triplicate in three different total liver samples from three mice. Data are expressed as means ± SEM (C). ∗P < 0.05 versus FVB/NJ mice; †P< 0.05 versus Mdr2−/− mice. Scale bars = 100 μm (A and B). Original magnification, ×20 (A and B). CK-19, cytokeratin-19.
The Effects of the SP/NK1R Axis Are Mediated by Activation of the TGF-β1/miR-31 Signaling
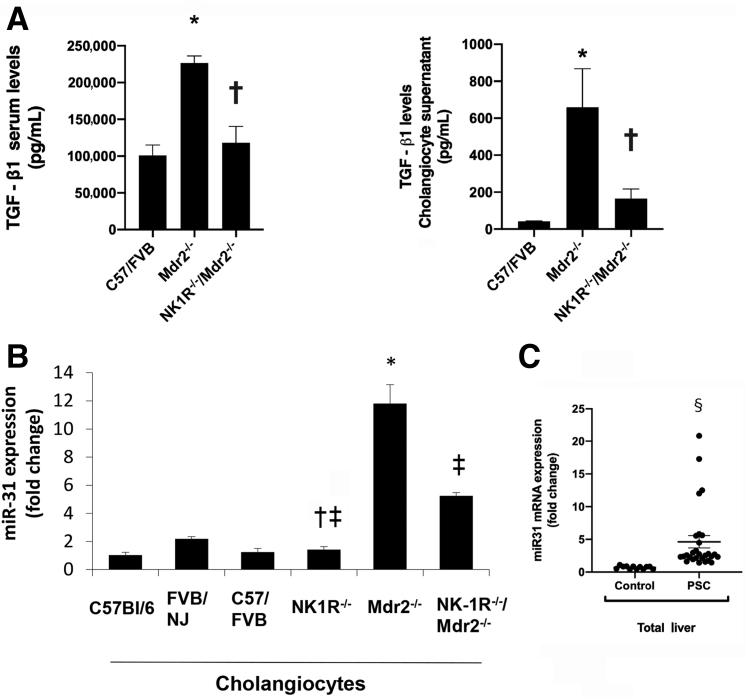
Next, it was determined whether TGF-β1 mediates the effects of the SP/NK1R axis on liver fibrosis by the activation of miR-31. According to Ingenuity Pathway Analysis, TGF-β1 modulates miR-31 expression (Figure 9), an miRNA that may regulate the effect of the SP/NK1R axis on changes in biliary mass/senescence and liver fibrosis.35 Increased TGF-β1 levels in serum and cholangiocyte supernatant were observed from Mdr2−/− mice (compared with WT mice), parameters that returned to values similar to that of WT mice in NK1R−/−/Mdr2−/− mice (Figure 10A). Furthermore, by real-time PCR, it was demonstrated that the expression of miR-31was higher in cholangiocytes from Mdr2−/− mice (compared with WT mice), but decreased in cholangiocytes from NK1R−/−/Mdr2−/− mice compared with Mdr2−/− mice (Figure 10B); increased expression of miR-31 was observed in total liver samples from late-stage PSC patients compared with healthy controls (Figure 10C).
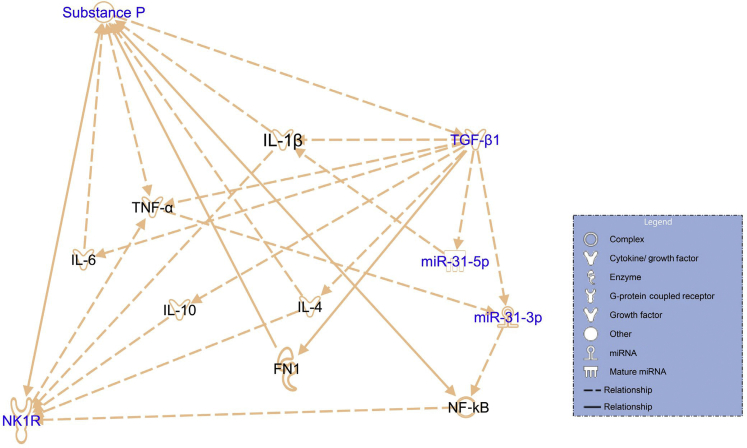
Figure 9.
