Abstract
Maintenance of functional β‐cell mass is critical to preventing diabetes, but the physiological mechanisms that cause β‐cell populations to thrive or fail in the context of obesity are unknown. High fat‐fed SM/J mice spontaneously transition from hyperglycemic‐obese to normoglycemic‐obese with age, providing a unique opportunity to study β‐cell adaptation. Here, we characterize insulin homeostasis, islet morphology, and β‐cell function during SM/J’s diabetic remission. As they resolve hyperglycemia, obese SM/J mice dramatically increase circulating and pancreatic insulin levels while improving insulin sensitivity. Immunostaining of pancreatic sections reveals that obese SM/J mice selectively increase β‐cell mass but not α‐cell mass. Obese SM/J mice do not show elevated β‐cell mitotic index, but rather elevated α‐cell mitotic index. Functional assessment of isolated islets reveals that obese SM/J mice increase glucose‐stimulated insulin secretion, decrease basal insulin secretion, and increase islet insulin content. These results establish that β‐cell mass expansion and improved β‐cell function underlie the resolution of hyperglycemia, indicating that obese SM/J mice are a valuable tool for exploring how functional β‐cell mass can be recovered in the context of obesity.
Keywords: diabetes, hyperglycemia, insulin, mouse model, obesity, β‐cell mass
High fat‐fed SM/J mice spontaneously transition from hyperglycemic‐obese to normoglycemic‐obese with age, providing a unique opportunity to study β‐cell adaptation. Here we characterize insulin homeostasis, islet morphology, and β‐cell function during SM/J's diabetic remission.

1. INTRODUCTION
Obesity and diabetes are a deadly combination, compounding risk for cardiovascular disease, cancer, and stroke (Centers for Disease Control and Prevention C, 2017; Esposito, Chiodini, Colao, Lenzi, & Giugliano, 2012; Ninomiya et al., 2004; Younis et al., 2016). Obesity raises the risk of developing type 2 diabetes 27‐ to 76‐fold, while approximately 60% of individuals with diabetes are obese (Abdullah, Peeters, de Courten, & Stoelwinder, 2010; Chatterjee, Khunti, & Davies, 2017; Colditz, Willett, Rotnitzky, & Manson, 1995). Chronic obesity exerts glycemic stress on pancreatic β‐cells, causing dysregulation and dysfunction, ultimately resulting in hyperglycemia (Kolb & Martin, 2017; Ojha, Ojha, Mohammed, Chandrashekar, & Ojha, 2019; Scheen, 2003; Stumvoll, Goldstein, & Van Haeften, 2005). Despite the stress obesity places on β‐cells, 10%–30% of obese individuals maintain glycemic control and are at low risk for developing diabetes (Meigs et al., 2006). These low‐risk obese individuals have elevated β‐cell mass and improved insulin secretion compared to BMI‐matched diabetic‐obese individuals (Aguayo‐Mazzucato & Bonner‐Weir,; Butler et al., 2003; Seino, Shibasaki, & Minami, 2011; Weir, Bonner‐Weir, & Leahy, 1990). Understanding the differences in β‐cell physiology between these populations may reveal therapeutic strategies for maintaining and improving glycemic control in obese individuals.
Recent work suggests that β‐cells do not respond uniformly to glycemic stress, rather they experience variable fates including dedifferentiation, replication, and apoptosis (Butler et al., 2003; Cinti et al., 2016; Georgia & Bhushan, 2004). Understanding how these changes mediate diabetic risk is complicated by β‐cell heterogeneity. β‐cell populations include subtypes that specialize in basal insulin secretion, β‐cell replication, coordinating “hub” cells, and β‐cells derived from transdifferentiated α‐cells, each of which differ in glycemic stress response (Farack et al., 2019; Johnston et al., 2016; Smukler et al., 2011). Thus, determining what differentiates nondiabetic‐obese and diabetic‐obese populations requires connecting β‐cell subtypes to their fate in prolonged glycemic stress.
Like in humans, diabetic risk in obese mice depends on genetic background (Kahle et al., 2013; Kobayashi, Ohno, Ihara, Murai, & Kumazawa, 2014; Meulen et al., 2017; Sims et al., 2013). Variation in β‐cell heterogeneity likely underlies variability in islet stress response, and thus needs to be accounted for when comparing nondiabetic‐obese and diabetic‐obese populations. Loss of function mutations in leptin (ob/ob) and leptin receptor (db/db) provide insight into β‐cell physiology in nondiabetic‐obese and diabetic‐obese states within individual mouse strains (Bock, Pakkenberg, & Buschard, 2003; Butler et al., 2003; Hummel, Coleman, & Lane, 1972; Keller et al., 2008; Leiter, Coleman, Eisenstein, & Strack, 1980); however, leptin and its receptor play a critical role in β‐cell function independent of obesity, limiting interpretations of these studies (Covey et al., 2006). No current mouse model is well‐suited to examine physiological differences in β‐cell health between nondiabetic‐obese and diabetic‐obese states.
The SM/J inbred mouse strain has traditionally been used to study interactions between diet and metabolism, and more recently has uncovered the genetic architecture underlying diet‐induced obesity and glucose homeostasis (Cheverud et al., 2011; Lawson, Cady, et al., 2011; Lawson & Cheverud, 2010; Lawson, Lee, et al., 2011; Lawson et al., 2010; Nikolskiy et al., 2015). After 20 weeks on a high fat diet, SM/J mice display characteristics of diabetic‐obese mice, including elevated adiposity, hyperglycemia, and glucose intolerance (Ehrich et al., 2003). We have previously shown that by 30 weeks of age, high fat‐fed SM/J mice enter diabetic remission, characterized by normalized fasting blood glucose, and greatly improved glucose tolerance and insulin sensitivity (Carson et al., 2019). Importantly, these changes occur in the context of sustained obesity. Given the central role of β‐cell health in susceptibility to diabetic‐obesity, we hypothesize that obese SM/J mice undergo restoration of functional β‐cell mass during the resolution of hyperglycemia. This study focuses on how insulin homeostasis, β‐cell morphology, and β‐cell function change during this remarkable transition and establishes SM/J mice as a useful model for teasing apart diabetic‐obese and nondiabetic‐obese states.
2. METHODS
2.1. Animal husbandry and tissue collection
SM/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Experimental animals were generated at the Washington University School of Medicine and all experiments were approved by the Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were weaned onto a high fat diet (42% kcal from fat; Envigo Teklad TD88137) or an isocaloric low fat diet (15% kcal from fat; Research Diets D12284), as previously described (Carson et al., 2019). At 20 or 30 weeks of age, mice were fasted for 4 hr, and blood glucose was measured via glucometer (GLUCOCARD). Mice were then injected with an overdose of sodium pentobarbital, followed by a toe pinch to ensure unconsciousness. Blood was collected via cardiac puncture and pancreas was detached from the spleen and duodenum.
2.2. Serum and pancreatic insulin measurements
Blood obtained via cardiac puncture was spun at 6,000 rpm at 4°C for 20 min to separate plasma, which was collected and stored at −80°C. Whole pancreas was homogenized in acid ethanol and incubated at 4°C for 48 hr, shaking. Homogenate was centrifuged at 2,500 rpm for 30 min at 4°C. Supernatant was collected and stored at −20°C. Protein content was measured using Pierce BCA Protein Assay kit (Thermo Scientific) according to manufacturer's instructions and read at 562 nm on the Synergy H1 Microplate Reader (Biotek). Insulin ELISA (ALPCO 80‐INSMR‐CH01) was used to measure plasma and pancreatic insulin levels following manufacturer's instructions.
2.3. Insulin tolerance test
At 19 or 29 weeks of age, mice were fasted for 4 hr prior to procedure. Insulin (humulin) was prepared by mixing 10‐ul insulin with 10‐ml sterile saline. Mice were injected with 3.75‐μl insulin mixture/g bodyweight. Blood glucose levels were assessed from a tail nick at times = 0, 15, 30, 60, and 120 min via glucometer (GLUCOCARD).
