Abstract
Background:
Recent studies showed that Macrophage migration inhibitory factor (MIF) is overexpressed and closely associated with prognosis in cancer patients. The present study was systematically evaluated the prognostic significance of MIF expression in cancer patients.
Methods:
PubMed, Cochrane library and Scopus were searched for eligible studies up to January 2020. Pooled hazard ratio with confidence interval (CI) was determined to assess the relationship between MIF expression and survival in cancer patients.
Results:
A total of 8 studies comprising 847 cancer patients were included in this meta-analysis. For overall survival, the pooled hazard ratio was 2.23 (95% CI 1.67–2.99, P < .001). For disease-free survival, the pooled hazard ratio was 2.24 (95% CI 1.69–2.96, P < .001). The results suggested that high expression of MIF was significantly related to poor overall survival and disease-free survival in cancer patients.
Conclusion:
MIF expression could be a valuable prognostic factor in cancer patients.
Keywords: cancer, macrophage migration inhibitory factor, meta-analysis, prognosis
1. Introduction
Cancer is a common cause of morbidity and mortality worldwide.[1] Due to the increasing prevalence of cancer risk factors, including overweight, physical inactivity, smoking, and changing reproductive patterns, the burden of cancer is still growing.[2] Despite intensive research efforts in genomics and drug development for cancer, the prognosis for patients with cancer remains poor. Therefore, a great deal of research is being carried out to discover clinical outcome and efficacy of treatment for cancer patients using biomarkers.
Macrophage migration inhibitory factor (MIF) was identified in the 1960s as a soluble, protein factor secreted by T cells with the ability to inhibit the random migration of macrophages.[3] MIF is a unique cytokine and critical mediator of host defenses with a role in chronic inflammatory and autoimmune disease.[4] Recent studies showed that MIF is overexpressed in many solid cancers and is closely related in the tumor cell proliferation, angiogenesis and tumorigenesis.[3,5,6] Moreover, the overexpression of MIF has been associated to tumor progression, metastasis and unfavorable prognosis in gastric, hepatocellular, pancreatic, and lung cancers, and oral squamous cell carcinoma and metastatic melanoma.[3,7,8]
However, the association between MIF expression and survival in patients with cancer is not systematically understood. In this study, we performed meta-analysis to comprehensively assess the prognostic significance of MIF expression in cancer patients.
2. Materials and methods
2.1. Search strategy
Eligible studies were identified by searching PubMed, Cochrane library and Scopus up to January 2020 using the following terms: (MIF or macrophage migration inhibitory factor) and (cancer or tumor or carcinoma or neoplasm or malignancy) and (prognostic or predict or prognosis or survival or outcome). All significant publications in the references of the reviewed articles were also manually searched to find qualified articles. This study was based on previously published studies, so ethical approval and informed consent were not required.
2.2. Inclusion and exclusion criteria
Eligible studies should be the following criteria:
-
(1)
the expression of MIF was detected in the tumor cells of human cancer tissue using immunohistochemistry;
-
(2)
the association between MIF expression and survival outcome was evaluated;
-
(3)
the hazard ratio (HR) and 95% confidence interval (CI) assessing multivariate Cox-regression for survival outcome were provided.
Studies were excluded if they met any of the following criteria:
-
(1)
duplicate studies;
-
(2)
conference abstracts, case reports, reviews, letters, and non-English articles;
-
(3)
studies of hematologic malignancy patients and children.
2.3. Data extraction and quality assessment
Two authors extracted necessary data independently and reached a consensus by discussion. The extracted data were as follows: first author, publication year, country, cancer type, sample size, sex, median age, tumor stage, study period, follow-up period, endpoints, MIF expression associated with poor prognosis, cut-off value of MIF expression, and adjusted HR with 95% CI for survival. The quality of eligible studies was assessed according to the Newcastle-Ottawa Scale (NOS). The scale included selection of the study group, comparability of groups and ascertainment of outcomes, and the NOS scores ranged from 0 to 9. The articles scored greater than 6 were considered as a high quality.
2.4. Statistical analysis
StataSE 12 (Stata, College Station, TX) was used for meta-analysis. The prognostic significance of MIF expression was evaluated using pooled HR and 95% CI. The heterogeneity among the enrolled studies was assessed by the Higgins’ I2 statistics and the chi-square Q test and an I2 statistic > 50% or a P value < .1 was considered as significant in a random effects model. Subgroup analysis was also performed to reveal the cause of heterogeneity. The publication bias was assessed by Funnel plot and Egger regression test. And the sensitivity analysis was conducted to estimate the consistency of the pooled results. P values <.05 were regarded as statistically significant.
