Abstract
Swedish national breast cancer guidelines recommend that all women diagnosed with breast cancer (BC) at the age of 35 years or younger should be referred to their regional oncogenetic clinic for genetic counseling and testing, regardless of family history of cancer. The main objective of this study was to evaluate whether place of residence at BC diagnosis and treating hospital were associated with the fact that not all BC patients diagnosed at ≤35 years in the southern part of Sweden have attended genetic counseling and testing. Between 2000 and 2013, 279 women in the South Swedish Health Care Region were diagnosed with BC at ≤35 years. Information regarding place of residence at BC diagnosis, treating hospital, time of registration and first meeting at the Oncogenetic Clinic in Lund, and genetic testing was collected. With a follow-up period until August 2018, 64% were registered at the clinic (60% underwent genetic testing) and 36% were not. BC patients from 2 counties and from rural settings with a population of <10,000 inhabitants were significantly less likely to be registered at the clinic. Our results suggest that place of residence at BC diagnosis and treating hospital were associated with the probability of referral for genetic counseling and testing for women diagnosed with BC at ≤35 years in the South Swedish Health Care Region. We propose, as a generalizable finding, that further educational and outreach activities within the health care system and the community may be needed to ensure that all women diagnosed with early-onset BC receive proper genetic counseling.
Keywords: Breast cancer, Early-onset, Genetic counseling, BRCA1, BRCA2
Introduction
Breast cancer (BC) is the most common cancer in women, both worldwide and in Sweden. Most women are diagnosed with BC late in life, and women diagnosed at the age of 35 years or younger accounted for <1.5% of all BC cases in Sweden in 2014 [1]. Unfortunately, young BC patients are more likely to present tumors with more aggressive biological characteristics and have higher mortality compared with older patients. Early-onset BC is also associated with heredity and young patients are more likely to harbor a genetic predisposition to BC, for example inherited pathogenic variants in the BC susceptibility genes BRCA1 and BRCA2. Among women who are already diagnosed with BC, the finding of a pathogenic BRCA1/2 variant is also associated with an increased risk for the development of new primary cancers. Therefore, the identification of germline pathogenic variants in these genes in early-onset BC patients is of great importance [2, 3].
Swedish national breast cancer guidelines recommend that all women diagnosed with invasive BC at the age of 35 years or younger should, regardless of family history of cancer, be referred for genetic counseling and given the option of genetic testing [4]. This service is funded by means of general taxation, meaning it is included in the Swedish health care system. After attending the first meeting with a physician, which costs less than EUR 30, subsequent genetic counseling and testing is free of charge for the patient. Genetic counseling is a process that involves a discussion about the benefits and limitations of genetic testing. The identification of a pathogenic variant could empower the patient to make informed decisions about clinical treatments [5], including the choice of surgical method and chemotherapy. Recently, poly (ADP-ribose) polymerase (PARP) inhibitors have become a treatment option for patients with pathogenic variants in BRCA1 and BRCA2 [6, 7]. A knowledge of carrier status is also important for cancer prevention strategies. Screening with annual mammography and magnetic resonance imaging is usually recommended and risk-reducing measures, including prophylactic contralateral mastectomy and bilateral salpingo-oophorectomy, can be discussed with the BC patient. Identifying a BC patient with a pathogenic variant in either BRCA1 or BRCA2 (or rarely both) may also strongly influence relatives to opt for genetic counseling and testing to identify healthy carriers at high risk and thereby be able to prevent cancer and cancer-related deaths through increased surveillance and prophylactic surgery [8, 9].
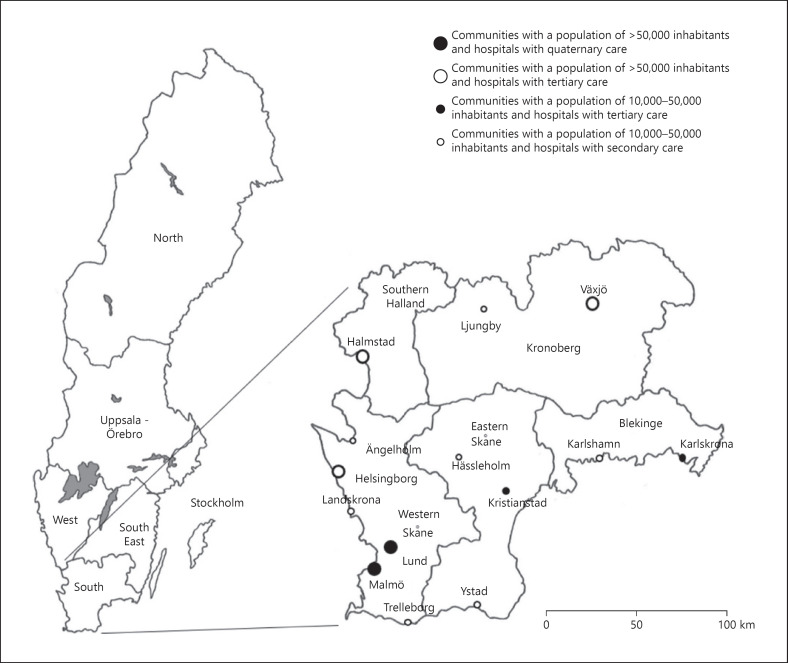
In a previous study, we reported that a large proportion of women diagnosed at 35 years or younger in the South Swedish Health Care Region (Fig. 1) between January 1, 2000 and December 31, 2013 were not registered at the Oncogenetic Clinic at Skåne University Hospital in Lund [10]. The Oncogenetic Clinic in Lund is the only such clinic in the South Swedish Health Care Region and thus serves the entire region, which encompasses approximately 20% of the total Swedish population, with genetic counseling and testing. Previous studies have reported different reasons as to why some early-onset BC patients do not receive genetic testing despite the clinical recommendations for this group. These reasons include the physicians' attitudes towards genetic counseling and testing, a lack of knowledge regarding hereditary aspects, a lack of discussion with the BC patients, and a lack of referral offers [11]. Other reasons include the BC patients' own decisions to attend genetic counseling and testing. One of the contributing factors is the patient's socioeconomic status, for example, marital status, education level, and income [12]. Other contributing factors are the expected benefits or limitations of genetic counseling and testing, general or cancer-related distress, a fear of negative information, guilt towards family members, an ability to contact the clinic, and the distance between the clinic and their homes [13].
