Abstract
Background
Identification of high-risk stage II colorectal cancer (CRC) patients, potential candidates for adjuvant chemotherapy, is challenging. Current clinical guidelines rely mainly on histopathological markers with relatively weak prognostic value. This motivates further search for prognostic markers.
Methods
This explorative study aimed to identify potential candidate gene mutations to facilitate differentiation between subgroups of patients with CRC stage II. Panel-based massive parallel sequencing was used to genetically characterize tumor tissues from 85 patients radically operated for CRC stage II, of which 12 developed recurrent cancer during follow-up. Genetic data was compared between patients with or without cancer recurrence, between tumors located in colon and in rectum, and for association with tumor differentiation grade.
Results
Genetic variation in ATM, C11ORF65 was associated with recurrence-free survival. Previous reports regarding the association between BRAF mutation and a higher age at diagnosis, and tumor location in colon were confirmed. APC, BRAF, or KRAS mutation was associated with tumor differentiation grade. Multiple correspondence analyses revealed no obvious clustering of patients with the studied clinical characteristics, indicating that the genetic signatures observed here were unique for each individual.
Conclusions
Taken together, we have demonstrated the utility of panel-based massive parallel sequencing to explore the pathogenesis of CRC stage II. We have identified promising candidate gene mutations associated with cancer recurrence, tumor location, and differentiation grade in patients with CRC stage II, which merit further investigation.
Keywords: Colorectal cancer stage II, Genomic profiling, ATM, BRAF, APC, KRAS
Introduction
Colorectal cancer (CRC) is the most common cancer form after lung cancer, female breast cancer, and prostate cancer [1]. Approximately 25% of the CRC patients are diagnosed as stage II. The majority of these patients are cured by surgery alone and prognosis is relatively good. However, 15–25% of stage II patients develop a more severe phenotype and may benefit from adjuvant chemotherapy [2, 3, 4, 5]. Identification of these patients is challenging.
The risk factors used today for identification of high-risk patients and for medical decision-making comprise histopathological low differentiation grade, lymphovascular or perineural invasion, perforation, T4 tumor invasion, and fewer than 12 lymph nodes removed and examined, in combination with microsatellite instability (MSI) status [2, 3, 4, 5]. An MSI stable tumor in CRC stage II is generally associated with a poor recurrence-free survival (RFS) rate [6]. However, the prognostic value of these risk factors is relatively weak [5, 7] leading to potential over- or undertreatment of certain patients.
Technological advances in molecular biology have improved our understanding of genomic changes and signal transduction pathways involved in CRC. Biomarkers at different biological levels have been investigated to distinguish between subgroups of patients within CRC stage II [8, 9, 10, 11, 12, 13, 14]. The use of mRNA profiling has shown potential as prognostic tool but still requires special handling to preserve sample stability. Biomarkers based on DNA are generally more stable. Although many of these markers show promising results, they are not included in clinical guidelines for medical decision-making.
Adjuvant chemotherapy in CRC stage II could probably be initiated on a more rational basis if objective and standardized molecular biomarkers were available and combined with the traditional risk factors. Recognizing the complexity of the colorectal carcinogenesis with multi-genetic events and pathways which interact with each other [15, 16, 17], we used a panel-based approach to explore whether genetic events could differentiate between subgroups of patients with CRC stage II.
Materials and Methods
Study Population
Eighty-five patients (33 female, 52 male) with radical operation for primary CRC stage II were identified from a local biobank of a total 401 CRC patients who underwent surgical resection for colorectal adenocarcinoma at the Department of Surgery, County Hospital Ryhov, Jönköping, south-eastern Sweden, between 1996 and 2013. Tumor location, differentiation grade, postoperative staging, and other histopathological characteristics were noted. Follow-up for date of recurrence and date and cause of death was obtained through the patients files. Follow-up ended on the date of death or on December 18, 2018. Tumors were classified as stage II (T3 or T4, N0, M0) according to The American Joint Committee on Cancer (AJCC) classification system v.7 [18].
Sampling and DNA Extraction
Tumor tissue samples were snap-frozen in liquid nitrogen and stored at −70°C. Extraction of DNA was done with QIAamp DNA Mini kit (Qiagen, Hilden, Germany). The concentration of DNA was determined with the Qubit dsDNA BR Assay kit and a Qubit 2.0 fluorometer (ThermoFisher Scientific, Waltham, MA, USA).