3. Results
3.1. Search results
Figure 1 shows the process flow diagram of study selection. After removing due to duplication and screening the titles and abstracts, 34 full-text articles are assessed for eligibility. Finally, 8 studies comprising 847 cancer patients were included for our meta-analysis.
Figure 1.
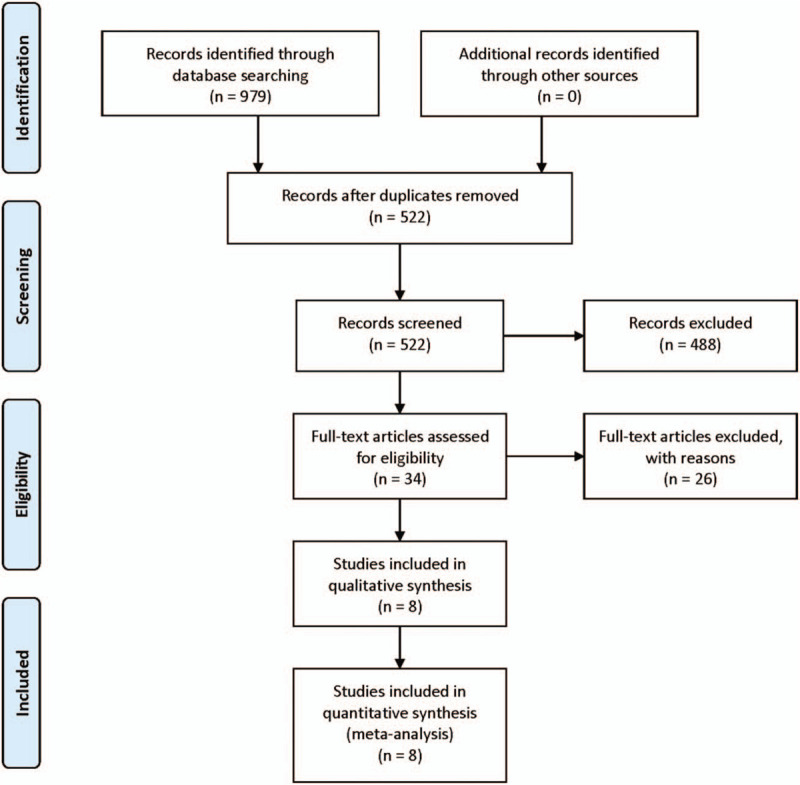
Flow diagram of study selection.
3.2. Study characteristics
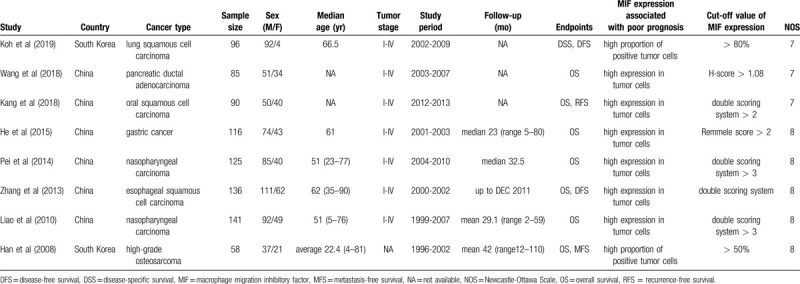
The basic characteristics of included studies are summarized in Table 1. Two studies were performed in South Korea, while the others were performed in China, published between 2008 and 2019. A total of 847 patients were included, and the enrolled studies were consisted of seven types of cancers, including lung squamous cell carcinoma (n = 1), pancreatic ductal adenocarcinoma (n = 1), oral squamous cell carcinoma (n = 1), gastric cancer (n = 1), nasopharyngeal carcinoma (n = 2), esophageal squamous cell carcinoma (n = 1), and high-grade osteosarcoma (n = 1). HR and 95% CI were obtained directly multivariate analysis from all of original articles. All the studies conducted immunohistochemistry to evaluate MIF expression in the tumor cells of human cancer tissue. NOS scores of all include studies ranges from 7 to 8.
Table 1.
Basic characteristics of the included studies.