Ingenuity Pathway Analysis (IPA) demonstrates the relationship between the substance P (SP)/neurokinin 1 receptor (NK1R) axis and transforming growth factor (TGF)-β1/miR-31/NF-κB signaling. The SP/NK1R axis and TGF-β1/miR-31/NF-κB signaling was generated with Qiagen IPA with permission to reproduce from Qiagen Silicon Valley. FN1, fibronectin-1; TNF-α, tumor necrosis factor-α.
Figure 10.
A: There is enhanced transforming growth factor (TGF)-β1 levels in serum and cholangiocyte supernatant from Mdr2−/− (Abcb4−/−) mice [compared with wild-type (WT) mice], parameters that returned to values similar to that of WT mice in NK1R−/−/Mdr2−/− (Tacr1−/−/Abcb4−/−) mice. Data related to TGF-β1 serum and cholangiocyte supernatant are from three different samples from three different mice and from cumulative preparations of cholangiocyte supernatant from six mice, respectively. B: By real-time PCR, the biliary expression of miR-31 is higher in Mdr2−/− mice (compared with WT mice), but decreased in cholangiocytes from NK1R−/−/Mdr2−/− compared with Mdr2−/− mice. Data are of three PCRs performed in triplicate from cumulative preparations of cholangiocytes from six mice. C: There is increased expression of miR-31 in total liver RNA samples from late-stage primary sclerosing cholangitis (PSC) patients compared with healthy controls. Data are of quadruplicate values. Data are expressed as means ± SEM (A–C). n = 7 PSC patients (C); n = 4 healthy controls (C). ∗P < 0.05 versus WT; †P < 0.05 versus Mdr2−/− mice; ‡P < 0.05 versus FVB/NJ mice; §P < 0.05 versus healthy controls.
Discussion
This study describes the development and functional characterization of a double-knockout model, where NK1R (a receptor expressed by both intrahepatic cholangiocytes and HSCs)9 expression is inhibited in the Mdr2−/− mouse model of PSC.9,22 This NK1R−/−/Mdr2−/− mouse model was characterized by decreased Tac1 mRNA expression and serum SP levels, but enhanced Mme mRNA expression compared with Mdr2−/− mice. In NK1R−/−/Mdr2−/− mice, we demonstrated: i) reduced periportal inflammation, biliary senescence/IBDM, and liver fibrosis; ii) decreased mRNA expression of Tgfb1 and TGF-β1 serum levels; and iii) reduced miR-31 expression compared with Mdr2−/− mice. The mouse data were supported by human findings showing a decrease in MME and an increase in miR-31 expression in late-stage PSC samples compared with healthy controls.
There is increasing information regarding the role of sensory innervation (α-CGRP and β-CGRP and the SP/NK1R axis) in the modulation of cholestatic liver diseases in animal models (eg, BDL and Mdr2−/−) as well as diseased human samples (eg, PSC).9,12,18,36 For example, several studies have shown that there is an increase in Calcα (encoding α-CGRP; and its receptor components calcitonin receptor like receptor, receptor activity modifying protein1, and CGRP receptor component) and α-CGRP serum levels; and enhanced expression of the SP/NK1R axis and SP serum levels in cholestatic animal models, such as Mdr2−/− and BDL, and human PSC samples and patients with cirrhosis.9,12,37 Furthermore, in cholestatic BDL α-CGRP−/− mice, there was decreased biliary mass and liver fibrosis as well as amelioration of liver damage compared with BDL WT mice.18,36 Other studies have shown that SP increases biliary mass and liver fibrosis through increased biliary senescence9; and knockout of NK1R decreases biliary proliferation/mass in BDL mice by down-regulation of cAMP/protein kinase A signaling.9,12,18 SP has also been shown to activate HSCs by TGF-β1/Smad-3–dependent signaling pathway.38 In addition, NK1R antagonists have been shown to prevent CD95- and cytokine-mediated liver injury.39,40 Moreover, overexpression of MME decreases SP-induced cholangiocarcinoma growth both in vitro (in cholangiocarcinoma cell lines) and in vivo in athymic mice.33
It is noteworthy that we developed a novel double-knockout model that allowed us to begin to study the intracellular mechanisms related to the phenotypes of human PSC, because this model maintained the normal morphologic phenotypes in other organs, except mild pathology in the lung. In this context, Mdr2−/− mice mimic some phenotypes of human PSC because they develop severe peribiliary inflammation, biliary senescence, and liver fibrosis.4,23 Parallel to previous studies performed in cholestatic mouse models, where lack of sensory innervation (α-CGRP and NK1R knockout) reduces biliary damage and liver fibrosis,12,18,36 our double-knockout model displays amelioration of liver damage, biliary hyperplasia/damage, and liver fibrosis. This model is also relevant to the inflammatory phenotypes of human PSC,2,5 because the enhanced peribiliary and liver inflammation (typical of Mdr2−/−) is decreased in NK1R−/−/Mdr2−/− mice, as evidenced by reduced number of macrophages and inflammation levels.
