Abstract
Estrogens are key signaling molecules that regulate various physiological processes such as cell growth, development, and differentiation. They also play a major role in many pathological conditions, such as hormone-dependent cancer. The importance of inhibiting estrogen receptor signaling in diseases of estrogen target tissues, such as breast cancer, is well documented. However, the role of estrogen signaling in diseases of nontarget tissues, such as lung cancer, is not well characterized. The aim of the current study is to examine the expression of estrogen receptor β (ERβ) and the roles of estradiol (E2) and fulvestrant on the progression of lung cancer. Tissue microarray (TMA) and immunohistochemistry (IHC) analyses were used to detect the expression of aromatase, ERα, and ERβ in 198 patients. We performed analyses to determine if there was any correlation among these three proteins. A mouse model of urethane-induced lung adenocarcinoma was used in the study. Mice were divided into three treatment groups: blank control, E2 alone, and E2 + fulvestrant (ERβ antagonist). Western blot analysis and fluorescence quantitative PCR (FQ-PCR) were used to measure expression of ERβ protein and mRNA levels, respectively. ERβ, but not ERα, was overexpressed in NSCLC samples. Lung cancer progression in mice treated with E2 was significantly increased compared to either the control group or the E2 + fulvestrant group. Mice in the E2 treatment group had significantly increased expression of ERβ at both the mRNA and protein levels compared to mice treated with E2 + fulvestrant or control. Our data suggest that ERβ promotes lung cancer progression in mice and that this progression can be inhibited with fulvestrant. These findings may help elucidate the role of ERβ in lung cancer and suggest that estrogen receptor antagonists, such as fulvestrant, may be therapeutically beneficial for the treatment of the disease.
Key words: Lung cancer, Mouse model, Estrogen receptor β (ERβ), Fulvestrant, Tissue microarray, Immunohistochemistry
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Overall, the prognosis for this disease is poor, with a 5-year survival rate of only 16%. Thus, improvements in treatment strategies for lung cancer are of great interest. Early studies on factors responsible for driving lung carcinogenesis concentrated on smoking and environment. Interestingly, recent studies have confirmed that estrogen not only promotes cancer progression in estrogen target organs (e.g., breast cancer) but also influences tumorigenesis in nontarget organs (e.g., lung cancer) (2–5). However, the mechanism of action in these nontarget tissues is not as well defined. Aromatase is the specific rate-limiting enzyme that converts testosterone to estrogen. Estrogens exert their biological effect through two estrogen receptor (ER) subtypes, ERα and ERβ (6,7). In the classical mode of ER action, these receptors regulate cancer cell growth by binding to estrogen response elements in the promoter region of target genes (8). Since many of the genes that ER regulates promote cellular proliferation, ERβ may be a useful therapeutic target in certain cancers. Although discovered in the mid-1990s, the molecular mechanisms of ERβ action in lung cancer are poorly understood, and effect of antiestrogen therapy for the treatment of lung cancer is of great interest (9,10).
We have previously shown that estrogen promotes the proliferation of lung cancer cells (11). For a more comprehensive understanding, we employed tissue microarray (TMA) and immunohistochemistry (IHC) analyses to measure expression of aromatase, ERα, and ERβ in non-small cell lung cancer (NSCLC) tissues. We evaluated the correlation among these proteins and clinicopathological characteristics. We used a mouse model of urethane-induced lung adenocarcinoma. Mice were treated with control, E2, or a combination of E2 + fulvestrant. Treatment was maintained for 16 weeks, and then mice were sacrificed. Lung tissues were removed and evaluated for cancer progression. Western blot and fluorescence quantitative PCR (FQ-PCR) were used to detect the expression of ERβ at both the protein and mRNA levels, respectively. In our mouse model, we examined the effect of the estrogen signaling molecule, ERβ, and estrogen inhibition on lung cancer progression. These findings may not only help elucidate the mechanism of estrogen signaling in lung cancer but may also support the use of antiestrogen therapies for the treatment of lung cancer.
