Supplemental Digital Content is Available in the Text.
Key Words: HIV drug resistance, clustering, men having sex with men, female sex workers
Background:
In low HIV prevalence settings, understanding the transmission dynamics and the impact of drug resistance is critical to curb down the epidemic. This study aims to explore the prevalence and dynamics of transmission of HIV drug-resistance mutations (DRMs) among key populations in Haiti.
Settings:
Eligible participants (naive, treated) were selected from 7 key population friendly health care centers in Port-au-Prince, Haiti, from September 2018 to July 2019.
Methods:
A total of 119 HIV-1 pol sequences were analyzed from men having sex with men (MSM), female sex workers (FSWs), and their sexual partners. Screening for HIV DRMs was performed using the Stanford University Drug Resistance Database. Phylogenetic and network analyses using HIV-TRACE software were performed to infer putative relationships and shared DRMs.
Results:
Of the 119 participants, 62.2% were men (74/119), and 75.7% of them (56/74) reported MSM as a main risk factor. The overall DRM prevalence was 58.8% (70/119). A DRM was observed in 37.5% of MSM (21/56), 82.2% of FSWs (37/45), and 66.7% (12/18) among FSWs' clients. In a multivariate model, age and FSWs were significant predictors for DRMs (P = 0.001). Transmission network analysis found 24 of the 119 (20.2%) genetically linked individuals forming 8 clusters. Clustering participants were mostly MSM (15/24; 62.5%). Five clusters (62.5%) had shared DRMs, and K103N and M184V were the main shared mutations.
Conclusions:
High prevalence of HIV DRMs was observed among MSM, FSWs, and their clients in Port-au-Prince, Haiti. Network analysis revealed frequent DRM transmission among genetically linked individuals, highlighting the need for appropriate interventions to limit HIV transmission in these high-risk populations.
INTRODUCTION
In low HIV prevalence settings, identifying hotspots of HIV transmission and understanding the burden of drug resistance are critical to curb the epidemic.1,2 Although the overall HIV prevalence in Haiti has been stable around 2% for the past 15 years, the prevalence in high-risk groups, such as men having sex with men (MSM) and female sex workers (FSWs), is much higher, 12.9% and 8.7%, respectively.3,4 Unprotected transactional and commercial sexual activities among MSM and FSWs have been reported as main drivers of new HIV infections in Haiti.5 With major support from the United States President's Emergency Plan For AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria, more than 91,000 individuals were receiving antiretroviral therapy (ART) as of March 2019; these patients on ART represent approximately 80% of people with HIV (PWH), who know their status.5 As the country continue to scale-up ART with the goal to achieve the UNAIDS 95-95-95 target, HIV drug resistance might pose a growing threat toward epidemic control. HIV drug-resistance mutations (DRMs) can emerge in HIV-infected individuals with suboptimal adherence to ART and in individuals who become HIV infected while taking preexposure prophylaxis (PrEP).6–8 In the face of a global rise in emerging HIV drug resistance, the World Health Organization recommended the use of integrase strand transfer inhibitor such as dolutegravir (DTG)-based therapy as the preferred first-line because of moderate-quality evidence that showed a generally more effective regimen, higher viral suppression and CD4 cell recovery rates, and lower risk of treatment discontinuation.9–11 The Haitian National AIDS Control Program launched in November 2018 the use of DTG-based therapy as the preferred first-line following the 2016 World Health Organization guidelines on ART.9,12 As of September 2019, 75.4% of the PWH were either placed or transitioned on DTG.12 The National AIDS Control Program also launched the PrEP in 2019, as part of HIV prevention services offered to key populations and other high-risk groups in Haiti.12 These prevention services face several challenges: the key populations are hard to reach because they are highly stigmatized, are often victims of violence, and the dynamics of HIV transmission in these groups is not well understood.13 Understanding the dynamics of HIV transmission in these high-risk populations and identifying the drivers of the epidemic are key to streamline prevention and treatment efforts. Phylogenetic analysis of viral sequences, combined with epidemiological, demographic, and behavioral data has provided a valuable method of elucidating transmission dynamics in high-risk populations.14,15 Limited data are available on HIV DRM prevalence and HIV transmission dynamics in key populations in Haiti.
This study aims to explore the overall prevalence of HIV DRMs and to identify transmission networks and factors associated with DRMs among key populations in Port-au-Prince, Haiti.
