Background:
Long-acting (LA) injectable regimens are a potential therapeutic option in people living with HIV-1.
Setting:
ATLAS (NCT02951052) and FLAIR (NCT02938520) were 2 randomized, open-label, multicenter, multinational phase 3 studies.
Methods:
Adult participants with virologic suppression (plasma HIV-1 RNA <50 copies/mL) were randomized (1:1) to continue with their current antiretroviral regimen (CAR) or switch to the long-acting (LA) regimen of cabotegravir (CAB) and rilpivirine (RPV). In the LA arm, participants initially received oral CAB + RPV once-daily for 4 weeks to assess individual safety and tolerability, before starting monthly injectable therapy. The primary endpoint of this combined analysis was antiviral efficacy at week 48 (FDA Snapshot algorithm: noninferiority margin of 4% for HIV-1 RNA ≥50 copies/mL). Safety, tolerability, and confirmed virologic failure (2 consecutive plasma HIV-1 RNA ≥200 copies/mL) were secondary endpoints.
Results:
The pooled intention-to-treat exposed population included 591 participants in each arm [28% women (sex at birth), 19% aged ≥50 years]. Noninferiority criteria at week 48 were met for the primary (HIV-1 RNA ≥50 copies/mL) and key secondary (HIV-1 RNA <50 copies/mL) efficacy endpoints. Seven individuals in each arm (1.2%) developed confirmed virologic failure; 6/7 (LA) and 3/7 (CAR) had resistance-associated mutations. Most LA recipients (83%) experienced injection site reactions, which decreased in incidence over time. Injection site reactions led to the withdrawal of 6 (1%) participants. The serious adverse event rate was 4% in each arm.
Conclusion:
This combined analysis demonstrates monthly injections of CAB + RPV LA were noninferior to daily oral CAR for maintaining HIV-1 suppression.
Key Words: long-acting, antiretroviral therapy, injectable, cabotegravir, rilpivirine, HIV
INTRODUCTION
HIV remains a major global health concern, with the Joint United Nations Programme on HIV/AIDS and the World Health Organization (WHO) estimating the number of people living with HIV (PLWH) to be ∼37.9 million in 2018.1,2 Improvements in HIV treatment efficacy are reflected in the increases in longevity of PLWH.3 Despite this progress, the effectiveness of current antiretroviral therapy (ART) is challenged by the need to maintain high levels of adherence to sustain virologic suppression.4 Factors related to regimen complexity, such as dosing frequency and pill burden, food considerations, stigma, and drug–drug interactions between oral antiretrovirals and commonly prescribed non-ART medications, are reported to contribute to this challenge.4–6 Furthermore, the emotional burden of living with HIV may be compounded by daily oral pill taking and additional complexities.7 Therefore, there is considerable interest in developing long-acting (LA) treatments that can address many of these issues while simplifying ART for PLWH.8
The recommended ART regimen for PLWH generally consists of daily doses of either an oral 2-drug or 3-drug combination.9,10 This combination typically involves an integrase strand transfer inhibitor (INSTI) (dolutegravir as the sole INSTI preferred for use in adults in the WHO guidelines9 and either dolutegravir, bictegravir, or raltegravir preferred in the US Department of Health and Human Services guidelines10), in combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs); although, a single NRTI (lamivudine) in combination with dolutegravir has recently been recommended as a 2-drug regimen in HIV treatment guidelines.10
Cabotegravir (CAB) (GSK1265744) is an investigational INSTI and structural analog of dolutegravir.11 Rilpivirine (RPV) is a next-generation non-NRTI (NNRTI) currently approved as a once-daily oral tablet to be used in combination with other antiretrovirals for the treatment of HIV infection.12,13 Both compounds are formulated as LA agents to be administered intramuscularly (IM), with oral formulations of RPV and a new formulation of CAB in development.13 These oral formulations have been used during an oral lead-in phase in the ATLAS14 and FLAIR15 studies to assess safety and tolerability before study participants transitioning to LA therapy with CAB and RPV. This 2-drug oral combination therapy of CAB and RPV was assessed in the LATTE (NCT01641809) study and shown to provide similar antiviral activity compared with efavirenz plus dual NRTIs.16
A single IM injection of CAB LA 800 mg or RPV LA 1200 mg demonstrated sustained mean or geometric mean plasma concentrations above their respective in vitro protein-adjusted 90% inhibitory concentrations (PA-IC90) at 32 weeks postdose for CAB LA17 (PA-IC90 0.166 μg/mL), and 24 weeks postdose for RPV LA18 (PA-IC90 12 ng/mL), providing rationale to investigate the 2 drugs as an LA combination regimen. In the LATTE-2 (NCT02120352) randomized, open-label phase 2b clinical trial, the injectable LA combination of CAB and RPV (CAB + RPV LA) as a 2-drug HIV-1 maintenance therapy, administered every 4 or 8 weeks, was similar to daily 3-drug oral therapy of CAB plus abacavir/lamivudine in maintaining viral suppression (plasma HIV-1 RNA <50 copies per/mL; FDA Snapshot algorithm) through 96 weeks.19 Together, these results supported phase 3 investigation of CAB + RPV LA.