3.3. Relationship between MIF expression and overall survival (OS)
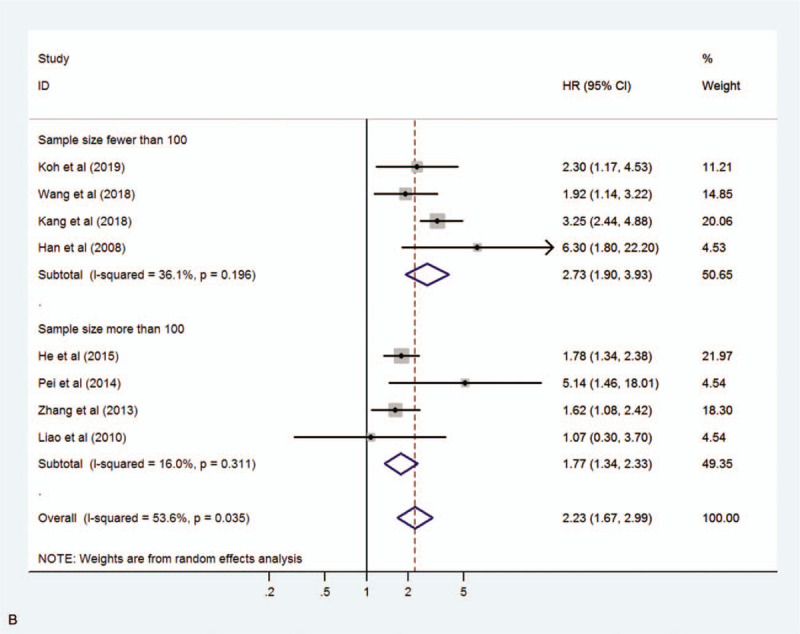
We assessed 8 studies to reveal the relationship between MIF expression and OS including disease-specific survival. A random-effect model was used due to significant heterogeneity (I2 = 53.6%, P = .035). The pooled HR for OS in cancer patients with high expression of MIF compared with low expression was 2.23 (95% CI 1.67–2.99, P < .001), indicating that high expression of MIF was significantly associated with poor OS in cancer patients (Fig. 2). Moreover, subgroup analyses according to cancer type (digestive system cancer or non-digestive system cancer) and sample size (fewer than 100 or more than 100) were conducted to reveal sources of heterogeneity (Fig. 3 A, B). The results showed that the cancer type may be the source of heterogeneity, however this did not affect the overall results.
Figure 2.
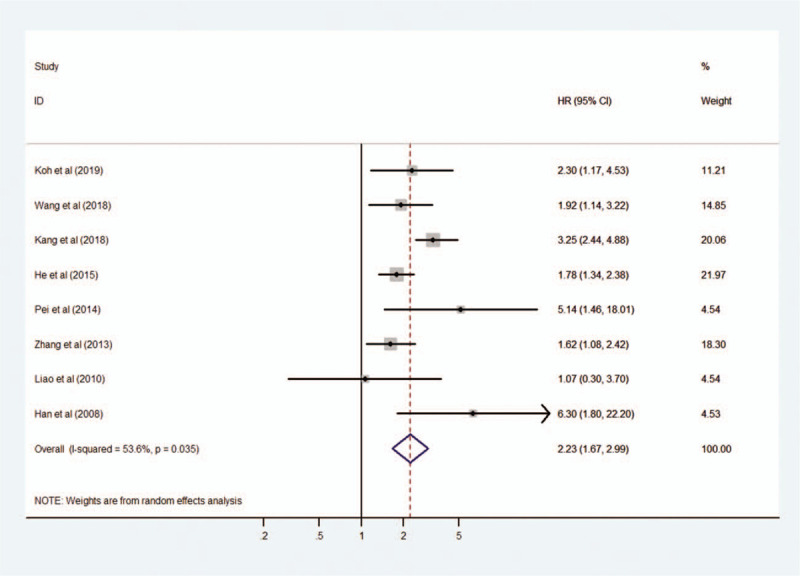
Forest plot reflecting the relationship between MIF expression and overall survival in cancer patients. MIF = macrophage migration inhibitory factor.
Figure 3.