MATERIALS AND METHODS
Human Tissue Samples
A total of 150 patients with pathologically confirmed primary NSCLC who underwent thoracic surgical procedures at the Department of Thoracic Surgery (Tongji Hospital, Affiliated Tongji Medical College, Huazhong University of Science and Technology) were prospectively recruited (date range October 2008 to November 2009) for this study. Patients had a mean age of 50 ± 6 years (range, 40–75 years), and none had received chemotherapy or radiotherapy. All female patients had reached menopause. NSCLC was categorized based on the p-TNM staging system (UICC, 7th edition, 2009). Tissues analyzed included primary lung tumors and, where possible, matched non-neoplastic lung tissue from patients with outcome data. At the same time, an additional 48 patients with benign pulmonary lesions who had received surgical intervention in our department were recruited as controls. This group consisted of 27 males and 21 females with a mean age of 50 ± 8 years (range, 43–62 years). All of the females had reached menopause. Tuberculosis tumors, bronchiectasis, and sclerosing hemangiomas were found in 26, 19, and 3 of these patients, respectively. None of these patients had dysfunction of the liver, kidney, or endocrine system, nor had they received steroids within the past month. Ethical approval for this investigation was obtained from the Research Ethics Committee (Tongji Medical College, Huazhong University of Scisence and Technology).
Reagents and Drugs
17β-Estradiol (≥98%; Sigma-Aldrich, St. Louis, MO, USA), fulvestrant (Sigma-Aldrich), rabbit anti-human ERα polyclonal antibody (sc-542; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human ERβ polyclonal antibody for human tissue (AB1410; Chemicon International, Temecula, CA, USA), ERβ rabbit anti-rat polyclonal antibody for mouse tissue (14007-1-AP; epitope in N-term-323aa; Wuhan Sanying Biotechnology, Wuhan, China), rabbit anti-human aromatase polyclonal antibody (sc-30086, Santa Cruz Biotechnology), urethane (50 mg/ml prepared in saline; Shanghai Zhanyun Chemical Co., Ltd., Shanghai, China), sodium pentobarbital (1% prepared in saline; Sigma-Aldrich), and olive oil (Local Chinese Department Store, imported from Italy). Preparation of E2 (0.036 mg/ml) and fulvestrant (0.8 mg/ml) solutions (in olive oil) and administration of these compounds were performed as previously reported (12).
Tissue Microarrays
TMAs were constructed using the selected archival tumor specimens (human and mouse). The selected core was extracted from 10% formalin-fixed, paraffin-embedded tissue blocks from each specimen. Each sample was independently examined by at least two pathologists and arrayed on recipient blocks (TMAs were prepared by Shanghai Outdo Biotech, Shanghai, China).
Immunohistochemistry
Sections were deparaffinized, hydrated, and immersed in 10 mmol/L citrate buffer (pH 6) using a pressure cooker. This was then followed by heat-induced antigen retrieval for 5 min. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 10 min. Samples were then blocked in 5% BSA for 20 min. Subsequently, sections were incubated with primary antibodies diluted in PBS [aromatase/ERα/ERβ, 1:200 for human and mouse lung cancer tissue; PBS alone was used as a negative control; many previous experiments, including our studies, have confirmed antibody specificity (6,11,13,14)], overnight at 4°C. Following washing in PBS, the sections were incubated with secondary antibody at room temperature (RT) for 20 min. After washing again in PBS, sections were treated with SABC for 20 min at RT. Additional washing in PBS was performed. Visualization was performed with DAB followed by counterstaining with hematoxylin and then dehydration, making them transparent, and mounting. Sections were then observed under a microscope.
IHC Evaluation
IHC was performed by a single board-certified pathologist blinded to clinical outcome (double-blind manner). All tissues (human and mouse) were negative for ERα. Aromatase and ERβ showed positive staining in either the membrane or cytoplasm; in some samples, yellow-brown granules were also observed. Pathological examination was performed on five randomly selected fields (400×) as previously described (15), and a total of 200 cancer cells were counted in each field (1,000 cells in total). The percentage of immunoreactive cells was rounded to the nearest fifth percentage. To obtain a proportion score, the following cutoffs for positive staining were utilized (16,17): 1 (0–20%), 2 (21–50%), 3 (51–75%), and 4 (76–100%). Intensity was scored on a scale of 1 to 4 with 1 being negative, 2 being weak, 3 being moderate, and 4 being strong. The final value was determined by multiplying the two scores and denoted as (−) ≤4, (+) >4 and ≤8, (+ +) >8 and ≤12, (+ + +) >12 and ≤16.