Fig. 1.
A map of the 6 national health care regions in Sweden and the South Swedish Health Care Region. The inhabitants have access to 1 hospital in each of the included communities.
The main objective of this study was to evaluate whether place of residence at BC diagnosis and treating hospital were associated with the fact that not all BC patients diagnosed at the age of 35 years or younger in the South Swedish Health Care Region have attended genetic counseling and testing.
Materials and Methods
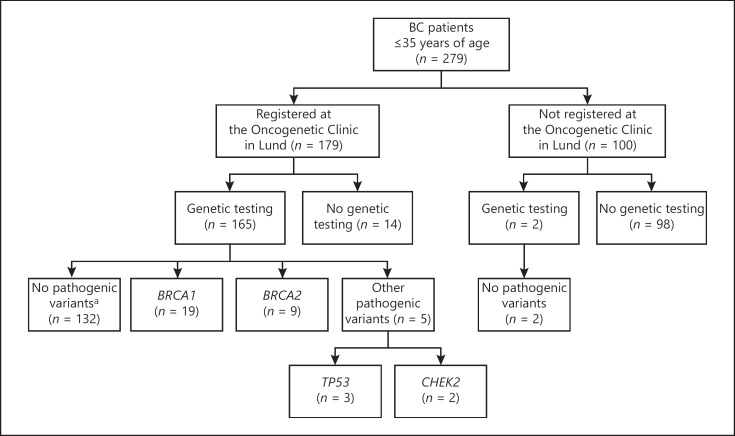
This retrospectively ascertained cohort study was composed of all the 279 female patients diagnosed with invasive BC at the age of 35 years or younger in the South Swedish Health Care Region between January 1, 2000 and December 31, 2013 (Fig. 2), with a follow-up period until August 31, 2018. The study was conducted in accordance with the Declaration of Helsinki and with national legislation. Ethical approval for scientific studies regarding clinical monitoring and genetic diagnostics was previously obtained from the Ethical Review Board at Lund University (reference No. LU 349-00).
Fig. 2.
A flow chart of all the patients who were diagnosed with BC between 2000 and 2013 in the South Swedish Health Care Region and of carrier status of the 167 BC patients who underwent genetic testing. aIncluding variants of uncertain significance in BRCA1 (n = 2), BRCA2 (n = 1), TP53 (n = 1), CHEK2 (n = 1), and CDH1 (n = 1).
Personal identification numbers and information regarding treating hospitals for the 279 BC patients were retrieved from the Southern Swedish Regional Tumor Registry at Skåne University Hospital in Lund. Information regarding place of residence for the 279 patients at BC diagnosis was extracted from the Swedish Population Register, administered by the Swedish Tax Agency. Information regarding time of registration and first meeting at the Oncogenetic Clinic in Lund, and genetic testing, was retrieved from the OnkGen Register at Skåne University Hospital in Lund in October 2017. The number of BC patients who were registered at the clinic was confirmed from the OnkGen Register in August 2018.
The South Swedish Health Care Region comprises the counties of Skåne, Blekinge, Kronoberg, and the southern part of Halland. Information regarding the population in the communities in these counties for the year 2017 was retrieved from Statistics Sweden [14]. Due to differences in the distribution of the population, Skåne County was divided into 2 subregions in this study: Western Skåne and Eastern Skåne (Fig. 1). The communities were divided into 3 groups: communities with more than 50,000 inhabitants, communities with 10,000–50,000 inhabitants, and communities with <10,000 inhabitants.