ATLAS (NCT02951052)14 and FLAIR (NCT02938520)15 are ongoing randomized, open-label, multinational phase 3 studies. These studies demonstrated that monthly injections of CAB + RPV LA were noninferior based on the primary endpoint (participants with plasma HIV-1 RNA ≥50 copies/mL at week 48) compared with a control group who continued their oral current antiretroviral regimen (CAR). The individual results of these studies are published elsewhere.14,15 Here, we present the preplanned pooled analyses of the efficacy (noninferiority), viral resistance, pharmacokinetic analysis, safety and tolerability, and preference findings observed in participants enrolled in the ATLAS and FLAIR studies.
METHODS
Study Design and Participants
ATLAS (NCT02951052) and FLAIR (NCT02938520) are randomized, multicenter, parallel-group, open-label phase 3 studies comparing CAB + RPV LA with CAR in ART-experienced PLWH-1 infection. Both studies comprised a screening phase, maintenance phase, and extension phase; participants in the FLAIR study initially received an induction antiretroviral regimen to achieve viral suppression before the maintenance phase (Fig. 1A). Details of the respective study designs are reported elsewhere.14,15
FIGURE 1.
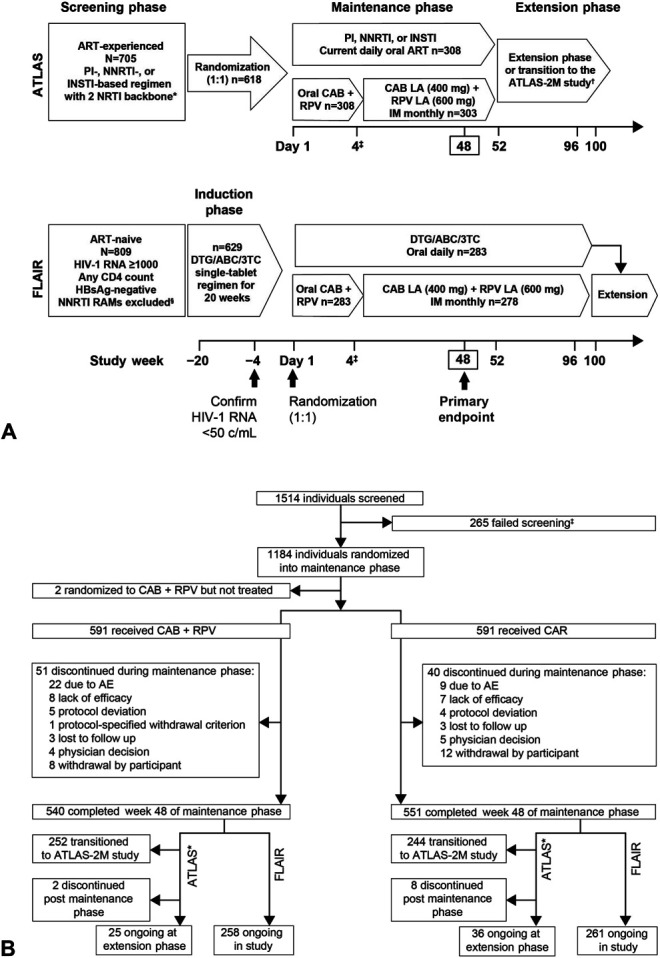
Study design and participant disposition. A, Study design. *Uninterrupted ART 6 months and VL <50 copies/mL at screening, 2 × VL <50 copies/mL ≤12 months (1 within the 6–12-month window, and 1 within 6 months prior to screening). †Optional switch to CAB + RPV LA at week 52 for those on CAR. ‡Participants received an initial loading dose of CAB LA (600 mg) and RPV LA (900 mg) at week 4. From week 8 onward, participants received CAB LA (400 mg) + RPV LA (600 mg) injections every 4 weeks. §The presence of any major INSTI or NNRTI resistance-associated mutation (except K103N) from prior genotype assay results were exclusionary. Figure adapted from Murray M, Antela A, Mills A, et al. Patient-Reported Outcomes in ATLAS and FLAIR Participants on Long-Acting Regimens of Cabotegravir and Rilpivirine Over 48 Weeks. AIDS Behav. 2020. https://doi.org/10.1007/s10461-020-02929-8, under a Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/. 3TC, lamivudine; ABC, abacavir; CAB, cabotegravir; CAR, current antiretroviral therapy; DTG, dolutegravir; IM, intramuscular; HBsAg, hepatitis B surface antigen; PI, protease inhibitor; RAM, resistance-associated mutation; RPV, rilpivirine; VL, viral load. B, Participant disposition. *Participants who completed study participation at week 52 had the option to continue by entering the extension phase, transitioning to the ATLAS-2M study, or leaving the study (no withdrawal visit needed). The latter was not captured in this figure. ‡The remaining 65 participants in FLAIR were withdrawn from the study prior to randomization into the maintenance phase. CAR, current antiretroviral therapy; CAB, cabotegravir; RPV, rilpivirine.
Enrolled study participants were ≥18 years old, or ≥19 years where required by local regulations. In the FLAIR study, all participants received an initial fixed-dose oral regimen of 600 mg abacavir, 50 mg dolutegravir, and 300 mg lamivudine once-daily for 20 weeks before randomization (non-abacavir–based backbone was administered for participants positive for HLA-B*5701, and an alternative backbone was chosen for those intolerant to or who developed toxicity to any component of the backbone regimen). Participants with suppressed plasma HIV-1 RNA (<50 copies/mL) in both studies were randomized (1:1) to receive either CAB + RPV LA monthly IM injections or to continue their daily CAR. Randomization was stratified by: sex at birth and baseline HIV-1 RNA (<100,000 or ≥100,000 copies/mL) for FLAIR; sex at birth and baseline regimen third-agent class (protease inhibitor, INSTI, or NNRTI) for ATLAS. Participants in the LA arm received oral CAB + RPV until week 4 or longer (oral lead-in) before switching to the IM regimen. Oral therapy (bridging) with CAB + RPV was available for participants who were unable to make a scheduled visit within the injection window.