In Vivo Experiments
Animals
Four-week-old female Kunming mice (body weights between 17 and 22 g) were provided by the Laboratory Animal Research Center of Hubei Province (No. 00015433; Laboratory Animal Research Center of Hubei Province, Wuhan, China). They were housed on hardwood bedding with 12-h light/dark cycles, fed standard rodent diet (Rat and Mice No. 3 Breeding Autoclavable; Shanghai Servanimal Bio-Tech Inc., Shanghai, China), and maintained in specific pathogen-free (SPF) animal quarters of the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology (No. 00021082, Wuhan, China). The animal protocol for this experiment was approved by the Institute of Laboratory Animals of Hubei (Hubei Y20110230; Wuhan, China). Body weights of all mice were measured once per week. Briefly, the urethane-induced lung cancer mouse model can be described as follows: 1 week after arrival in our facility, Kunming mice were treated with freshly prepared urethane as a single intraperitoneal (IP) injection of 0.8 ml; this procedure was performed six times per week, as previously described (12,18).
Experimental Design
Mice received urethane injections over the course of 1 week. Oophorectomy was performed to minimize effects of endogenous estrogen. One percent pentobarbital sodium was used as a general anesthesia (0.009 ml/g, IP), and disinfection was performed with penicillin (0.16 pan-unit/g). One week after surgery, the remaining mice (a total of 18) were randomized into the following three treatment groups with six mice in each group: Control, E2, and E2 + fulvestrant. E2 (dissolved in olive oil, 0.036 mg/ml) and fulvestrant (dissolved in olive oil, 0.800 mg/ml) were administered by subcutaneous injection. The final working concentration of E2 was 0.05 ml/mouse, and the final concentration of fulvestrant was 0.06 ml/mouse (18–20). The experiment was terminated at 16 weeks, and all mice were sacrificed by neck exarticulation (12,18,21). At necropsy, lungs were removed. Both wet weight and the number of surface tumors were recorded, and lungs were inflated with neutral buffer formalin (10%). After fixation, lung tissue was embedded in paraffin, cut into sections, stained with H&E, and reexamined under an optical microscope. Both gross and histologic analyses of the lungs were done in a blinded fashion by two independent investigators.
Western Blot Analysis
Mouse lung cancer tissue samples were lysed in RIPA buffer, and protein concentration was measured; 50 μg of total protein was used for 10% PAGE electrophoresis. Protein was transferred from gel to membrane (wet transfer method), which was then subjected to incubation with the appropriate antibodies (22). The primary antibody against ERβ was prepared at a 1:1,000 dilution. Many previous experiments, including our studies, have confirmed antibody specificity (6,11,13,14). Optical densities of each band were quantified. The protein of interest was normalized against an internal loading control.
FQ-PCR Analysis
Mouse lung tissues were treated with 1 ml Trizol and then homogenized. Next, 200 μl of chloroform was added, and samples were gently mixed. Samples were incubated at room temperature for 5 min, and then spun at 12,000 rpm for 15 min at 4°C. The aqueous phase (approximately 400 ul) was transferred to a new 1.5-ml tube, and 400 μl of isopropyl alcohol was added. Samples were incubated at room temperature for 10 min. This was followed by centrifugation at 12,000 rpm for 10 min at 4°C. The precipitate was then washed three times with ethanol, and the pellet was allowed to air dry for 5–10 min. Finally, the pellet was dissolved in 20 μl DEPC water. Detailed FQ-PCR analysis has been previously described (22). The primer sequences are as follows: mouse ERβ, F: 5′-CTGCAATTCCTGAACCGAAA-3′; R: 5′-GTGGTAATTTGGGGGCTCTT-3′ (216-bp-sized fragments); mouse β-actin, F: 5′-CACGATGGAGGGGCCGGACTCATC-3′; R: 5′-TAAAGACCTCTATGCCAACACAGT-3′ (164 bp).
Statistical Analysis
Pearson chi-square and Spearman correlation were performed for statistical analyses using SPSS 19.0.1 (SPSS, Chicago, IL, USA). Differences in expression between aromatase and ERβ in NSCLC and benign pulmonary lesions (BPL) were determined using the chi-square test. Comparisons between animal body weight and tumor weights were determined using t test analysis. Comparisons tumor index (23) and organ (lung) index between the different groups were determined by chi-square test analysis. Mouse tumor index was analyzed via independent samples t test. p Values were two-sided, and p < 0.05 was considered to be statistically significant.
RESULTS
Expression of Aromatase, ERα, and ERβ in NSCLC and BPL (Table 1)
Table 1.