METHODS
Study Population
Enrolled individuals included PWH who were newly diagnosed or on ART for 1 year or less. All eligible participants were tested for HIV viral loads, and we sequenced samples from those who had viral loads at least 250 copiesper milliliter at study entry. Demographic, clinical, and behavioral data were collected at the screening visit. A total of 119 HIV-1 pol sequences were sampled and analyzed from MSM, FSWs, and their sexual partners, enrolled in 7 key population friendly health care centers in Port-au-Prince, Haiti, from September 2018 to July 2019.
Laboratory Testing
HIV viral load was performed using the RealTime HIV-1 Viral Load Assay (Abbott Molecular, Abbott Park, IL; lower limit of quantification of 40 copies per milliliter). To identify individuals who were likely to be recently infected at the time of sample collection, we performed the HIV-1 Limiting Antigen Avidity EIA (LAg) (Lag-Avidity assay; SEDIA Biosciences Corporation, Portland, OR and Maxim Biomedical, Bethesda, MD), according to their respective product inserts.16,17 Bulk sequencing of the partial HIV-1 pol coding region was performed (Viroseq v.2.0; Celera Diagnostics, Alameda, CA).
DRMs and Genetic Network Analysis
Genotypic analysis was performed to detect DRMs in the HIV-1 pol gene fragment encoding protease and reverse transcriptase (RT), as previously described.18 Screening for major DRMs was performed according to the Stanford University Genotypic Resistance Interpretation (http://hivdb.stanford.edu). We assessed potential confounding effects of convergent evolution for drug resistances by repeating our analysis after we have excluded 48 codon positions in protease and RT, which are associated with drug resistance.19,20 Phylogenetic and genetic network analyses were performed to infer putative transmission links and shared DRMs. We used HIV-TRACE software (HIV TRAnsmission Cluster Engine: www.hivtrace.org) to infer transmission links between sequences.21 We performed a sensitivity analysis across genetic distance thresholds, varying from 0.5% to 5%, to determine the most accurate threshold to detect recent transmission clusters. Putative transmission links (ie, edges) were inferred when 2 sequences (ie, nodes) had a Tamura-Nei 9322 genetic distance of ≤1.5%. We defined a shared DRM as any DRM present in 2 or more genetically linked individuals (≤1.5% genetic distance). All nucleotide ambiguities were resolved, and only sequences with less than 1.5% diversity were retained. Multiple linkages were then combined into putative clusters.
Statistical Analysis
Descriptive statistics were generated for demographic, clinical, and behavioral parameters comparing participants with and without DRMs. The prevalence of DRM in the different risk groups was estimated. Unadjusted and adjusted logistic regressions were used to describe the association between behavioral, clinical, and demographic factors with the odds of harboring any DRM. Only variables tested in the univariable analysis with P value <0.05 were included in the multivariable model. Characteristics between clustering and nonclustering participants in the transmission network were compared using the Fisher exact or χ2 tests and calculating odds ratios (ORs) for categorical variables. Values of P < 0.05 were considered statistically significant. SPSS version 26.0 (SPSS Inc., Chicago, IL) was used for analyses.
Ethical Considerations
The Haiti National Bioethics Committee (Ref: 1718-46) and the Institutional Review Board of the University of California San Diego (Project# 180456) approved the protocol for this project. Data were fully anonymized before they were extracted for analysis. All participants signed an informed consent form.
RESULTS
Samples Included in the Resistance Study
This study included analysis of samples from 267 HIV-infected individuals screened for participation. Among participants, 130 (48.7%) had a viral load of at least 250 copies per milliliter at study entry, and among them 117 had a viral load higher or equal to 1000 copies per milliliter. HIV genotyping was performed, and protease/RT genotyping results were obtained for 119 (91.5%) of the samples; 11 failed genotyping.
Demographic, Clinical, and Behavioral Characteristics
Demographic, clinical, and risk factor characteristics of the sampled population are displayed in Table 1. The majority of participants were men (74/119; 62.2%), and 75.7% of them (56/74) reported MSM as main risk factor. Almost all participants were already on ART at the time of enrollment (115/119; 96.6%). About 75% of FSWs (33/44, 1 missing), 56.6% of MSM (30/53, 3 missing), and 61% of FSWs' clients (11/18) have been on ART for more than 6 months, and 77.4% (89/115) were on Tenofovir-Disoproxil Fumarate (TDF)-Lamivudine (3TC)-Efavirenz (EFV) and 22.6% (26/115) were on TDF-3TC-DTG.
TABLE 1.
