Abstract
Background
Attention deficit/hyperactivity disorder (ADHD), conduct disorder (CD), and sensation seeking (SS) have been consistently related to a higher risk of substance use (SU) and substance use disorder (SUD).
Objectives
To investigate the relationship between ADHD and prevalence rates in males at age 20 and age 25, the initiation of SU and SUD after age 20, and the escalation of SU from age 20 to age 25, and to explore the role of CD and SS in the relation of ADHD with SU and SUD initiation and escalation.
Method
Data were obtained as part of the Cohort Study on Substance Use Risk Factors (C-SURF), which focused on young Swiss men aged 20 years at baseline and 25 years at follow-up.
Results
Participants who screened positive for ADHD at baseline exhibited a higher rate of SU and SUD than participants who screened negative. The presence of ADHD symptoms at age 20 predicted initiation of all SU between age 20 and age 25, except for alcohol and smoking. After controlling for self-reported CD and SS, ADHD still predicted this late initiation of use of hallucinogens, meth-/amphetamines, and ecstasy/MDMA; non-medical use of ADHD medication and sedatives, and alcohol use disorder (AUD). No escalation of weekly drinking and smoking or annual cannabis use was observed from age 20 to age 25.
Conclusion
Screened-positive ADHD is an independent predictor of late SU and AUD, along with self-reported CD and SS. From a public health perspective, identifying ADHD is not only important in childhood and adolescence but also in early adulthood to guide specific interventions to lower risks of drug use initiation and the development of AUD in early adulthood.
Keywords: Attention deficit/hyperactivity disorder, Conduct disorder, Sensation seeking, Substance use, Substance use disorders, Longitudinal study, Early adulthood, Young men
Introduction
Various risk factors for substance use (SU) and substance use disorder (SUD) have been identified, including attention deficit/hyperactivity disorder (ADHD) and conduct disorder (CD) [1, 2]. Personality traits such as sensation seeking (SS) [3] also play an important role in initiation of SU and escalation into SUD. However, the association between these factors and SU has been explored primarily in cross-sectional studies; prospective studies on the course of SU are scarce.
ADHD, which involves inattention, hyperactivity, and impulsivity [4], is a common childhood disorder with an estimated worldwide prevalence of 3.4% in children and adolescents [5]. In approximately half to two-thirds of cases, childhood ADHD persists into adulthood [6, 7]. Both childhood and persistent ADHD are associated with a higher prevalence and a more severe and chronic course of SUD in adolescence and adulthood [7, 8, 9, 10, 11], indicating that ADHD contributes to earlier onset [12, 13] and longer duration [13, 14] of SUD. Biederman et al. [15] and Molina et al. [12] have reported a more rapid progression within 4 years from substance abuse to dependence among male adolescents with ADHD compared to those without ADHD. Further studies suggest that when individuals with ADHD develop SUD in adolescence, its prevalence remains stable until early adulthood [16, 17, 18, 19, 20]. Finally, it has been suggested that if individuals with ADHD do not develop SUD prior to early adulthood, their risk later in life is not elevated [19, 21]. However, only few studies have prospectively investigated the course of ADHD and SU in early adulthood, and therefore, it is not known whether ADHD is associated with (late) initiation or (adult) escalation of SU after age 20. Molina and Pelham [22] observed an association between ADHD persistence and higher rates of daily cigarette smoking, repetitive drunkenness, and alcohol-related problems in adolescents diagnosed with childhood ADHD. These adolescents were also 3 times more likely to have used inhalants, hallucinogens, cocaine, and non-prescribed stimulant medication. Sibley et al. [23] showed that individuals diagnosed with ADHD in early childhood who began to use tobacco and cannabis in early adolescence were 4–5 times more likely than people without ADHD to escalate to heavy use by the age of 18. These findings indicate that individuals with ADHD show comparatively earlier initiation and/or greater escalation in the use of certain substances between late adolescence and early adulthood than individuals without ADHD.
In general population and clinical samples, ADHD and CD were found to co-occur in 30–50% of cases, likely as a result of shared genetic and common environmental influences [24, 25]. Some studies found that the association between ADHD and SUD is partly mediated by comorbid CD [23, 26, 27]. However, most studies show additive effects of ADHD and CD on SU outcomes, suggesting that ADHD and CD likely contribute independently to a higher risk of SUD; furthermore, in some cases, ADHD could be a risk factor for development of CD [14, 17, 20, 22, 28, 29, 30]. Therefore, studies that explore associations between ADHD and the course of SU must control for CD.
Unlike ADHD and CD, SS is not a disorder but a personality trait. SS individuals willingly take physical, social, legal, and financial risks in pursuit of varied, novel, complex, and intense sensations and experiences [31]. Two recent longitudinal studies on predictors of SU initiation in adolescence and early adulthood have shown that SS contributes significantly to the onset of alcohol, cannabis, or other drug use [32, 33].
Literature that specifically explores the relationship between ADHD, CD, SS, and the course of SU is scarce. To our knowledge, only one cross-sectional study has explored these 3 factors as predictors of stimulant use (i.e., caffeine, cocaine, amphetamines, or non-prescribed ADHD medication) in a large sample of college students between 18 and 25 years of age [34]. ADHD, CD, and the Disinhibition subscale of the Brief Sensation Seeking Scale (BSSS) [35] were found to be related to non-prescribed use of ADHD medication; only CD was associated with illegal drug use.
The present prospective study has the following aims: (1) to compare prevalence rates of SU and SUD in men screened positive for ADHD (ADHD S+) with those in men screened negative for ADHD (ADHD S−) at baseline (age 20) and at 5-year follow-up (age 25); (2) to identify late initiation of SU and late development of SUD, that is, between age 20 and age 25; and (3) to examine the course/escalation of SU among all participants in a large sample of Swiss men in their early 20s, that is, from age 20 to age 25. This extends a previous study by Vogel et al. [30], which used the same dataset, by adding SS to ADHD and CD as a predictor and prolonging the observation period from 15 months to 5 years. Based on the results of Vogel et al., we expected that between age 20 and age 25, non-using ADHD S+ men will show increased late initiation of cannabis and other drug use (particularly stimulants, including all types of amphetamines, non-prescribed ADHD medication, and cocaine), but not late initiation of alcohol use or smoking compared to ADHD S− men. We also expected that ADHD S+ at age 20 would independently predict late SU initiation, particularly late initiation of stimulant use, even after controlling for the possible effects of CD and SS on this kind of late SU initiation. Finally, we expected that there would be no escalation in alcohol, tobacco, or cannabis use between age 20 and age 25 in the ADHD S+ nor in the ADHD S− group, because both the ADHD S+ and ADHD S− groups would most likely have already developed problematic use of these substances in late adolescence, that is, before age 20.