Expression of Aromatase and ERβ in NSCLC and BPL
| Staining | Aromatase | ERβ | ||
|---|---|---|---|---|
| NSCLC | BPL | NSCLC | BPL | |
| − | 31 | 42 | 34 | 35 |
| + | 22 | 5 | 27 | 12 |
| ++ | 41 | 1 | 41 | 1 |
| +++ | 56 | 0 | 48 | 0 |
| Positive (%) | 79.33% | 12.50% | 77.33% | 27.08% |
| χ2 | 69.783 | 40.442 | ||
| p Value | 0.000 | 0.000 | ||
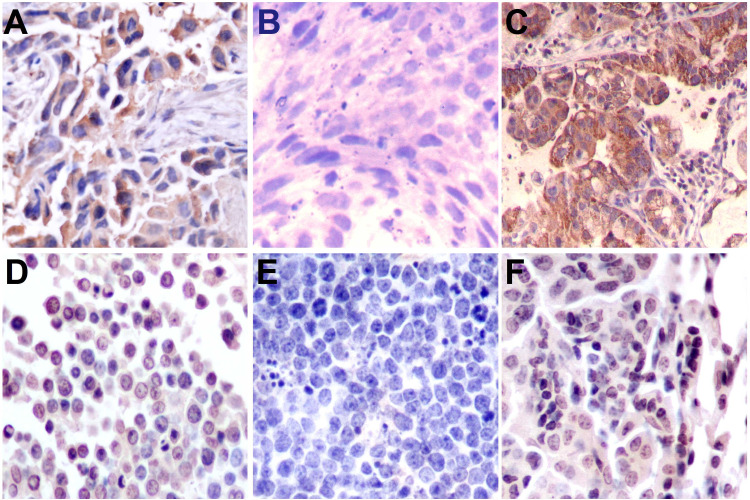
ERβ-positive granules were primarily detected in the membrane and sometimes in the cytoplasm of lung cancer cells (Fig. 1C). In BPL, only a few cells were mildly to moderately positive for ERβ (ranging from + to ++) (Table 1). In the NSCLC group, about 77.33% (116/150) of the samples were positive for ERβ. This was significantly higher than the positive staining rate in BPL, which was only 27.08% (13/48) (p = 0.000).
Figure 1.
Representative immunohistochemical staining of aromatase, ERα, and ERβ in human NSCLC (A, B, C) and mouse lung adenocarcinoma (D, E, F) samples. Aromatase staining is highly cytoplasmic in cancer cells and also detected at the membrane (A, D). Immunopositive staining of ERβ is observed in the membrane and cytoplasmic region of the cells, with strong positive staining of the membrane (C, F). In human NSCLC and mouse lung adenocarcinoma samples, ERα staining is negative (B, E). Original magnification: 400×.
Aromatase-positive granules, staining yellow or yellow-brown in color, were primarily detected in the cytoplasm and on the membrane in NSCLC samples (Fig. 1A). In BPL, cells were mildly to moderately positive (ranging from + to ++) for aromatase (Table 1). In NSCLC tissue, 79.33% (119/150) were positive for aromatase, and this was significantly higher than aromatase staining in BPL (12.50%; 6/48) (p = 0.000).
Interestingly, both NSCLC and BPL samples were negative for ERα based on IHC analysis. ERα was also not detected in mouse lung cancer tissue (Fig. 1B, E).
Correlation Between the Expression of Aromatase and ERβ and the Clinicopathological Characteristics of NSCLC (Table 2)
Table 2.
Correlation Between the Expression of Aromatase and ERβ and the Clinicopathological Characteristics of NSCLC
| Aromatase | ERβ | |||||
|---|---|---|---|---|---|---|
| − | + | p | − | + | p | |
| Gender | 0.160 | 0.709 | ||||
| Male | 26 | 85 | 26 | 85 | ||
| Female | 5 | 34 | 8 | 31 | ||
| Age | 0.689 | 0.776 | ||||
| <55 | 14 | 49 | 15 | 48 | ||
| ≥55 | 17 | 70 | 19 | 68 | ||
| Smoking index | 0.818 | 0.372 | ||||
| <400 | 14 | 51 | 17 | 48 | ||
| ≥400 | 17 | 68 | 17 | 68 | ||
| Histological type | 0.217 | 0.368 | ||||
| SCC | 10 | 53 | 12 | 51 | ||
| AD | 21 | 66 | 22 | 65 | ||
| Lymph node metastasis | 0.207 | 0.432 | ||||
| N0 | 7 | 41 | 9 | 39 | ||
| N1–3 | 24 | 78 | 25 | 77 | ||
| Stage | 0.406 | 0.181 | ||||
| IA–IIB | 12 | 56 | 12 | 56 | ||
| IIIA–IV | 19 | 63 | 22 | 60 | ||
| Degree of differentiation | 0.553 | 0.001* | ||||
| M to W | 21 | 87 | 32 | 76 | ||
| Poor | 10 | 32 | 2 | 40 | ||
AD, adenocarcinoma; SCC, squamous cell carcinoma; M, moderate, W, well.
p < 0.05.