All hospitals in the South Swedish Health Care Region are publicly owned. These hospitals were divided into groups based on regions and subregions, and categorized based on level of care (secondary, tertiary, and quaternary). In Western Skåne, a further subgrouping was applied for the 2 university hospitals in Lund and Malmö and the remaining 4 hospitals (Fig. 1). The median travel distance from the hospitals in the South Swedish Health Care Region to the Oncogenetic Clinic in Lund (when excluding the University Hospital in Lund) was 77 km, ranging from 18 to 187 km (Table 1).
Table 1.
Travel distance from the hospitals in the South Swedish Health Care Region to the Oncogenetic Clinic in Lund
| Hospitals | Distance, km |
|---|---|
| University hospitals with quaternary care | |
| Lund | − |
| Malmö | 18 |
| County hospitals with tertiary care | |
| Halmstad | 129 |
| Helsingborg | 55 |
| Karlskrona | 187 |
| Kristianstad | 80 |
| Växjö | 184 |
| Local hospitals with secondary care | |
| Hässleholm | 75 |
| Karlshamn | 136 |
| Landskrona | 34 |
| Ljungby | 174 |
| Trelleborg | 50 |
| Ystad | 61 |
| Ängelholm | 77 |
All statistical analyses were performed using the IBM SPSS statistical computing package (version 25.0; SPSS Inc., Chicago, IL, USA). Logistic regression analyses were performed to examine the association between place of residence at BC diagnosis and treating hospital and the probability of registration at the Oncogenetic Clinic at Skåne University Hospital in Lund and the trend of registration over time (from 2000 to 2013). The results are given as odds ratios (ORs) and 95% CIs. Statistical significance was considered with a two-tailed p value <0.05.
Results
All 279 BC patients who were included in this study fulfilled the Swedish national breast cancer guidelines for consideration of referral for genetic counseling and testing. However, 100 (36%) of the patients were not registered at the Oncogenetic Clinic at Skåne University Hospital in Lund (Table 2). Out of these 100 patients, 2 had undergone genetic testing without being registered at the clinic. Both these BC patients tested negative for inherited BRCA1/2 pathogenic variants (Fig. 2; Table 3). For one of these patients, the reason for not being registered was due to a clerical error. Regarding the other patient, she chose to visit one of the other regional oncogenetic clinics in Sweden, even though she lived in our region. Further detailed information regarding the BC patients who were registered at the Oncogenetic Clinic in Lund is shown in Figure 2 and Table 3.
Table 2.
The proportion of BC patients registered versus not registered at the Oncogenetic Clinic in Lund in relation to age and year of diagnosis
| All (n = 279) |
≤25 years (n = 15) |
26–30 years (n = 70) |
31–35 years (n = 194) |
|||||
|---|---|---|---|---|---|---|---|---|
| registered (n = 179) | not registered (n = 100) | registered (n = 13) | not registered (n = 2) | registered (n = 51) | not registered (n = 19) | registered (n = 115) | not registered (n = 79) | |
| 2000–2004 | 45 (51.1) | 43 (48.9) | 3 (100.0) | 0 (0.0) | 10 (43.5) | 13 (56.5) | 32 (51.6) | 30 (48.4) |
| 2005–2009 | 62 (62.0) | 38 (38.0) | 4 (100.0) | 0 (0.0) | 20 (83.3) | 4 (16.7) | 38 (52.8) | 34 (47.2) |
| 2010–2013 | 72 (79.1) | 19 (20.9) | 6 (75.0) | 2 (25.0) | 21 (91.3) | 2 (8.7) | 45 (75.0) | 15 (25.0) |
Data are presented as n (%).
Table 3.
Time of registration and first meeting at the Oncogenetic Clinic in Lund and genetic testing of the BC patients
| Registered (n = 179) | First meeting (n = 165) | Genetic testing |
|||||
|---|---|---|---|---|---|---|---|
| alla(n = 167) | no pathogenic variantsa, b(n = 134) | BRCA1 (n = 19) | BRCA2 (n = 9) | other pathogenic variantsc(n = 5) | |||
| Before BC diagnosis | |||||||
| <–15 years | 11 (6.1) | 6 (3.6) | 4 (2.4) | 0 | 3 (15.8) | 1 (11.1) | 0 |
| After BC diagnosis | |||||||
| 0–1 years | 73 (40.8) | 73 (44.2) | 53 (31.7) | 42 (31.3) | 6 (31.6) | 4 (44.4) | 1 (20.0) |
| 1–2 years | 39 (21.8) | 42 (25.5) | 52 (31.1) | 46 (34.3) | 3 (15.8) | 2 (22.2) | 1 (20.0) |
| 2–3 years | 12 (6.7) | 12 (7.3) | 18 (10.8) | 16 (11.9) | 1 (5.3) | 1 (11.1) | 0 |
| 3–4 years | 7 (3.9) | 6 (3.6) | 10 (6.0) | 6 (4.5) | 2 (10.5) | 0 | 2 (40.0) |
| 4–5 years | 5 (2.8) | 4 (2.4) | 4 (2.4) | 3 (2.2) | 1 (5.3) | 0 | 0 |
| 5–10 years | 21 (11.7) | 16 (9.7) | 18 (10.8) | 15 (11.2) | 1 (5.3) | 1 (11.1) | 1 (20.0) |
| 10–15 years | 11 (6.1) | 6 (3.6) | 7 (4.2) | 6 (4.5) | 1 (5.3) | 0 | 0 |
| Missing | 0 | 0 | 1 (0.6) | 0 | 1 (5.3) | 0 | 0 |
Data are presented as n (%).