Participants with confirmed virologic failure (CVF; 2 consecutive plasma HIV-1 RNA measurements ≥200 copies/mL) were discontinued from the study treatment. Participants who withdrew from LA therapy entered long-term follow-up (52 weeks). After completion of the maintenance phase of the study, participants had the option to continue/switch to the LA therapy for the duration of the extension phase, which is ongoing. ATLAS participants also had the option to rollover into the ongoing ATLAS-2M randomized study (NCT03299049), comparing CAB + RPV LA administered every 4 weeks vs every 8 weeks.20
ATLAS and FLAIR were conducted in accordance with the Declaration of Helsinki.21 All participants provided written informed consent. The study protocol, any amendments, the informed consent, and other information that required pre-approval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board.
Endpoints and Assessments of Pooled Analysis
The primary endpoint of this pooled analysis was the proportion of participants with plasma HIV-1 RNA levels ≥50 copies/mL at week 48 by FDA Snapshot algorithm. The key secondary endpoint was the proportion of participants with plasma HIV-1 RNA <50 copies/mL at week 48. Selected secondary and exploratory endpoints included virologic outcomes by randomization strata and baseline subgroups, resistance analysis of participants with CVF through week 48, pharmacokinetic analysis, safety and tolerability, laboratory abnormalities, and participant preference.
Statistical Methods and Populations for Analysis
The planned participant number of 285 participants per arm in each study was chosen to appropriately power the statistical analyses of the primary and key secondary noninferiority endpoints of the individual studies. The overall sample size provided sufficient power to perform the pooled analysis. With the combined sample size of 570 participants per treatment arm, the study would have 90% power to demonstrate noninferiority for the primary endpoint of the proportion of participants with virologic failure at week 48 by the Snapshot intention-to-treat exposed (ITT-E) approach using a prespecified 4% noninferiority margin. The ITT-E population consisted of randomized participants who received at least 1 dose of study drug during the maintenance phase of the study (on or after day 1 visit). Participants were analyzed according to the randomized treatment arm. The safety population consisted of all randomized participants who received at least 1 dose of study drug during the maintenance phase of the study (on or after day 1 visit).
The primary and key secondary efficacy analyses were based on the ITT-E population using the Snapshot algorithm. For the primary analysis, treatment with CAB + RPV LA was declared noninferior to CAR if the upper end limit of the 2-sided 95% confidence interval (CI) for the difference in proportions of participants with plasma HIV-1 RNA ≥50 copies/mL between the 2 groups [(CAB + RPV LA)—CAR] at week 48 was less than 4%. For the key secondary efficacy analysis, treatment with CAB + RPV LA was declared noninferior to CAR if the lower limit of the 2-sided 95% CI for the difference in virologic suppression rates (HIV-1 viral load <50 copies/mL) between the 2 groups [(CAB + RPV LA)—CAR] at week 48 was greater than −10% (prespecified).
RESULTS
Participants
A total of 1514 individuals were screened (ATLAS, n = 705; FLAIR, n = 809) and 1184 were randomized (2 participants were randomized but did not receive treatment: 1 for noncompliance with study treatment, 1 for noncompliance with protocol procedures). Participant disposition is shown in Figure 1B. The baseline characteristics of participants in the LA and CAR arms were similar (Table 1 and see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/B518). Overall, 591 participants in each arm initiated the maintenance phase (n = 308 from ATLAS and n = 283 from FLAIR). Most participants were of white race (LA, 73%; CAR, 69%), with a median (range) age of 38 years (LA, 19–74 years; CAR, 18–82 years). Women accounted for >25% of participants (LA, 27%; CAR, 28%).
TABLE 1.
Pooled Participant Baseline Characteristics: ITT-E Population
| LA (n = 591) | CAR (n = 591) | |
| Median age, yr (range) | 38 (19–74) | 38 (18–82) |
| Age ≥50 yr, n (%) | 99 (17) | 125 (21) |
| Women (sex at birth), n (%) | 162 (27) | 168 (28) |
| Race, n (%) | ||
| White | 430 (73) | 408 (69) |
| Black or African American | 109 (18) | 133 (23) |
| Other | 52 (9) | 50 (8)* |
| Mean weight (SD), kg† | 77 (15.30) | 77 (16.98) |
| Median BMI (range), kg/m2† | 25 (15–51) | 25 (13–58) |
| BMI ≥30, (%)† | 100 (17) | 103 (17) |
| Median CD4+ cell count (IQR), cells/mm3 | 645 (487–824) | 641 (480–821) |
| HIV-1–HCV co-infection, n (%)† | 42 (7) | 40 (7) |
Baseline for FLAIR was day 1 (maintenance phase).
Two participants' data are missing.
Collected at induction baseline (week −20) for FLAIR.
BMI, body mass index; CAR, current antiretroviral therapy; IQR, interquartile range; HCV, hepatitis C.