Massive Parallel Sequencing
DNA libraries were prepared with the TruSeq Amplicon Cancer panel, which targets 48 cancer-related genes (212 amplicons), and the TruSeq Custom Amplicon Index kit (Illumina, San Diego, CA, USA). Library preparation was done according to the manufacturers' instructions with 250 ng of DNA as template. The pooled libraries were then sequenced on a MiSeq sequencer (Illumina). Genes included in the panel are listed in online supplementary Table S1 (see www.karger.com/doi/10.1159/000507118 for all online suppl. material).
Variant Calling and Filtering
Variants were called using MiSeq Reporter version 2.4 (Illumina) and the Human Genome Build 19 (hg19, GRCh37) as the reference genome. Further filtering was done with Variant Studio version 3.0 (Illumina), to meet the following criteria:
-
-
amplicon coverage >300
-
-
allele frequency >5%
-
-
keep all but synonymous variants
The Integrative Genomics Viewer (IGV; Broad Institute, Cambridge, MA, USA) was used to evaluate variants when judged necessary.
Statistical Analysis
Basic statistic is presented as median (lower and upper quartile). For group comparisons, the Mann-Whitney U test was used. For categorical variables, Fisher's exact test of independence was used. Odds ratios (OR) were expressed with 95% confidence intervals (CI). RFS and cancer-specific survival rates were visualized by Kaplan-Meier curves and compared using log-rank tests. Hazard ratios were calculated using Cox-regression analysis. For group comparisons, two-sided tests were used and considered statistically significant if p < 0.05. p values were not corrected for multiple testing due to the explorative approach of this pilot study, but also as the gene panel used was designed to cover cancer-related genes only. Many of these genes are involved in signaling pathways of the carcinogenesis which are known to interact with each other and are therefore not considered to be completely independent [15, 16, 17].
Multiple correspondence analysis (MCA) was used to study gene signatures. The analyses were based on the pattern of gene mutations per patient.
Mutation count was defined as the sum of all genetic variants detected per gene and patient.
Analysis was done on CRC-associated genes as defined by the gene panel: AKT1, APC, BRAF, CTNNB1, EGFR, FBXW7, KRAS, NRAS, MET, PIK3CA, PTEN, TP53, SRC, and on all 48 genes combined.
Statistical analyses were done using Statistica version 13.3 (Statsoft, Inc., Tulsa, OK, USA) and Rstudio version 1.0.143 [19], with the package FactoMineR [20]. Linkage disequilibrium (LD) calculation was done in LD-link version 3.2.0 (National Cancer Institute, Bethesda, MD, USA) [21].
Results
The median age at diagnosis in the studied population was 72 years (interquartile range 62–78 years). Some 12 patients developed recurrent cancer during follow-up, 8 of which died of a cancer-related cause. There was no difference in the distribution of gender between patients with and without cancer recurrence, between tumor locations, or between tumor differentiation grades (Table 1 and data not shown). All gene mutations found were similarly distributed between gender (data not shown).
Table 1.
Patient characteristics stratified according to cancer recurrence at follow-up
| Cancer recurrence (n = 12) | No cancer recurrence (n = 73) | p value | |
|---|---|---|---|
| Gender (female/male) | 7/5 | 26/47 | 0.20 |
| Age at diagnosis (years) | 76.5 (66.5–78.5) | 72 (62–78) | 0.52 |
| Tumor location (colon/rectum) | 8/4 | 43/30 | 0.76 |
| <12 lymph nodes examined | 5 (42%) | 32 (44%) | 1.00 |
| Poor tumor differentiation | 5 (42%) | 15 (21%) | 0.14 |
| Mucinous tumor | 2 (17%) | 13 (18%) | 1.00 |
| T4 tumor | 3 (25%) | 4 (5.5%) | 0.05 |
| Postoperative adjuvant therapy planned | 1 (8.3%) | 3 (4.1%) | 0.46 |
| Recurrence-free survival (years) | 2.07 (0.89–3.64) | ||
| Survival (years) | 6.05 (3.18–9.04) | 8.74 (5.87–13.28) | 0.07 |
The risk factors used today for identification of high-risk stage II patients were similarly distributed between patients with and without cancer recurrence at follow-up (Table 1). Overall, 66% of patients carried one or more risk factors (1 risk factor: 42%; 2 risk factors: 20%; 3 risk factors: 4%). None of the risk factors was associated with RFS, except for tumor T4 (Table 2).