Materials and Methods
Participants and Procedures
This study analysed data from the longitudinal Cohort Study on Substance Use Risk Factors (C-SURF) [36], which examined SU patterns in young Swiss men. Participants were recruited from 3 of Switzerland's 6 army recruitment centres. In Switzerland, army recruitment is mandatory for men nearing 19 years of age, leading to a quasi-census of all young Swiss men. There were no preselection criteria (e.g., affluence or education), as in college student samples. In total, 15,066 Swiss men attended army recruitment in the 3 target centres between August 2010 and November 2011. Participants were assured of confidentiality and were assessed outside the military environment. Those who provided written informed consent received the baseline questionnaire at their home address approximately 2 weeks later, which has to be completed either online or on paper. They were sent the follow-up questionnaire approximately 5 years after completing the baseline questionnaire.
Among all participants who were informed of the study during army recruitment, 7,556 (50.1% of the eligible population) consented to participate. Of these, 5,987 (79.2%) filled out the baseline questionnaire, and 4,923 of these participated at the 5-year follow-up (82.2%). Participants with missing data on ADHD (n = 12) and nicotine dependence (n = 309) were excluded from the analysis. The final sample included 4,602 participants (76.9%). Two published reports showed that differences between respondents, non-respondents, and non-consenters at baseline were small and there were some different SU trends [37, 38]. The study protocol (Protocol No. 15/07) was approved by the Lausanne University Medical School Clinical Research Ethics Committee.
Measures
Sociodemographic Characteristics, ADHD, CD, and SS
All assessment instruments can be found on the C-SURF website [36]. ADHD, CD, SS, and sociodemographic characteristics, such as marital status, education, employment, and native language, were assessed at baseline.
Current ADHD was assessed using the Adult ADHD Self-Report Scale (ASRS-v1.1), a short screening tool developed by the World Health Organisation for use in the general population [39]. The ASRS includes 6 questions concerning the frequency of recent ADHD symptoms, based on the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, fourth Edition (DSM-IV) [40]. For each question, the respondents rate how often a symptom occurred over the preceding 12 months on a 5-point scale from 0 (never) to 4 (very often). Total scores range from 0 to 24. As proposed by Kessler et al. [41], a score of 14 was defined as the cut-off point for ADHD S+ versus ADHD S−. In a sample of 218 participants from the USA, Kessler et al. [41] found an estimated sensitivity of 64.9% and an estimated specificity of 93.9% using this cut-off value.
CD in early adolescence was assessed retrospectively using the self-report version of the Mini International Neuropsychiatric Interview Plus, which measures 6 behaviours exhibited before age 15 (e.g., destruction of property) [42] as part of the diagnostic criteria for antisocial personality disorder. In early adolescence, if two or more of the dichotomous items (no = 0; yes = 1) receive a response of “yes,” the participants were considered screened positive for CD.
SS was measured using the BSSS, an 8-item self-report scale derived from form V of the Sensation Seeking Scale (SSS-V) [35]. Its 4 subscales include “Thrill and Adventure,” “Experience Seeking,” “Disinhibition,” and “Boredom Susceptibility.” Participants rate all items on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). Only the full scale mean was used in the analyses. Hoyle et al. [35] identified acceptable internal consistency (α = 0.76), similar to that of this study (α = 0.81).
Substance Use
SU was measured at baseline and follow-up with a recall period of 12 months. Weekly alcohol use was assessed by asking participants whether they had consumed any type of alcohol in the preceding 12 months, the number of days per week they consumed alcohol, and the number of standard drinks (i.e., 10–12 g pure alcohol) they consumed on a typical day of drinking. “Drinking alcohol” was defined as having consumed at least one standard alcoholic drink within the previous 12 months. “Binge drinking” was defined as consuming six or more standard drinks on one occasion at least monthly [43]. Alcohol use disorder (AUD) was assessed by a self-report questionnaire [44] based on the diagnostic criteria of the DSM-5 [4], which included 12 dichotomous items (yes/no) adapted from the Semi-Structured Assessment for the Genetics of Alcoholism that asked about problems related to alcohol use over the past 12 months [45, 46]. Participants who answered “yes” to 4 or more of the questions were considered to have an AUD.
Weekly tobacco use was assessed by asking participants whether they had smoked in the preceding 12 months, the number of days per week they smoked, and the number of cigarettes they smoked on a typical smoking day. “Smoking” was defined as having smoked more than 50 cigarettes, 15 cigars, or 10 pipes within the previous 12 months. Nicotine dependence was assessed using the Fagerström Test (FTND) [47]; participants with a total score of 4 or higher were considered to have a nicotine dependence.
Annual cannabis use was assessed by asking participants whether they had used cannabis in the preceding 12 months, the frequency of cannabis use, and the number of hours spent under the influence of cannabis on typical days of use. “Cannabis use” was defined as having consumed cannabis at least once within the previous 12 months. Cannabis use disorder (CUD) was identified using the Cannabis Use Disorder Identification Test (CUDIT) [48]. Participants with a total score of 8 or higher were considered to have a CUD.
Use of any amount of any other illicit drug within the preceding 12 months was measured by one question (yes or no). Illicit drugs included hallucinogens/psychedelics (e.g., mushrooms, psilocybin, peyote, LSD, phencyclidine [“PCP/angel dust”], 2C-B, and 2C-I), meth-/amphetamines (“speed”), MDMA/ecstasy, heroin, and cocaine/crack/freebase.
Non-medical use of prescription drugs was assessed by asking participants whether they had taken ADHD medication (e.g., methylphenidate [e.g., Ritalin®] or amphetamine sulphate [Adderall®]) or sedatives (e.g., hypnotics or tranquilisers) without a prescription or for any reason other than those for which the substance is prescribed by a physician within the preceding 12 months.
Statistical Analyses
Statistical analyses were performed using IBM SPSS Version 26. Sociodemographic characteristics, CD, SS, SU outcomes at baseline, and SU outcomes at 5-year follow-up were compared between the ADHD S+ and ADHD S− groups using Pearson's χ2 tests with continuity correction where appropriate (for dichotomous variables) and independent t tests (for continuous variables). Partial correlation analyses were conducted to assess associations between ADHD, CD, and SS (ra1, ra2, and ra3; see Fig. 1 for the analysis structure), and bootstrap tests were conducted to obtain confidence intervals (CIs) for the correlation coefficients (bias corrected and accelerated bootstrap CI; BCa CI).