There was no apparent correlation between aromatase expression and gender, age, smoking index, histological subtype, lymph node metastasis, or tumor stage. ERβ expression was related to the degree of differentiation of NSCLC. For example, ERβ expression was significantly higher in poorly differentiated NSCLC (5.88%; 2/34) compared to moderately to well-differentiated NSCLC (94.12%; 32/34) (p = 0.001).
E2 Promotes Lung Cancer Progression in the Urethane-Induced Lung Adenocarcinoma Mouse Model
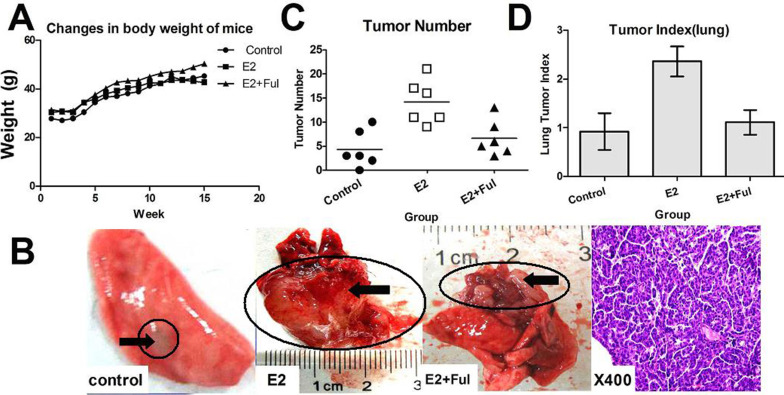
There were no significant changes in body weight among mice in the three treatment groups (p > 0.05), indicating that the administered drugs were not generally toxic and that the selection of dose was reasonable (Fig. 2A). Lung tumors were identified both on the lung surface and beneath the pulmonary pleura (Fig. 2B). The number of tumors arising in the E2 group was significantly higher than the number of tumors in either the E2 + fulvestrant group (p = 0.012) or the control group (p = 0.006) (Fig. 2C). The lung tumor index in the E2 group was also significantly higher than the E2 + fulvestrant group (p = 0.012) and the control group (p = 0.018) (Fig. 2D). These data support a role for ERβ in lung cancer progression in mice.
Figure 2.
Effect of ERβ and its inhibitor (fulvestrant) on mouse lung tumorigenesis. (A) Body weights of Kunming mice exposed to three treatment conditions were unchanged (p > 0.05). (B) Images of representative lung tissues from control mice and mice treated with drugs (control, E2, and E2 + Ful) were obtained by direct observation and by use of a microscope. (C) Tumor number in the E2 group was significantly higher than in the other groups (p < 0.05), and the numbers of tumors and positive adenocarcinoma samples were consistent. (D) Index of mouse lung tumors in the E2-treated group was statistically higher than in the other groups (p < 0.05).
Expression of Aromatase, ERα, and ERβ in Mouse Lung Cancer Tissue
Immunohistochemistry revealed clear expression of aromatase and ERβ in mouse lung cancer tissue. Staining was characterized by yellow-brown granules and was primarily detected in the cytoplasm (Fig. 1D, F). No staining for ERα was detected in mouse lung cancer tissue (Fig. 1E).