Table 2.
Recurrence-free survival versus clinical characteristics and versus gene mutation status (univariate analysis)
| Clinical characteristics and gene mutations1 | HR (95% CI) | p value |
|---|---|---|
| Age at diagnosis | 1.02 (0.97–1.07) | 0.45 |
| Location (colon vs. rectum) | 1.56 (0.47–5.21) | 0.47 |
| <12 lymph nodes examined | 0.91 (0.29–2.88) | 0.87 |
| Poor tumor differentiation | 2.93 (0.93–9.25) | 0.07 |
| Mucinous tumor | 0.91 (0.20–4.18) | 0.91 |
| T4 tumor | 4.50 (1.21–16.65) | 0.024 |
| APC2 | 1.33 (0.42–4.20) | 0.62 |
| ATM, C11ORF65 | 0.19 (0.06–0.61) | 0.005 |
| BRAF2 | 2.49 (0.75–8.30) | 0.14 |
| CTNNB12, 4 | 1.32 (0.17–10.25) | 0.79 |
| FBXW72 | 0.49 (0.06–3.84) | 0.50 |
| FGFR1 | 1.95 (0.58–6.48) | 0.28 |
| FGFR3 | 0.49 (0.06–3.84) | 0.50 |
| GNA11 | 0.83 (0.18–3.83) | 0.81 |
| GNAQ | 0.58 (0.18–1.94) | 0.37 |
| HNF1A | 1.11 (0.24–5.09) | 0.89 |
| HRAS4 | 3.66 (0.80–16.73) | 0.09 |
| KRAS2 | 0.78 (0.21–2.89) | 0.71 |
| Chr22 rs358934283 | 0.75 (0.24–2.37) | 0.63 |
| Chr2 rs10595243 | 1.84 (0.58–5.79) | 0.30 |
| PIK3CA2 | 0.47 (0.10–2.16) | 0.33 |
| PTEN2 | 0.40 (0.12–1.32) | 0.13 |
| RB1 | 1.73 (0.52–5.76) | 0.37 |
| APC or CTNNB12 | 1.07 (0.34–3.39) | 0.90 |
| KRAS, BRAF, or NRAS2 | 1.34 (0.43–4.22) | 0.62 |
| KRAS, BRAF, NRAS, or APC2 | 3.90 (0.50–30.22) | 0.19 |
Genes with gene mutation present in at least 10 patients were included in the analysis, unless otherwise stated.
Classified as CRC-associated gene according to the gene panel.
No gene assigned.
Five patients with gene mutation, CTNNB1 included based on its involvement in the Wnt signaling pathway, HRAS based on the significant result in relation to cancer-specific survival (online suppl. Table S5), although few patients with gene mutation. HR, hazard ratio; CI, confidence interval.
BRAF Mutation Was Associated with Tumor Location
Colon cancer was documented in 51 patients and rectal cancer in 34 patients. BRAF mutation was more frequent in colon tumors than in rectal tumors (Table 3) (OR = 15.08 [95% CI; 1.89–120.21], p = 0.010). rs113488022 (BRAF p.V600E) was detected in all BRAF-positive colon tumors but one (rs121913351; BRAF p.G466E).
Table 3.
Gene mutations significantly associated with clinical characteristics
| Gene mutation | Samples with gene mutation | % | Samples with gene mutation | % | p value |
|---|---|---|---|---|---|
| Colon (n = 51) | Rectum (n = 34) | ||||
| BRAF1 | 16 | 31.4 | 1 | 2.9 | 0.002 |
| Poor differentiation (n = 20) | Moderate/well differentiation (n = 65) | ||||
| ABL1 | 3 | 15.0 | 1 | 1.5 | 0.039 |
| APC1 | 5 | 25.0 | 39 | 60.0 | 0.010 |
| BRAF1 | 13 | 65.0 | 4 | 6.2 | <0.001 |
| KRAS1 | 2 | 10.0 | 23 | 35.4 | 0.047 |
| Cancer recurrence (n = 12) | No cancer recurrence (n = 73) | ||||
| ATM, C11ORF65 | 5 | 41.7 | 59 | 80.8 | 0.008 |
Classified as CRC-associated gene according to the gene panel.