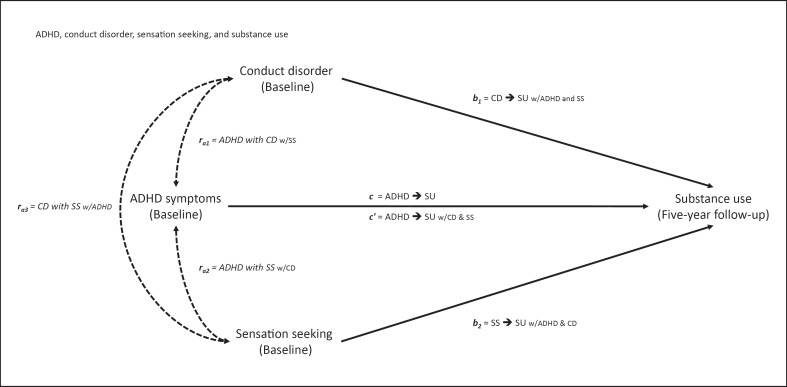
Fig. 1.
Theoretical model of attention deficit/hyperactivity disorder (ADHD) as a predictor of substance use (SU), controlling for conduct disorder (CD) and sensation seeking (SS). Thick lines indicate the direct paths c from ADHD at baseline to SU at 5-year follow-up and c′ from ADHD to SU controlled for CD and SS (noted as ADHD → SU w/CD & SS; “w/” = “with”), path b1 from CD to SU controlled for ADHD and SS, and path b2 from SS to SU controlled for ADHD and CD. Dotted lines indicate partial correlations of predictors: ra1 of ADHD with CD (w/SS), ra2 of ADHD with SS (w/CD), and ra3 of CD with SS (w/ADHD).
Multiple logistic regressions were performed to obtain regression coefficients B and odds ratios (ORs) for late SU initiation (dichotomous outcomes) and development of an AUD, a nicotine dependence, or a CUD. Only baseline non-users of the given substance were included in these analyses. Consequently, the samples were different for each substance. Bivariate association was calculated to estimate ADHD's predictive contribution to SU initiation (c), and adjusted association to estimate the direct effect of ADHD on SU (c′), statistically controlling for both CD and SS as covariates. A significant value for c′ would indicate that ADHD at age 20 is a significant independent predictor for SU initiation at age 25. The contributions of CD (b1) and SS (b2) to SU initiation were estimated after statistically controlling for the other 2 variables (i.e., ADHD and SS for b1, ADHD and CD for b2). Cohen's d effect sizes were computed from the ORs according to the calculation formula of Borenstein et al. [49].
Escalation in standard drinks per week, cigarettes per week, and annual days of cannabis use was assessed using analysis of covariance, with differences in SU between 5-year follow-up and baseline as the dependent variables, ADHD as the factor, and CD, SS, and language as covariates. All participants (baseline users and non-users) were included in the analyses. Escalation of each substance was estimated based on the measures of standard drinks per week, cigarettes per week, and days of cannabis use per year. Language was included as a covariate in all analyses because there was a significant language difference between the ADHD S+ and ADHD S− groups.
Results
Sociodemographic Characteristics and Prevalence of SU and SUD at Age 20 and Age 25
The ADHD S+ (4.2%) and ADHD S− (95.8%) groups had no statistically significant differences in sociodemographic characteristics, except that French-speaking participants were significantly more often screened positive for ADHD than German-speaking participants. The ADHD S+ group was significantly more likely to have screened positive for CD and scored higher on SS than the ADHD S− group (see Table 1).
Table 1.
Comparison of sociodemographic characteristics, CD, SS, SU, and SUDs at baseline by screened ADHD status
| ADHD S− n = 4,410 (95.8%) | ADHD S+ n = 192 (4.2%) | t or χ2 | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age, years (mean ± SD) | 19.96±1.21 | 20.10±1.27 | 1.28 |
| Language, % | |||
| French | 94.8 | 5.2 | 14.74*** |
| German | 97.2 | 2.8 | |
| Marital status, % | |||
| Single | 95.1 | 97.4 | 1.54 |
| Married/in a relationship | 4.9 | 2.6 | |
| Education, % | |||
| Compulsory education | 49.6 | 48.4 | 4.47 |
| Vocational/high school | 48.9 | 49.0 | |
| College | 1.5 | 2.6 | |
| Employment status, % | |||
| Unemployed | 7.1 | 8.0 | 2.29 |
| Employed/self-employed | 17.4 | 14.8 | |
| High school/college student | 75.5 | 77.2 | |
| CD, % | 18.2 | 31.2 | 19.20*** |
| SS (mean ± SD) | 3.02±0.86 | 3.39±0.78 | 5.79*** |
| SU characteristics | |||
| Alcohol | |||
| Drinking alcohol | 92.4 | 92.2 | 0.01 |
| Binge drinking | 45.1 | 54.5 | 6.06* |
| AUD | 9.2 | 26.3 | 57.68*** |
| Tobacco | |||
| Cigarette smoking | 44.6 | 56.3 | 9.58** |
| Nicotine dependence | 9.4 | 18.2 | 15.46*** |
| Cannabis | |||
| Cannabis use | 29.1 | 49.5 | 35.58*** |
| CUD | 7.4 | 19.8 | 36.92*** |
| Illicit drugs | |||
| Hallucinogens | 3.3 | 9.9 | 21.95*** |
| Meth-/amphetamines | 2.9 | 6.8 | 8.42** |
| Ecstasy/MDMA | 3.1 | 8.4 | 13.97*** |
| Cocaine/crack/freebase | 2.6 | 8.9 | 24.12*** |
| Non-medical use of prescription drugs | |||
| ADHD medication | 1.5 | 6.3 | 22.01*** |
| Sedatives | 4.0 | 12.6 | 31.18*** |
Hallucinogens include psilocybin (“magic mushrooms”), LSD, and PCP; ADHD medication includes methylphenidate and amphetamine sulphate; sedatives include tranquilisers and hypnotics. Heroin was excluded due to small sample sizes. CD, conduct disorder; SS, sensation seeking; SU, substance use; SUD, substance use disorder; SD, standard deviation; AUD, alcohol use disorder CUD, cannabis use disorder; ADHD, attention deficit/hyperactivity disorder; ADHD S–, ADHD screened negative group (ASRS score <14); ADHD S+, ADHD screened positive group (ASRS score ≥14); ASRS, Adult ADHD Self-Report Scale.
p < 0.05
p < 0.01
p < 0.001.
At baseline, an equivalent percentage of participants in both groups drank alcohol. However, the ADHD S+ group exhibited significantly higher rates of binge drinking and AUD than the ADHD S− group. The ADHD S+ group was more likely than the ADHD S− group to use all other substances, to have a nicotine dependence, and to have a CUD.
ADHD symptoms were significantly related to CD (ra1 = 0.06, 95% BCa CI [0.024, 0.096], p < 0.001) and SS (ra2 = 0.23, 95% BCa CI [0.201, 0.260], p < 0.001), and CD was significantly related to SS (ra3 = 0.06, 95% BCa CI [0.034, 0.092], p < 0.001). Effect sizes were none to small (d = 0.12–0.48).