2.4. Islet Histology and Analyses
At the time of tissue collection, whole pancreas was placed in 3 ml of neutral buffered formalin. These samples were incubated at 4°C while gently shaking for 24 hr. Immediately afterwards, samples were placed into plastic cages and acclimated to 50% EtOH for 1 hr. Samples were then processed into paraffin blocks using a Leica tissue processor with the following protocol: 70% EtOH for 1 hr × 2, 85% EtOH for 1 hr, 95% EtOH for 1 hr × 2, 100% EtOH for 1 hr × 2, Xylenes for 1 hr × 2, paraffin wax. Pancreas blocks were sectioned into 4‐μm thick sections. Four samples per individual were randomly selected, at least 100 μm apart.
Slides were incubated at 60°C for 1 hr, then placed in xylenes to remove remaining paraffin wax. Slides were then rehydrated using successive decreasing EtOH concentrations (xylenes × 2, 50% EtOH in xylenes, 100% EtOH × 2, 95% EtOH, 70% EtOH, 50% EtOH, and H2O). Slides were incubated in sodium citrate (pH 6) at 85°C for 30 min, then submerged in running water for 5 min. Slides were washed with 0.025% Triton X‐100 in TBS and blocked in 10% normal donkey serum for 1 hr (Abcam ab7475), followed by incubation with primary antibody overnight at 4°C [primary antibodies: rat anti‐insulin (1:100, R&D MAB1417), mouse anti‐glucagon (1:100, Abcam ab10988), and rabbit anti‐phospho‐histone H3 (1:100, Sigma SAB4504429)]. After an additional wash, secondary antibody was applied for 1 hr at room temperature [secondary antibodies: donkey anti‐rabbit 488 (1:1000, Abcam ab150061), donkey anti‐mouse 647 (1:1000, Abcam ab150107), and donkey anti‐rat 555 (1:1000, Abcam ab 150154)]. Fluoroshield Mounting Medium with DAPI (Abcam) was applied to seal the coverslip and slides were stored at 4°C. Imaging was performed using the Zeiss AxioScan.Z1 at 20X magnification and 94.79% laser intensity.
Background was subtracted from DAPI, insulin, glucagon, and phospho‐histone H3 images using ImageJ. DAPI channel was used to identify total nuclei in CellProfiler. Insulin and glucagon channels were combined and overlaid on the DAPI image to identify islet nuclei. Insulin (INS+) staining overlaid with DAPI identified β‐cells and glucagon (GCG+) staining overlaid with DAPI identified α‐cells, based on the proximity of the signal to a given nuclei. Phosphohistone H3 (PHH3+) staining identified mitotic nuclei. Total nuclei, islet cells, β‐cells, α‐cells, and mitotic nuclei were summed across four slides for each individual. Islet, β‐cell, and α‐cell mass are reported as fraction of total nuclei. Mitotic islet index is reported as proportion of β‐cells and α‐cells positive for phosphohistone H3. Mean β‐cell area was calculated by dividing the total INS+ area by the number of INS+ cells. Mean β‐cell area is reported for each individual. Islets with diameter <50 µm were discarded.
2.5. Islet isolation
Pancreas was removed and placed in 8‐mL HBSS buffer on ice. Pancreas was then thoroughly minced. Collagenase P (Roche) was added to a final concentration of 0.75 mg/ml. Mixture was then shaken in a 37°C water bath for 10–14 min. Mixture was spun at 1,500 rpm for 2 min. The pellet was washed twice with HBSS. The pellet was re‐suspended in HBSS and transferred to a petri dish. Hand‐selected islets were placed in sterile‐filtered RPMI with L‐glutamine (Gibco) containing 11‐mM glucose, supplemented with 5% pen/strep and 10% fetal bovine serum (Gibco). Islets were rested overnight in a cell culture incubator set to 37°C with 5% CO2.
2.6. Glucose‐stimulated insulin secretion and islet insulin content
Islets of roughly equal size were equilibrated in KRBH buffer containing 2.8‐mM glucose for 30 min at 37°C. Five islets were hand‐selected and placed in 150‐µl KRBH containing either 2.8‐ or 11‐mM glucose. Tubes were placed in a 37°C water bath for 45 min. Islets were then spun at 2000 × g, hand‐picked with a pipette, and transferred from the secretion tube and placed in the content tube with acid ethanol. The content and secretion tubes were stored at −20°C overnight. Each condition was performed in duplicate for each individual. Mouse insulin ELISA (ALPCO 80‐INSMU‐E01) was performed according to the manufacturer's instructions, with the secretion tubes diluted 1:5, and content tubes diluted 1:100. Normalized insulin secretion was calculated by dividing the secreted value by the content value. Glucose‐stimulated insulin secretion was calculated by dividing the normalized insulin secretion at 11‐mM glucose by the normalized insulin secretion at 2.8‐mM glucose. Each sample was measured in duplicate. Total islet protein within each content tube was measured using Pierce BCA Protein Assay kit (Thermo Scientific) according to the manufacturer's instructions and read at 562 nm on the Synergy H1 Microplate Reader (Biotek). Islet insulin content was calculated by dividing the insulin level in the content tubes by the total protein value.
2.7. Statistical analyses
Phenotypes were assessed for normality by a Shapiro‐Wilk test, and outliers were removed. ANOVA revealed that sex‐by‐diet‐by‐age interactions did not contribute meaningful variance to any phenotype, so males and females were pooled at each time point. A student's t‐test was used to assess significance between two cohorts, while a one‐way ANOVA with Tukey's post hoc test was used to assess significance among multiple cohorts. Pearson's correlation was used to determine strength of correlation among variables. P < .05 were considered significant.
3. RESULTS
3.1. Obese SM/J mice increase insulin levels and improve insulin sensitivity
The resiliency of β‐cells distinguishes nondiabetic‐obese and diabetic‐obese individuals (Bock et al., 2003; Keller et al., 2008; Klöppel, Löhr, Habich, Oberholzer, & Heitz, 1985; Mezza et al., 2014; Ogilvie, 1933; Rahier et al.,; Saisho et al., 2013; Shirakawa et al., 2017). While both groups develop hyperinsulinemia, diabetic‐obese individuals become insulin resistant, leading to β‐cell dysfunction, hypoinsulinemia, and hyperglycemia. Our previous work shows that obese SM/J mice spontaneously transition from hyperglycemic to normoglycemic with age (Carson et al., 2019). Principle to this is a 40 mg/dl decrease in fasting glucose levels in high fat‐fed SM/J mice between 20 and 30 weeks (Figure 1a). We first sought to characterize how insulin homeostasis changes during this transition. Interestingly, 20‐week high fat‐fed SM/J mice have comparable levels of plasma and pancreatic insulin levels compared to age‐matched low fat‐fed mice (Figure 1b‐c). By 30 weeks, high fat‐fed SM/J mice increase circulating insulin levels 5.3‐fold and pancreatic insulin levels 1.9‐fold, in line with other models of hyperinsulinemic nondiabetic‐obesity (Fransson et al., 2013; Gupta et al., 2017; Liu, Jetton, & Leahy, 2002). We sought to test for peripheral insulin resistance via an insulin tolerance test (ITT), as insulin resistance is a known mechanism for increasing circulating and pancreatic insulin levels. Surprisingly, 20‐week high fat‐fed SM/J mice display insulin resistance compared to low fat‐fed mice; however, insulin sensitivity is restored by 30 weeks (Figure 1d‐e). The simultaneous increase in insulin production and improved insulin sensitivity is unprecedented and suggests a novel mechanism beyond insulin resistance for enhancing β‐cell insulin secretion.
FIGURE 1.