BRAF mutation was more common in women than in men (30% vs. 13%), but the difference did not reach statistical significance (p = 0.09). The age at diagnosis was higher in BRAF mutation carriers (median 78, range 73–81 years) than in BRAF wild-type patients (median 71, range 61–77 years), (p = 0.025). Accordingly, the age at diagnosis was higher in patients with colon cancer (median 75, range 67–80 years) compared with rectal cancer (median 71, range 61–74 years), (p = 0.048).
None of the genes frequently studied in CRC, such as KRAS, NRAS, or APC, were associated with tumor location (online suppl. Table S2). The distribution of 0–1 versus 2 APC mutations did not differ between tumor locations (p = 1.00).
BRAF, KRAS, and APC Gene Mutations Were Associated with Tumor Differentiation Grade
The tumor was of poor differentiation grade in 20 patients (24%) and of moderate/well grade in 65 patients (76%). BRAF mutation was more common in poorly differentiated tumors compared with moderate/well-differentiated tumors (OR = 28.32 [95% CI; 7.22–111.07], p < 0.001) (Table 3).
KRAS mutation was more frequent in moderate/well-differentiated tumors compared with tumors with poor differentiation grade (OR = 4.92 [95% CI; 1.05–23.15], p = 0.043) (Table 3). Overall, KRAS mutation was identified in 25 patients (29.4%). The majority of mutations detected were in codons 12 and 13 (84%), whereas sporadic mutations were detected in codons 5, 61, and 117.
APC mutation was noticed in 44 patients (51.8%) and was overrepresented in tumors with moderate/well differentiation grade compared with poorly differentiated tumors (OR = 4.50 [95% CI; 1.46–13.89], p = 0.009) (Table 3). The frequency of 0–1 versus 2 APC mutations was similar over differentiation grades (p = 0.44). Frameshift mutations and stop-gained mutations expected to result in a truncated protein dominated mutations found (91%).
ABL1 mutation was also associated with tumor differentiation grade but identified in a few cases only (Table 3). The distribution of all gene mutations explored in the study population, stratified according to tumor differentiation, is described in online supplementary Table S3.
ATM, C11ORF65 Gene Mutation Was Associated with Cancer Recurrence
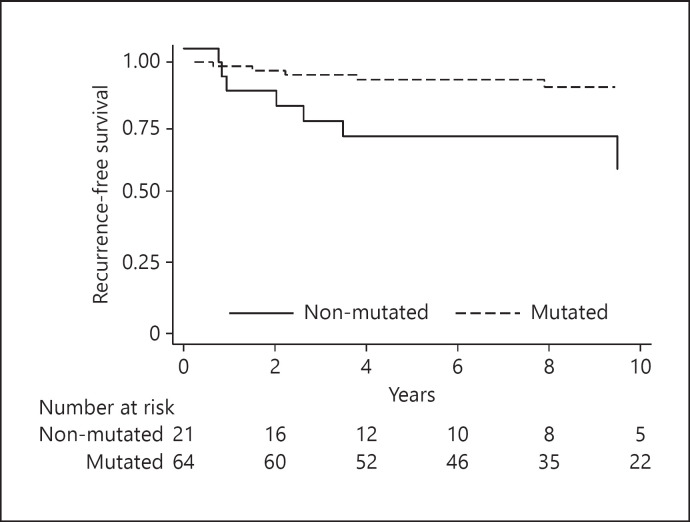
ATM, C11ORF65 mutation was detected in 64 patients (75%) and was differently distributed between patients with and without cancer recurrence at follow-up (Table 3). At a median follow-up of 8.33 years (5.26–12.74 years), patients with ATM, C11ORF65 mutated tumors showed a better RFS than patients with ATM, C11ORF65 wild-type tumors (Fig. 1; Table 2).
Fig. 1.
Kaplan-Meier curve illustrating a better recurrence-free survival rate in ATM, C11ORF65 mutation carriers (log-rank p = 0.003).
The genetic variants contributing to the ATM, C11ORF65 results were mainly the intronic variants rs227075 and rs664143, detected in 63 patients. In all but one patient, the same genotype was observed for rs227075 and rs664143. The variants were detected by different PCR products. However, it could be confirmed that the G allele of rs664143 and the C allele of rs227075 were in LD (r2 = 1.0, p < 0.001) and redundant based on genotype data of a European dataset originating from Phase 3 (Version 5) of the 1000 Genomes Project, available via LD-link.