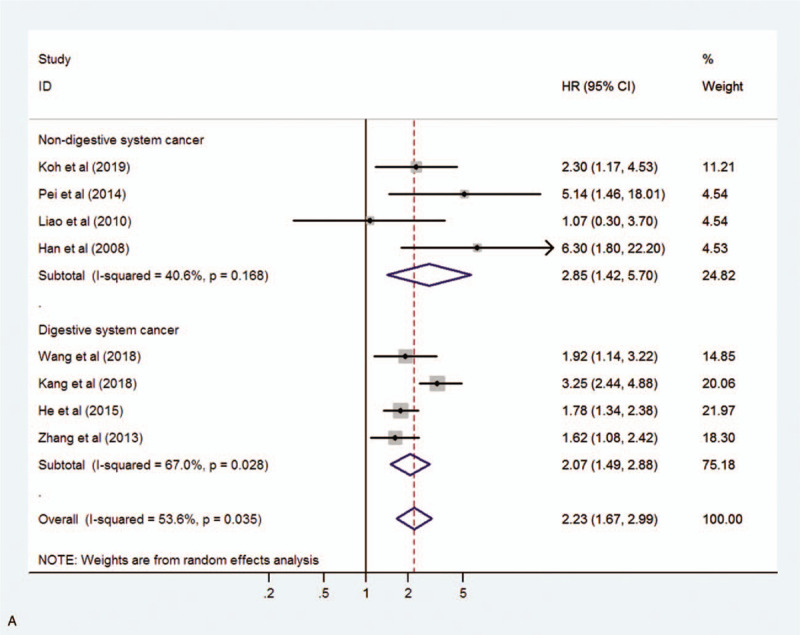
Forest plot showing the subgroup analysis stratified by cancer type (A) and sample size (B).
3.4. Relationship between MIF expression and disease-free survival (DFS)
We evaluated 4 studies to reveal the relationship between MIF expression and DFS including recurrence-free survival and metastasis-free survival. A fixed-effect model was used because of no obvious heterogeneity (I2 = 12.6%, P = .330). The pooled HR for DFS in cancer patients with high expression of MIF compared with low expression was 2.24 (95% CI 1.69–2.96, P < .001), indicating that high expression of MIF was significantly related with worse DFS in cancer patients (Fig. 4). Subgroup analysis was not conducted for DFS due to the limited number of studies and the uniformity among studies.
Figure 3 (Continued).
Forest plot showing the subgroup analysis stratified by cancer type (A) and sample size (B).
3.5. Publication bias
We performed Funnel plot and Egger regression test to assess the publication bias. For OS, the Funnel plot seemed asymmetrical (Fig. 5A). However, Egger regression test did not demonstrate the publication bias (P = .503). We also performed the trim and fill analysis. The result revealed that there is no basis for questioning the validity of the overall results in this study (P < .001) (Fig. 5B). For DFS, the Funnel plot also seemed asymmetrical (Fig. 5C). But, Egger test did not show the publication bias (P = .140). In addition, trim and fill analysis showed a similar finding (P < .001) as shown in the analysis for OS (Fig. 5D).
Figure 4.
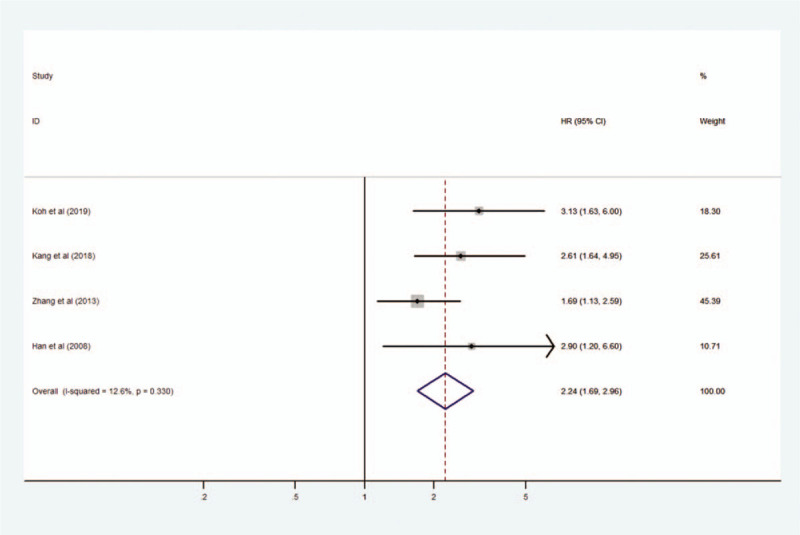
Forest plot reflecting the relationship between MIF expression and disease-free survival in cancer patients. MIF = macrophage migration inhibitory factor.