At 5-year follow-up, the significant differences between the 2 groups in SU, except alcohol use, remained similar to the baseline, with participants in the ADHD S+ group being more likely to binge drink and to use all types of substances. AUD, nicotine dependence, and CUD also showed results similar to baseline (see Table 2).
Table 2.
Comparison of SU and SUDs at 5-year follow-up by screened ADHD status
| ADHD S− % (n/N)a | ADHD S+ % (n/N)a | χ2 | |
|---|---|---|---|
| Alcohol | |||
| Drinking alcohol | 93.2 (4,109/4,410) | 94.3 (181/192) | 0.20 |
| Binge drinking | 37.2 (1,641/4,406) | 45.3 (87/192) | 4.76* |
| AUD | 8.2 (359/4,401) | 20.8 (40/192) | 35.69*** |
| Tobacco | |||
| Cigarette smoking | 39.7 (1,749/4,410) | 54.2 (104/192) | 15.50*** |
| Nicotine dependence | 9.5 (413/4,361) | 16.1 (31/192) | 8.57** |
| Cannabis | |||
| Cannabis use | 28.8 (1,268/4,406) | 46.4 (89/192) | 26.48*** |
| CUD | 7.8 (345/4,407) | 15.6 (30/192) | 13.91*** |
| Illicit drugs | |||
| Hallucinogens | 4.3 (190/4,404) | 10.4 (20/192) | 14.34*** |
| Meth-/amphetamines | 3.7 (163/4,405) | 10.4 (20/192) | 19.99*** |
| Ecstasy/MDMA | 7.3 (321/4,405) | 16.7 (32/192) | 21.53*** |
| Cocaine/crack/freebase | 5.8 (256/4,406) | 14.1 (27/192) | 20.29*** |
| Non-medical use of prescription drugs | |||
| ADHD medication | 1.7 (74/4,402) | 7.3 (14/192) | 28.15*** |
| Sedatives | 5.2 (231/4,402) | 14.1 (27/191) | 25.63*** |
Hallucinogens include psilocybin (“magic mushrooms”), LSD, and PCP; ADHD medication includes methylphenidate and amphetamine sulphate; sedatives include tranquilisers and hypnotics. Heroin was excluded due to small sample sizes. ADHD, attention deficit/hyperactivity disorder; ADHD S–, ADHD screened negative group (ASRS score <14); ADHD S+, ADHD screened positive group (ASRS score ≥14); ASRS, Adult ADHD Self-Report Scale; SU, substance use; SUD, substance use disorder; AUD, alcohol use disorder; CUD, cannabis use disorder.
p < 0.05
p < 0.01
p < 0.001.
n/N = number of participants who use the substance/total number of participants of the corresponding subgroup.
Initiation of SU from Age 20 to Age 25
Table 3 presents the bivariate and adjusted associations of ADHD with late SU initiation, AUD, nicotine dependence, and CUD, with corresponding B coefficients and ORs and their CIs. CD and SS also had direct effects (for an overview of the different paths, see Fig. 1). ADHD predicted all outcomes except initiation of alcohol use, binge drinking, smoking, and nicotine dependence, with ORs ranging from 1.70 for cannabis use to 4.29 for ADHD medication use and effect sizes varying from small to large (d = 0.29–0.80). After controlling for CD and SS, ADHD's associations with beginning to use hallucinogens, meth-/amphetamines, ecstasy/MDMA, ADHD medication, and sedatives decreased but remained significant; its association with AUD increased. ORs ranged from 1.81 for ecstasy/MDMA to 3.41 for ADHD medication, with corresponding effect sizes varying from small to medium (d = 0.33–0.68). Thus, except for initiation of cannabis and cocaine use and development of CUD, ADHD significantly predicted all illegal SUs independent of CD and SS.
Table 3.
Initiation of alcohol drinking, binge drinking, cigarette smoking, cannabis use, and drug use and development of related use disorders from baseline to 5-year follow-up
| B | OR | 95% CI | Wald | d | |
|---|---|---|---|---|---|
| Drinking alcohol: ADHD S− (161/333), ADHD S+ (9/15)a | |||||
| b1(CD → SU w/ADHD & SS) | 0.36 | 1.43 | 0.77–2.65 | 1.27 | 0.20 |
| b2(SS → SU w/ADHD & CD) | −0.01 | 1.00 | 0.78–1.27 | 0.01 | 0.00 |
| c(ADHD → SU) | 0.47 | 1.60 | 0.56–4.60 | 0.76 | 0.26 |
| c‘(ADHD → SU w/CD & SS) | 0.42 | 1.52 | 052–4.42 | 0.59 | 0.23 |
| Binge drinking: ADHD S− (446/2,410), ADHD S+ (19/87) | |||||
| b1(CD → SU w/ADHD & SS) | 0.01 | 1.00 | 0.75–1.35 | 0.01 | 0.00 |
| b2(SS → SU w/ADHD & CD) | 0.21 | 1.23 | 1.08–1.39 | 10.46*** | 0.11 |
| c(ADHD → SU) | 0.19 | 1.21 | 0.72–2.03 | 0.51 | 0.11 |
| c‘(ADHD → SU w/CD & SS) | 0.14 | 1.14 | 0.68–1.93 | 0.25 | 0.07 |
| AUD: ADHD S− (240/3,992), ADHD S+ (19/140) | |||||
| b1(CD → SU w/ADHD & SS) | 0.67 | 1.96 | 1.46–2.61 | 20.52*** | 0.37 |
| b2(SS → SU w/ADHD & CD) | 0.46 | 1.59 | 1.34–1.87 | 29.66*** | 0.26 |
| c(ADHD → SU) | 0.95 | 2.59 | 1.56–4.28 | 13.70*** | 0.53 |
| c‘(ADHD → SU w/CD & SS) | 0.82 | 2.78 | 1.36–3.80 | 9.87** | 0.45 |
| Cigarette smoking: ADHD S− (329/2,441), ADHD S+ (17/84) | |||||
| b1(CD → SU w/ADHD & SS) | 0.07 | 1.07 | 0.76–1.50 | 0.14 | 0.04 |
| b2(SS → SU w/ADHD & CD) | 0.31 | 1.36 | 1.18–1.57 | 18.00*** | 0.17 |
| c(ADHD → SU) | 0.48 | 1.62 | 0.94–2.79 | 2.98 | 0.27 |