ABL1, APC, BRAF, or KRAS mutations were not associated with cancer recurrence (Table 2; online suppl. Table S4). The frequency of 0–1 versus 2 APC mutations were similar in patients with and without cancer recurrence at follow-up (p = 0.15).
Gene Mutations and Cancer-Specific Survival
Neither gene mutation of ABL1, APC, BRAF, KRAS, nor of ATM, C11ORF65 was associated with cancer-specific survival (online suppl. Table S5). However, HRAS mutation was significantly associated with cancer-specific survival, but was detected in five patients only (online suppl. Table S5).
Combined Analysis of Gene Mutations and Mutation Count in Relation to Clinical Characteristics
Central pathways in the development of CRC are, among others, the Wnt signaling pathway, the RAS-RAF-MEK-ERK mitogen-activated protein kinase (MAPK) signaling pathway, and the PI3K-PTEN-AKT pathway. These pathways interact with each other in different ways [15, 16, 17]. We found no overlap between KRAS, BRAF, and NRAS mutations, involved in the MAPK pathway, or between APC and CTNNB1 (except for one patient), involved in the Wnt pathway. We analyzed whether defects in any of these pathways separately or combined were associated with RFS (Table 2). KRAS, BRAF, or NRAS mutation and APC mutation co-occurred in 27% of patients, but this combination was not more frequent than expected by chance (p = 1.00).
Due to the complex nature of interacting gene products in CRC, we next investigated the total number of gene mutations and the total mutation count per patient in relation to RFS, tumor location, and tumor differentiation. No significant results were noticed (online suppl. Table S6a–c).
Analysis of Gene Signatures
In MCA, based on the gene mutation status of each CRC-associated gene per patient, the three first MC dimensions explained 16, 13, and 12% of the total variation in data, respectively (online suppl. Fig. S1a). The gene signatures were not associated with cancer recurrence or with tumor location, based on Mann-Whitney U test of sample coordinates. However, the sample coordinates of the first MC dimension were associated with tumor differentiation grade (p < 0.0001). BRAF, APC, and KRAS contributed the most to this dimension, confirming their association with tumor differentiation grade in the univariate analyses. Coordinates of the second dimension were associated with gender (p = 0.014). The genes contributing the most to dimension two were PTEN, CTNNB1, and MET. No correlation between samples coordinates and age at inclusion was noticed in any dimension (data not shown).
Including only the six genes contributing the most to dimension one and two, based on correlation, increased the percentage of variation in data explained by the three first MC dimensions to 28, 24, and 16%, respectively, but the statistical results remained the same as with all CRC-associated genes included in the analysis (online suppl. Fig. S1b, and data not shown).
The association between RFS and gene mutation status of the four genes strongest correlated with MC dimension one and two in the MCA, including also genes and clinical characteristics which showed statistical significance in other comparisons in this study, was investigated in a multivariate Cox regression analysis (Table 4). Again, ATM, C11ORF65 mutation and T4 tumor, the only conventional risk factor associated with RFS, were associated with RFS when considering the effect of the other variables.
Table 4.
Analysis of recurrence-free survival associated with gene mutation status of the four genes strongest correlated with dimension one and two in the MCA, including also genes and clinical characteristics which showed statistical significance in other comparisons in this study (multivariate analysis)
| HR (95% CI) | p value | |
|---|---|---|
| APC | 4.56 (0.94–22.12) | 0.06 |
| ATM, C11ORF65 | 0.14 (0.034–0.53) | 0.004 |
| BRAF | 2.76 (0.53–14.30) | 0.23 |
| CTNNB1 | 0.96 (0.10–9.38) | 0.97 |
| KRAS | 0.62 (0.13–3.03) | 0.56 |
| PTEN | 0.24 (0.06–1.00) | 0.05 |
| T4 tumor | 7.80 (1.42–42.85) | 0.02 |
HR, hazard ratio; CI, confidence interval; MCA, multiple correspondence analysis.
Discussion
Development in molecular diagnostics holds promise for the delivery of precision medicine in the context of cancer management. In this explorative study we determined the genetic profiles of CRC stage II tumors by panel-based massive parallel sequencing, and evaluated identified gene mutations, and the mutation count, in relation to cancer recurrence, tumor location, and tumor differentiation grade.