Efficacy
In the LA and CAR arms, 11 (2%) and 10 (2%) participants, respectively, had HIV-1 RNA ≥50 copies/mL at week 48, with an adjusted treatment difference [LA—CAR (95% CI)] of 0.16% (−1.35 to 1.67), meeting the noninferiority criteria of the primary endpoint (4% noninferiority margin). The secondary endpoint was also met, with 550 (93%) LA participants and 558 (94%) CAR participants having HIV-1 RNA <50 copies/mL at week 48, with an adjusted treatment difference [LA—CAR (95% CI)] of −1.37% (−4.12 to 1.39), meeting the prespecified −10% noninferiority margin (Snapshot) (Table 2).
TABLE 2.
Pooled Efficacy Outcomes at Week 48: ITT-E Population
| Outcome | LA, n = 591, n (%) | CAR‡, n = 591, n (%) | Difference, % (95% CI)§ | Adjusted Difference, % (95% CI)║ |
| HIV-1 RNA <50 copies/mL at week 48* (key secondary endpoint) | 550 (93.1) | 558 (94.4) | −1.35 (−4.11 to 1.41) | −1.37 (−4.12 to 1.39) |
| HIV-1 RNA ≥50 copies/mL at week 48* (primary endpoint) | 11 (1.9) | 10 (1.7) | 0.17 (−1.34 to 1.68) | 0.16 (−1.35 to 1.67) |
| Data in window not below threshold | 3 (0.5) | 3 (0.5) | ||
| Discontinued for lack of efficacy | 7 (1.2) | 5 (0.8) | ||
| Discontinued for other reason while not below threshold | 1 (0.2) | 2 (0.3) | ||
| Change in background therapy | 0 | 0 | ||
| No virologic data | 30 (5.1) | 23 (3.9) | ||
| Discontinued study because of AE or death† | 19 (3.2) | 7 (1.2) | ||
| Discontinued study for other reasons | 11 (1.9) | 16 (2.7) | ||
| On study but missing data in window | 0 | 0 |
Per FDA Snapshot algorithm; 4% and −10% noninferiority margins prespecified for primary and key secondary endpoints, respectively.
One death occurred in the CAR arm because of a methamphetamine overdose (ATLAS). This was determined to be unrelated to study treatment.
In ATLAS, CAR = standard 3-drug oral ART regimen; in FLAIR, CAR = ABC/DTG/3TC (a non-abacavir–based backbone was administered for participants positive for HLA-B*5701, and an alternative backbone was chosen for those intolerant to or who developed toxicity to any component of the backbone regimen).
Difference = proportion on LA minus proportion on CAR.
Based on Cochran–Mantel–Haenszel stratified analysis, adjusted for 10 strata.
3TC, lamivudine; ABC, abacavir; CAR, current antiretroviral therapy; DTG, dolutegravir.
In the LA arm, 98% of the 6920 expected injection visits across the 2 studies occurred within the allowed ±7 days of the planned visit day. No participant who received an injection outside of the allowable dosing window met CVF criteria. In total, oral bridging ranging 4–61 days was utilized in 16 participants to cover missed (n = 7) or delayed (n = 9) injection visits.
Efficacy results were generally consistent between subgroups (see Figures S2 and S3, Supplemental Digital Content, http://links.lww.com/QAI/B518). However, a slightly higher proportion of women (sex at birth) and participants with a body mass index ≥30 kg/m2 in the LA arm had HIV-1 RNA ≥50 copies/mL at week 48, with a difference in proportion (95% CI) of 2.49 (−0.64 to 6.54) and 3.06 (−2.54 to 9.72), respectively. Also, the LA arm was favored by the African American subgroup for the proportion of participants with HIV-1 RNA <50 copies/mL at week 48 [difference in proportion (95% CI) of 6.86 (0.12 to 13.90)].
Resistance Analysis
At week 48, 7 participants in each arm had CVF (2 consecutive plasma HIV-1 RNA measurements ≥200 copies/mL). This includes 1 participant in the LA arm of FLAIR who temporarily discontinued the oral lead-in dosing during a work-up for an apparent positive pregnancy test that was later confirmed to be a false positive result and, upon re-initiation, met the criteria for CVF; this participant, however, never received LA injections and was withdrawn from the study.
Of the 6 participants in the LA arm who received CAB + RPV LA, NNRTI (n = 6) and/or INSTI (n = 4) resistance mutations were present at the time of suspected virologic failure (Table 3). Four of these participants had HIV-1 subtype A1, 1 had subtype A, and 1 had subtype AG at baseline. Of the 6 participants, NNRTI (n = 2) resistance mutations and the INSTI L74I polymorphism (n = 5) were present at baseline; all retained full susceptibility to dolutegravir at suspected virologic failure timepoint (below clinical cut-off of 4.0-fold change). The participant who met CVF criteria during the oral lead-in phase had HIV-1 subtype AG with no resistance mutations detected.
TABLE 3.