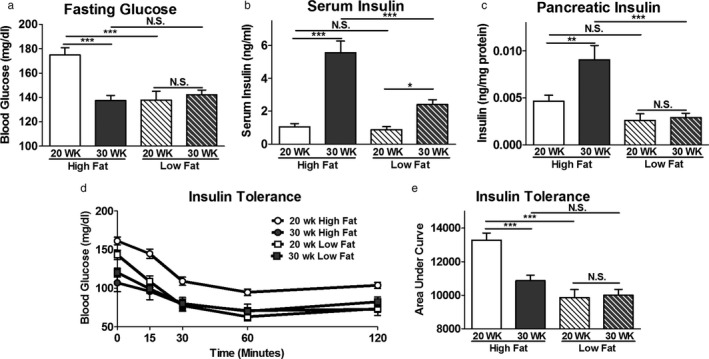
Insulin homeostasis during the resolution of hyperglycemia in obese SM/J mice. Blood glucose levels measured after 4‐hr fast from high and low fat‐fed, 20‐ and 30‐week SM/J mice (a). Plasma insulin (b) and pancreatic insulin levels (c) assessed via insulin ELISA, collected after 4‐hr fast. Insulin tolerance test performed via intraperitoneal insulin injection following 4‐hr fast (d), summarized in the area under the curve (e). N = 38–50 mice per cohort for panel A, C, D. N = 10–24 mice per cohort for panel B‐C, equal numbers of males and females. Bar represents group means, error bars represent SEM. *p < .05, **p < .01, ***p < .001, N.S. – Not Significant
3.2. Obese SM/J mice increase islet mass during resolution of hyperglycemia
In humans and mice, obesity initially increases islet mass, and maintenance of that mass in part differentiates nondiabetic‐obese individuals from diabetic‐obese individuals (Aguayo‐Mazzucato & Bonner‐Weir,; Butler et al., 2003; Dimas et al., 2014; Meier et al., 2008; Seino et al., 2011; Teta, Long, Wartschow, Rankin, & Kushner, 2005). To understand the source of increased insulin production in obese SM/J mice, we examined islet morphology during the resolution of hyperglycemia. To quantify islet mass and number, β‐cell mass, α‐cell mass, and mitotic index, we randomly selected four sections per fixed pancreas and stained with antibodies against insulin, glucagon, and phosphohistone H3. Representative images of immuno‐stained pancreatic sections for 30‐week high fat‐fed mice and 30‐week low fat‐fed mice are shown in Figure 2a‐b. Consistent with other mouse models of obesity, 20‐week high fat‐fed SM/J mice have a 2.75‐fold increase in total islet mass compared to low fat‐fed mice (Figure 2c). This increased mass is driven by an increase in both median islet area and number of islets (Figure 2d‐e). Islet mass is further elevated 2‐fold between 20‐ and 30 weeks in high fat‐fed mice, while the islet population remains unchanged in low fat‐fed mice. A full summary of the islet quantification is presented in Table S1. Distribution of islet size is shown in Figure S1, along with corresponding density plot for each cohort. Islet mass correlates with BMI in obese humans (Dybala et al., 2019), a similar correlation is seen between islet mass and body weight in high fat‐fed SM/J mice (Figure 2f).
FIGURE 2.
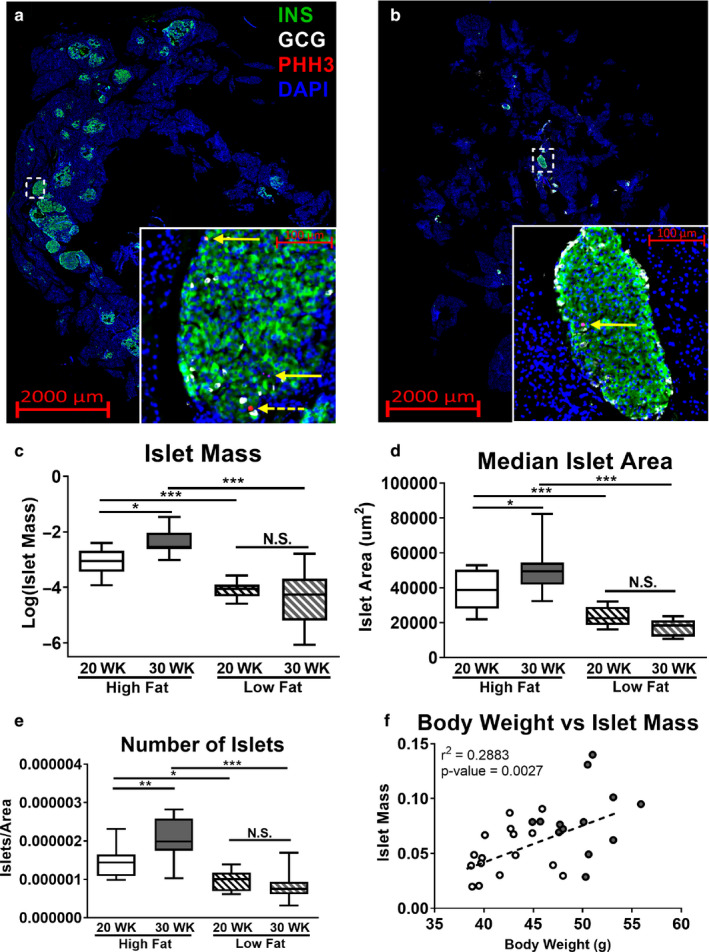
Changes in islet mass during the resolution of hyperglycemia. Representative pancreatic cross sections from 30‐week high fat‐fed mice (a) and 30‐week low fat‐fed mice (b) stained for insulin (green), glucagon (white), and phosphohistone H3 (red). Dashed white box identifies location of image in inset. Solid yellow arrows within inset identify INS+:PHH3+ cells, and dashed yellow arrow identifies GCG+:PHH3+ cell. Islet mass reported as ratio of islet cells over total cells, summed across four pancreatic sections (c). Median islet area calculated for each individual across four sections (d). Total number of islets quantified per individual, normalized by total DAPI area (e). Correlation between body weight and β‐cell mass in high fat‐fed mice (f), open circles – 20‐week high fat‐fed, filled circles – 30‐week high fat‐fed. N = 12–16 mice per cohort for panels C‐F, equal number of males and females. *p < .05, **p < .01, ***p < .001, N.S. – Not Significant
3.3. Obese SM/J mice increase β‐cell mass and α‐cell replication
To identify the source of the increased islet mass in high fat‐fed SM/J mice, we quantified the relative representation of β‐cells and α‐cells within each cohort. Increased islet mass in 20‐week high fat‐fed mice is driven by a 3.3‐fold increase in the number of β‐cells and a 2.5‐fold increase in the number of α‐cells compared to low fat mice, while growth between 20‐ and 30‐week high fat‐fed mice is driven by a further 2.2‐fold increase in β‐cell number (Figure 3a‐b). In obesity, islet mass expands primarily through β‐cell hyperplasia (Georgia & Bhushan, 2004; Porat et al., 2011; Teta et al., 2005; Zhong & Jiang, 2019). Mean β‐cell area is not different across age and dietary cohorts (Figure S2). We quantified mitotic index of β‐ and α‐cells in our model using phosphohistone H3 and assessed how mitotic index relates to β‐cell mass during the resolution of hyperglycemia in obese SM/J mice. Surprisingly, calculation of β‐cell mitotic index reveals similar rates of β‐cell replication across cohorts (Figure 3c), while α‐cell mitotic index is elevated 6‐fold in high fat‐fed mice compared to low fat‐fed controls (Figure 3d). Examining the relationship between β‐cell mitotic index and β‐cell mass in high fat‐fed mice reveals that β‐cell replication correlates with β‐cell mass in 20‐week mice, but not in 30‐week mice (Figure 3e‐f).
FIGURE 3.