With a less stringent filtering approach, our main finding was an association between genetic variation in ATM, C11ORF65 and RFS. The main genetic variants contributing to this result were the intronic variants rs227075 T/C and rs664143 A/G. The two variants were in LD and therefore considered to be redundant. Mutation carriers, as defined by the reference genome (G/A+G/G), had a better RFS. The genomic region of the ATM gene harboring these variants overlaps with the 3′-terminal noncoding region of C11ORF65 on the minus strand of DNA. In silico analysis of rs664143 indicates that genetic variation here may affect a protein binding motif of importance in exon 61 splicing of the ATM gene [22]. The putative role of rs227075 remains unclear. C11ORF65 encodes an uncharacterized protein, with high expression mainly in testis and lower expression in other tissues [23].
ATM (ataxia telangiectasia mutated, a serine/threonine kinase) is widely expressed in vivo [23]. The protein is crucial for maintaining genomic integrity and is a key regulator of cell cycle checkpoint, apoptosis, and a main transducer and sensor in DNA double-strand break repair [24, 25, 26, 27, 28]. Reduced protein expression of ATM has been suggested as a biomarker of poor RFS in CRC stage II/III [29], supporting the potential importance of ATM, C11ORF65 as a prognostic marker noticed here. In the context of metastatic disease reduced ATM protein expression, as well as genetic variants of ATM, have been related with increased chemosensitivity to oxaliplatin-based therapy and overall survival [30, 31]. However, the concordance between protein loss and presence of genetic variation, when described, was relatively weak [30].
In summary, ATM, C11ORF65 mutation was associated with RFS in early stage CRC, but not with tumor differentiation or location, and merits further investigation.
Mutations in KRAS, BRAF and APC are important genetic events in the aberrant activation of the MAPK and the Wnt signaling pathways, respectively [15, 32]. The frequency of gene mutations of BRAF (20%), KRAS (29%), and of APC (52%) observed here were of similar magnitude as those published by others [32, 33, 34, 35, 36, 37, 38]. These genes have been studied extensively.
Our results confirm the association between BRAF mutation and age at diagnosis, with tumor location in colon [37, 39, 40], and with poor tumor differentiation in CRC stage II [36, 41]. The observed association between KRAS and a higher degree of tumor differentiation has been described both in CRC stage II and III [35]. The different associations noticed between BRAF and KRAS and tumor differentiation are difficult to explain, but suggest that the genes may have different roles although present in a common signaling pathway.
As has been reported by others in CRC stage II [36, 42], BRAF mutation here was not associated with RFS or with cancer-specific survival. However, BRAF is generally considered an independent predictor of a poor prognosis, and growing evidence suggests that this is particularly true for MSI stable tumors [12, 14, 35, 36, 39, 41].
KRAS seems to be of no prognostic value in CRC stage II, but controversy exists [10, 12, 34, 35, 36, 38, 40, 42].
APC is involved in many signaling pathways and its role in the neoplastic process and in different stages of CRC is not fully understood [32]. By stimulation of proteasomal degradation of ß-catenin (encoded by CTNNB1) APC is a main negative regulator of the Wnt signaling pathway. Genetic variation in APC or CTNNB1 may therefore lead to the deregulation of ß-catenin/T-cell factor-dependent transcription. Studies have shown that the Wnt and the MAPK signaling pathways interact. Defects in one pathway may enhance the activity in the other [15, 16, 17]. Central gene mutations (KRAS, NRAS, BRAF, APC, and CTNNB1) in these pathways are almost mutually exclusive [33, 43]. Separate and combined analysis of these genes has been associated with RFS in MSI stable stage III patients, but not in MSI stable stage II patients [38, 42]. Similar observations were done here in stage II, although MSI status was unknown in our study population.
Over the last decades, analytical technologies have evolved dramatically allowing for the simultaneous analysis of several markers, for the comparison of genetic signatures, gene-expression profiles, or affected pathways between patient categories. Comparing results between studies based on these new technologies is challenging, as results will depend not only on the study design, the technological platform used, or genomic/proteomic content included, but also on the bioinformatic approaches applied on the data. In our analysis based on gene signatures, no obvious clustering of patients with cancer recurrence, tumor location, or differentiation grade was noticed, indicating that gene signatures here were unique for each patient or that the population included was too small to find such patterns.