Of which 2 BC patients underwent genetic testing without being registered at the Oncogenetic Clinic in Lund.
Genetic testing without findings, including variants of uncertain significance.
Positive for a germline pathogenic variant in TP53 (n = 3) or CHEK2 (n = 2).
The median travel distance from the 279 BC patients' homes to the Oncogenetic Clinic in Lund was 54 km. However, the distances ranged widely (from 2 to 221 km). For BC patients who were registered at the clinic (n = 179), the median travel distance was 37 km (with a range from 2 to 199 km) and for BC patients who were not registered at the clinic (n = 100), the median travel distance was 72.5 km (with a range from 2 to 221 km).
A logistic regression analysis was performed to analyze the association between place of residence of the patients at BC diagnosis and the probability of registration at the Oncogenetic Clinic in Lund. This indicated that BC patients from Blekinge (OR 0.12, p < 0.001) and the southern part of Halland (OR 0.32, p = 0.02) were significantly less likely to be registered at the clinic compared with patients from the western part of Skåne (Table 4). The trend of registration from 2000 to 2013 for the entire South Swedish Health Care Region indicated significant improvement over time (OR 1.16, 95% CI 1.08–1.24, p < 0.001). However, there were variations in the trend of registration over time in the 5 different regions. Both the western part of Skåne and Kronoberg indicated significant improvement over time (OR 1.16, p = 0.001, and OR 1.64, p = 0.03, respectively), in contrast to Blekinge, where no improvement over time was indicated (OR 0.99, p = 0.94; Table 4).
Table 4.
Registration at the Oncogenetic Clinic in Lund (full study duration) and trend of registration over time (from 2000 to 2013) in relation to the patients' places of residence at BC diagnosis and treating hospitals
| Registered at the oncogenetic clinic |
Trend over time |
||||
|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | p value | OR (95% CI) | p value | |
| Regions | |||||
| Western Skåne | 132/184 (71.7) | 1.00 (ref.) | 1.16(1.06–1.26) | 0.001 | |
| Eastern Skåne | 20/30 (66.7) | 0.79 (0.35–1.80) | 0.57 | 1.06 (0.87–1.30) | 0.55 |
| Blekinge | 6/26 (23.1) | 0.12(0.05–0.31) | <0.001 | 0.99 (0.78–1.25) | 0.94 |
| Kronoberg | 13/21 (61.9) | 0.64 (0.25–1.63) | 0.35 | 1.64(1.07–2.53) | 0.03 |
| Southern Halland | 8/18 (44.4) | 0.32(0.12–0.84) | 0.02 | 1.25 (0.94–1.67) | 0.12 |
| Population | |||||
| >50,000 | 85/117 (72.6) | 1.00 (ref.) | 1.09 (0.98–1.21) | 0.12 | |
| 10,000–50,000 | 45/67 (67.2) | 0.79 (0.41–1.51) | 0.47 | 1.18(1.02–1.37) | 0.02 |
| <10,000 | 49/95 (51.6) | 0.39(0.22–0.70) | 0.001 | 1.21(1.08–1.36) | 0.001 |
| Hospitals | |||||
| Lund | 43/65 (66.2) | 1.00 (ref.) | 1.17(1.01–1.36) | 0.04 | |
| Malmö | 50/62 (80.6) | 2.13 (0.95–4.81) | 0.07 | 1.10 (0.92–1.31) | 0.30 |
| Helsingborg/Ängelholm/Landskrona/Trelleborg | 27/43 (62.8) | 0.86 (0.39–1.93) | 0.72 | 1.25(1.04–1.50) | 0.02 |
| Kristianstad/Hässleholm/Ystad | 24/33 (72.7) | 1.36 (0.54–3.43) | 0.51 | 1.04 (0.86–1.27) | 0.67 |
| Karlskrona/Karlshamn | 6/21 (28.6) | 0.21(0.07–0.60) | 0.004 | 1.02 (0.81–1.29) | 0.85 |
| Växjö/Ljungby | 13/21 (61.9) | 0.83 (0.30–2.31) | 0.72 | 1.64(1.07–2.53) | 0.03 |
| Halmstad | 8/18 (44.4) | 0.41 (0.14–1.18) | 0.10 | 1.25 (0.94–1.67) | 0.12 |
| Missing | 8/16 (50.0) | ||||
Statistically significant associations are indicated in bold.