Subtypes and Mutations in ATLAS and FLAIR*
| Study | Sex at Birth, Country, HIV-1 Subtype (Day 1/SVF) | Baseline RAMs† | Viral Load at SVF/CVF (Copies/mL) | SVF Timepoint RAMs | Drug Sensitivity at SVF (Fold Change)§ | ||
| NNRTI | INSTI‡ | NNRTI | INSTI‡ | ||||
| ATLAS | W, Russia, A1/A | E138E/A | None | 79,166/25,745 | E138A | None | RPV (2.4) CAB (0.8) DTG (0.9) |
| W, France, AG/AG | V108V/I, E138K | None | 695/258 | V108I, E138K | None | RPV (3.7) CAB (1.2) DTG (1.0) |
|
| M, Russia, A/A1 | None | None | 544/1841 | E138E/K | N155H | RPV (6.5) CAB (2.7) DTG (1.2) |
|
| FLAIR║ | W, Russia, A1/A1 | None | None | 373/456 | E138E/A/K/T | Q148R | RPV (7.1) CAB (5.2) DTG (1.0) |
| M, Russia, A1/A1¶ | None | None | 287/299 | K101E | G140R | RPV (2.6) CAB (6.>7) DTG (2.2) |
|
| W, Russia, A1/A | None | None | 488/440 | E138K | Q148R | RPV (1.0) CAB (9.4) DTG (1.1) |
|
In the CAR arm, there were 7 CVFs. In ATLAS, there were 4 CVFs in the CAR arm, 3 of whom had reverse transcriptase mutations (M184I; M184V + G190S; and M230M/I) detected in HIV-1 RNA samples from 1 participant each, and 1 had no mutations. In FLAIR, there were 3 CVFs in the CAR arm, all without treatment-emergent resistance mutations or phenotypic changes.
Baseline genotype sequences were derived from peripheral blood mononuclear cell HIV-1 DNA in ATLAS and plasma HIV-1 RNA in FLAIR.
L74I was present in 5 out of 6 participants at baseline and at SVF timepoint. It is not considered an INSTI RAM by IAS–USA guidelines and has no impact on CAB activity.28
Monogram biological cut-offs: RPV = 2.0, CAB = 2.5; Monogram clinical cut-off: DTG = 4.0.
FLAIR had 1 participant who had oral CAB + RPV dosing interrupted because of a false-positive pregnancy test and, upon re-initiation of oral therapy, had SVF that was confirmed.
INSTI genotype and INSTI phenotype could not be generated at SVF timepoint (week 28). Therefore, week 24 plasma was sent for virology analyses, shown here.
CAB, cabotegravir; CAR, current antiretroviral therapy; DTG, dolutegravir; RAM, resistance-associated mutation; RPV, rilpivirine; SVF, suspected virologic failure.
Pharmacokinetics
Mean plasma CAB and RPV concentrations were well above their respective PA-IC90 (CAB PA-IC90, 0.166 μg/mL; RPV PA-IC90, 12 ng/mL)19 during the maintenance phase. At week 8, 4 weeks after the initial IM loading dose (initial trough concentration), pooled geometric mean (95% CI) plasma concentrations were 1.38 μg/mL (1.31 to 1.46) for CAB and 39.88 ng/mL (38.05 to 41.80) for RPV, 8-fold and 3-fold above their respective PA-IC90. At week 48, geometric mean (95% CI) plasma concentrations were 2.97 μg/mL (2.85 to 3.10) for CAB and 86.42 ng/mL (82.87 to 90.14) for RPV, approximately 18-fold and 7-fold above their respective PA-IC90 values and similar to those reported in the LATTE-2 study.19 Individual plasma CAB and RPV concentrations in participants with CVF at the time of failure were below the population means but, for most participants, were within the overall range of exposures for individuals who maintained virologic suppression. CAB/RPV plasma concentrations of those with CVF are presented in the individual ATLAS and FLAIR supplementary sections.14,15
Safety and Tolerability
Adverse events (AEs) through week 48 are presented in Table 4. When excluding injection site reactions (ISRs), AEs were reported by 86% (n = 506) and 75% (n = 444) of participants in the LA and CAR arms, respectively. Most of these were grade 1 or 2, with 7% (n = 44) and 6% (n = 35) of participants in the LA and CAR arms, respectively, reporting an AE of grade ≥3. Drug-related AEs (excluding ISRs) occurred in 28% (n = 165) of participants in the LA arm compared with 6% (n = 35) in the CAR arm. AEs leading to withdrawal were reported for 22 (4%) participants in the LA arm and 9 (2%) participants in the CAR arm (specific reasons for withdrawal are listed in Table 4). Serious AEs were similar in both arms. Two serious AE occurrences were considered by investigators to be related to the study treatment: right knee mono-arthritis in the LA arm and suicidal ideation in the CAR arm. One death occurred during the maintenance phase due to a methamphetamine overdose (CAR arm). This was determined to be unrelated to study treatment. AEs during the oral lead-in period occurred in 32% (n = 187) of participants; the 3 most commonly occurring were headache [3% (n = 17)], nasopharyngitis, and vitamin D deficiency [both 3% (n = 16)]. Mean (SD) weight change at week 48 from baseline was an increase of 2.34 kg (5.67) and 1.17 kg (5.22) in the LA and CAR arms, respectively.
TABLE 4.