Next, in vivo (in Mdr2−/− mice and human PSC samples) experiments were performed to demonstrate that the effects of the SP/NK1R axis on biliary senescence/IBDM and liver fibrosis are mediated by changes in the expression/activation of the TGF-β1/miR-31 signaling, which is important in the progression of the phenotypes in several cholestatic liver diseases, including PSC.10,41 For example, the miR-31/hypoxia-inducible factor-1 pathway is associated with TGF-β1/Smad3-induced activation of HSCs and liver fibrosis induced by carbon tetrachloride treatment.35 As well, miRNA-31-5p has been shown to regulate chemosensitivity of hepatocellular carcinoma by preventing the nuclear location of poly [ADP-ribose] polymerase 1 (PARP1), the gene encoding the chromatin-associated enzyme, poly(ADP-ribosyl)transferase.42 The rationale for studying the TGF-β1/miR-31 signaling is also supported by the fact that SP has been shown to activate HSCs increasing liver fibrosis by activation of the TGF-β1/Smad2 signaling and mediate experimental colitis via miRNA-31-3p.10,43 In other gastrointestinal organs, SP regulates miR-31 expression in the pathophysiology of colitis, because NK1R-deficient mice had reduced colonic miR-31 levels after trinitrobenzenesulfonic acid (TNBS)-induced colitis.43 miR-31 is overexpressed in colorectal cancer,44 and it plays an important role in multipotent mammary stem cell self-renewal and tumorigenesis by regulating Wnt pathway activation.45 In the present study, the effects of the SP/NK1R axis are mediated by increased levels of TGF-β1 (in serum and cholangiocyte supernatant) expression in both Mdr2−/− mice and late-stage PSC samples, leading to changes in miR-31 expression. In support of our current finding, it has been shown that cholangiocytes and HSCs have a differential profile of cellular senescence with enhanced biliary senescence and reduced HSC senescence in rodent models of cholestasis.9 In support of this concept, SP has been shown to increase biliary senescence/IBDM and liver fibrosis (decreasing HSC senescence) by enhanced biliary TGF-β1 expression/secretion.9 Supporting the key role of the TGF-β1/miR-31 axis in modulating liver fibrosis, a study showed35 that miR-31/factor inhibiting hypoxia-inducible factor 1α may be a regulator of TGF-β1 signaling in HSC-mediated liver fibrosis, suggesting also a feedback loop between TGF-β1/miR-31; further studies are necessary to determine this loop during the progression of biliary senescence.