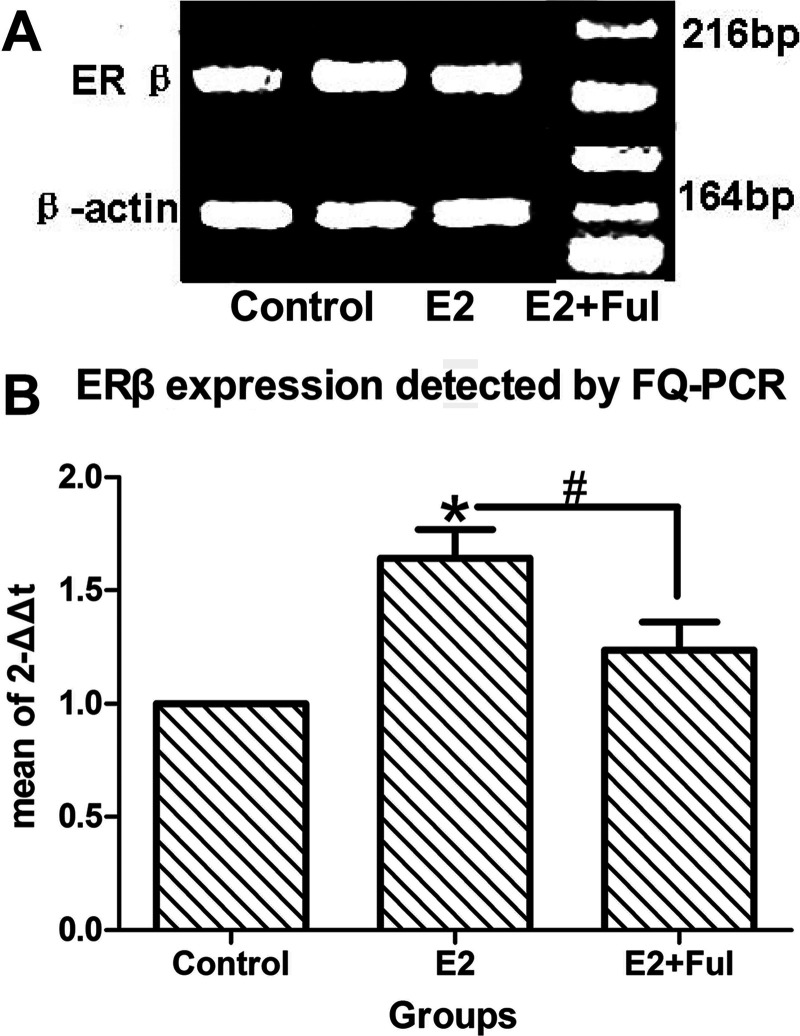
Western blot and FQ-PCR analyses were used to measure expression of ERβ in mouse lung cancer. However, these methods were not used to measure expression of ERα since ERα was not detected in either NSCLC or BPL (Figs. 3 and 4) samples. Expression of ERβ was higher in the E2-treated group compared to the E2 + fulvestrant group, supporting the fact that ERβ signaling may play an important role in promoting mouse lung adenocarcinoma.
Figure 3.
Expression of ERβ protein in the three groups of mice with urethane-induced lung adenocarcinoma. (A) Western blot analysis and (B) detection of optical density in mouse lung cancer samples show that the expression of ERβ in the E2 group is significantly higher than in either the control (*p = 0.001 ) or the E2 + Ful (#p = 0.039) groups.
Figure 4.
Expression of ERβ mRNA in the three groups of mice with urethane-induced lung adenocarcinoma. (A) FQ-PCR analysis and (B) detection of ERβ mRNA in mouse lung cancer samples show that the expression of ERβ mRNA in the E2 group was significantly higher than that in either the control (*p = 0.004 ) or the E2 + Ful (#p = 0.045) groups.
DISCUSSION
Poor clinical outcome associated with lung cancer has prompted the need for new therapeutic strategies for its treatment. However, since the beginning of the 1990s, while lung cancer incidence has stabilized in men, it has steadily increased in women. Epidemiology has maintained that the primary risk factor for lung cancer is still smoking (24,25). Interestingly, however, the smoking danger to females is markedly higher than the risk to males. Moreover, among nonsmokers, women are found to be twice as likely to suffer from lung cancer compared to men. Male patients with NSCLC have higher levels of E2 than females. Specifically, the local concentration of estrogen is 3.7 times higher than it is in postmenopausal women (1,26). These data suggest that lung cancer may be an estrogen-related carcinoma and that ER may be a driver of the disease (3,27,28). However, the mechanism by which ER signaling promotes lung cancer progression and the receptor (ERα/ERβ) that is primarily responsible for this effect are poorly understood.
Earlier studies have shown that estrogen promotes the proliferation of lung cancer cells (11). Here we show that the expression of ERβ in NSCLC is markedly higher than it is in BPL. Furthermore, expression correlates with clinicopathological characteristics of NSCLC. Both NSCLC samples and mouse lung adenocarcinoma tissue stain positive for ERβ but do not express ERα, which is consistent with previous reports (5,29). Additionally, we find that estrogen treatment significantly enhances lung cancer progression in our mouse model. This suggests that estrogen may mediate the development of lung cancer specifically via ERβ and that the ERβ signaling pathway may be a promising target for the treatment of lung cancer. Poorly differentiated NSCLC shows increased aromatase and ERβ expression compared to moderately to well-differentiated NSCLC. In our animal studies, the tumor index of the E2-treated group was significantly higher than the tumor index of the E2 + fulvestrant group, indicating that significant inhibition by fulvestrant may slow lung cancer progression. Based on these findings at both the tissue and whole animal levels, we hypothesize that ERβ significantly promotes malignant progression of lung cancer and that inhibition of ER action may be a useful therapeutic strategy to combat the disease.