Baseline Demographic, Risk, and Clinical Characteristics of the Participants Comparing the Presence of or Not of DRMs
| Characteristics at Time of Sampling | DRM | |||
| Present (n = 70) | Absent (n = 49) | |||
| n (%) | 95% CI | n (%) | 95% CI | |
| Risk | ||||
| MSM | 21 (17.6) | 11.6 to 25.2 | 35 (29.4) | 21.8 to 38.0 |
| FSW | 37 (31.1) | 23.3 to 39.8 | 8 (6.7) | 3.2 to 12.3 |
| FSWs' clients | 12 (10.1) | 5.6 to 16.4 | 6 (5.0) | 2.1 to 10.1 |
| Age, yrs | ||||
| ≤24 | 8 (6.7) | 3.2 to 12.3 | 24 (20.2) | 13.7 to 28.0 |
| 25–29 | 14 (11.8) | 6.9 to 18.5 | 17 (14.3) | 8.9 to 21.4 |
| 30–34 | 18 (15.1) | 9.6 to 22.4 | 4 (3.4) | 1.1 to 7.8 |
| >35 | 30 (25.2) | 18.1 to 33.5 | 4 (3.4) | 1.1 to 7.8 |
| Gender | ||||
| Male | 33 (27.7) | 20.3 to 36.2 | 41 (34.5) | 26.4 to 43.3 |
| Female | 37 (31.1) | 23.3 to 39.8 | 8 (6.7) | 3.2 to 12.3 |
| Education | ||||
| Never attended/some primary school | 27 (22.7) | 15.9 to 30.8 | 9 (7.6) | 3.8 to 13.3 |
| Secondary school or higher | 41 (34.5) | 26.4 to 43.3 | 40 (33.6) | 25.6 to 42.4 |
| Marital status | ||||
| Single | 44 (37.0) | 28.7 to 45.9 | 33 (27.7) | 20.3 to 36.2 |
| Others (married or living with partner) | 23 (19.3) | 13.0 to 27.1 | 16 (13.4) | 8.2 to 20.4 |
| No. of sex partners past 6 mo | ||||
| 5 or less | 45 (37.8) | 29.5 to 46.7 | 38 (31.9) | 24.1 to 40.7 |
| More than 5 | 23 (19.3) | 13.0 to 27.1 | 11 (9.2) | 5.0 to 15.4 |
| ART regimen | ||||
| TDF-3TC-EFV | 56 (47.1) | 38.3 to 56 | 33 (27.7) | 20.3 to 36.2 |
| TDF-3TC-DTG | 11 (9.2) | 5.0 to 15.4 | 15 (12.6) | 7.6 to 19.4 |
| Time on ART | ||||
| 6 mo or less | 20 (17.4) | 11.3 to 25.1 | 21 (18.3) | 12.0 to 26.1 |
| More than 6 mo | 47 (40.9) | 32.2 to 50.0 | 27 (23.5) | 16.5 to 31.8 |
| Viral load | ||||
| ≤1000 copies/mL | 7 (5.9) | 2.7 to 11.2 | 6 (5.0) | 2.1 to 10.1 |
| >1000 copies/mL | 63 (54.3) | 44 to 61.7 | 43 (36.1) | 27.9 to 45.0 |
| Recently infected | ||||
| Yes | 1 (0.8) | 0.1 to 3.9 | 4 (3.4) | 1.1 to 7.8 |
| No | 69 (58.0) | 49.0 to 66.6 | 45 (37.8) | 29.5 to 46.7 |
| Cluster | ||||
| Yes | 15 (12.6) | 7.6 to 19.4 | 9 (7.6) | 3.8 to 13.3 |
| No | 55 (46.2) | 37.4 to 55.2 | 40 (33.6) | 25.6 to 42.4 |
Values in bold in the same row are significantly different at P < 0.05 in the 2-sided test of equality for column proportions. Cells with no subscript are not included in the test. Tests assume equal variances. Tests are adjusted for all pairwise comparisons within a row of each innermost subtable using the Bonferroni correction.