Pooled Adverse Events Through Week 48
| Event category, n (%) | LA (n = 591) | CAR (n = 591) |
| Any AE | 561 (95%) | 444 (75%) |
| Excluding ISRs | 506 (86%) | 444 (75%) |
| Any grade ≥3 AE* | 63 (11%) | 35 (6%) |
| Excluding ISRs | 44 (7%) | 35 (6%) |
| Any AEs leading to withdrawal† | 22 (4%) | 9 (2%) |
| Any SAE | 24 (4%) | 25 (4%) |
| SAEs related to study treatment (excluding ISRs)‡ | 1 (<1%) | 1 (<1%) |
| Any drug-related AE | 490 (83%) | 35 (6%) |
| Excluding ISRs | 165 (28%) | 35 (6%) |
| Any grade ≥3 drug-related AE* | 28 (5%) | 1 (<1%) |
| Excluding ISRs | 8 (1%) | 1 (<1%) |
| Any injection site pain§ | 458 (79%) | N/A |
| Grade ≥3 severity§ | 21 (4%) | N/A |
| Leading to withdrawal§ | 6 (1%) | N/A |
| Common AEs (≥5% in either arm) excluding ISRs | ||
| Nasopharyngitis | 108 (18%) | 88 (15%) |
| Headache | 71 (12%) | 38 (6%) |
| Upper respiratory tract infection | 66 (11%) | 52 (9%) |
| Diarrhea | 52 (9%) | 38 (6%) |
| Pyrexia | 43 (7%) | 13 (2%) |
| Back pain | 40 (7%) | 23 (4%) |
| Influenza | 41 (7%) | 34 (6%) |
| Vitamin D deficiency | 31 (5%) | 24 (4%) |
| Nausea | 30 (5%) | 16 (3%) |
| AEs of special interest | ||
| Anxiety | 27 (5%) | 20 (3%) |
| Depression | 16 (3%) | 14 (2%) |
| Suicidal ideation/behavior | 4 (<1%) | 5 (<1%) |
There was only 1 (<1%) participant with a grade 5 AE (death) in the CAR arm during the maintenance phase, which was due to a methamphetamine overdose that was determined to be unrelated to study treatment.
AEs leading to withdrawal in >1 participant in the LA arm were injection site pain (n = 6); hepatitis (hepatitis A, n = 4; acute hepatitis B and C, n = 3 and n = 1, respectively); headache (n = 2); and diarrhea (n = 2). No single AE leading to withdrawal was reported in >1 participant in the CAR arm.
Serious AEs related to study treatment: LA arm, right knee mono-arthritis; CAR arm, suicidal ideation.
Percentages based on number of participants who received at least 1 injection (n = 581).
CAR, current antiretroviral therapy; SAE, serious AE.
Seventeen (3%) participants in the LA arm and 6 (1%) participants in the CAR arm had alanine aminotransferase elevations ≥3 times the upper limit of normal. Eleven (2%) and 3 (<1%) participants in the LA and CAR arms, respectively, met protocol-defined liver-related stopping criteria. Of these, 7 were diagnosed with hepatitis A, 3 with hepatitis B, 2 with hepatitis C, and 1 with hepatitis E; none were considered to represent drug-induced liver injury. Other laboratory evaluations were unremarkable.
Throughout the course of the maintenance phase, 14,682 injections were given to 581 participants. A total of 3663 ISRs were reported, accounting for 25% of all injections administered across the study period, <1% (n = 34) of which were grade ≥3 (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/B518). No grade 4 or 5 ISRs were reported. Most ISRs (88%) resolved within 7 days (median 3 days). Injection site pain was the most common event, reported with 21% (n = 3087) of injections, 1% of which (n = 32) were grade 3. Nodule, induration, and swelling were other reported ISRs, occurring in 140 (<1%), 136 (<1%), and 86 (<1%) of administered injections. Six (1%) participants discontinued treatment because of ISRs, comprising injection site pain in all cases (Table 4) either alone or with injection site nodule (n = 1) and/or injection site swelling (n = 1). The incidence of ISRs was highest at week 4, with ISRs experienced by 70% of participants. The incidence decreased over the course of the 48 weeks, occurring in 16% of participants at Week 48 (see Figure S4, Supplemental Digital Content, http://links.lww.com/QAI/B518).
Rates of AEs in the LA arm were similar between men and women (sex at birth), with any AEs and drug-related AEs reported in 91% (n = 148) and 78% (n = 126) of women, respectively, compared with 96% (n = 413) and 85% (n = 365) in men, respectively. Of those who received at least 1 injection, ISRs were reported in 81% (n = 128/159) of women and 86% (n = 361/422) of men. Discontinuation because of ISRs was 1% (n = 2) in women and <1% (n = 4) in men.
Preference
At weeks 24 and 44, there was a significant increase from baseline in treatment satisfaction of injectable therapy in participants in the LA arm compared with the CAR arm (see Figure S5, Supplemental Digital Content, http://links.lww.com/QAI/B518). Of the participants who responded to the preference survey, 98% (523/532) preferred the LA regimen.
DISCUSSION
The pooled results of the ATLAS and FLAIR studies described here demonstrate that monthly IM injections of CAB + RPV LA are noninferior to CAR in maintaining HIV-1 virologic suppression in treatment-experienced participants. The results of the primary and secondary endpoints were generally similar between subgroups. The findings are consistent with the individual results of the ATLAS and FLAIR studies,14,15 with this analysis gaining increased power because of the pooling of the data. The high proportion of participants with virologic success (HIV-1 RNA <50 copies/mL) with LA therapy at week 48 (93%) in this analysis is similar to previous phase 3 noninferiority switch studies such as SWORD-1 and -2.22
The number of participants with CVF in the LA arm was low, occurring in 7 (1%) participants through week 48. This proportion is comparable to other large switch studies, which also reported incidences of ≤1%.22,23 Excluding the 1 participant who discontinued oral lead-in dosing because of a false-positive pregnancy test, all of these participants had NNRTI (n = 6) and/or INSTI (n = 4) resistance-associated mutations at time of suspected virologic failure. All except 1 (ATLAS, subtype AG) of these participants were subtype A1 or A. All but the ATLAS participant with subtype AG had the polymorphism L74I. However, subsequent in vitro investigations of viruses from those participants with CVF in the FLAIR study compared the observed INSTI resistance-associated mutations with or without the L74I polymorphism, and concluded that subtype A1 or B did not differ with respect to sensitivity to CAB.24 Despite the presence of INSTI resistance-associated mutations, all participants retained full susceptibility to dolutegravir and 5/6 were subsequently resuppressed on alternative ART (HIV-1 RNA <50 copies/mL).