Using urban settings with a population of 50,000 inhabitants or more as reference, BC patients from rural settings with a population of <10,000 inhabitants were significantly less likely to be registered at the Oncogenetic Clinic in Lund (OR 0.39, p = 0.001). However, the trend of registration at the clinic for BC patients diagnosed from 2000 to 2013 indicated significant improvement over time for patients from both communities with <10,000 inhabitants and communities with 10,000–50,000 inhabitants (OR 1.21, p = 0.001, and OR 1.18, p = 0.02, respectively; Table 4).
When analyzing the association between treating hospital and the probability of registration at the Oncogenetic Clinic in Lund, BC patients from the hospitals in Karlskrona and Karlshamn (OR 0.21, p = 0.004) were significantly less likely to be registered at the clinic compared with patients from Lund (Table 4). The result regarding BC patients treated at the hospital in Malmö was not statistically significant (OR 2.13, p = 0.07), nevertheless indicating that BC patients from Malmö were more likely to be registered at the clinic compared with patients from Lund. The result regarding BC patients from Kristianstad/Hässleholm/Ystad was also not statistically significant (OR 1.36, p = 0.51). However, in this group of hospitals, all BC patients from Ystad were registered at the clinic (data not shown). The trend of registration over time at the Oncogenetic Clinic in Lund indicated significant improvement for the hospitals in Lund (OR 1.17, p = 0.04), Växjö/Ljungby (OR 1.64, p = 0.03), and Helsingborg/Ängelholm/Landskrona/Trelleborg (OR 1.25, p = 0.02; Table 4), despite none of the BC patients from the hospital in Trelleborg being registered at the clinic (data not shown).
Discussion
In the present study, we evaluated why not all women diagnosed with BC at the age of 35 years or younger in the South Swedish Health Care Region were registered at the Oncogenetic Clinic at Skåne University Hospital in Lund. All the patients in our cohort fulfilled the Swedish national breast cancer guidelines for consideration of referral for genetic counseling and testing. However, more than one-third of the BC patients were not registered at the clinic. One explanation as to why some of the early-onset BC patients were not registered at the Oncogenetic Clinic in Lund could be that the patients did not wish to attend genetic counseling and undergo genetic testing because they did not want to know if they were carriers of a pathogenic variant in a BC predisposing gene. Another explanation could be that the BC patients experienced postdiagnosis distress and that the information regarding genetic counseling and testing was too much information at that time, and was therefore disregarded. However, the hypothesis of this study was that place of residence of the patients at BC diagnosis and the hospitals where they were treated were plausible contributing factors as to why not all patients were registered at the clinic.
It should be noted that prior to the year 2012, the lower age limit for consideration of genetic counseling and testing, regardless of family history of cancer, was 30 years. The lower age limit of 35 years was mentioned in older state-of-the-art documents maintained by the Swedish Oncogenetic Network, but not explicitly stated in the Swedish national breast cancer guidelines until 2012 [4]. Nevertheless, for BC patients diagnosed at 30 years or younger between 2000 and 2013, approximately 25% were still not registered at the Oncogenetic Clinic in Lund at the end of August in 2018. In 2018, the lower age limit in the Swedish national breast cancer guidelines was once again altered, so that all BC patients diagnosed at 40 years or younger are now recommended genetic counseling and testing [15]. However, this revision does not affect the results in our retrospective study.
In a previous study, the authors reported that 59% of the patients diagnosed at 40 years or younger were referred for genetic counseling, 37% attended the genetic consultation, and 24% completed genetic testing within 2 years after diagnosis [16]. In our study, 42% of the 279 BC patients were registered at the Oncogenetic Clinic, 41% had their first meeting at the Oncogenetic Clinic, and 39% underwent genetic testing within 2 years after diagnosis (when excluding the 11 BC patients who were registered at the clinic before diagnosis). In another study, the authors described that 40% of the BC patients diagnosed at 40 years or younger underwent genetic testing, and in most cases (99%) after diagnosis. Out of these patients, 25% tested positive for inherited BRCA1/2 pathogenic variants [17]. In our study, 17% of the 167 BC patients who underwent genetic testing tested positive for germline pathogenic variants in BRCA1 or BRCA2.
Previously published studies have reported an increase in referrals due to the so-called Angelina Jolie effect [18, 19]. The BC patients in our study were diagnosed between 2000 and 2013. However, follow-up was conducted until August 2018 in order to provide time for the patients to be referred to, and attend meetings at, the Oncogenetic Clinic in Lund. No evident peaks were seen in the number of BC patients attending genetic counseling and testing after Angelina Jolie's decisions to undergo mastectomy in 2013 and oophorectomy in 2015, respectively. Rather, a continuous steady increase was seen from 2000 and onwards (data not shown).