Analysis of HIV Drug Resistance
Determination of genetic linkage was robust to the inclusion or exclusion of sites associated with drug resistance. The percentage of participants who harbored at least 1 DRM was 58.8% [70/119; 95% confidence interval (CI): 49.4% to 67.8%]. At least 1 DRM was observed in 37.5% of MSM (21/56, 95% CI: 24.9% to 51.5%), 82.2% of FSWs (37/45, 95% CI: 67.9% to 92.0%), and 66.7% among FSWs' clients (12/18, 95% CI: 41.0%–86.7%) (Table 1). The most common DRMs identified were associated with nonnucleoside reverse transcriptase inhibitors (NNRTIs) resistance at 58.0% (69/119; 95% CI: 48.6% to 67.0%), followed by NRTI at 33.6% (40/119; 95% CI: 25.2% to 42.8%) and protease inhibitors (PIs) resistance at 4.2% (5/119; 95% CI: 1.4% to 9.5%). Multiple class DRM were found in 32.8% of participants (39/119; 95% CI: 24.4 to 42.0). Of the DRM, K103N (46/119; 38.6%), which causes high-level resistance to efavirenz and nevirapine, and M184V (35/119; 29.4%), associated with TDF and Emtricitabine (FTC) resistance, were the most frequently observed (Fig. 1). We also analyzed cases of possible transmitted drug resistance. About 67% of participants (80/119) reported been infected within 0–3 months of sample collection. Nevertheless, the results of the LAg-Avidity assay revealed 114 individuals with long-term infections and only 5 recently infected individuals. Resistance mutations were detected in 1 of the 5 individuals classified as recently infected (NNRTI: K103N; K101E).
FIGURE 1.
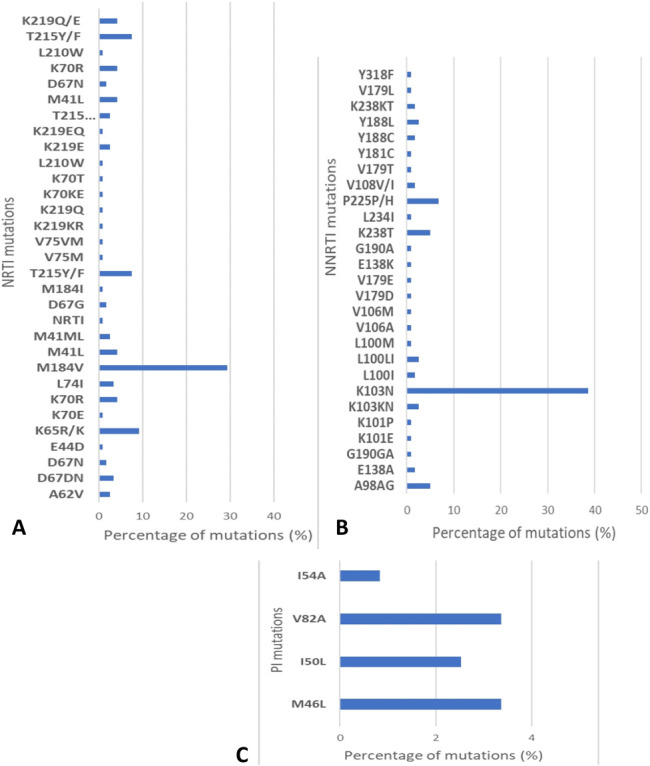
Proportion of resistance mutations in sequences. A, Proportion of NRTI mutations. B, Proportion of NNRTI mutations. C, Proportions of PI mutations.
Factors Associated With HIV Drug Resistance
We next evaluated the association of drug resistance with behavioral, demographic, and clinical factors (Table 2). In univariable analyses, older age (30–34 and ≥35 years) was significantly associated with any HIV DRM (OR: 4.85, 95% CI: 1.89 to 12.44 and OR: 5.53, 95% CI: 2.20 to 13.90, respectively). FSWs and participants who never attended schools or had some primary education had an increased odd of DRM (OR: 3.43, 95% CI: 1.78 to 6.60 and OR: 2.16, 95% CI: 1.12 to 4.18, respectively). In multivariable analyses, older age (30–34 and ≥35 years) and being a FSW remained significant predictors for HIV DRM [adjusted OR (aOR): 9.53, 95% CI: 2.21 to 41.06; aOR: 19.88, 95% CI: 4.68 to 84.42, and aOR: 7.13, 95% CI: 2.39 to 21.26, respectively].
TABLE 2.