CONCLUSION
Our study provides direct and comprehensive evidence for ERβ in lung cancer. The results suggest that estrogen signaling molecule ERβ promotes lung cancer in a mouse model and that fulvestrant may attenuate this progression. This study may help elucidate the mechanisms underlying estrogen action on lung cancer and provide evidence for the advancement of molecular targeted therapy for lung cancer treatment.
ACKNOWLEDGMENTS
Lung tissue samples were prepared at the Department of Thoracic Surgery, Tongji Hospital (Wuhan, China). The authors thank the Research Center of Experimental Medicine and Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology (China). This study was funded by the National Natural Science Foundation of China (NSFC, No. 81272590).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A.; Bray F.; Center M. M.; Ferlay J.; Ward E.; Forman D. Global cancer statistics. CA Cancer J. Clin. 61:69–90; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Henschke C. I.; Yip R.; Miettinen O. S. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 296:180–184; 2006. [DOI] [PubMed] [Google Scholar]
- 3. Liao Y. D.; Zhao J. P.; Ma L. C.; Zhou S.; Huang Q.; Doris M. Expression of new type estrogen receptor β in human adenocarcinoma and squamous cell carcinoma of lung and its correlation with peripheral serum estradiol level [in Chinese]. Acta Med. Univ. Sci. Technol. Huazhong 34:572–581; 2005. [Google Scholar]
- 4. Niikawa H.; Suzuki T.; Miki Y.; Suzuki S.; Nagasaki S.; Akahira J.; Honma S.; Evans D. B.; Hayashi S.; Kondo T.; Sasano H. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 14:4417–4426; 2008. [DOI] [PubMed] [Google Scholar]
- 5. Hammoud Z.; Tan B.; Badve S.; Bigsby R. M. Estrogen promotes tumor progression in a genetically defined mouse model of lung adenocarcinoma. Endocr. Relat. Cancer 15:475–483; 2008. [DOI] [PubMed] [Google Scholar]
- 6. Zhang G.; Liu X.; Farkas A. M.; Parwani A. V.; Lathrop K. L.; Lenzner D.; Land S. R.; Srinivas H. Estrogen receptor beta functions through nongenomic mechanisms in lung cancer cells. Mol. Endocrinol. 23:146–156; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stabile L. P.; Siegfried J. M. Estrogen receptor pathways in lung cancer. Curr Oncol. Rep. 6:259–267; 2004. [DOI] [PubMed] [Google Scholar]
- 8. Navaratnam S.; Skliris G.; Qing G.; Banerji S.; Badiani K.; Tu D.; Bradbury P. A.; Leighl N. B.; Shepherd F. A.; Nowatzki J.; Demers A.; Murphy L. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm. Cancer 3:93–100; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davydov M. I.; Bogush T. A.; Polotskii B. E.; Tiuliandin S. A. [Estrogen receptors beta--new target in cellular lung cancer treatment]. Vestn. Ross. Akad. Med. Nauk. 2:16–22; 2012. [PubMed] [Google Scholar]
- 10. Verma M. K.; Miki Y.; Abe K.; Nagasaki S.; Niikawa H.; Suzuki S.; Kondo T.; Sasano H. Co-expression of estrogen receptor beta and aromatase in Japanese lung cancer patients: Gender-dependent clinical outcome. Life Sci. 91(15–16):800–808; 2012. [DOI] [PubMed] [Google Scholar]
- 11. Tang H.; Liao Y.; Chen G.; Xu L.; Zhang C.; Ju S.; Zhou S. Estrogen upregulates the IGF-1 signaling pathway in lung cancer through estrogen receptor-beta. Med. Oncol. 29:2640–2648; 2012. [DOI] [PubMed] [Google Scholar]
- 12. Li S. Q.; Xiao B. L.; Dong Q. N.; Guo J. Y. Experimental conditions of mouse lung tumors induced by short-term trials [in Chinese]. Chin. J. Pharmacol. Toxicol. 7:68–72; 1993. [Google Scholar]
- 13. Zheng K.; Yang C.; Zhang Y.; Li N.; Li S.; Yan Y. Study on the expression of ERβ and p-ERβ on mouse ovary during the oestrous cycle [in Chinese]. J. Northeast Agric. Univ. 40:85–89; 2009. [Google Scholar]