Analysis of the association between place of residence at BC diagnosis and treating hospital and the probability of registration at the Oncogenetic Clinic in Lund revealed that patients from 2 of the counties in the South Swedish Health Care Region were less likely to have been registered at the clinic. Consistent with previous studies, improvement was indicated regarding the trend of registration over time (from 2000 to 2013) [20, 21]. This was true for all counties in the South Swedish Health Care Region except for 1 of the 2 counties where the BC patients were also less likely to have been registered at the Oncogenetic Clinic in Lund. One explanation could be that the distance between this county and the clinic in Lund was considered too far and the patients did not appreciate the benefits of taking the time and effort to go to the clinic. In a previous study, the authors presented different reasons as to why patients affected with breast and/or ovarian cancer, or non-affected patients with a family history of cancer, did not pursue genetic counseling after their first informative genetic consultation [13]. One of the reasons given for discontinuing counseling was that the hospital was too far away. Another reason was that they did not have the time to do all the visits required for the genetic testing program. However, in this previous study, the patients discontinued counseling after their first meeting. In our study, the patients were never referred. Because of their young ages, all the BC patients in our study should have been referred and subsequently given the option of attending or declining genetic counseling and testing. Hence, the distance between the residence at BC diagnosis and the Oncogenetic Clinic in Lund should not have been an important factor regarding the probability of registration. In addition, other counties with a similar distance to the clinic showed significant improvement over time (Fig. 1; Table 1).
Another previous study has reported the patients' socioeconomic status, for example, marital status, education levels, and income, as a reason to why some early-onset BC patients do not receive genetic testing despite the clinical recommendations for this group [12]. In Sweden, the genetic counseling sessions and genetic testing are included in the health care system. A visit to a physician costs less than EUR 30 and the maximum cost for health care per year is approximately EUR 110 per patient. Subsequent health care is free of charge. After attending a meeting with a physician, the physician can refer the BC patient to the regional oncogenetic clinic, where the genetic counseling session and the genetic testing are free of charge for the patient.
Participants who accept genetic counseling are more often better educated than those who decline [22, 23]. If BC patients with a higher educational background in general have a higher knowledge of genetics and hereditary cancer, thus more often discussing the subject with their physicians early in the process, this might lead to a higher probability of referral. A limitation of our study is that information regarding educational background is missing. However, in Sweden, 9 years of elementary school is mandatory, and all citizens have access to 3 years of high school free of charge.
The analysis regarding the association between the population setting at BC diagnosis and the probability of registration at the Oncogenetic Clinic in Lund revealed that patients from rural settings were less likely to be registered at the clinic than patients from urban settings, which correspond well with results from a previous study [24]. However, the trend of registration at the clinic over time (from 2000 to 2013) indicated significant improvement for BC patients from both communities with <10,000 inhabitants and communities with 10,000–50,000 inhabitants, which is an encouraging trend.
Some previously published studies have addressed the association between ethnicity and the probability of receiving genetic counseling and testing [22, 25]. It should be mentioned that while our study relies heavily on the high quality of Swedish health care registries and the possibility to link data from different registries through personal identification numbers, ethnicity is considered sensitive information and could not be extracted on an individual basis for this project. However, Malmö is the largest of all the communities in the South Swedish Health Care Region and in 2018 approximately 34% of the inhabitants in Malmö were immigrants [26]. Nevertheless, >80% of the BC patients from Malmö were registered at the Oncogenetic Clinic in Lund. Even though the result from the logistic regression analysis was not statistically significant (OR 2.13, p = 0.07), it indicated that the BC patients from Malmö were more likely to be registered at the clinic compared with patients from Lund, where 22% of the inhabitants were immigrants in 2018. In addition, BC patients from Karlskrona and Karlshamn were significantly less likely to be registered (OR 0.21, p = 0.004) at the clinic compared with patients from Lund. In 2018, 13% of the inhabitants in Karlskrona and 14% in Karlshamn were immigrants [26]. Both abovementioned results indicate that ethnical background might not be a large confounder in our study, quite the opposite.
Among high-risk BC patients, previously published studies demonstrate that the clinical setting can also be associated with the probability of referral for cancer genetic counseling. One factor influencing the probability of referral is a lack of discussion regarding the possibility of carrying a germline pathogenic variant with the physician [21]. However, most medical specialists appear to attach value to investigating whether patients have an inherited predisposition for BC or not, and have positive attitudes towards genetic counseling and testing in general [11]. Since we cannot explain the difference in referrals with general confounders, it appears that either structural differences at different regional hospitals, or alternatively the physicians' attitudes or awareness of referral criteria, most likely could be plausible explanations for the low referral rates in our study too. In a recent paper, the author argues that while the potential value of personalized medicine in general may be overrated, there is evidence that universal testing for BRCA1 and BRCA2 may be of benefit on a population level. The current criteria for genetic counseling and testing may be an obstacle for women to receive genetic testing, and the author and co-workers have initiated a Canadian project (TheScreenProject), which provides testing to all Canadian women for a relatively low fee [27]. However, the accrual to this project is described as low, exemplifying that even in the context of population testing the complexities of dissemination of information to increase uptake should not be forgotten.