Factors Associated With HIV Drug Resistance
| Characteristics at Time of Sampling | Total | DRM | Univariable Analysis | Multivariable Analysis | |||||
| Present | Absent | OR | 95% CI | P | aOR | 95% CI | P | ||
| Age, yrs | |||||||||
| 18–24 | 32 | 8 | 24 | — | — | ||||
| 25–29 | 31 | 14 | 17 | 1.54 | 0.95 to 2.48 | 0.12 | 2.12 | 0.64 to 7.02 | 0.22 |
| 30–34 | 22 | 18 | 4 | 4.85 | 1.89 to 12.44 | <0.001 | 9.53 | 2.21 to 41.06 | 0.002 |
| 35 and older | 34 | 30 | 4 | 5.53 | 2.20 to 13.90 | <0.001 | 19.88 | 4.68 to 84.42 | <0.001 |
| Gender | |||||||||
| Male | 74 | 33 | 41 | — | |||||
| Female | 45 | 37 | 8 | 3.24 | 1.65 to 6.33 | <0.001 | |||
| Risk | |||||||||
| MSM | 56 | 21 | 35 | — | — | ||||
| FSW | 45 | 37 | 8 | 3.43 | 1.78 to 6.60 | <0.001 | 7.13 | 2.39 to 21.26 | <0.001 |
| FSWs' clients | 18 | 12 | 6 | 2.48 | 1.04 to 5.91 | 0.055 | 1.55 | 0.53 | |
| Marital status | |||||||||
| Single | 77 | 44 | 33 | — | |||||
| Others (married or living with partner) | 39 | 23 | 16 | 1.05 | 0.62 to 1.77 | 1.00 | |||
| No. of sex partners in the past 6 mo | |||||||||
| 5 or less | 83 | 45 | 38 | — | |||||
| More than 5 | 34 | 23 | 11 | 1.51 | 0.81 to 2.79 | 0.22 | |||
| Education | |||||||||
| Never attended/some primary school | 36 | 27 | 9 | 2.16 | 1.12 to 4.18 | 0.015 | 2.47 | 0.82 to 7.43 | 0.11 |
| Secondary school or higher | 81 | 41 | 40 | — | — | ||||
| ART regimen | |||||||||
| TDF-3TC-EFV | 89 | 56 | 33 | 1.22 | 0.98 to 1.51 | 0.07 | |||
| TDF-3TC-DTG | 26 | 11 | 15 | — | |||||
| Time on ART | |||||||||
| ≤6 mo | 41 | 20 | 21 | — | |||||
| >6 mo | 74 | 47 | 27 | 1.25 | 0.93 to 1.67 | 0.17 | |||
| Viral load | |||||||||
| ≤1000 copies/mL | 13 | 7 | 6 | — | |||||
| >1000 copies/mL | 106 | 63 | 43 | 1.14 | 0.61 to 2.14 | ||||
| Recently infected | |||||||||
| Yes | 5 | 1 | 4 | — | |||||
| No | 114 | 69 | 45 | 2.03 | 1.24 to 3.32 | 0.16 | |||
| Cluster | |||||||||
| Yes | 24 | 15 | 9 | 1.17 | 0.56 to 2.45 | 0.82 | |||
| No | 95 | 55 | 40 | — | |||||
Reference category denoted by “—”.
OR and aOR values in bold type indicate significance of <0.05.
Cluster Analyses
Sensitivity analysis across genetic distance thresholds varying from 0.5% to 5% showed that the 1.5% threshold was accurate in detecting the most recent transmission clusters (see Fig. S1, Supplemental Digital Content, http://links.lww.com/QAI/B522). However, a 3% threshold allowed the detection of 5 additional clusters that are more likely reflective of more historical genetic linkage (see Fig. S2, Supplemental Digital Content, http://links.lww.com/QAI/B522). Transmission network analysis, using a 1.5% genetic distance threshold, found 24 of the 119 (20.2%) genetically linked individuals forming 8 clusters (size 2–6 individuals). Clustering participants were significantly more likely to be men (87.5%; χ2 = 8.19, P = 0.004), reporting MSM behavior as their main risk factor (62.5%; χ2 = 8.56, P = 0.01) (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/B522). Five clusters had shared resistance mutations, and K103N (14/24) and M184V (12/24) were the main DRM shared (Fig. 2). Of all clustering individuals, 62.5% contained at least 1 DRM. The frequency of DRMs did not differ between clustering and nonclustering individuals [62.5% (15/24) versus 56.8% (54/95); P = 0.65]. Among participants with DRMs, men were significantly more likely to cluster within the network than women (OR: 2.1, 95% CI: 1.38 to 3.20); and MSM were significantly more likely to cluster within the network than FSWs (OR: 2.63, 95% CI: 1.46 to 4.73) (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/B522). Only one of the sequences from the 5 individuals identified as recently infected clustered with other study sequences.
FIGURE 2.