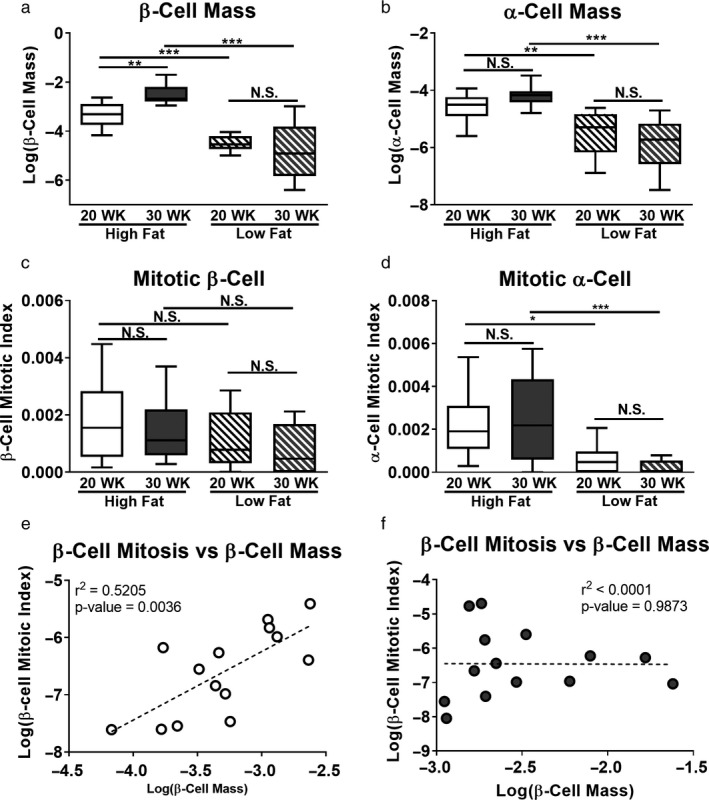
Islet cell mass and mitotic index in obese SM/J mice. β‐cell mass reported as ratio of INS+ cells divided by total cells summed across four slides per individual (a). α‐cell mass reported as GCG+ cells divided by total cells summed across four slides per individual (b). β‐cell mitotic index calculated by dividing INS+:PHH3+ cells by total INS+ cells summed across four slides per individual (c). α‐cell mitotic index calculated by dividing GCG+:PHH3+ cells by total GCG+ cells summed across four slides (d). Correlation between β‐cell mitotic index and β‐cell mass in 20‐week high fat‐fed mice (e) and 30‐week high fat‐fed mice (f). Open circles – 20‐week high fat‐fed, filled circles – 30‐week high fat‐fed. N = 12–16 mice per cohort for all panels, equal males and females. *p < .05, **p < .01, ***p < .001, N.S. – Not Significant
3.4. Obese SM/J mice increase islet insulin secretion and insulin content
In conjunction with changing β‐cell morphology, diabetic‐obesity is associated with altered β‐cell function, including diminished first phase insulin secretion, increased basal insulin secretion, and decreased β‐cell insulin production (Cheng et al., 2013; Deng et al., 2004; Marchetti et al., 2004; Peyot et al., 2010). We sought to examine if changes in β‐cell insulin secretion and content corresponded with the resolution of hyperglycemia and expanded β‐cell mass we observe. To test this, we isolated islets from high and low fat‐fed 20‐ and 30‐week SM/J mice. After allowing islets to rest overnight, we performed a glucose‐stimulated insulin secretion assay by subjecting islets to low (2.8 mM) or high (11 mM) glucose conditions. We find that high fat‐fed SM/J mice dramatically improve glucose‐stimulated insulin secretion between 20 and 30 weeks of age. This includes transitioning from blunted insulin secretion under high glucose conditions to appropriately elevated secretion (Figure 4a), and improvement in the ratio of insulin secreted in response to high versus low glucose conditions (Figure 4b). Normalized insulin secreted in response to elevated glucose does not differ between cohorts (Figure S3a). Twenty‐week high fat‐fed mice have elevated insulin secretion in response to low glucose (Figure 4c), consistent with other studies of islets in type 2 diabetic humans and mice. Correspondingly, 20‐week high fat‐fed SM/J mice have decreased islet insulin content (Figure 4d), which increases 3‐fold by 30 weeks. Consistent with current understanding of the β‐cell maturation process (Salinno et al., 2019), there is a positive correlation between islet insulin content and glucose‐stimulated insulin secretion in high fat‐fed (Figure 4e) and low fat‐fed (Figure S3b) SM/J mice. This suggests that obese SM/J mice experience β‐cell maturation between 20 and 30 weeks, characterized by increased insulin content and improved insulin secretion in response to high glucose. This spontaneous improvement in β‐cell health and function in the context of obesity has not been reported in other mouse strains, suggesting a genetic basis unique to SM/J.
FIGURE 4.
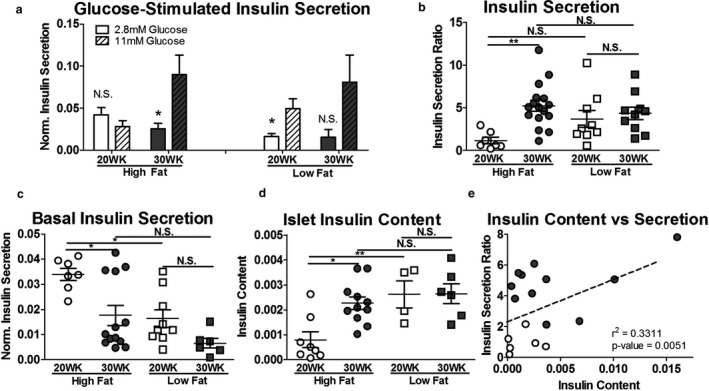
Islet insulin secretion and insulin content. Islet insulin secretion in response to low (2.8 mM) and high (11 mM) glucose conditions, normalized by islet insulin content (a), reported as a ratio of high glucose to low glucose insulin secretion (b). Comparison of islet insulin secretion under low glucose conditions in 20‐ and 30‐week, high and low fat‐fed mice (c). Islet insulin content normalized by total protein measured via protein BCA (d). Correlation between insulin secretion ratio and islet insulin content (e). Open circles – 20‐week high fat‐fed, closed circles – 30‐week high fat‐fed. N = 8–19 mice per cohort for panels A‐C, n = 5–11 mice per cohort for panel D, n = 22 mice for panel E. *p < .05, **p < .01, ***p < .001, N.S. – Not Significant
4. DISCUSSION
The ability to maintain appropriate insulin production and secretion, termed functional β‐cell mass, is a central determinant of diabetic risk. In this study, we describe insulin homeostasis, islet morphology, and β‐cell function in obese SM/J mice as they transition from hyperglycemic to normoglycemic. We determine that increased insulin production and insulin sensitivity accompany improved glycemic control, driven by expanded β‐cell mass and improved glucose‐stimulated insulin secretion. Our results show that obese SM/J mice undergo restoration of functional β‐cell mass, providing an opportunity to explore how compensatory insulin production can be achieved in the context of obesity.
Susceptibility to high fat diet‐induced diabetes in mice depends on genetic background. Several strains and sub‐strains develop diabetic‐obesity, including hyperglycemia, glucose intolerance, and insulin resistance, consistent with the diabetic phenotypes observed in obese SM/J mice at 20 weeks (Andrikopoulos et al., 2005; Kahle et al., 2013; Surwit, Kuhn, Cochrane, McCubbin, & Feinglos, 1988). Remarkably, by 30 weeks, obese SM/J mice have characteristics of diabetic‐resistant obese strains, retaining glycemic control by dramatically increasing insulin production and improving insulin sensitivity (Andrikopoulos et al., 2005; Meulen et al., 2017; Sims et al., 2013; Surwit et al., 1988). To our knowledge, this is the first report of transient hyperglycemia in an inbred strain, although similar phenomena have been reported in mice with the leptin receptor (db/db) mutation. C57bl/6J (db/db) and 129/J (db/db) mice are obese and initially develop mild hyperglycemia at 8–10 weeks of age, but this resolves by 20–30 weeks, concurrent with increased insulin production and β‐cell mass (Hummel et al., 1972; Leiter et al., 1980). Unfortunately, leptin and its receptor play an important role in β‐cell growth and function independent of obesity, which confounds understanding of how genetic background mediates diabetic risk in obesity (Covey et al.,). Interestingly, low fat‐fed mice increase circulating insulin levels between 20‐ and 30 weeks, despite no change in pancreatic insulin levels, insulin sensitivity, or β‐cell mass.