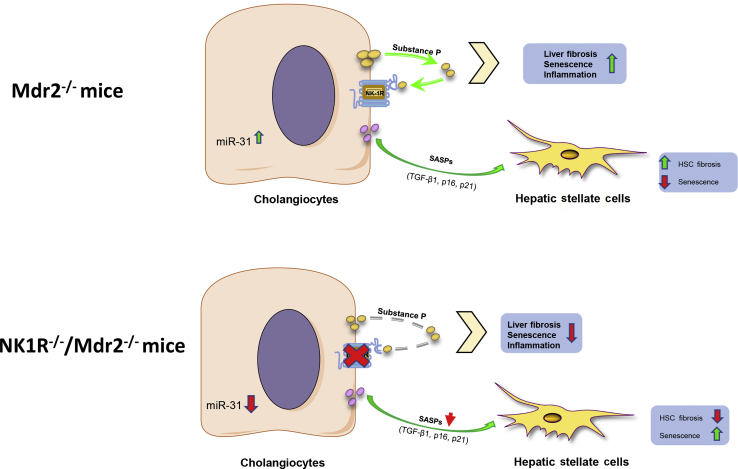
In conclusion, we developed a new cholestatic mouse model that allows us to study the pathologic mechanisms of human PSC and demonstrated the multiple aspects of NK1R in biliary damage/senescence, peribiliary inflammation, and liver fibrosis. Mdr2−/− mice are characterized by increased levels of SP, which promote biliary senescence, peribiliary inflammation, and liver fibrosis through changes in TGF-β1/miR-31 signaling.9,10 We propose that the release of senescence-associated secretory phenotypes (eg, TGF-β) by cholangiocytes increases fibrosis but decreases cellular senescence of HSCs through changes in miR-31. When the NK1R is knocked out, there is reduced biliary senescence, peribiliary inflammation, and liver fibrosis as well as decreased release of senescence-associated secretory phenotype (eg, TGF-β) secretion, leading to decreased miR-31 levels (Figure 11). Finally, we have identified TGF-β1/miR-31 axis as an important downstream modulator of biliary senescence and liver fibrosis during cholestasis. A shortcoming of these studies is that we did not provide direct evidence that changes in miR-31 axis modulate biliary senescence and liver fibrosis; in this project (currently undergoing in our laboratory), Mdr2−/− mice will be treated with control or miR-31 vivo morpholinos before evaluating biliary damage/senescence, liver fibrosis, and SP/TGF-β1 signaling. Down-regulation of the SP/NK1R/TGF-β1/miR-31 axis may be an important therapeutic target for the management of PSC.
Figure 11.
In Mdr2−/− (Abcb4−/−) mice, cholangiocytes secrete substance P that promotes biliary senescence, peribiliary inflammation, and liver fibrosis by increased miR-31, which, in turn, stimulates the release of senescence-associated secretory phenotypes (SASPs) and transforming growth factor (TGF)-β1. Both SASPs and TGF-β1 are able to activate hepatic stellate cells (HSCs) by increasing HSC fibrosis and reduced HSC senescence. When NK1R (Tacr1) is inhibited, there is less biliary senescence, peribiliary inflammation, and liver fibrosis, as well as decreased miR-31 levels, leading to reduced SASP secretion, which determined an increase of HSC senescence.
Footnotes
Supported by the Hickam Endowed Chair, Gastroenterology, Medicine, Indiana University; the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative; US Department of Veteran's Affairs, Biomedical Laboratory Research and Development Service VA Merit awards 5I01BX000574 (G.A.), 1I01BX003031 (H.F.) and 1I01BX001724 (F.M.); NIH grants DK108959 (H.F.), DK119421 (H.F.), DK054811, DK115184, DK076898, DK107310, DK110035, DK062975, AA025997, and AA025157 (all to G.A., F.M. and S.G.); the PSC Partners Seeking a Cure (G.A. and F.M.); and Sapienza University funds RM1181642BEF570E (P.O.). This material is the result of work supported by resources at the Central Texas Veterans Health Care System (Temple, TX), the Richard L. Roudebush VA Medical Center (Indianapolis, IN), and the Medical Physiology, Medical Research Building (Temple, TX).
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
S.G. and G.A. contributed equally to this work as senior authors.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2020.07.007.
Supplemental Data
Supplemental Figure S1.