One limitation that must be considered in the interpretation of the results in our study is that the number of observed BC patients was relatively small. It should be noted, however, that we were able to include all 279 women who were diagnosed with BC at the age of 35 years or younger in the South Swedish Health Care Region between the years 2000 and 2013. Because all patients diagnosed with early-onset BC in the South Swedish Health Care Region, which encompasses approximately 20% of the Swedish population, would be referred to the Oncogenetic Clinic at Skåne University Hospital in Lund, this study can be considered population based. The results regarding the differences in the possibility of being offered genetic counseling and testing at an oncogenetic clinic may therefore be valid for the entire country. Regarding the generalizability to other countries, a previous study compared findings from BC genetic counseling in the UK and the Netherlands and found that there is reason to be cautious when attempting to transfer research findings between different cultures [23]. At the same time, the authors found that core components in information needs were very similar in the 2 countries, demonstrating that knowledge propagation and education are of vital importance to improve BC genetic counseling.
Conclusion
The results of this study suggest that there is an association between place of residence at BC diagnosis and treating hospital and the probability of referral for genetic counseling and testing for patients diagnosed at the age of 35 years or younger in the South Swedish Health Care Region. Because of the benefits of genetic testing, including surveillance, risk-reducing prophylactic surgery, and the potential for targeted treatment, for example PARP inhibitors for carriers of BRCA1/2 pathogenic variants, our results indicate a need for extended oncogenetic service and educational outreach in regional hospitals to improve care. Further efforts are required to ensure the integration of the Swedish national breast cancer guidelines regarding referral for genetic counseling and testing into clinical practice for women diagnosed with BC at an early age. Recently, Regional Cancer Centers (RCCs) have been initiated in all the Swedish health care regions. The RCCs work towards improving cancer care by defining areas for improvement, setting clear goals for the regions' cancer care, following up results, and ensuring spread of knowledge and that national guidelines and cancer care guidelines are applied across the regions. Therefore, the RCCs are important players also in disseminating knowledge regarding hereditary predisposition to cancer. On a general level, further educational and outreach activities within different health care systems may be needed to ensure that all women diagnosed with early-onset BC receive proper genetic counseling and access to testing.
Statement of Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the European Research Council under Advanced Grant ERC-2011-294576, the Swedish Cancer Society, the Hospital Funds of Skåne University Hospital, and the Mrs. Berta Kamprad Foundation.
Author Contributions
The authors hereby declare that all illustrations and figures are entirely original and do not require reprint permission. A.A., C.E., U.K., and H.O. contributed to the study concept and design. A.A., C.E., U.K., H.O., and H.E. contributed to the acquisition, analysis, and interpretation of data, drafting of the manuscript, and made critical revisions and approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We wish to thank Karin Henriksson, manager at the OnkGen Register at Skåne University Hospital in Lund, Bo Midberg, data manager at the Division of Cancer Epidemiology at Lund University, and Philippe Wagner, statistician at the Division of Cancer Epidemiology at Lund University.
References
- 1.The National Board of Health and Welfare [Internet] Cancer incidence in Sweden. 2014. [cited 2019 Nov 25]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2015-12-26.pdf.
- 2.Paluch-Shimon S, Pagani O, Partridge AH, Bar-Meir E, Fallowfield L, Fenlon D, et al. Second international consensus guidelines for breast cancer in young women (BCY2) Breast. 2016 Apr;26:87–99. doi: 10.1016/j.breast.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Pagani O, Abulkhair O, Aebi S, Amant F, Azim HA, Jr, et al. First international consensus guidelines for breast cancer in young women (BCY1) Breast. 2014 Jun;23((3)):209–20. doi: 10.1016/j.breast.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Regionala cancercentrum i samverkan. Nationellt vårdprogram Bröstcancer. 2014. [cited 2019 Nov 25]. Available from: http://www.swebcg.se/vardprogram/
- 5.Henriksson K, Olsson H, Kristoffersson U. The need for oncogenetic counselling. Ten years' experience of a regional oncogenetic clinic. Acta Oncol. 2004;43((7)):637–49. doi: 10.1080/02841860410018520. [DOI] [PubMed] [Google Scholar]
- 6.Domchek SM. The evolution of poly(ADP-ribose) polymerase inhibitors in the treatment of breast cancer. Clin Adv Hematol Oncol. 2018 May;16((5)):330–2. [PubMed] [Google Scholar]