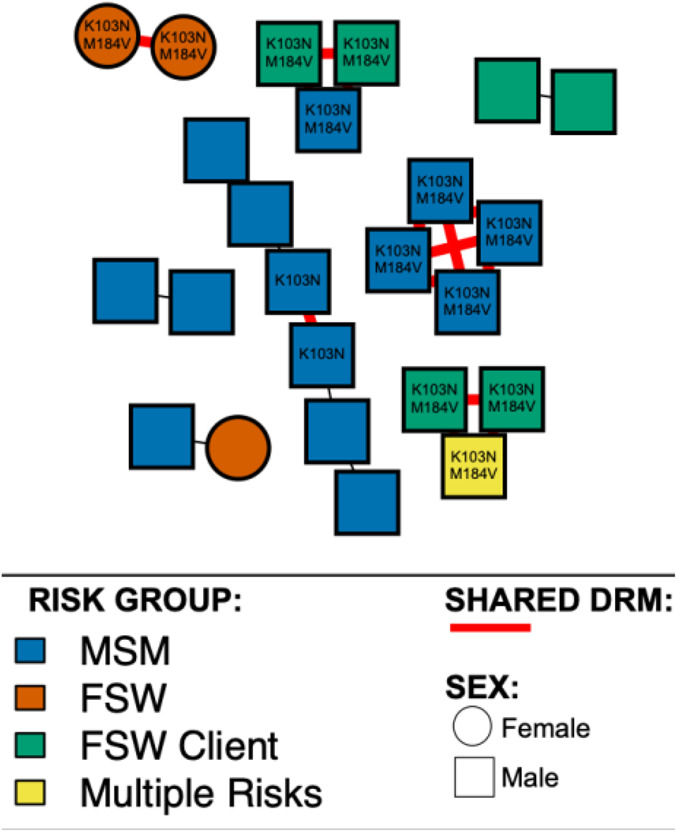
HIV drug resistance transmission among genetically linked individuals. Individuals (nodes) are shaped as square (men) and circle (women). Nodes are colored according to risk factors: blue for MSM, orange for FSWs, green for FSWs' clients, and yellow for multiple risks (MSM and FSWs' client). Shared DRMs are showed in red edges. All edges represent a genetic distance of ≤1.5% separating nodes.
DISCUSSION
To the best of our knowledge, this is the first study to date looking at HIV-1 transmission network and drug resistance in key populations in Haiti. This study showed a very high prevalence of HIV DRMs of approximately 58.8% in key populations in Port-au-Prince, Haiti. In 2008, Charles et al23 raised the alarm on high levels of HIV DRMs (86%; 26/29) in a small cohort of Haitian adolescents and young adults on antiretrovirals. We previously showed high rates of resistance to NRTIs and NNRTIs among newly HIV-diagnosed children in Haiti, suggesting a high vertical HIV DRM transmission rate, which have led the National HIV Program to revise the pediatric guidelines to include PIs in first-line regimens for all HIV-positive newborns.24 Nevertheless, the dynamics of transmission of HIV and the risk factors associated with DRMs are different in key populations. Reports from the Latin America and Caribbean region showed low to high levels of transmitted HIV drug resistance in key populations (9.0 among MSM and 10.3 among FSWs in Salvador, 3.7% among MSM in Peru, 12% among MSM in Chile, and 28% among MSM in Jamaica).25–28 In this study, women had a significantly higher risk for DRMs than men in general, and FSWs were more likely to harbor DRMs compared with MSM. Unreported data from FSWs in Haiti show that they are less likely to adhere to ART because of reported side effects that might interfere with their sex work, as also reported by others.29,30 In addition, FSWs had more previous treatment exposure (75% on ART for more than 6 months vs 57% for MSM). This very high burden of HIV drug resistance in a population with a high background prevalence of HIV is worrisome and requires routine monitoring of HIV drug resistance as well as targeted prevention and treatment interventions. We also found older age (30–34 and ≥35 years) to be significantly associated with HIV DRMs in our study; this is due to the fact that 46.4% of the study population who were older than 29 years were FSWs, and this subgroup presented a 92% DRM rate. The prevalence of DRM to NNRTI was higher than DRM to NRTI and PI in this cohort, which is consistent with other studies.23,27,31 The overall NNRTI DRM prevalence was 58%, and this high rate of resistance justified the decision of the National AIDS Control program to switch from NNRTI-based first-line regimens to DTG. Resistance to TDF/FTC was also observed, which could impact use of PrEP in key populations in Haiti.
Findings from this study suggest that some participants may have been infected with drug-resistant HIV. We detected DRMs in 1 of 5 individuals who were classified as recently infected using the LAg-Avidity assay that reflects a case of transmitted drug resistance. Time on ART was not associated with drug resistance. This suggests that the mutations identified may be the result of poor adherence to ART regimens or treatment interruptions (acquired drug resistance); unfortunately, adherence is often self-reported and poorly documented at these settings, which makes the data difficult to analyze.