Hematoxylin and eosin staining in liver sections from C57/Bl6, FVB/NJ, C57/FVB, NK1R−/−(Tacr1−/−), Mdr2−/−(Abcb4−/−), and NK1R−/−/Mdr2−/− mice. Data analyzed from three different animals for each group. Black arrows indicate inflammatory infiltrate and foamy cytoplasm in the lobules. Original magnification, ×40.
References
- 1.Kanno N., LeSage G., Glaser S., Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 2.Guicciardi M.E., Trussoni C.E., LaRusso N.F., Gores G.J. The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology. 2020;71:741–748. doi: 10.1002/hep.31067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroni L., Haibo B., Ray D., Zhou T., Wan Y., Meng F., Marzioni M., Alpini G. Functional and structural features of cholangiocytes in health and disease. Cell Mol Gastroenterol Hepatol. 2015;1:368–380. doi: 10.1016/j.jcmgh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis: a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Rizvi S., Eaton J.E., Gores G.J. Primary sclerosing cholangitis as a premalignant biliary tract disease: surveillance and management. Clin Gastroenterol Hepatol. 2015;13:2152–2165. doi: 10.1016/j.cgh.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchioni Beery R.M., Vaziri H., Forouhar F. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women's health perspective. J Clin Transl Hepatol. 2014;2:266–284. doi: 10.14218/JCTH.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazaridis K.N., Strazzabosco M., Larusso N.F. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Tabibian J.H., O'Hara S.P., Splinter P.L., Trussoni C.E., LaRusso N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Y., Meng F., Wu N., Zhou T., Venter J., Francis H., Kennedy L., Glaser T., Bernuzzi F., Invernizzi P., Glaser S., Huang Q., Alpini G. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology. 2017;66:528–541. doi: 10.1002/hep.29138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., Onori P., Marzioni M., Alvaro D., Franchitto A., Gaudio E., Glaser S., Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in TGF-beta1 biliary secretion. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L., Quezada M., Levine P., Han Y., McDaniel K., Zhou T., Lin E., Glaser S., Meng F., Francis H., Alpini G. Functional role of cellular senescence in biliary injury. Am J Pathol. 2015;185:602–609. doi: 10.1016/j.ajpath.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Y., Ceci L., Wu N., Zhou T., Chen L., Venter J., Francis H., Bernuzzi F., Invernizzi P., Kyritsi K., Baker P., Huang Q., Wu C., Sybenga A., Alpini G., Meng F., Glaser S. Knockout of alpha-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes. Lab Invest. 2019;99:764–776. doi: 10.1038/s41374-018-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killingsworth C.R., Shore S.A., Alessandrini F., Dey R.D., Paulauskis J.D. Rat alveolar macrophages express preprotachykinin gene-I mRNA-encoding tachykinins. Am J Physiol. 1997;273:L1073–L1081. doi: 10.1152/ajplung.1997.273.5.L1073. [DOI] [PubMed] [Google Scholar]
- 14.Inoue T., Ito Y., Nishizawa N., Eshima K., Kojo K., Otaka F., Betto T., Yamane S., Tsujikawa K., Koizumi W., Majima M. RAMP1 in Kupffer cells is a critical regulator in immune-mediated hepatitis. PLoS One. 2018;13:e0200432. doi: 10.1371/journal.pone.0200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock J.V., Blum A., Walder J., Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141:961–966. [PubMed] [Google Scholar]