High fat diet‐induced obesity in mice can result in increased islet mass, no change, or decreased mass (Andrikopoulos et al., 2005; Hull et al., 2005; Meulen et al., 2017; Peyot et al., 2010; Sims et al., 2013). Across these studies, inability to expand islet mass is associated with hyperglycemia. In humans, islet mass correlates with BMI in nondiabetic‐obese individuals, while diabetic‐obese individuals have low islet mass compared to nondiabetic individuals (Dybala et al., 2019; Klöppel et al., 1985; Lencioni, Lupi, & Del Prato, 2008). High fat‐fed SM/J mice are unique because they have expanded islet mass at 20 weeks, yet normal insulin levels and insulin resistance. By 30 weeks, islet mass continues to expand, driven by increased islet area and increased islet number, corresponding with increased insulin production and improved insulin sensitivity. Islet neogenesis may contribute to the increased islet number, and fission of large islets has been reported during development, suggesting that islets have mechanisms to maintain an appropriate size (Jo et al., 2011; Seymour, Bennett, & Slack, 2004).
β‐cell hyperplasia is the primary driver of islet expansion in mouse models of obesity (Bock et al., 2003; Keller et al., 2008). Some nondiabetic‐obese mice experience increased β‐cell number, but do not show evidence for elevated β‐cell replication in immunostaining of pancreatic sections (Hull et al., 2005; Sims et al., 2013). This has been attributed to islets in the tail of the pancreas being substantially more proliferative in response to high fat diet than the body and head regions (Ellenbroek et al., 2013); thus, technical artifacts in sampling could result in inflated variances which mask biological differences. This could be the case here, given that high fat‐fed SM/J’s β‐cell number is far above low fat‐fed controls, that their β‐cell number expands 2‐fold during the resolution of hyperglycemia, yet we find no evidence for increased β‐cell replication. However, α‐cell number also expands in obesity (Ellenbroek et al., 2017; Henquin & Rahier, 2011; Merino et al., 2015). While α‐cell mass is elevated in high fat‐fed SM/J mice compared to low fat‐fed controls, we find that it does not change between 20 and 30 weeks, despite substantial elevation of α‐cell mitotic index.
Retention of β‐cell function separates diabetic‐obesity and nondiabetic‐obesity (Basu et al., 2009; Guillausseau et al., 2008; Kahn, 1998). Twenty‐week high fat‐fed SM/J mice have an insulin secretion profile similar to diabetic‐obese mice and humans, including blunted glucose‐stimulated insulin release, elevated basal insulin secretion, and low islet insulin content, which resolves by 30 weeks. Underscoring this transition is the positive correlation between glucose‐stimulated insulin release and islet insulin content. Care was taken to select normal‐sized islets across all cohorts for functional assessment (~100 µm in diameter), indicating this robust improvement in β‐cell functional mass is due to changes to β‐cell physiology.
Three current, nonmutually exclusive components of β‐cell stress response may shed light on the perplexing improvement in glycemic control seen in SM/J mice: β‐cell dedifferentiation, nascent β‐cell maturation, and changes in β‐cell subtype proportions. While early studies concluded that overworked β‐cells undergo apoptosis (Butler et al., 2003; Maclean & Ogilvie, 1955; Pipeleers & Ling, 1992; Sakuraba et al., 2002), recent studies have suggested that β‐cells dedifferentiate into a dysfunctional, progenitor‐like state, potentially as a defense mechanism against prolonged glycemic stress (Cinti et al., 2016; Jonas et al., 1999; Marselli et al., 2014; Talchai et al., 2012). These dedifferentiated β‐cells have low insulin content and poor glucose‐stimulated insulin secretion. Further, the dedifferentiated state is reversible in cultured conditions, revealing potential for therapeutic intervention (Diedisheim et al., 2018). It is feasible that obese SM/J mice have β‐cells in the dedifferentiated state at 20 weeks, which would explain the low insulin content and poor functionality despite the elevated β‐cell mass. Improvement in insulin sensitivity could ease glycemic stress, allowing dedifferentiated β‐cells to redifferentiate by 30 weeks, reestablishing insulin production and secretion.
Work from several groups suggests that β‐cells can be divided into two broad categories: functionally immature and functionally mature cells. Immature β‐cells have greater proliferative potential and are resistant to stress, at the expense of functional maturity (Bader et al., 2016; Blum et al., 2012; Puri et al., 2018). These immature β‐cells have low insulin content, high basal insulin secretion, and a lack of glucose‐stimulated insulin secretion. The large β‐cell expansion seen in obese SM/J mice suggests that nascent β‐cells must undergo maturation at some point. We have no evidence of enhanced β‐cell replication at 20 weeks, but it is possible that these β‐cells are still functionally immature and reach maturity by 30 weeks. This could explain why islets from these mice lack glucose‐stimulated insulin release, show elevated basal insulin secretion, and have low insulin content, despite elevated mass.
Recent advances in single‐cell technology have allowed for identification of β‐cell subtypes, based on functional characteristics and gene expression. These include β‐cells that specializes in basal insulin secretion, characterized by low mature insulin content, and enriched in db/db diabetic islets (Farack et al., 2019). While these cells are not equipped to respond to elevated glucose, they are enriched for maturity markers, including Ucn3 and Glut2, distinguishing them from immature β‐cells. Pancreatic multipotent progenitors (PMPs) are rare insulin‐positive cells capable of generating endocrine cells in vivo including functionally mature β‐cells (Razavi et al., 2015; Smukler et al., 2011). These cells resemble immature β‐cells, with low insulin content and Glut2 expression, whose proliferation is stimulated by glycemic stress in STZ‐treated and NOD mouse models. Lastly, β‐cell hub cells coordinate calcium signaling and insulin release of surrounding β‐cells (Johnston et al., 2016). These cells have markers for both mature and immature β‐cells, including expression of Gck and Pdx1, but low insulin content, and are especially sensitive to glycemic and inflammatory stress. Ablation of these cells results in loss of coordinated insulin release, suggesting that they are necessary for mature islet function. Given the elevated β‐cell mass, poor insulin secretion, and low insulin content in 20‐week high fat‐fed SM/J mice, it is possible that islets are enriched for basal insulin secretors and PMP’s, while devoid of hub cells. At 30 weeks, as glycemic stress diminishes, basal insulin secretors and PMP populations decline, while hub cells rise, reestablishing β‐cell functionality.
Clearly, the interplay between β‐cell dedifferentiation, nascent β‐cell maturation, and β‐cell subtype identity in diabetic‐obesity needs to be clarified. SM/J mice are a useful model because they allow for appropriate comparisons across diabetic‐obese, nondiabetic‐obese, and nondiabetic‐lean populations. The improvement in β‐cell function and increase in insulin production in obese SM/J mice could be explained by a combination of these innate and stress response β‐cell mechanisms. Future studies interrogating how SM/J β‐cells change over time will provide insight into the physiological mechanisms that allow β‐cell functionality to be maintained and improved in the context of obesity.
DISCLOSURE
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
HAL and MAM designed the experiments. MAM, CC, HS, and JPW performed mouse phenotyping and ELISAs. MAM, JMV, and MK performed histological assays and analyses. MAM, JWH, and CLP performed experiments on isolated islets. MAM and HAL wrote the manuscript. All authors edited and approved the final draft.
Supporting information
Supplemental Figure 1 Final
Supplemental Figure 2 Final
Supplemental Figure 3 Final
Supplemental Table 1
Miranda MA, Carson C, St. Pierre CL, et al. Spontaneous restoration of functional β‐cell mass in obese SM/J mice. Physiol Rep. 2020;8:e14573 10.14814/phy2.14573
Funding information
This work was supported by the Washington University Department of Genetics, the Diabetes Research Center at Washington University (P30DK020579), the NIH NIDDK (K01 DK095003) to HAL and NIH NIGM (T32 GM007067) and NIH NIDDK (T32 DK108742) to MAM.