| c‘(ADHD → SU w/CD & SS) | 0.33 | 1.27 | 0.79–2.44 | 1.27 | 0.13 |
| Nicotine dependence: ADHD S− (203/3,951), ADHD S+ (9/157) | |||||
| b1(CD → SU w/ADHD & SS) | 0.31 | 1.36 | 0.96–1.92 | 2.99 | 0.17 |
| b2(SS → SU w/ADHD & CD) | 0.16 | 1.17 | 0.99–1.39 | 3.20 | 0.09 |
| c(ADHD → SU) | 0.13 | 1.14 | 0.57–2.27 | 0.14 | 0.07 |
| c‘(ADHD → SU w/CD & SS) | 0.04 | 1.05 | 0.52–2.09 | 0.02 | 0.03 |
| Cannabis use: ADHD S− (461/3,127), ADHD S+ (22/97) | |||||
| b1(CD → SU w/ADHD & SS) | 0.40 | 1.49 | 1.15–1.93 | 9.04** | 0.22 |
| b2(SS → SU w/ADHD & CD) | 0.46 | 1.58 | 1.39–1.79 | 50.83*** | 0.25 |
| c(ADHD → SU) | 0.53 | 1.70 | 1.05–2.77 | 4.59* | 0.29 |
| c‘(ADHD → SU w/CD & SS) | 0.41 | 1.50 | 0.92–2.47 | 2.60 | 0.22 |
| CUD: ADHD S− (164/4,080), ADHD S+ (11/154) | |||||
| b1(CD → SU w/ADHD & SS) | 0.75 | 2.11 | 1.50–2.97 | 18.36*** | 0.41 |
| b2(SS → SU w/ADHD & CD) | 0.45 | 1.56 | 1.28–1.91 | 19.20*** | 0.25 |
| c(ADHD → SU) | 0.65 | 1.91 | 1.01–3.60 | 3.96* | 0.36 |
| c‘(ADHD → SU w/CD & SS) | 0.43 | 1.54 | 0.81–2.94 | 1.73 | 0.24 |
| Hallucinogens: ADHD S− (139/4,256), ADHD S+ (15/172) | |||||
| b1(CD → SU w/ADHD & SS) | 0.50 | 1.65 | 1.12–2.38 | 7.12** | 0.28 |
| b2(SS → SU w/ADHD & CD) | 0.86 | 2.35 | 1.87–2.96 | 53.55*** | 0.47 |
| c(ADHD → SU) | 1.03 | 2.80 | 1.60–4.89 | 13.06*** | 0.57 |
| c‘(ADHD → SU w/CD & SS) | 0.69 | 2.00 | 1.12–3.56 | 5.55* | 0.38 |
| Meth-/amphetamines: ADHD S− (118/4,276), ADHD S+ (16/178) | |||||
| b1(CD → SU w/ADHD & SS) | 0.68 | 1.97 | 1.35–2.88 | 12.17*** | 0.37 |
| b2(SS → SU w/ADHD & CD) | 0.78 | 2.17 | 1.70–2.78 | 37.98*** | 0.43 |
| c(ADHD → SU) | 1.33 | 3.78 | 2.18–6.55 | 22.43*** | 0.73 |
| c‘(ADHD → SU w/CD & SS) | 1.04 | 2.83 | 1.60–4.99 | 12.83*** | 0.57 |
| Ecstasy/MDMA: ADHD S− (256/4,264), ADHD S+ (25/175) | |||||
| b1(CD → SU w/ADHD & SS) | 0.54 | 1.72 | 1.30–2.27 | 14.62*** | 0.30 |
| b2(SS → SU w/ADHD & CD) | 0.94 | 2.57 | 2.16–3.06 | 111.13*** | 0.52 |
| c(ADHD → SU) | 0.94 | 2.56 | 1.64–3.98 | 17.22*** | 0.52 |
| c‘(ADHD → SU w/CD & SS) | 0.59 | 1.81 | 1.14–2.87 | 2.65* | 0.33 |
| Cocaine/crack/freebase: ADHD S− (201/4,289), ADHD S+ (17/174) | |||||
| b1(CD → SU w/ADHD & SS) | 0.90 | 2.46 | 1.82–3.32 | 34.54*** | 0.50 |
| b2(SS → SU w/ADHD & CD) | 0.71 | 2.02 | 1.67–2.45 | 52.95*** | 0.39 |
| c(ADHD → SU) | 0.77 | 2.17 | 1.29–3.65 | 8.45** | 0.43 |
| c‘(ADHD → SU w/CD & SS) | 0.43 | 1.54 | 0.90–2.65 | 2.46 | 0.24 |
| ADHD medication: ADHD S− (58/4,328), ADHD S+ (9/177) | |||||
| b1(CD → SU w/ADHD & SS) | 0.20 | 1.25 | 0.70–2.16 | 0.49 | 0.12 |
| b2(SS → SU w/ADHD & CD) | 0.64 | 1.90 | 1.36–2.65 | 14.14*** | 0.35 |
| c(ADHD → SU) | 1.46 | 4.29 | 2.08–8.86 | 15.12*** | 0.80 |
| c‘(ADHD → SU w/CD & SS) | 1.23 | 3.41 | 1.63–7.16 | 10.55** | 0.68 |
| Sedatives: ADHD S− (196/4,220), ADHD S+ (17/165) | |||||
| b1(CD → SU w/ADHD & SS) | 0.41 | 1.51 | 1.08–2.12 | 5.65* | 0.23 |
| b2(SS → SU w/ADHD & CD) | 0.03 | 1.03 | 0.87–1.22 | 0.12 | 0.02 |
| c(ADHD → SU) | 0.81 | 2.24 | 1.33–3.78 | 9.10** | 0.45 |
| c‘(ADHD → SU w/CD & SS) | 0.78 | 2.17 | 1.28–3.40 | 8.19** | 0.43 |
Language was included as a covariate; the Wald statistic follows a χ2 distribution; d = Cohen's d. Hallucinogens include psilocybin (“magic mushrooms”), LSD, and PCP; ADHD medication includes methylphenidate and amphetamine sulphate; and sedatives include tranquilisers and hypnotics. Heroin was excluded due to small sample sizes. See Figure 1 for the structure of the statistical analyses. ADHD, attention deficit/hyperactivity disorder; ADHD S–, ADHD screened negative group (ASRS score <14); ADHD S+, ADHD screened positive group (ASRS score ≥14); ASRS, Adult ADHD Self-Report Scale; CD, conduct disorder; CI, confidence interval; OR, odds ratio; SS, sensation seeking; SU, substance use; AUD, alcohol use disorder; CUD, cannabis use disorder.
p < 0.05.
p < 0.01.
p < 0.001.
Numbers in parentheses following the ADHD S− and ADHD S+ groups indicate the number of participants who initiated use or developed the disorder/total number of non-users or participants who did not have the disorder at baseline.
Further analysis of the late initiation hypothesis revealed the following results for CD and SS: After controlling for ADHD and SS, CD predicted development of AUD and CUD and beginning to use all other drugs except non-prescribed ADHD medication. Significant ORs ranged from 1.49 to 2.46 and effect sizes from small to medium (d = 0.22–0.50). After controlling for ADHD and CD, SS also predicted development of AUD and CUD, binge drinking, and beginning to use all other substances except sedatives. Significant ORs ranged from 1.36 to 2.57 and effect sizes varied from non-significant to medium (d = 0.17–0.52).