- 16.Alpini G., Franchitto A., Demorrow S., Onori P., Gaudio E., Wise C., Francis H., Venter J., Kopriva S., Mancinelli R., Carpino G., Stagnitti F., Ueno Y., Han Y., Meng F., Glaser S. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeSage G., Alvaro D., Benedetti A., Glaser S., Marucci L., Baiocchi L., Eisel W., Caligiuri A., Phinizy J.L., Rodgers R., Francis H., Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 18.Glaser S., Gaudio E., Renzi A., Mancinelli R., Ueno Y., Venter J., White M., Kopriva S., Chiasson V., DeMorrow S., Francis H., Meng F., Marzioni M., Franchitto A., Alvaro D., Supowit S., DiPette D.J., Onori P., Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G297–G305. doi: 10.1152/ajpgi.00418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor T.M., O'Connell J., O'Brien D.I., Goode T., Bredin C.P., Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 20.Peng L., Agogo G.O., Guo J., Yan M. Substance P and fibrotic diseases. Neuropeptides. 2019;76:101941. doi: 10.1016/j.npep.2019.101941. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy L., Francis H., Invernizzi P., Venter J., Wu N., Carbone M., Gershwin M.E., Bernuzzi F., Franchitto A., Alvaro D., Marzioni M., Onori P., Gaudio E., Sybenga A., Fabris L., Meng F., Glaser S., Alpini G. Secretin/secretin receptor signaling mediates biliary damage and liver fibrosis in early-stage primary biliary cholangitis. FASEB J. 2019;33:10269–10279. doi: 10.1096/fj.201802606R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baghdasaryan A., Claudel T., Gumhold J., Silbert D., Adorini L., Roda A., Vecchiotti S., Gonzalez F.J., Schoonjans K., Strazzabosco M., Fickert P., Trauner M. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2-/- (Abcb4-/-) mouse cholangiopathy model by promoting biliary HCO(-)(3) output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popov Y., Patsenker E., Fickert P., Trauner M., Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Mariotti V., Cadamuro M., Spirli C., Fiorotto R., Strazzabosco M., Fabris L. Animal models of cholestasis: an update on inflammatory cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 2019;1865:954–964. doi: 10.1016/j.bbadis.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T., Wu N., Meng F., Venter J., Giang T.K., Francis H., Kyritsi K., Wu C., Franchitto A., Alvaro D., Marzioni M., Onori P., Mancinelli R., Gaudio E., Glaser S., Alpini G. Knockout of secretin receptor reduces biliary damage and liver fibrosis in Mdr2(-/-) mice by diminishing senescence of cholangiocytes. Lab Invest. 2018;98:1449–1464. doi: 10.1038/s41374-018-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaser S., Lam I.P., Franchitto A., Gaudio E., Onori P., Chow B.K., Wise C., Kopriva S., Venter J., White M., Ueno Y., Dostal D., Carpino G., Mancinelli R., Butler W., Chiasson V., DeMorrow S., Francis H., Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser S., Meng F., Han Y., Onori P., Chow B.K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J.M., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808.e12. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Xu N., Xu J., Kong B., Copple B., Guo G.L., Wang L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology. 2014;60:919–930. doi: 10.1002/hep.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salguero Palacios R., Roderfeld M., Hemmann S., Rath T., Atanasova S., Tschuschner A., Gressner O.A., Weiskirchen R., Graf J., Roeb E. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest. 2008;88:1192–1203. doi: 10.1038/labinvest.2008.91. [DOI] [PubMed] [Google Scholar]
- 30.Morini S., Carotti S., Carpino G., Franchitto A., Corradini S.G., Merli M., Gaudio E. GFAP expression in the liver as an early marker of stellate cells activation. Ital J Anat Embryol. 2005;110:193–207. [PubMed] [Google Scholar]