Because of the small sample size of participants with CVF, no definitive conclusions can be made on factors that could have contributed to CVF. No single factor alone (eg, sex at birth, body mass index, region, race, age, or prior NNRTI use) seemed to drive Snapshot or CVF (Table 2; see Figure S2, Supplemental Digital Content, http://links.lww.com/QAI/B518; data on file). The roles of certain patient and viral factors, such as pharmacokinetics, HIV-1 subtype, and L74I polymorphism, are being evaluated to further define their contributions toward virologic failure development.
Overall, excluding ISRs, the observed AE profile with CAB + RPV LA therapy was similar to that in the CAR arm and is in line with the previously reported safety profile of CAB + RPV oral therapy.16 Despite ISRs being common (25% of administered injections), most were mild (99% grade 1 or 2). Overall incidence of ISRs steadily decreased throughout the study (week 4, 70%; week 48, 16%) and the duration of ISRs was short (median 3 days). These data suggest the initial high incidence of ISRs may have been because of initiation of a novel treatment administration route (IM injection), with a reduced incidence over the longer term as participants became more familiar with the injection procedure.
Both studies enrolled a substantial number of women (sex at birth) [ITT-E populations: ATLAS, 33% (n = 203); FLAIR, 22% (n = 127)], exceeding that targeted for inclusion in the individual studies (25% in ATLAS, 20% in FLAIR). This is an important achievement given that women are often under-represented in ART clinical trials,25 despite accounting for the majority of adults living with HIV globally (WHO estimates, 2018).1,2 In this analysis, efficacy was comparable between men and women.
In the era of highly active ART, developing therapies that cause minimal disruption to the daily lives of PLWH is a key goal of current research efforts. Along with reducing exposure to the number of ART agents compared with traditional 3-drug regimens, CAB + RPV LA reduces the dosing frequency from daily to monthly. Some populations of PLWH may therefore find this a preferable option to their daily CAR, potentially facilitating improved adherence in those who report factors such as pill burden and forgetfulness as barriers to adherence.4 The Positive Perspectives study involving over 1000 PLWH found that most regarded daily ART as a “reminder of their HIV status,” whereas 25% felt “being tied to daily medication limited their day to day life.”7 Monthly CAB + RPV LA injections may therefore represent a more discreet regimen that could help some PLWH avoid the stigma associated with their HIV status. CAB + RPV LA would also enable the first option for directly observed therapy because of the monthly clinic visits required. This would result in more frequent interactions with health care professionals, which could potentially improve retention in care. Therefore, CAB + RPV LA may be a welcome treatment option for such populations of PLWH who are virally suppressed and see monthly injections as a beneficial trade-off for freedom from daily ART; however, the impact of monthly visits on HIV clinics will need to be considered.
Further to CAB + RPV LA being a welcomed treatment option compared with daily ART, nearly all of the participants who completed the preference question preferred the LA regimen over the oral regimen (98%). This is consistent with findings from the LATTE-2 study, in which participants also reported a preference for CAB + RPV LA therapy compared with daily oral ART.26
Although the reduced dosing frequency of CAB + RPV LA may be beneficial to some PLWH, this needs to be specifically assessed in different populations, particularly those with adherence challenges. LATITUDE (NCT03635788), a study investigating CAB + RPV LA in PLWH with a history of suboptimal adherence, will provide further clarity in this regard.27 In addition, the present analysis is limited by the relatively short duration of treatment (48 weeks). Longer-term data will be provided by the 96-week results. In addition, there was likely a selection bias with the ATLAS and FLAIR studies, whereby only PLWH who were interested in an LA IM injectable regimen were likely to enroll. However, CAB + RPV LA is intended to add a novel therapy option to the treatment armamentarium for those willing to receive injections who may benefit from reduced dosing frequency.
In summary, the once-monthly regimen of CAB + RPV LA was noninferior to CAR in maintaining HIV-1 suppression in individuals with a prior treatment history of 20 weeks to many years of suppressive ART. These efficacy and safety results indicate CAB + RPV LA can be a potential therapeutic approach for virologically suppressed PLWH-1.
ACKNOWLEDGMENTS
The authors thank everyone who has contributed to the success of the studies: all study participants and their families, and the ATLAS and FLAIR clinical investigators and their staff. ATLAS and FLAIR are funded by ViiV Healthcare and Janssen. Professional medical writing and editorial assistance was provided by Daniel Williams, MSc, at SciMentum (Nucleus Global), and funded by ViiV Healthcare.
Footnotes
Supported by ViiV Healthcare and Janssen. The funders participated in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. All authors vouch for the accuracy and completeness of the data, data analyses and interpretation, and fidelity to the protocol, and approved the final manuscript for submission.