Escalation of Alcohol, Tobacco, and Cannabis Use from Age 20 to Age 25
Similar to baseline (Table 1), at 5-year follow-up, the ADHD S+ group showed significantly more binge drinking and tobacco and cannabis use than the ADHD S− group (Table 2). However, there was no escalation in usage. Instead, after controlling for CD and SS, participants in the ADHD S+ group significantly reduced their tobacco use from baseline to 5-year follow-up, with a minor effect size (d = 0.17). The large standard deviations may prevent significant results, however (Table 4).
Table 4.
Escalation of alcohol, tobacco, and cannabis use from baseline to 5-year follow-up
| Substance use | ADHD S− (mean ± SD) |
ADHD S+ (mean ± SD) |
F | d | ||||
|---|---|---|---|---|---|---|---|---|
| baseline | 5-year follow-up | Δ5-year follow-up − baseline | baseline | 5-year follow-up | Δ 5-year follow-up − baseline | |||
| Standard drinks per week | 7.0±11.75 | 7.0±10.77 | 0.08±13.12 | 10.3±17.55 | 10.8±16.22 | 0.49±15.56 | 0.19 | 0.03 |
| Cigarettes per week | 20.0±41.96 | 18.3±40.90 | −1.63±32.14 | 37.3±60.86 | 29.2±55.29 | −8.10±38.16 | 4.89* | 0.17 |
| Days of cannabis use per year | 24.3±75.12 | 24.5±77.42 | 0.24±70.04 | 50.2±106.33 | 48.2±108.74 | −2.06±105.76 | 0.02 | 0.01 |
d = Cohen's d. ns differ slightly due to missing data (i.e., ADHD S–, n = 4,346 to 4,368; ADHD S+, n = 188–189). Language, CD, and SS were included as covariates. ADHD, attention deficit/hyperactivity disorder; ADHD S–, ADHD screened negative group (ASRS score <14); ADHD S+, ADHD screened positive group (ASRS score ≥14); ASRS, Adult ADHD Self-Report Scale; SD, standard deviation; CD, conduct disorder; SS, sensation seeking.
p < 0.05.
Discussion
This longitudinal study compared SU patterns in a large sample of young adult Swiss men, including 192 who screened positive for ADHD (4.2%) and 4,410 who screened negative (95.8%). Both at age 20 and at age 25, the ADHD S+ group had a higher prevalence of alcohol, cigarette, and cannabis use and related SUDs, illicit drug use, and non-medical use of prescription drugs. These findings are consistent with many studies on the relationship between SU, SUD, and ADHD in early adulthood, which have reported higher rates of use and misuse of a wide range of potentially addictive substances and a higher risk of SUD among individuals with ADHD [8, 11].
ADHD at age 20 did not predict late initiation (between age 20 and age 25) of alcohol use, binge drinking, smoking, or cannabis use but was significantly associated with late initiation of 3 other drugs (hallucinogens, meth-/amphetamines, and ecstasy/MDMA) and non-prescribed medications (ADHD medication and sedatives), with a medium effect size for meth-/amphetamines and a large effect size for non-prescribed ADHD medication. Molina et al. [12] found that adolescents with ADHD were more likely to begin using alcohol, tobacco, cannabis, and some illicit drugs earlier than their peers, typically before the age of 15 or 16. Only a minority began using illegal drugs before the age of 17. Sibley et al. [23] also reported that adolescents began to use alcohol, tobacco, and cannabis around the age of 14–15 years. Because the mean age of the present study's sample at baseline was 20 years, the sample may have been too old to observe subjects' initial use of these substances.
ADHD remained a significant predictor of late initiation of both illegal (except for cocaine) and non-medical prescription drugs with low to medium effect sizes, even after statistically controlling for both CD and SS. As reported in numerous other studies, CD and SS also increased the risk of beginning to use almost all substances independent of ADHD [15, 17, 20, 22, 28, 29, 32, 33]. In other words, both CD and SS exert additive effects on ADHD, rendering young men with both ADHD and CD and/or SS even more prone to SU initiation and SUD (i.e., AUD or CUD). Our findings suggest that ADHD carries a unique risk for SU initiation in early adulthood, independent of self-reported CD and SS [27]. Importantly, the risk of developing AUD after age 20 was almost 3 times higher in the ADHD S+ group than in the ADHD S− group, suggesting rapid progression to alcohol dependence even if having started drinking after age 20 [12, 15].
Due to methodological reasons (i.e., cross-sectional assessment of predictors), no formal mediator analyses were performed in this study with the 3, only slightly, correlating predictors ADHD, CD, and SS. To date, only two prospective studies have revealed CD as a mediator between ADHD and SU. Brook et al. [26] examined only SUD, not SU initiation; Sibley et al. [23] investigated use of alcohol, tobacco, and cannabis but not other substances, such as stimulants or non-prescribed ADHD medication. Moreover, to our knowledge, no existing prospective study on the relationship of ADHD with SU initiation has controlled for SS. A cross-sectional study by van Eck et al. [34] reported that SS (i.e., higher scores on the Disinhibition subscale of the BSSS) moderated between ADHD and increased misuse of ADHD medication. However, no moderating effect was found for other stimulants, and no other substances were examined.
An important finding of the present study was that after adjusting for CD and SS, alcohol and cannabis use remained stable in both groups from baseline to 5-year follow-up. This indicates that the ADHD S+ group continued to use these substances more heavily than the ADHD S− group. The ADHD S+ group only very slightly decreased their cigarette smoking compared to the ADHD S− group, perhaps because they switched to using other substances examined in the study. Alternatively, they might have reached their peak use of alcohol, tobacco, and cannabis before baseline, and, at least with respect to smoking, begun to grow out of these consumption patterns by the 5-year follow-up. Molina et al. [12] observed an escalation of alcohol, tobacco, cannabis, and illicit drug use throughout adolescence until the age of 21, and a decline in alcohol, tobacco, and cannabis use thereafter. Another study by Estévez-Lamorte et al. [50], using the same sample as Vogel et al. [30], found that a subsample of the ADHD S+ group showed maturation out of alcohol, nicotine, and CUDs and risky cannabis use. Breyer et al. [16] and Levy et al. [19] did not observe any change in SUD prevalence rates over time. However, they examined only substance dependence as an outcome and did not consider use patterns.
This study has important clinical implications. Use of the ASRS, an easily applicable screening instrument for ADHD, could help to identify young adult men at high risk for the initiation of various drugs, including stimulants and sedatives, and for persistent heavy use of alcohol, tobacco, and cannabis. SS contributes independently to an elevated risk of initiating use of any substance except alcohol and sedatives, and CD to an elevated risk of any substance except alcohol, tobacco, and non-prescribed ADHD medication. These findings may be of specific interest within the context of secondary prevention programmes or primary care, allowing a focus on men at high risk in early adulthood.