- 31.Raven A., Lu W.Y., Man T.Y., Ferreira-Gonzalez S., O'Duibhir E., Dwyer B.J., Thomson J.P., Meehan R.R., Bogorad R., Koteliansky V., Kotelevtsev Y., Ffrench-Constant C., Boulter L., Forbes S.J. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B., Wu X., Liu J., Song L., Song Q., Wang L., Yuan D., Wu Z. beta-Actin: not a suitable internal control of hepatic fibrosis caused by Schistosoma japonicum. Front Microbiol. 2019;10:66. doi: 10.3389/fmicb.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng F., DeMorrow S., Venter J., Frampton G., Han Y., Francis H., Standeford H., Avila S., McDaniel K., McMillin M., Afroze S., Guerrier M., Quezada M., Ray D., Kennedy L., Hargrove L., Glaser S., Alpini G. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2014;306:G759–G768. doi: 10.1152/ajpgi.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanino T., Bando T., Nojiri Y., Okada Y., Nagai N., Ueda Y., Sakurai E. Hepatic cytochrome P450 metabolism suppressed by mast cells in type 1 allergic mice. Biochem Pharmacol. 2018;158:318–326. doi: 10.1016/j.bcp.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Hu J., Chen C., Liu Q., Liu B., Song C., Zhu S., Wu C., Liu S., Yu H., Yao D., Kang J., Zhu L. The role of the miR-31/FIH1 pathway in TGF-beta-induced liver fibrosis. Clin Sci (Lond) 2015;129:305–317. doi: 10.1042/CS20140012. [DOI] [PubMed] [Google Scholar]
- 36.Glaser S.S., Ueno Y., DeMorrow S., Chiasson V.L., Katki K.A., Venter J., Francis H.L., Dickerson I.M., DiPette D.J., Supowit S.C., Alpini G.D. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87:914–926. doi: 10.1038/labinvest.3700602. [DOI] [PubMed] [Google Scholar]
- 37.Lee F.Y., Lin H.C., Tsai Y.T., Chang F.Y., Lu R.H., Hou M.C., Li C.P., Chu C.J., Wang S.S., Lee S.D. Plasma substance P levels in patients with liver cirrhosis: relationship to systemic and portal hemodynamics. Am J Gastroenterol. 1997;92:2080–2084. [PubMed] [Google Scholar]
- 38.Peng L., Jia X., Zhao J., Cui R., Yan M. Substance P promotes hepatic stellate cell proliferation and activation via the TGF-beta1/Smad-3 signaling pathway. Toxicol Appl Pharmacol. 2017;329:293–300. doi: 10.1016/j.taap.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Bang R., Biburger M., Neuhuber W.L., Tiegs G. Neurokinin-1 receptor antagonists protect mice from CD95- and tumor necrosis factor-alpha-mediated apoptotic liver damage. J Pharmacol Exp Ther. 2004;308:1174–1180. doi: 10.1124/jpet.103.059329. [DOI] [PubMed] [Google Scholar]
- 40.Bang R., Sass G., Kiemer A.K., Vollmar A.M., Neuhuber W.L., Tiegs G. Neurokinin-1 receptor antagonists CP-96,345 and L-733,060 protect mice from cytokine-mediated liver injury. J Pharmacol Exp Ther. 2003;305:31–39. doi: 10.1124/jpet.102.043539. [DOI] [PubMed] [Google Scholar]
- 41.Ikenaga N., Liu S.B., Sverdlov D.Y., Yoshida S., Nasser I., Ke Q., Kang P.M., Popov Y. A new Mdr2(-/-) mouse model of sclerosing cholangitis with rapid fibrosis progression, early-onset portal hypertension, and liver cancer. Am J Pathol. 2015;185:325–334. doi: 10.1016/j.ajpath.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Que K.T., Zhou Y., You Y., Zhang Z., Zhao X.P., Gong J.P., Liu Z.J. MicroRNA-31-5p regulates chemosensitivity by preventing the nuclear location of PARP1 in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:268. doi: 10.1186/s13046-018-0930-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Fang K., Law I.K.M., Padua D., Sideri A., Huang V., Kevil C.G., Iliopoulos D., Pothoulakis C. MicroRNA-31-3p is involved in substance P (SP)-associated inflammation in human colonic epithelial cells and experimental colitis. Am J Pathol. 2018;188:586–599. doi: 10.1016/j.ajpath.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M.H., Yu J., Chen N., Wang X.Y., Liu X.Y., Wang S., Ding Y.Q. Elevated microRNA-31 expression regulates colorectal cancer progression by repressing its target gene SATB2. PLoS One. 2013;8:e85353. doi: 10.1371/journal.pone.0085353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv C., Li F., Li X., Tian Y., Zhang Y., Sheng X., Song Y., Meng Q., Yuan S., Luan L., Andl T., Feng X., Jiao B., Xu M., Plikus M.V., Dai X., Lengner C., Cui W., Ren F., Shuai J., Millar S.E., Yu Z. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8:1036. doi: 10.1038/s41467-017-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]