REFERENCES
- Abdullah, A. , Peeters, A. , de Courten, M. , & Stoelwinder, J. (2010). The magnitude of association between overweight and obesity and the risk of diabetes: A meta‐analysis of prospective cohort studies. Diabetes Research and Clinical Practice, 89, 309–319. 10.1016/j.diabres.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Aguayo‐Mazzucato, C. , & Bonner‐Weir, S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metabolism, 27(1), 57–67. 10.1016/j.cmet.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrikopoulos, S. , Massa, C. M. , Aston‐Mourney, K. , Funkat, A. , Fam, B. C. , Hull, R. L. , … Proietto, J. (2005). Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. Journal of Endocrinology, 187(1), 45–53. 10.1677/joe.1.06333 [DOI] [PubMed] [Google Scholar]
- Bader, E. , Migliorini, A. , Gegg, M. , Moruzzi, N. , Gerdes, J. , Roscioni, S. S. , … Lickert, H. (2016). Identification of proliferative and mature β‐cells in the islets of Langerhans. Nature, 535, 430–434. 10.1038/nature18624 [DOI] [PubMed] [Google Scholar]
- Basu, A. , Dalla Man, C. , Basu, R. , Toffolo, G. , Cobelli, C. , & Rizza, R. A. (2009). Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care, 32(5), 866–872. 10.2337/dc08-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, B. , Hrvatin, S. , Schuetz, C. , Bonal, C. , Rezania, A. , & Melton, D. A. (2012). Functional beta‐cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nature Biotechnology, 30(3), 261–264. 10.1038/nbt.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, T. , Pakkenberg, B. , & Buschard, K. (2003). Increased islet volume but unchanged islet number in ob/ob mice. Diabetes, 52(7), 1716–1722. 10.2337/diabetes.52.7.1716 [DOI] [PubMed] [Google Scholar]
- Butler, A. E. , Janson, J. , Bonner‐Weir, S. , Ritzel, R. , Rizza, R. A. , & Butler, P. C. (2003). β‐cell deficit and increased β‐cell apoptosis in humans with type 2 diabetes. Diabetes, 52, 102–110. 10.2337/diabetes.52.1.102 [DOI] [PubMed] [Google Scholar]
- Carson, C. , Miranda, M. A. , Macias‐Velasco, J. F. , Gunawardana, S. , Hughes, J. , Oyama, S. , … Lawson, H. A. (2019). Natural brown adipose expansion and remission of hyperglycemia in obese SM/J mice. bioRxiv ( August 5, 2019). 10.1101/724369 [DOI]
- Centers for Disease Control and Prevention C . (2017). National diabetes statistics report: Estimates of diabetes and its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2017. US Dep Heal Hum Serv: 2009–2012, 2017. [Google Scholar]
- Chatterjee, S. , Khunti, K. , & Davies, M. J. (2017). Type 2 diabetes. Lancet (London, England), 389, 2239–2251. 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- Cheng, K. , Andrikopoulos, S. , & Gunton, J. E. (2013). First phase insulin secretion and type 2 diabetes. Current Molecular Medicine, 13(1), 126–139. 10.2174/156652413804486287 [DOI] [PubMed] [Google Scholar]
- Cheverud, J. M. , Lawson, H. A. , Fawcett, G. L. , Wang, B. , Pletscher, L. S. , Fox, R. , … Semenkovich, C. F. (2011). Diet‐dependent genetic and genomic imprinting effects on obesity in mice. Obesity (Silver Spring), 19, 160–170. 10.1038/oby.2010.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti, F. , Bouchi, R. , Kim‐Muller, J. Y. , Ohmura, Y. , Sandoval, P. R. , Masini, M. , … Accili, D. (2016). Evidence of β‐cell dedifferentiation in human type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism, 101, 1044–1054. 10.1210/jc.2015-2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz, G. A. , Willett, W. C. , Rotnitzky, A. , & Manson, J. E. (1995). Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine, 122(7), 481– 10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- Covey, S. D. , Wideman, R. D. , Mcdonald, C. , Unniappan, S. , Huynh, F. , Asadi, A. , … Kieffer, T. J. (2006). The pancreatic b cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metabolism, 4(4), 291–302. 10.1016/j.cmet.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Deng, S. , Vatamaniuk, M. , Huang, X. , Doliba, N. , Lian, M. M. , Frank, A. , … Markmann, J. F. (2004). Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes, 53(3), 624–632. 10.2337/diabetes.53.3.624 [DOI] [PubMed] [Google Scholar]
- Diedisheim, M. , Oshima, M. , Albagli, O. , Huldt, C. W. , Ahlstedt, I. , Clausen, M. , … Scharfmann, R. (2018). Modeling human pancreatic beta cell dedifferentiation. Molecular Metabolism, 10, 74–86. 10.1016/j.molmet.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimas, A. S. , Lagou, V. , Barker, A. , Knowles, J. W. , Mägi, R. , Hivert, M.‐F. , … Prokopenko, I. (2014). Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes, 63(6), 2158–2171. 10.2337/db13-0949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybala, M. P. , Olehnik, S. K. , Fowler, J. L. , Golab, K. , Millis, J. M. , Golebiewska, J. , … Hara, M. (2019). Pancreatic beta cell/islet mass and body mass index. Islets, 11, 1–9. 10.1080/19382014.2018.1557486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich, T. H. , Kenney, J. P. , Vaughn, T. T. , Pletscher, L. S. , & Cheverud, J. M. (2003). Diet, obesity, and hyperglycemia in LG/J and SM/J mice. Obesity Research, 11(11), 1400–1410. 10.1038/oby.2003.189 [DOI] [PubMed] [Google Scholar]
- Ellenbroek, J. H. , Töns, H. A. , de Graaf, N. , Loomans, C. J. , Engelse, M. A. , Vrolijk, H. , … de Koning, E. J. (2013). Topologically heterogeneous beta cell adaptation in response to high‐fat diet in mice. PLoS One, 8(2), e56922 10.1371/journal.pone.0056922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek, J. H. , Töns, H. A. M. , Hanegraaf, M. A. J. , Rabelink, T. J. , Engelse, M. A. , Carlotti, F. , & de Koning, E. J. P. (2017). Pancreatic α‐cell mass in obesity. Diabetes, Obesity and Metabolism, 19(12), 1810–1813. 10.1111/dom.12997 [DOI] [PubMed] [Google Scholar]
- Esposito, K. , Chiodini, P. , Colao, A. , Lenzi, A. , & Giugliano, D. (2012). Metabolic syndrome and risk of cancer: A systematic review and meta‐analysis. Diabetes Care, 35, 2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farack, L. , Golan, M. , Egozi, A. , Dezorella, N. , Bahar Halpern, K. , Ben‐Moshe, S. , … Itzkovitz, S. (2019). Transcriptional heterogeneity of beta cells in the intact pancreas. Developmental Cell, 48(1), 115–125.e4. 10.1016/j.devcel.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Fransson, L. , Franzén, S. , Rosengren, V. , Wolbert, P. , Sjöholm, Å. , & Ortsäter, H. (2013). β‐cell adaptation in a mouse model of glucocorticoid‐induced metabolic syndrome. Journal of Endocrinology, 219(3), 231–241. 10.1530/JOE-13-0189. [DOI] [PubMed] [Google Scholar]
- Georgia, S. , & Bhushan, A. (2004). β cell replication is the primary mechanism for maintaining postnatal β cell mass. Journal of Clinical Investigation, 114(7), 963–968. 10.1172/JCI200422098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillausseau, P. J. , Meas, T. , Virally, M. , Laloi‐Michelin, M. , Médeau, V. , & Kevorkian, J. P. (2008). Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes & Metabolism, 34, S43–S48. 10.1016/S1262-3636(08)73394-9 [DOI] [PubMed] [Google Scholar]