Some parts of the data have been presented at: the 10th International AIDS Conference; July 21–24, 2019; Mexico City, Mexico. IDWeek; October 2–6, 2019; Washington, DC. European AIDS Conference; November 6–9, 2019; Basel, Switzerland.
G.R. has received grants and personal fees from MSD, Gilead, and ViiV Healthcare; grants from Janssen, outside of the submitted work. E.T.O. has received research support to institution during the conduct of this study, and has served as a consultant for ViiV, Merck, Theratechnologies, and Gilead outside of the submitted work. C.O. reports grants, personal fees, and non-financial support from ViiV Healthcare during the conduct of the study, and grants, personal fees, non-financial support, and other from Gilead, MSD, Janssen, ViiV, and GlaxoSmithKline outside of the submitted work. S.S. reports grants to her institution from ViiV Healthcare during the conduct of this study. K.A. reports grants from ViiV Healthcare and GlaxoSmithKline outside of the submitted work. M.G.H.-M. reports personal fees from ViiV Healthcare, during the conduct of the study; grants and personal fees from ViiV Healthcare, personal fees from Gilead, personal fees from Janssen, outside the submitted work. V.P. has received personal fees from GlaxoSmithKline, Gilead, MSD, and ViiV Healthcare and non-financial support from GlaxoSmithKline outside of the submitted work. S.O. reports grants from MSD and ViiV Healthcare; honorarium for lectures from MSD, ViiV Healthcare, Gilead, Torii Pharmaceutical, and Janssen; non-financial support from Japan Tobacco/Torii Pharmaceutical, outside of the submitted work. G.J.R. has received grants from Gilead, ViiV Healthcare, TaiMed outside of the submitted work. A.B. has received personal fees (honoraria) from AbbVie, Gilead, Janssen, Sanofi, MSD, and ViiV Healthcare outside of the submitted work. M.M. has received consulting fees for advisory boards from ViiV Healthcare, Janssen and Gilead, and travel grants from ViiV Healthcare and Janssen, outside of the submitted work. S.G., C.H., K.H., M.S.C., C.T., P.P., A.C., R.D., J.M., K.S., W.S., and D.M. are employed by ViiV Healthcare. V.V.E., S.V., R.V.S.-R., and H.C. are employed by Janssen. S.F., S.W., K.C., J.R., A.W., and N.W. are employed by GlaxoSmithKline. K.S., D.M., J.M., W.S., C.T., S.F., A.C., S.G., K.H., P.P., and R.D. are stockholders of GlaxoSmithKline. M.S.C. reports company stock options at GlaxoSmithKline. C.H. is a shareholder of GlaxoSmithKline. S.V. and H.C. are shareholders of Johnson & Johnson. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS Statistics—2019 Fact Sheet. 2019. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed October 31, 2019. [Google Scholar]
- 2.World Health Organization. Summary of the Global HIV Epidemic. 2018. Available at: https://www.who.int/gho/hiv/en/. Accessed October 31, 2019. [Google Scholar]
- 3.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachega JB, Hsu AJ, Uthman OA, et al. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012;26(suppl 1):S39–S53. [DOI] [PubMed] [Google Scholar]
- 6.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Los Rios P, Young B, Marcotullio S, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the Positive Perspectives Study. Open Forum Infect Dis. 2019;6(suppl 2):S481. [Google Scholar]
- 8.Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine. 2013;8:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. Accessed October 31, 2019.
- 10.U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2020. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/virologic-failure. Accessed February 25, 2020. [Google Scholar]
- 11.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIV/AIDS. 2019;11:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen CJ, Molina JM, Cahn P, et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr. 2012;60:33–42. [DOI] [PubMed] [Google Scholar]
- 13.Janssen Therapeutics. Edurant (Rilpivirine) Prescribing Information. 2019. Available at: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/EDURANT-pi.pdf. Accessed March 17, 2020.
- 14.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 15.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 16.Margolis DA, Brinson CC, Smith GHR, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145–1155. [DOI] [PubMed] [Google Scholar]
- 17.Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67:481–486. [DOI] [PubMed] [Google Scholar]
- 18.McGowan I, Dezzutti CS, Siegel A, et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV. 2016;3:e569–e578. [DOI] [PubMed] [Google Scholar]
- 19.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 20.ClinicalTrialsgov. Efficacy, Safety and Tolerability Study of Long-Acting Cabotegravir Plus Long-Acting Rilpivirine (CAB LA + RPV LA) in Humanimmunodeficiency Virus-1 (HIV-1) Infected Adults. 2019. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03299049. Accessed October 31, 2019.
- 21.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 22.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. [DOI] [PubMed] [Google Scholar]
- 23.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose two-drug regimen versus continuing a tenofovir alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults with HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis. 2020:doi: 10.1093/cid/ciz1243 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffrey J, St Clair M, Wang P, et al. HIV A1 or B Do Not Differentially Impact Cabotegravir in Vitro Potency or Durability [Abstract 532]. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 8–11, 2020; Boston, MA.
- 25.Curno MJ, Rossi S, Hodges-Mameletzis I, et al. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71:181–188. [DOI] [PubMed] [Google Scholar]
- 26.Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One. 2018;13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov. The LATITUDE Study: Long-Acting Therapy to Improve Treatment Success in Daily Life (NCT03635788). 2019. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03635788. Accessed March 18, 2020.