This study's results should be interpreted in the context of some strengths and limitations. The sample's age homogeneity from 20 to 25 is a considerable strength, as this age group is at particularly high risk for SU and the prevalence of SU varies greatly across diverse age groups [51]. Considering that only a minority (about 4%) of individuals both have ADHD and use illegal drugs, the study's large sample size allowed for collection of more information to make reliable observations. Another strength is the study's longitudinal design, which observed the course of SU from baseline to later follow-up measures [36].
The study also has limitations. First, the sample included only men, who are more likely to have ADHD, CD, and SS, and generally exhibit higher rates of SU than women. Thus, the results may not be generalised to the female population. However, in a meta-analysis, Lee et al. [8] did not observe sex differences in the increased risk of individuals with ADHD developing SUD. Second, recruiting in military centres implies that only men with a Swiss passport were included in the sample. Non-Swiss permanent residents, who represent about one-fourth of the Swiss population, or individuals with dual citizenship, who fulfilled their military obligations in the other country, were thus not considered. Third, ADHD was assessed using the ASRS screening instrument rather than the structured diagnostic interviews; thus, the diagnosis of ADHD was not confirmed via extensive clinical examination. ADHD may be underreported since the most frequently used cut-off in comparable studies with a score of 14 points may set too high, leading to a very conservative estimate of the ADHD prevalence due to false-negative cases. Luderer et al. [52] suggested that lowering the cut-off among individuals with AUD decreases the rate of false-negative cases. However, their results are based on a sample of patients attending residential treatment for alcohol dependence, a sample that is considerably different from our general population sample of young adult Swiss men. Likewise, CD, SS, and SU were based on self-report questionnaires, meaning some men may have under- or over-reported their ADHD, CD, SS, SU, or SUD for a variety of reasons (e.g., difficulty in recalling quantity and frequency of SU). However, the assessment instruments have been validated [35, 41, 43, 47, 48, 53], and previous studies have demonstrated, in particular, the reliability and validity of using self-report screening instruments, for example, to assess ADHD, in the context of SUD in adults [54]. Fourth, prevalence rates of CD were quite high, most likely of non-clinical assessment and wording of questions, permitting rather common behaviours in adolescents to be rated as CD symptoms, which may have resulted in false-positive cases. However, CD was used as a control variable and non-differential misclassification of false-positive should bias the OR of ADHD towards the null and may have resulted in conservative estimates.
Conclusions
ADHD is a risk factor for late initiation of stimulant use (meth-/amphetamines and non-medical use of ADHD medication), hallucinogens, and non-prescribed sedatives as well as for the development of new AUD after age 20 and is associated with heavy (but not escalating) alcohol, tobacco, and cannabis use. CD and SS contribute independently to the risk of new AUD or CUD and late initiation of any substance except for alcohol, in case of CD except for smoking and non-medical use of ADHD medication, and in case of SS except for sedatives. From a public health perspective, identifying ADHD in childhood or adolescence and continuous treatment and support would be best to lower risks of SU and SUD, but identification of ADHD in early adulthood may be still relevant for that start of specific ADHD and SU interventions.
Statement of Ethics
All subjects provided written informed consent. The study protocol (Protocol No. 15/07) was approved by the Lausanne University Medical School Clinical Research Ethics Committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
Funding for this study was provided by the Swiss National Science Foundation (SNSF), grant numbers FN 33CSC0_122679, FN 33CS30_139467, and FN 33CS30_148493. The SNSF had no further role in the study design; collection, analysis, or interpretation of data; report writing; or decision to submit the paper for publication.
Author Contributions
G.G. and M.M.-K. were the principal investigators who wrote the study protocol, acquired the SNSF Grant, and coordinated the project. J.S. was involved in the data collection, management, and analyses. F.M. and D.S. analysed the data, and F.M. wrote the first draft of the manuscript. F.M., D.S., L.M.S., M.M.-K., N.E.-L., J.S., and G.G. interpreted the data, provided comments on various versions of the paper, and approved the final version of the manuscript for publication.
Acknowledgement
We would like to thank the young Swiss men who participated in this study. We would also like to express our gratitude to the C-SURF research team (see www.c-surf.ch/en) for making the data available to examine the research question posed in this article. We thank Wim van den Brink and Arnt Schellekens for their valuable comments on earlier versions of this paper.
References
- 1.Erskine HE, Norman RE, Ferrari AJ, Chan GC, Copeland WE, Whiteford HA, et al. Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2016;55((10)):841–50. doi: 10.1016/j.jaac.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. 2011;24((4)):280–5. doi: 10.1097/YCO.0b013e328345c956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuckerman M. Leary MR, Hoyle RH. Handbook of individual differences in social behavior. New York: Guildford Press; 2009. Sensation seeking; pp. p.455–65. [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-5) 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56((3)):345–65. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 6.Kaye S, Ramos-Quiroga JA, van de Glind G, Levin FR, Faraone SV, Allsop S, et al. Persistence and subtype stability of ADHD among substance use disorder treatment seekers. J Atten Disord. 2019;23((12)):1438–53. doi: 10.1177/1087054716629217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122((5)):97–109. doi: 10.3810/pgm.2010.09.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31((3)):328–41. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina BS, Pelham WE. Attention-deficit/hyperactivity disorder and risk of substance use disorder: developmental considerations, potential pathways, and opportunities for research. Annu Rev Clin Psychol. 2014;10:607–39. doi: 10.1146/annurev-clinpsy-032813-153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163((4)):716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50((1)):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Molina BSG, Howard AL, Swanson JM, Stehli A, Mitchell JT, Kennedy TM, et al. Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: findings from the MTA longitudinal study. J Child Psychol Psychiatry. 2018;59((6)):692–702. doi: 10.1111/jcpp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. Am J Addict. 1998;7((2)):156–63. [PubMed] [Google Scholar]
- 14.Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44((4)):269–73. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36((1)):21–9. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychol Addict Behav. 2014;28((1)):238–46. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108((8)):1503–11. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- 18.Lambert N. The contribution of childhood ADHD, conduct problems, and stimulant treatment to adolescent and adult tobacco and psychoactive substance abuse. Ethical Hum Psychol Psychiatry. 2005;7((3)):197–221. [Google Scholar]