- Gupta, D. , Jetton, T. L. , LaRock, K. , Monga, N. , Satish, B. , Lausier, J. , … Leahy, J. L. (2017). Temporal characterization of β cell‐adaptive and ‐maladaptive mechanisms during chronic high‐fat feeding in C57BL/6NTac mice. Journal of Biological Chemistry, 292(30), 12449–12459. 10.1074/jbc.M117.781047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin, J. C. , & Rahier, J. (2011). Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia, 54(7), 1720–1725. 10.1007/s00125-011-2118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, R. L. , Kodama, K. , Utzschneider, K. M. , Carr, D. B. , Prigeon, R. L. , & Kahn, S. E. (2005). Dietary‐fat‐induced obesity in mice results in beta cell hyperplasia but not increased insulin release: Evidence for specificity of impaired beta cell adaptation. Diabetologia, 48, 1350–1358. 10.1007/s00125-005-1772-9 [DOI] [PubMed] [Google Scholar]
- Hummel, K. P. , Coleman, D. L. , & Lane, P. W. (1972). The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL/KsJ and C57BL/6J strains. Biochemical Genetics, 7(1), 1–13. 10.1007/BF00487005 [DOI] [PubMed] [Google Scholar]
- Jo, J. , Kilimnik, G. , Kim, A. , Guo, C. , Periwal, V. , & Hara, M. (2011). Formation of pancreatic islets involves coordinated expansion of small islets and fission of large interconnected islet‐like structures. Biophysical Journal, 101(3), 565–574. 10.1016/j.bpj.2011.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, N. R. , Mitchell, R. K. , Trauner, D. , Rutter, G. A. , Hodson, D. J. , Haythorne, E. , … Broichhagen, J. (2016). Beta cell hubs dictate pancreatic islet responses to glucose cell metabolism article beta cell hubs dictate pancreatic islet responses to glucose. Cell Metabolism, 24, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas, J. C. , Sharma, A. , Hasenkamp, W. , Ilkova, H. , Patanè, G. , Laybutt, R. , … Weir, G. C. (1999). Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. Journal of Biological Chemistry, 274(20), 14112–14121. 10.1074/jbc.274.20.14112 [DOI] [PubMed] [Google Scholar]
- Kahle, M. , Horsch, M. , Fridrich, B. , Seelig, A. , Schultheiß, J. , Leonhardt, J. , … Neschen, S. (2013). Phenotypic comparison of common mouse strains developing high‐fat diet‐induced hepatosteatosis %. Molecular Metabolism, 2(4), 435–446. 10.1016/j.molmet.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, B. B. (1998). Type 2 diabetes: When insulin secretion fails to compensate for insulin resistance. Cell, 92, 593–596. [DOI] [PubMed] [Google Scholar]
- Keller, M. P. , Choi, Y. , Wang, P. , Davis, D. B. , Rabaglia, M. E. , Oler, A. T. , … Attie, A. D. (2008). A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Research, 18(5), 706–716. 10.1101/gr.074914.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel, G. , Löhr, M. , Habich, K. , Oberholzer, M. , & Heitz, P. U. (1985). Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Survey and Synthesis of Pathology Research, 4(2), 110–125. [DOI] [PubMed] [Google Scholar]
- Kobayashi, M. , Ohno, T. , Ihara, K. , Murai, A. , & Kumazawa, M. (2014). Searching for genomic region of high‐fat diet‐induced type 2 diabetes in mouse chromosome 2 by analysis of congenic strains. PLoS One, 9, e96271 10.1371/journal.pone.0096271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, H. , & Martin, S. (2017). Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Medicine, 15, 131 10.1186/s12916-017-0901-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, H. A. , Cady, J. E. , Partridge, C. , Wolf, J. B. , Semenkovich, C. F. , & Cheverud, J. M. (2011). Genetic effects at pleiotropic loci are context‐dependent with consequences for the maintenance of genetic variation in populations. PLoS Genetics, 7, e1002256 10.1371/journal.pgen.1002256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, H. A. , & Cheverud, J. M. (2010). Metabolic syndrome components in murine models. Endocrine, Metabolic and Immune Disorders Drug Targets, 10, 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, H. A. , Lee, A. , Fawcett, G. L. , Wang, B. , Pletscher, L. S. , Maxwell, T. J. , … Cheverud, J. M. (2011). The importance of context to the genetic architecture of diabetes‐related traits is revealed in a genome‐wide scan of a LG/J × SM/J murine model. Mammalian Genome, 22(3‐4), 197–208. 10.1007/s00335-010-9313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, H. A. , Zelle, K. M. , Fawcett, G. L. , Wang, B. , Pletscher, L. S. , Maxwell, T. J. , … Cheverud, J. M. (2010). Genetic, epigenetic, and gene‐by‐diet interaction effects underlie variation in serum lipids in a LG/JxSM/J murine model. Journal of Lipid Research, 51, 2976–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter, E. H. , Coleman, D. L. , Eisenstein, A. B. , & Strack, I. (1980). A new mutation (db3J) at the diabetes locus in strain 129/J mice ‐ I. Physiological and histological characterization. Diabetologia, 19, 58–65. 10.1007/BF00258313 [DOI] [PubMed] [Google Scholar]
- Lencioni, C. , Lupi, R. , & Del Prato, S. (2008). β‐cell failure in type 2 diabetes mellitus. Current Diabetes Reports, 8(3), 179–184. 10.1007/s11892-008-0031-0 [DOI] [PubMed] [Google Scholar]
- Liu, Y. Q. , Jetton, T. L. , & Leahy, J. L. (2002). β‐cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate‐pyruvate shuttle activity in islets of nondiabetic zucker fatty rats. Journal of Biological Chemistry, 277(42), 39163–39168. 10.1074/jbc.M207157200 [DOI] [PubMed] [Google Scholar]
- Maclean, N. , & Ogilvie, R. F. (1955). Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes, 4(5), 367–376. 10.2337/diab.4.5.367 [DOI] [PubMed] [Google Scholar]
- Marchetti, P. , Del Guerra, S. , Marselli, L. , Lupi, R. , Masini, M. , Pollera, M. , … Del Prato, S. (2004). Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. The Journal of Clinical Endocrinology & Metabolism, 89(11), 5535–5541. 10.1210/jc.2004-0150 [DOI] [PubMed] [Google Scholar]
- Marselli, L. , Suleiman, M. , Masini, M. , Campani, D. , Bugliani, M. , Syed, F. , … Marchetti, P. (2014). Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia, 57(2), 362–365. 10.1007/s00125-013-3098-3 [DOI] [PubMed] [Google Scholar]
- Meier, J. J. , Butler, A. E. , Saisho, Y. , Monchamp, T. , Galasso, R. , Bhushan, A. , … Butler, P. C. (2008). β‐cell replication is the primary mechanism subserving the postnatal expansion of β‐cell mass in humans. Diabetes, 57(6), 1584–1594. 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs, J. B. , Wilson, P. W. F. , Fox, C. S. , Vasan, R. S. , Nathan, D. M. , Sullivan, L. M. , & D'Agostino, R. B. (2006). Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism, 91(8), 2906–2912. 10.1210/jc.2006-0594 [DOI] [PubMed] [Google Scholar]