- 7.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018 Dec;379((26)):2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Flores-Ramos L, Álvarez-Gómez RM, Villarreal-Garza C, Wegman-Ostrosky T, Mohar A. Breast cancer genetics in young women: what do we know? Mutat Res. 2017 Oct;774:33–45. doi: 10.1016/j.mrrev.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Llort G, Chirivella I, Morales R, Serrano R, Sanchez AB, Teulé A, et al. SEOM Hereditary Cancer Working Group SEOM clinical guidelines in Hereditary Breast and ovarian cancer. Clin Transl Oncol. 2015 Dec;17((12)):956–61. doi: 10.1007/s12094-015-1435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustinsson A, Ellberg C, Kristoffersson U, Borg Å, Olsson H. Accuracy of self-reported family history of cancer, mutation status and tumor characteristics in patients with early onset breast cancer. Acta Oncol. 2018 May;57((5)):595–603. doi: 10.1080/0284186X.2017.1404635. [DOI] [PubMed] [Google Scholar]
- 11.Van Riel E, Wárlám-Rodenhuis CC, Verhoef S, Rutgers EJ, Ausems MG. BRCA testing of breast cancer patients: medical specialists' referral patterns, knowledge and attitudes to genetic testing. Eur J Cancer Care (Engl) 2010 May;19((3)):369–76. doi: 10.1111/j.1365-2354.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 12.Von Wachenfeldt A, Brandberg Y, Johansson H, Fornander T. Socioeconomic status and quality of life of women with family history of breast cancer attending an oncogenetic counseling clinic: a comparison with general population. Acta Oncol. 2009;48((1)):86–92. doi: 10.1080/02841860802343182. [DOI] [PubMed] [Google Scholar]
- 13.Caruso A, Vigna C, Maggi G, Sega FM, Cognetti F, Savarese A. The withdrawal from oncogenetic counselling and testing for hereditary and familial breast and ovarian cancer. A descriptive study of an Italian sample. J Exp Clin Cancer Res. 2008 Nov;27((1)):75. doi: 10.1186/1756-9966-27-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Sweden [Internet] Folkmängd och landareal i tätorter, per tätort. Vart femte år. 1960 - 2018. [cited 2019 Nov 25]. Available from: https://www.scb.se/hitta-statistik/statistik-efter-amne/miljo/markanvandning/tatorter/#_Rapporter.
- 15.Regionala cancercentrum i samverkan. Nationellt vårdprogram Bröstcancer. 2018. [cited 2019 Nov 25]. Available from: http://www.swebcg.se/vardprogram/
- 16.Powell CB, Littell R, Hoodfar E, Sinclair F, Pressman A. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer. 2013 Mar;23((3)):431–6. doi: 10.1097/IGC.0b013e318280f2b4. [DOI] [PubMed] [Google Scholar]
- 17.Samphao S, Wheeler AJ, Rafferty E, Michaelson JS, Specht MC, Gadd MA, et al. Diagnosis of breast cancer in women age 40 and younger: delays in diagnosis result from underuse of genetic testing and breast imaging. Am J Surg. 2009 Oct;198((4)):538–43. doi: 10.1016/j.amjsurg.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Evers C, Fischer C, Dikow N, Schott S. Familial breast cancer: genetic counseling over time, including patients´ expectations and initiators considering the Angelina Jolie effect. PLoS One. 2017 May;12((5)):e0177893. doi: 10.1371/journal.pone.0177893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts MC, Dusetzina SB. The effect of a celebrity health disclosure on demand for health care: trends in BRCA testing and subsequent health services use. J Community Genet. 2017 Apr;8((2)):141–6. doi: 10.1007/s12687-017-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehl KL, Shen C, Litton JK, Arun B, Giordano SH. Rates of BRCA1/2 mutation testing among young survivors of breast cancer. Breast Cancer Res Treat. 2016 Jan;155((1)):165–73. doi: 10.1007/s10549-015-3658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SM, Ruddy KJ, Tamimi RM, Gelber S, Schapira L, Come S, et al. BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer. JAMA Oncol. 2016 Jun;2((6)):730–6. doi: 10.1001/jamaoncol.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Riel E, van Dulmen S, Ausems MG. Who is being referred to cancer genetic counseling? Characteristics of counselees and their referral. J Community Genet. 2012 Oct;3((4)):265–74. doi: 10.1007/s12687-012-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albada A, Werrett J, Van Dulmen S, Bensing JM, Chapman C, Ausems MG, et al. Breast cancer genetic counselling referrals: how comparable are the findings between the UK and the Netherlands? J Community Genet. 2011 Dec;2((4)):233–47. doi: 10.1007/s12687-011-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivers KF, Baldwin LM, Miller JW, Matthews B, Andrilla CH, Lishner DM, et al. Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: a vignette-based study. Cancer. 2011 Dec;117((23)):5334–43. doi: 10.1002/cncr.26166. [DOI] [PubMed] [Google Scholar]
- 25.Stamp MH, Gordon OK, Childers CP, Childers KK. Painting a portrait: analysis of national health survey data for cancer genetic counseling. Cancer Med. 2019 Mar;8((3)):1306–14. doi: 10.1002/cam4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics Sweden [Internet] Utrikes födda efter län, kommun och födelseland 31 december. 2018. [cited 2019 Nov 25]. Available from: https://www.scb.se/hitta-statistik/statistik-efter-amne/befolkning/befolkningens-sammansattning/befolkningsstatistik/
- 27.Narod SA. Personalised medicine and population health: breast and ovarian cancer. Hum Genet. 2018 Oct;137((10)):769–78. doi: 10.1007/s00439-018-1944-6. [DOI] [PubMed] [Google Scholar]