Taken together, of the traditional risk factors used for the identification of high-risk CRC stage II patients, only T4 tumor was associated with RFS. This supports the need for additional objective markers to facilitate identification of these patients. Here, we have demonstrated the utility of panel-based massive parallel sequencing to explore the pathogenesis of CRC stage II. Our results indicate that genetic variation in ATM, C11ORF65 may be prognostic in CRC stage II. HRAS mutation was associated with cancer-specific survival, but was detected in few patients only. Previous reports regarding the association between BRAF mutation and clinical characteristics were confirmed. Gene mutation of APC, BRAF, or KRAS was associated with tumor differentiation grade. These promising results motivate further studies, including larger cohorts and including associated markers at different biological levels, to assess the true prognostic value and usefulness to refine medical decision-making.
Statement of Ethics
The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. It was approved by the Regional Ethical Review Board in Linköping, Sweden, Dnr 2013/271-31, and informed consent was obtained from the participants.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by grants from FUTURUM − the Academy for Health and Care, Region Jönköping County, Sweden.
Author Contributions
Conception and design: S.H., J.D.; analysis of data: S.H.; interpretation of data: S.H., J.D., R.E.A.; clinical data: R.E.A.; writing − original draft: S.H.; writing − revising, editing for intellectual content: S.H., J.D., R.E.A.; final approval of manuscript: S.H., J.D., R.E.A.; funding acquisition: S.H.
Data Availability
Research materials supporting this publication are not publicly available but may be accessed after reasonable motivation by contacting the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
The authors thank Linda Berglind, Marita Skarstedt, and Viktor Wadskog, Department of Laboratory Medicine, Jönköping, Region Jönköping County, for excellent technical assistance. We also thank Jan Söderman, for valuable advices regarding data analysis, and Andreas Matussek, former head of operation, for approval and encouragement of this study, Department of Laboratory Medicine, Jönköping, Region Jönköping County, Sweden.
References
- 1.WHO and the International Agency for Research on Cancer, Lyon, France: The Globocan Project [accessed January 7, 2019] Available from http://gco.iarc.fr/
- 2.Engstrom PF, Arnoletti JP, Benson AB, 3rd, Chen YJ, Choti MA, Cooper HS, et al. NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Canc Netw. 2009 Sep;7((8)):778–31. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 3.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A, Group EGW. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010 May;21 Suppl 5((Suppl 5)):v70–7. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- 4.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24 Suppl 6((Suppl 6)):vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 5.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015 Jan;54((1)):5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 6.Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013 May;24((5)):1274–82. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer. 2014 May 16;14:336. doi: 10.1186/1471-2407-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol. 2010 Sep 1;28((25)):3937–44. doi: 10.1200/JCO.2010.28.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011 Jan 1;29((1)):17–24. doi: 10.1200/JCO.2010.30.1077. [DOI] [PubMed] [Google Scholar]
- 10.Akiyoshi T, Kobunai T, Watanabe T. Recent approaches to identifying biomarkers for high-risk stage II colon cancer. Surg Today. 2012 Nov;42((11)):1037–45. doi: 10.1007/s00595-012-0324-4. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Hu M, Chen C, Hou D. Computational analysis of mRNA expression profiles identifies a novel triple-biomarker model as prognostic predictor of stage II and III colorectal adenocarcinoma patients. Cancer Manag Res. 2018;10:2945–2. doi: 10.2147/CMAR.S170502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo E, Camps C, Kaisaki PJ, Parsons MJ, Mouradov D, Pentony MM, et al. Mutation burden and other molecular markers of prognosis in colorectal cancer treated with curative intent: results from the QUASAR 2 clinical trial and an Australian community-based series. Lancet Gastroenterol Hepatol. 2018 Sep;3((9)):635–43. doi: 10.1016/S2468-1253(18)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Li Y, Huang D, Cai S, Peng J. HER2 as a potential biomarker guiding adjuvant chemotherapy in stage II colorectal cancer. Eur J Surg Oncol. 2018 Oct 19;45((2)):167–73. doi: 10.1016/j.ejso.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Murcia O, Juárez M, Rodríguez-Soler M, Hernández-Illán E, Giner-Calabuig M, Alustiza M, et al. Colorectal cancer molecular classification using BRAF, KRAS, microsatellite instability and CIMP status: prognostic implications and response to chemotherapy. PLoS One. 2018;13((9)):e0203051. doi: 10.1371/journal.pone.0203051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arends MJ. Pathways of colorectal carcinogenesis. Appl Immunohistochem Mol Morphol. 2013 Mar;21((2)):97–102. doi: 10.1097/PAI.0b013e31827ea79e. [DOI] [PubMed] [Google Scholar]