- Merino, B. , Alonso‐Magdalena, P. , Lluesma, M. , Ñeco, P. , Gonzalez, A. , Marroquí, L. , … Quesada, I. (2015). Pancreatic alpha‐cells from female mice undergo morphofunctional changes during compensatory adaptations of the endocrine pancreas to diet‐induced obesity. Scientific Reports, 5(1), 1–13. 10.1038/srep11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezza, T. , Muscogiuri, G. , Sorice, G. P. , Clemente, G. , Hu, J. , Pontecorvi, A. , … Kulkarni, R. N. (2014). Insulin resistance alters islet morphology in nondiabetic humans. Diabetes, 63(3), 994–1007. 10.2337/db13-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolskiy, I. , Conrad, D. F. , Chun, S. , Fay, J. C. , Cheverud, J. M. , & Lawson, H. A. (2015). Using whole‐genome sequences of the LG/J and SM/J inbred mouse strains to prioritize quantitative trait genes and nucleotides. BMC Genomics, 16, 415 10.1186/s12864-015-1592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya, J. K. , L'Italien, G. , Criqui, M. H. , Whyte, J. L. , Gamst, A. , & Chen, R. S. (2004). Association of the metabolic syndrome with history of myocardial infarction and stroke in the third national health and nutrition examination survey. Circulation, 109(1), 42–46. 10.1161/01.CIR.0000108926.04022.0C [DOI] [PubMed] [Google Scholar]
- Ogilvie, R. F. (1933). The islands of langerhans in 19 cases of obesity. Journal of Pathology and Bacteriology, 37(3), 473–481. 10.1002/path.1700370314. [DOI] [Google Scholar]
- Ojha, A. , Ojha, U. , Mohammed, R. , Chandrashekar, A. , & Ojha, H. (2019). Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. Clinical Pharmacology: Advances and Applications, 11, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyot, M. L. , Pepin, E. , Lamontagne, J. , Latour, M. G. , Zarrouki, B. , Lussier, R. , … Prentki, M. (2010). β‐cell failure in diet‐induced obese mice stratified according to body weight gain: Secretory dysfunction and altered islet lipid metabolism without steatosis or reduced β‐cell mass. Diabetes, 59(9), 2178–2187. 10.2337/db09-1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers, D. , & Ling, Z. (1992). Pancreatic beta cells in insulin‐dependent diabetes. Diabetes/Metabolism Reviews, 8(3), 209–227. 10.1002/dmr.5610080303 [DOI] [PubMed] [Google Scholar]
- Porat, S. , Weinberg‐Corem, N. , Tornovsky‐Babaey, S. , Schyr‐Ben‐Haroush, R. , Hija, A. , Stolovich‐Rain, M. , … Dor, Y. (2011). Control of pancreatic β cell regeneration by glucose metabolism. Cell Metabolism, 13(4), 440–449. 10.1016/j.cmet.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Puri, S. , Roy, N. , Russ, H. A. , Leonhardt, L. , French, E. K. , Roy, R. , … Hebrok, M. (2018). Replication confers β cell immaturity. Nature Communications, 9(1), 485 10.1038/s41467-018-02939-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier, J. , Guiot, Y. , Goebbels, R. M. , Sempoux, C. , & Henquin, J. C. (2008). Pancreatic β‐cell mass in European subjects with type 2 diabetes. Diabetes, Obesity and Metabolism, 10, 32–42. 10.1111/j.1463-1326.2008.00969.x [DOI] [PubMed] [Google Scholar]
- Razavi, R. , Najafabadi, H. S. , Abdullah, S. , Smukler, S. , Arntfield, M. , & van der Kooy, D. (2015). Diabetes enhances the proliferation of adult pancreatic multipotent progenitor cells and biases their differentiation to more β‐cell production. Diabetes, 64, 1311–1323. 10.2337/db14-0070 [DOI] [PubMed] [Google Scholar]
- Saisho, Y. , Butler, A. E. , Manesso, E. , Elashoff, D. , Rizza, R. A. , & Butler, P. C. (2013). β‐Cell mass and turnover in humans: Effects of obesity and aging. Diabetes Care, 36(1), 111–117. 10.2337/dc12-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba, H. , Mizukami, H. , Yagihashi, N. , Wada, R. , Hanyu, C. , & Yagihashi, S. (2002). Reduced beta‐cell mass and expression of oxidative stress‐related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia, 45(1), 85–96. 10.1007/s125-002-8248-z [DOI] [PubMed] [Google Scholar]
- Salinno, C. , Cota, P. , Bastidas‐Ponce, A. , Tarquis‐Medina, M. , Lickert, H. , & Bakhti, M. (2019). β‐cell maturation and identity in health and disease. International Journal of Molecular Sciences, 20(21), 5417– 10.3390/ijms20215417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen, A. J. (2003). Pathophysiology of type 2 diabetes. Acta Clinica Belgica, 58(6), 335–341. 10.1179/acb.2003.58.6.001 [DOI] [PubMed] [Google Scholar]
- Seino, S. , Shibasaki, T. , & Minami, K. (2011). Dynamics of insulin secretion and the clinical implications for obesity and diabetes. Journal of Clinical Investigation, 121(6), 2118–2125. 10.1172/JCI45680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour, P. A. , Bennett, W. R. , & Slack, J. M. W. (2004). Fission of pancreatic islets during postnatal growth of the mouse. Journal of Anatomy, 204(2), 103–116. 10.1111/j.1469-7580.2004.00265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa, J. , Fernandez, M. , Takatani, T. , El Ouaamari, A. , Jungtrakoon, P. , Okawa, E. R. , … Kulkarni, R. N. (2017). Insulin signaling regulates the FoxM1/PLK1/CENP‐a pathway to promote adaptive pancreatic β cell proliferation. Cell Metabolism, 25(4), 868–882.e5. 10.1016/j.cmet.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, E. K. , Hatanaka, M. , Morris, D. L. , Tersey, S. A. , Kono, T. , Chaudry, Z. Z. , … Evans‐Molina, C. (2013). Divergent compensatory responses to high‐fat diet between C57Bl6/J and c57BlKS/J inbred mouse strains. American Journal of Physiology‐Endocrinology and Metabolism, 305(12), E1495–E1511. 10.1152/ajpendo.00366.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smukler, S. R. , Arntfield, M. E. , Razavi, R. , Bikopoulos, G. , Karpowicz, P. , Seaberg, R. , … van der Kooy, D. (2011). The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell, 8, 281–293. 10.1016/j.stem.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Stumvoll, M. , Goldstein, B. J. , & Van Haeften, T. W. (2005). Type 2 diabetes: Principles of pathogenesis and therapy. The Lancet, 365(9467), 1333–1346. 10.1016/S0140-6736(05)61032-X [DOI] [PubMed] [Google Scholar]
- Surwit, R. S. , Kuhn, C. M. , Cochrane, C. , McCubbin, J. A. , & Feinglos, M. N. (1988). Diet‐induced type II diabetes in C57BL/6J mice. Diabetes, 37(9), 1163–1167. 10.2337/diab.37.9.1163 [DOI] [PubMed] [Google Scholar]
- Talchai, C. , Xuan, S. , Lin, H. V. , Sussel, L. , & Accili, D. (2012).Pancreatic β Cell Dedifferentiation as a Mechanism of Diabetic β Cell Failure. 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed]
- Teta, M. , Long, S. Y. , Wartschow, L. M. , Rankin, M. M. , & Kushner, J. A. (2005). Very slow turnover of β‐cells in aged adult mice. Diabetes, 54, 2557–2567. [DOI] [PubMed] [Google Scholar]
- van der Meulen, T. , Mawla, A. M. , DiGruccio, M. R. , Adams, M. W. , Nies, V. , Dólleman, S. , … Huising, M. O. (2017). Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metabolism, 25(4), 911–926.e6. 10.1016/j.cmet.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, G. C. , Bonner‐Weir, S. , & Leahy, J. L. (1990). Islet mass and function in diabetes and transplantation. Diabetes, 39, 401–405. 10.2337/diabetes.39.4.401 [DOI] [PubMed] [Google Scholar]
- Younis, A. , Younis, A. , Tzur, B. , Peled, Y. , Shlomo, N. , Goldenberg, I. , … Klempfner, R. (2016). Metabolic syndrome is independently associated with increased 20‐year mortality in patients with stable coronary artery disease. Cardiovascular Diabetology, 15, 149 10.1186/s12933-016-0466-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, F. , & Jiang, Y. (2019). Endogenous pancreatic β cell regeneration: A potential strategy for the recovery of β cell deficiency in diabetes. Frontiers in Endocrinology, 10, 101 10.3389/fendo.2019.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Final
Supplemental Figure 2 Final
Supplemental Figure 3 Final
Supplemental Table 1