3.6. Sensitivity analysis
We conducted sensitivity analysis to detect the influences of individual studies and the consistency of our results by omitting single study consecutively. For OS, the sensitivity analysis revealed that omitting the study of Kang et al, the pooled HR appears to be 1.88 (95% CI 1.55–2.29), indicating a significant change compared to the result including all studies (the pooled HR, 2.23; 95% CI, 1.67–2.99). On the other hand, other studies showed that each does not differ much from the overall HR (Fig. 6A). For DFS, no individual study influenced the overall results (Fig. 6B).
Figure 5.
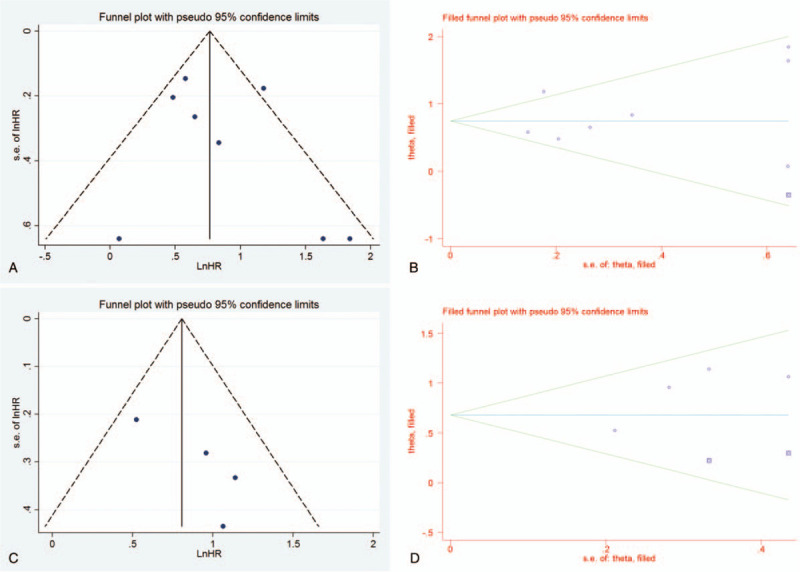
Funnel plot and trim and fill analysis for overall survival (A, B) and for disease-free survival (C, D).
Figure 6.
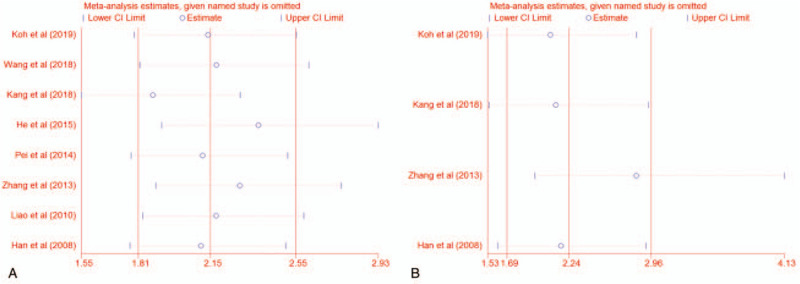
Sensitivity analysis for overall survival (A) and disease-free survival (B).
4. Discussion
MIF is a pleiotropic inflammatory mediator that is considered the first cytokine activity to be reported.[9] MIF plays a central role in acute and chronic inflammatory diseases as well as autoimmune diseases. Recently, it is known not only to play a role in inflammatory diseases, but also to involve in tumorigenesis and tumor progression. MIF contributes to tumor development, progression and tumor cell survival through inhibition of p53-mediated apoptosis.[5] MIF also functions as a pro-survival and anti-apoptotic factor for cancer cells through the activation of PI3K/Akt cascade.[5] Moreover, MIF enables cancer cell growth and metastasis by activating HIF-1α under hypoxic condition.[10] Furthermore, MIF has been suggested as a potential prognostic factor for various cancers including gastric, hepatocellular, pancreatic, and lung cancers, and oral squamous cell carcinoma and metastatic melanoma.[3,7,8] Therefore, we conducted this meta-analysis to calculate the prognostic significance of MIF expression in cancer patients.