In this study, 20% of participants were involved in transmission clusters. More than 87% of transmission clusters identified in this study consisted exclusively of men, and 71% of them are younger than 35 years. Young men, specially MSM, represent a particular target for the Haitian National AIDS Control program because of the higher prevalence of HIV in this subgroup of the population.3,5 Because sampling was not population based, more linkages between MSM were observed because they tend to enroll in studies with their sex partners. FSWs rarely enroll with their specific male clients, and in fact, it is challenging to reach these men due to the stigma and the illegal status of prostitution in Haiti. It is worth noting the absence of links between FSWs and FSWs' clients in our study. Because of the low sample size and the limited sampling density in this study, additional unsampled individuals could have been intermediary members of the transmission chain linking the 2 linked individuals.32–34 Unobserved intermediaries or misrepresentation of risk potentially related to stigma that prevents men from self-identifying as MSM might explain the presence of a triad of MSM and FSWs' client, as also reported by others.33 There are several limitations to our findings; the most important of which is sampling bias. The participants were selected from 7 key population friendly health facilities in the metropolitan area of Port-au-Prince; thus, our study population might not be representative of the overall local key populations. More comprehensive sample from other regions in Haiti and higher sampling density would likely have resulted in more putatively identified transmission links and a higher overall clustering rate, and thus, a better picture of the transmission network throughout the country.
CONCLUSIONS
The high prevalence of HIV drug resistance was observed among key populations in Port-au-Prince, Haiti. Network analysis revealed frequent DRM transmission among genetically linked individuals. These findings provide a framework for prioritization and allocation of treatment and prevention resources. These results can be used to design tailored HIV prevention and intervention strategies to key populations in Haiti.
ACKNOWLEDGMENTS
The authors thank the Haitian National Public Health Laboratory for helping with specimen collection, transport, and storage for this study.
Footnotes
Supported by a USAID grant through EQUIP-MatCH. Grant Number: AID-OAA-A-15-00070 and through NIH Grant Research (San Diego Center for AIDS Research) and the funders had no role in study design, data collection and analysis, and decision to publish.
Presented in part at CROI 2020; Boston, MA; March 11, 2020. https://www.iasusa.org/wp-content/uploads/2020/04/28-1-3.pdf.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Poon AF, Gustafson R, Daly P, et al. Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: an implementation case study. Lancet HIV. 2016;3:e231–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta SR, Wertheim JO, Brouwer KC, et al. HIV transmission networks in the San Diego–Tijuana border region. EBioMedicine. 2015;2:1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institut Haïtien de l'Enfance (IHE) et ICF. 2018. Enquête Mortalité, Morbidité et Utilisation des Services (EMMUS-VI 2016-2017) Pétion-Ville, Haïti, et Rockville, Maryland, USA : IHE et ICF. Available at https://www.dhsprogram.com/pubs/pdf/FR326/FR326.pdf. Accessed April 2020. [Google Scholar]
- 4.Ministère de la Sante Publique et de la Population Programme National de Lutte Contre le VIH/Sida. Rapport REDES 2014 et 2015 Estimation du flux des ressources et dépenses liées au VIH/sida. 2016. Available at : https://www.unaids.org/sites/default/files/media/documents/Haiti_NASA_2016.pdf. Accessed April 2020. [Google Scholar]
- 5.U.S. Department of State PEPFAR Country and Regional Operational Plans. Haiti Country Operational Plan (COP) 2019 Strategic Direction Summary June 11, 2019. Available at: https://www.state.gov/wp-content/uploads/2019/09/Haiti_COP19-Strategic-Directional-Summary_public.pdf. Accessed March 2020. [Google Scholar]
- 6.Clutter DS, Jordan MR, Bertagnolio S, et al. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogel JM, Sivay MV, Cummings V, et al. HIV drug resistance in a cohort of HIV-infected MSM in the United States. AIDS. 2020;34:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 10.Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. [DOI] [PubMed] [Google Scholar]
- 12.Ministère de la Sante Publique et de la Population Programme National de Lutte contre les IST/VIHSIDA. Bulletin de Surveillance Épidémiologique VIH/Sida no 20. 2019. Accessed March 2020. [Google Scholar]