- 16.Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/β-catenin pathways. J Biol Chem. 2013 Dec 13;288((50)):35651–9. doi: 10.1074/jbc.M112.437913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Geel RM, Beijnen JH, Bernards R, Schellens JH. Treatment individualization in colorectal cancer. Curr Colorectal Cancer Rep. 2015;11((6)):335–44. doi: 10.1007/s11888-015-0288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010 Jun;17((6)):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.RStudio-Team . RStudio: Integrated Development for R. RStudio. Inc.: Boston, MA; 2015. http://www.rstudio.com/ [Google Scholar]
- 20.Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;1((25)):1–18. [Google Scholar]
- 21.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015 Nov 1;31((21)):3555–7. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Kim H, Lee KY, Choe KH, Ryu JS, Yoon HI, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet. 2006 Apr 1;15((7)):1181–6. doi: 10.1093/hmg/ddl033. [DOI] [PubMed] [Google Scholar]
- 23.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014 Feb;13((2)):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998 Sep 11;281((5383)):1674–7. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 25.Petrini JH, Stracker TH. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003 Sep;13((9)):458–62. doi: 10.1016/s0962-8924(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 26.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007 Apr 15;6((8)):931–42. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 27.Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett. 2010 Sep 10;584((17)):3675–81. doi: 10.1016/j.febslet.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita M. Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death. J Radiat Res. 2010;51((5)):493–501. doi: 10.1269/jrr.10039. [DOI] [PubMed] [Google Scholar]
- 29.Beggs AD, Domingo E, McGregor M, Presz M, Johnstone E, Midgley R, et al. Loss of expression of the double strand break repair protein ATM is associated with worse prognosis in colorectal cancer and loss of Ku70 expression is associated with CIN. Oncotarget. 2012 Nov;3((11)):1348–55. doi: 10.18632/oncotarget.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar R, Miranda S, Rodrigues DN, Chénard-Poirier M, Dolling D, Clarke M, et al. Ataxia telangiectasia mutated protein loss and benefit from oxaliplatin-based chemotherapy in colorectal cancer. Clin Colorectal Cancer. 2018 Dec;17((4)):280–4. doi: 10.1016/j.clcc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Randon G, Fucà G, Rossini D, Raimondi A, Pagani F, Perrone F, et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci Rep. 2019 Feb 27;9((1)):2858. doi: 10.1038/s41598-019-39525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst. 2017 Aug 1;109((8)) doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fransén K, Klintenäs M, Osterström A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004 Apr;25((4)):527–33. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 34.Lüchtenborg M, Weijenberg MP, Wark PA, Saritas AM, Roemen GM, van Muijen GN, et al. Mutations in APC, CTNNB1 and K-ras genes and expression of hMLH1 in sporadic colorectal carcinomas from The Netherlands Cohort Study. BMC Cancer. 2005 Dec 15;5:160. doi: 10.1186/1471-2407-5-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010 Dec;21((12)):2396–402. doi: 10.1093/annonc/mdq258. [DOI] [PubMed] [Google Scholar]
- 36.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010 Jan 20;28((3)):466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 37.Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015 Dec;6((6)):660–7. doi: 10.3978/j.issn.2078-6891.2015.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Broek E, Krijgsman O, Sie D, Tijssen M, Mongera S, van de Wiel MA, et al. Genomic profiling of stage II and III colon cancers reveals APC mutations to be associated with survival in stage III colon cancer patients. Oncotarget. 2016 Nov 8;7((45)):73876–87. doi: 10.18632/oncotarget.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012 Feb;55((2)):128–33. doi: 10.1097/DCR.0b013e31823c08b3. [DOI] [PubMed] [Google Scholar]
- 40.Vacante M, Borzì AM, Basile F, Biondi A. Biomarkers in colorectal cancer: current clinical utility and future perspectives. World J Clin Cases. 2018 Dec 6;6((15)):869–81. doi: 10.12998/wjcc.v6.i15.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005 Jul 15;65((14)):6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 42.Birnbaum DJ, Laibe S, Ferrari A, Lagarde A, Fabre AJ, Monges G, et al. Expression profiles in stage II colon cancer according to APC gene status. Transl Oncol. 2012 Apr;5((2)):72–6. doi: 10.1593/tlo.11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998 Mar 15;58((6)):1130–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
Research materials supporting this publication are not publicly available but may be accessed after reasonable motivation by contacting the corresponding author.