We found 8 eligible studies. Han et al[11] showed that high expression of MIF was related with poor OS and metastasis-free survival in patients with high-grade osteosarcoma. Zhang et al[12] and Koh et al[8] demonstrated significantly poorer OS or disease-specific survival and DFS with high expression of MIF compared with low expression in patient with esophageal squamous cell carcinoma and lung squamous cell carcinoma, respectively. Kang et al[13] revealed the relationship between MIF expression and OS and recurrence-free survival in patients with oral squamous cell carcinoma. Liao et al[14] and Pei et al[15] showed that high expression of MIF was associated with poor OS in patients with nasopharyngeal carcinoma. He et al[6] and Wang et al[16] revealed that high expression of MIF was related with unfavorable OS in patients with gastric cancer and pancreatic ductal adenocarcinoma, respectively.
We finally identified that cancer patients with high expression of MIF tended to have worse OS and DFS than those with low expression through meta-analysis. The pooled HR was 2.23 (95% CI 1.67–2.99, P < .001) and 2.24 (95% CI 1.69–2.96, P < .001) for OS and for DFS, respectively. Therefore, MIF expression could be used as a prognostic biomarker for OS and for DFS in cancer patients. To the best of our knowledge, this is the first meta-analysis to evaluate the prognostic significance of MIF expression in cancer patients.
5. Limitations
There are several limitations to this study. First, all included studies were performed in Asia, which may affect the reliability of our results in western countries. Second, the diversity of included studies may have led to some heterogeneity although we selected eligible studies by uniform criteria. Third, the number and the sample size of eligible studies were limited with only 8 studies consisting 847 cancer patients. Thus, further studies should be designed on a large-scale and individual cancers.
6. Conclusion
This meta-analysis revealed that high expression of MIF was associated with poor survival in cancer patients and could be a useful prognostic factor.
Author contributions
Conceptualization: Hyun Min Koh, Dong Chul Kim
Data curation: Hyun Min Koh, Dong Chul Kim
Formal analysis: Hyun Min Koh, Dong Chul Kim
Investigation: Hyun Min Koh, Dong Chul Kim
Resources: Hyun Min Koh, Dong Chul Kim
Supervision: Dong Chul Kim
Validation: Hyun Min Koh, Dong Chul Kim
Writing – original draft: Hyun Min Koh
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HR = hazard ratio, MIF = macrophage migration inhibitory factor, NOS = Newcastle-Ottawa Scale, OS = overall survival, RFS = recurrence-free survival.
How to cite this article: Koh HM, Kim DC. Prognostic significance of macrophage migration inhibitory factor expression in cancer patients: a systematic review and meta-analysis. Medicine. 2020;99:32(e21575).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files.
References
- [1].Li X, Shu K, Wang Z, et al. Prognostic significance of KIF2A and KIF20A expression in human cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan H, Wang W, Yan J, et al. Prognostic significance of CXCR7 in cancer patients: a meta-analysis. Cancer Cell Int 2018;18:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol 2012;189:5533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lue H, Kleemann R, Calandra T, et al. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microb Infect 2002;4:449–60. [DOI] [PubMed] [Google Scholar]
- [5].Subbannayya T, Leal-Rojas P, Barbhuiya MA, et al. Macrophage migration inhibitory factor-a therapeutic target in gallbladder cancer. BMC Cancer 2015;15:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].He LJ, Xie D, Hu PJ, et al. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterol 2015;21:9916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kindt N, Journe F, Laurent G, et al. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett 2016;12:2247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koh HM, Kim DC, Kim Y, et al. Prognostic role of macrophage migration inhibitory factor expression in patients with squamous cell carcinoma of the lung. Thorac Cancer 2019;10:2209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grieb G, Merk M, Bernhagen J, et al. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect 2010;23:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Winner M, Koong AC, Rendon BE, et al. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res 2007;67:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Han I, Lee MR, Nam KW, et al. Expression of macrophage migration inhibitory factor relates to survival in high-grade osteosarcoma. Clin Orthop 2008;466:2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang L, Ye S, Ma G, et al. The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma. J Transl Med 2013;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kang Y, Zhang Y, Sun Y. Macrophage migration inhibitory factor is a novel prognostic marker for human oral squamous cell carcinoma. Pathol Res Pract 2018;214:1192–8. [DOI] [PubMed] [Google Scholar]
- [14].Liao B, Zhong B, Li Z, et al. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol 2010;102:844–51. [DOI] [PubMed] [Google Scholar]
- [15].Pei XJ, Wu TT, Li B, et al. Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int J Med Sci 2013;11:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang D, Wang R, Huang A, et al. Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma. Oncol Rep 2018;40:2628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