- 13.FHI 360 LINKAGES. Programmatic Mapping and Size Estimation of Key Populations in Haiti. 2017. Available at: https://www.fhi360.org/sites/default/files/media/documents/resource-linkages-haiti-size-key-populations-april-2017.pdf. Accessed April 2020. [Google Scholar]
- 14.Grabowski MK, Redd AD. Molecular tools for studying HIV transmission in sexual networks. Curr Opin HIV AIDS. 2014;9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis F, Hughes GJ, Rambaut A, et al. Episodic sexual transmission of HIV revealed by molecular phylodynamics. PLoS Med. 2008;5:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxim Biomedical. Maxim HIV-1 Limiting Antigen Avidity EIA: Single Well Avidity Enzyme Immunoassay for Detection of Recent HIV-1 Infection, Cat. No. 92001. 2013. Available at: http://www.maximbio.com/img/insert/920013-Product-Insert.pdf. Accessed April 2020 [Google Scholar]
- 17.Sedia Biosciences Corporation. Sedia HIV-1 LAg-Avidity EIA: Single Well Avidity Enzyme Immunoassay for Detection of Recent HIV-1 Infection, Cat. No. 1002. 2016. Available at: http://www.sediabio.com/LiteratureRetrieve.aspx?ID=134692. Accessed April 2020 [Google Scholar]
- 18.Little SJ, Pond SLK, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9:e98443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler WH, Ziebell RA, Zabina H, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, US–2006. AIDS. 2010;24:1203–1212. [DOI] [PubMed] [Google Scholar]
- 20.Wertheim JO, Kosakovsky Pond SL. Purifying selection can obscure the ancient age of viral lineages. Mol Biol Evol. 2011;28:3355–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosakovsky Pond SL, Weaver S, Leigh Brown AJ, et al. HIV-TRACE (TRAnsmission Cluster Engine): a tool for large scale molecular epidemiology of HIV-1 and other rapidly evolving pathogens. Mol Biol Evol. 2018;35:1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. [DOI] [PubMed] [Google Scholar]
- 23.Charles M, Noel F, Leger P, et al. Survival, plasma HIV-1 RNA concentrations and drug resistance in HIV-1-infected Haitian adolescents and young adults on antiretrovirals. Bull World Health Organ. 2008;86:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis FJ, Segaren N, Desinor O, et al. High levels of HIV-1 drug resistance in children who acquired HIV infection through mother to child transmission in the era of option B+, Haiti, 2013 to 2014. Pediatr Infect Dis J. 2019;38:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acevedo W, Gallardo AM, Galaz J, et al. Detection of primary antiretroviral resistance in Chilean patients recently infected with human immunodeficiency virus (HIV). Rev Med Chil. 2007;135:1406–1413. [PubMed] [Google Scholar]
- 26.Lama JR, Sanchez J, Suarez L, et al. Linking HIV and antiretroviral drug resistance surveillance in Peru: a model for a third-generation HIV sentinel surveillance. J Acquir Immune Defic Syndr. 2006;42:501–505. [DOI] [PubMed] [Google Scholar]
- 27.Murillo W, Lorenzana de Rivera I, Albert J, et al. Prevalence of transmitted HIV-1 drug resistance among female sex workers and men who have sex with men in El Salvador, Central America. J Med Virol. 2012;84:1514–1521. [DOI] [PubMed] [Google Scholar]
- 28.Collins-Fairclough AM, Dennis AM, Nelson JA, et al. HIV drug resistance surveillance among Jamaican men who have sex with men should be prioritized for reducing HIV transmission. AIDS Res Hum Retroviruses. 2015;31:841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg SM, Montaner J, Duff P, et al. Structural barriers to antiretroviral therapy among sex workers living with HIV: findings of a longitudinal study in Vancouver, Canada. AIDS Behav. 2016;20:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okoronkwo I, Okeke U, Chinweuba A, et al. Nonadherence factors and sociodemographic characteristics of HIV-infected adults receiving antiretroviral therapy in Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. ISRN AIDS. 2013;2013:743794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stecher M, Chaillon A, Eis-Hubinger AM, et al. Pretreatment human immunodeficiency virus type 1 (HIV-1) drug resistance in transmission clusters of the Cologne-Bonn region, Germany. Clin Microbiol Infect. 2019;25:253.e1–253.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta SR, Wertheim JO, Delport W, et al. Using phylogeography to characterize the origins of the HIV-1 subtype F epidemic in Romania. Infect Genet Evol. 2011;11:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stecher M, Chaillon A, Eberle J, et al. Molecular epidemiology of the HIV epidemic in three German metropolitan regions–Cologne/Bonn, Munich and Hannover, 1999–2016. Sci Rep. 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novitsky V, Moyo S, Lei Q, et al. Impact of sampling density on the extent of HIV clustering. AIDS Res Hum Retroviruses. 2014;30:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]