- 19.Levy S, Katusic SK, Colligan RC, Weaver AL, Killian JM, Voigt RG, et al. Childhood ADHD and risk for substance dependence in adulthood: a longitudinal, population-based study. PLoS One. 2014;9((8)):e105640. doi: 10.1371/journal.pone.0105640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50((6)):543–53. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina BSG, Walther CAP, Cheong J, Pedersen SL, Gnagy EM, Pelham WE. Heavy alcohol use in early adulthood as a function of childhood ADHD: developmentally specific mediation by social impairment and delinquency. Exp Clin Psychopharmacol. 2014;22((2)):110–21. doi: 10.1037/a0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina BS, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112((3)):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 23.Sibley MH, Pelham WE, Molina BSG, Coxe S, Kipp H, Gnagy EM, et al. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol. 2014;123((2)):362–74. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee SH, Willcutt EG, Hartman CA, Pennington BF, DeFries JC. Test of alternative hypotheses explaining the comorbidity between attention-deficit/hyperactivity disorder and conduct disorder. J Abnorm Child Psychol. 2008;36((1)):29–40. doi: 10.1007/s10802-007-9157-9. [DOI] [PubMed] [Google Scholar]
- 25.Sigfusdottir ID, Asgeirsdottir BB, Hall HA, Sigurdsson JF, Young S, Gudjonsson GH. An epidemiological study of ADHD and conduct disorder: does family conflict moderate the association? Soc Psychiatry Psychiatr Epidemiol. 2017;52((4)):457–64. doi: 10.1007/s00127-017-1352-6. [DOI] [PubMed] [Google Scholar]
- 26.Brook DW, Brook JS, Zhang C, Koppel J. Association between attention-deficit/hyperactivity disorder in adolescence and substance use disorders in adulthood. Arch Pediatr Adolesc Med. 2010;164((10)):930–4. doi: 10.1001/archpediatrics.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looby A. Childhood attention deficit hyperactivity disorder and the development of substance use disorders: valid concern or exaggeration? Addict Behav. 2008;33((3)):451–63. doi: 10.1016/j.addbeh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Carpentier PJ, Knapen LJ, van Gogh MT, Buitelaar JK, De Jong CA. Addiction in developmental perspective: influence of conduct disorder severity, subtype, and attention-deficit hyperactivity disorder on problem severity and comorbidity in adults with opioid dependence. J Addict Dis. 2012;31((1)):45–59. doi: 10.1080/10550887.2011.642756. [DOI] [PubMed] [Google Scholar]
- 29.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64((10)):1145–52. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 30.Vogel T, Dom G, van de Glind G, Studer J, Gmel G, Strik W, et al. Is attention deficit/hyperactivity disorder among men associated with initiation or escalation of substance use at 15-month follow-up? A longitudinal study involving young Swiss men. Addiction. 2016;111((10)):1867–78. doi: 10.1111/add.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- 32.Haug S, Núñez CL, Becker J, Gmel G, Schaub MP. Predictors of onset of cannabis and other drug use in male young adults: results from a longitudinal study. BMC Public Health. 2014;14:1202. doi: 10.1186/1471-2458-14-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen M, Chassin L, Gonzales NA. Neighborhood moderation of sensation seeking effects on adolescent substance use initiation. J Youth Adolesc. 2017;46:1953–67. doi: 10.1007/s10964-017-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Eck K, Markle RS, Flory K. Do conduct problems and sensation seeking moderate the association between ADHD and three types of stimulant use in a college population? Psychol Addict Behav. 2012;26((4)):939–47. doi: 10.1037/a0027431. [DOI] [PubMed] [Google Scholar]
- 35.Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personal Individual Differences. 2002;32((3)):401–14. [Google Scholar]
- 36.Cohort study on substance use risk factors (CSURF) Cohort study on substance use risk factors, 2019. https: //www.c-surf.ch/en/1.html.
- 37.Studer J, Baggio S, Mohler-Kuo M, Dermota P, Gaume J, Bertholet N, et al. Examining non-response bias in substance use research: are late respondents proxies for non-respondents? Drug Alcohol Depend. 2013;132((1–2)):316–23. doi: 10.1016/j.drugalcdep.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Studer J, Mohler-Kuo M, Dermota P, Gaume J, Bertholet N, Eidenbenz C, et al. Need for informed consent in substance use studies: harm of bias? J Stud Alcohol Drugs. 2013;74((6)):931–40. doi: 10.15288/jsad.2013.74.931. [DOI] [PubMed] [Google Scholar]
- 39.Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35((2)):245–56. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- 40.APA . Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- 41.Kessler RC, Adler LA, Gruber MJ, Sarawate CA, Spencer T, Van Brunt DL. Validity of the World Health Organization Adult ADHD Self-Report Scale (ASRS) screener in a representative sample of health plan members. Int J Methods Psychiatr Res. 2007;16((2)):52–65. doi: 10.1002/mpr.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecubrier Y, Weiller E, Hergueta T, Amorim P, Bonora LI, Lépine JP. Mini international neuropsychiatric interview (M.I.N.I. Plus version 5.0.0). INSERM, editor. Paris: Hôpital de la Salpétrière; 1998. [Google Scholar]
- 43.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption: II. Addiction. 1993;88((6)):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 44.Knight JR, Wechsler H, Kuo M, Seibring M, Weitzman ER, Schuckit MA. Alcohol abuse and dependence among U.S. college students. J Stud Alcohol. 2002;63((3)):263–70. doi: 10.15288/jsa.2002.63.263. [DOI] [PubMed] [Google Scholar]
- 45.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55((2)):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 46.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA: a comparison with the SCAN. Addiction. 1999;94((9)):1361–70. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 47.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86((9)):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 48.Adamson SJ, Sellman JD. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22((3)):309–15. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- 49.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: John Wiley & Sons; 2009. [Google Scholar]
- 50.Estévez-Lamorte N, Foster S, Eich-Höchli D, Moggi F, Gmel G, Mohler-Kuo M. Adult attention-deficit/hyperactivity disorder, risky substance use and substance use disorders: a follow-up study among young men. Eur Arch Psychiatry Clin Neurosci. 2019;269((6)):667–79. doi: 10.1007/s00406-018-0958-3. [DOI] [PubMed] [Google Scholar]
- 51.Gmel G, Kuendig H, Notari L, Gmel C. Monitorage suisse des addictions: consommation d'alcool, tabac et drogues illégales en Suisse en 2016 [Swiss addiction monitoring: consumption of alcohol, tobacco, and illegal drugs in Switzerland in 2016] Lausanne: Addiction Suisse; 2017. [Google Scholar]
- 52.Luderer M, Kaplan-Wickel N, Richter A, Reinhard I, Kiefer F, Weber T. Screening for adult attention-deficit/hyperactivity disorder in alcohol dependent patients: underreporting of ADHD symptoms in self-report scales. Drug Alcohol Depend. 2019;195:52–8. doi: 10.1016/j.drugalcdep.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98((Suppl 2)):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 54.van de Glind G, van den Brink W, Koeter MW, Carpentier PJ, van Emmerik-van Oortmerssen K, Kaye S, et al. Validity of the Adult ADHD Self-Report Scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. 2013;132((3)):587–96. doi: 10.1016/j.drugalcdep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]