- 14. Tremblay G. B.; Tremblay A.; Copeland N. G.; Gilbert D. J.; Jenkins N. A.; Labrie F.; Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol. 11:353–365; 1997. [DOI] [PubMed] [Google Scholar]
- 15. Kang K. F.; Wang X. W.; Chen X. W.; Shi X. C. Expression and clinical significance of Beclin1 and NF-κB p65 protein in primary human hepatocellular carcinoma [in Chinese]. World Chin. J. Digestol. 16:2244–2247; 2008. [Google Scholar]
- 16. Kawasaki H.; Altieri D. C.; Lu C. D.; Toyoda M.; Tenjo T.; Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 58:5071–5074; 1998. [PubMed] [Google Scholar]
- 17. Yang X. R.; Pfeiffer R. M.; Garcia-Closas M.; Rimm D. L.; Lissowska J.; Brinton L. A.; Peplonska B.; Hewitt S. M.; Cartun R. W.; Mandich D.; Sasano H.; Evans D. B.; Sutter T. R.; Sherman M. E. Hormonal markers in breast cancer: Coexpression, relationship with pathologic characteristics, and risk factor associations in a population-based study. Cancer Res. 67:10608–10617; 2007. [DOI] [PubMed] [Google Scholar]
- 18. Jiang Y. G.; Chen J. K.; Wu Z. L. Promotive effect of diethylstilbestrol on urethan-induced mouse lung tumorigenesis. Chemosphere 41:187–190; 2000. [DOI] [PubMed] [Google Scholar]
- 19. Hecht S. S.; Isaacs S.; Trushin N. Lung tumor induction in A/J mice by the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene: A potentially useful model for evaluation of chemopreventive agents. Carcinogenesis 15:2721–2725; 1994. [DOI] [PubMed] [Google Scholar]
- 20. Jassam N.; Bell S. M.; Speirs V.; Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes’ staging. Oncol. Rep. 14:17–21; 2005. [PubMed] [Google Scholar]
- 21. Yamagami H.; Matsumoto T.; Fujiwara N.; Arakawa T.; Kaneda K.; Yano I.; Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect. Immun. 69:810–815; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soda M.; Takada S.; Takeuchi K.; Choi Y. L.; Enomoto M.; Ueno T.; Haruta H.; Hamada T.; Yamashita Y.; Ishikawa Y.; Sugiyama Y.; Mano H. A mouse model for EML4-ALK-positive lung cancer. Proc. Natl. Acad. Sci. USA 105:19893–19897; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamasaki N.; Isowa K.; Kamada K.; Terano Y.; Matsumoto T.; Arakawa T.; Kobayashi K.; Yano I. In vivo administration of mycobacterial cord factor (Trehalose 6, 6′-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect. Immun. 68:3704–3709; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazieres J.; Rouquette I.; Brouchet L. [Lung cancer in women and pregnancy: Towards a hormonal origin?]. Rev. Mal. Respir. 24:983–997; 2007. [DOI] [PubMed] [Google Scholar]
- 25. Marquez-Garban D. C.; Chen H. W.; Fishbein M. C.; Goodglick L.; Pietras R. J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72:135–143; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miki Y.; Suzuki T.; Abe K.; Suzuki S.; Niikawa H.; Iida S.; Hata S.; Akahira J.; Mori K.; Evans D. B.; Kondo T.; Yamada-Okabe H.; Sasano H. Intratumoral localization of aromatase and interaction between stromal and parenchymal cells in the non-small cell lung carcinoma microenvironment. Cancer Res. 70:6659–6669; 2010. [DOI] [PubMed] [Google Scholar]
- 27. Ali G.; Donati V.; Loggini B.; Servadio A.; Dell’Omodarme M.; Prati M. C.; Camacci T.; Lucchi M.; Melfi F.; Mussi A.; Fontanini G. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum. Pathol. 39:1465–1473; 2008. [DOI] [PubMed] [Google Scholar]
- 28. Zhao G.; Zhao S.; Wang T.; Zhang S.; Lu K.; Yu L.; Hou Y. Estrogen receptor beta signaling regulates the progression of Chinese non-small cell lung cancer. J. Steroid Biochem. Mol. Biol. 124:47–57; 2011. [DOI] [PubMed] [Google Scholar]
- 29. Hershberger P. A.; Vasquez A. C.; Kanterewicz B.; Land S.; Siegfried J. M.; Nichols M. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 65:1598–1605; 2005. [DOI] [PubMed] [Google Scholar]