Abstract
Background:
We design this study to assess the efficacy and safety of Chinese herbal compound for allergic rhinitis in children.
Methods:
PubMed, EMbase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang, the Chongqing VIP Chinese Science and Technology Periodical Database, and China biomedical literature database (CBM) will be searched from the establishment of each database to July 2020. Randomized controlled trials of Chinese herbal compound for the treatment of allergic rhinitis in children will be included. Two researchers will screen the literature, extract data, and assess the risk of bias independently. Statistical analysis will be performed in RevMan 5.3.
Results:
This study will summarize high quality evidence of randomized controlled trials on exploring the efficacy and safety of Chinese herbal compound for allergic rhinitis in children.
Conclusions:
The findings of study will provide scientific evidence of the efficacy and safety of Chinese herbal compound for allergic rhinitis in children for clinician and further studies.
Ethics and dissemination:
The private information from individuals will not be published. This systematic review also will not involve endangering participant rights. Ethical approval is not required. The results may be published in a peer-reviewed journal or disseminated in relevant conferences.
OSF Registration number:
DOI 10.17605/OSF.IO/Q5TRZ.
Keywords: Chinese herbal compound, pediatric, allergic rhinitis, systematic review
1. Introduction
Allergic rhinitis in children is a non-infectious inflammatory disease of nasal mucosa caused by exposure to allergens and mediated mainly by immunoglobulin E (IgE) in susceptible children.[1] The 4 typical symptoms of allergic rhinitis in children are sneezing, watery nose, nasal itching, and nasal congestion. Infants can show nasal congestion, accompanied by mouth opening breathing, snoring, wheezing, feeding difficulties, nose, and eyes rubbing, etc.[2]. Allergic rhinitis has become a major respiratory inflammatory disease in children, mostly occurring between 8 and 13 years old, with an increasing incidence year by year.[3,4] Due to childrens difficulty in self-management, various symptoms could not only seriously affect childrens physical and mental health, academic performance and quality of life, but also lead to various complications, including asthma, conjunctivitis, and adenoid hypertrophy.[5,6] Studies have found that allergic rhinitis in children is related to allergy, low immunity, heredity, air pollution, and other factors.[7] The main pathological mechanism is the entry of antigens into the sensitized individuals, which causes the release of relevant inflammatory mediators and the aggregation of inflammatory cells, thus triggering a series of symptoms.[8] The treatment of allergic rhinitis in children requires the combination of prevention and treatment, which includes environmental control, drug therapy, immunotherapy, and health education.[9,10] Drug therapy mainly includes glucocorticoids, antihistamines, and leukotriene receptor antagonists.[11] Although those drugs can quickly relieve symptoms and slow down disease progression, it is difficult to fundamentally improve allergy, with poor long-term efficacy and high risk of recurrence. Moreover, long-term use of those drugs is prone to drug resistance and side effects in different levels (such as growth retardation, fatigue, impaired liver and kidney function, gastrointestinal reactions, etc.).[12]
The traditional Chinese medicine compound has the treatment characteristic of multi-pathways, multi-links and multi-targets, and has the potential prevention and treatment advantages. The treatment of allergic rhinitis in children with traditional Chinese medicine compound has the effect of regulating immunity and reducing allergic state, with the advantages of safety, reliability, stable efficacy, low recurrence rate, minimal toxicity and side effects, which has attracted wide attention.[13] However, there is no systematic review and meta-analysis evaluating the efficacy and safety of Chinese herbal compound in pediatric patients with allergic rhinitis. Thus, this study will evaluate the efficacy and safety of Chinese herbal compound in pediatric patients with allergic rhinitis.
2. Methods
2.1. Study registration
This protocol of systematic review and meta-analysis has been drafted under the guidance of the preferred reporting items for systematic reviews and meta-analyses protocols (PRISMA-P). Moreover, it has been registered on open science framework (OSF) on July 8, 2020. (Registration number: DOI 10.17605/OSF.IO/Q5TRZ)
2.2. Ethics
Ethical approval is not required because there is no patient recruitment and personal information collection, and the data included in our study are derived from published literature.
2.3. Inclusion criteria for study selection
2.3.1. Type of studies
Randomized controlled trials involving Chinese herbal compound for pediatric patients with allergic rhinitis will be included. The language will be limited to Chinese and English.
2.3.2. Type of participants
Participants conformed to Guidelines for diagnosis and treatment of pediatric allergic rhinitis[3] will be included, regardless of gender, age, and race.
2.3.3. Type of interventions
The control group was treated with western medicine alone, and the treatment group was treated with Chinese herbal compound, regardless of the dosages, and duration of drug treatment.
2.3.4. Type of outcome measures
The main outcome indicator was the clinical efficiency, and the efficacy was evaluated according to the score of symptoms and signs.[14] Treatment index ≥66% was significant, treatment index 65% to 26% was effective, and treatment index ≤25% was ineffective. Total efficiency = (significant effective cases + effective cases)/total number of cases.
Secondary outcome measures were sign score, symptom score, IgE level, recurrence rate, and occurrence of adverse reactions.
2.4. Exclusion criteria
-
1.
In addition to the traditional Chinese medicine compound, the treatment group also used other traditional Chinese medicine therapies, such as acupuncture and moxibustion, acupoint sticking, cupping, etc.;
-
2.
Studies with unsatisfactory outcome indicators;
-
3.
As for duplicate published literatures, select the literature with the most complete data;
-
4.
Missing main content, unable to conduct statistical analysis, and unable to obtain the literature after contacting the author;
-
5.
Literature with errors in random methods.
2.5. Search strategy
A systematic search was conducted on the randomized controlled trials of Chinese herbal compound for the treatment of allergic rhinitis in children. The databases include: PubMed, EMbase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang, the Chongqing VIP Chinese Science and Technology Periodical Database, and China biomedical literature database (CBM). The retrieval time was from the establishment of each database to July 2020. At the same time to retrieve Baidu, Google Scholar, International Clinical Trials Registry Platform (ICTRP), and Chinese Clinical Trials Registry (ChiCTR) and other databases for comprehensive literature search. The search terms were as follows: “Chinese herbal compound”, “allergic rhinitis”, “pediatric”, etc. Search strategy in PubMed is shown in Table 1.
Table 1.
Search strategy in PubMed database.
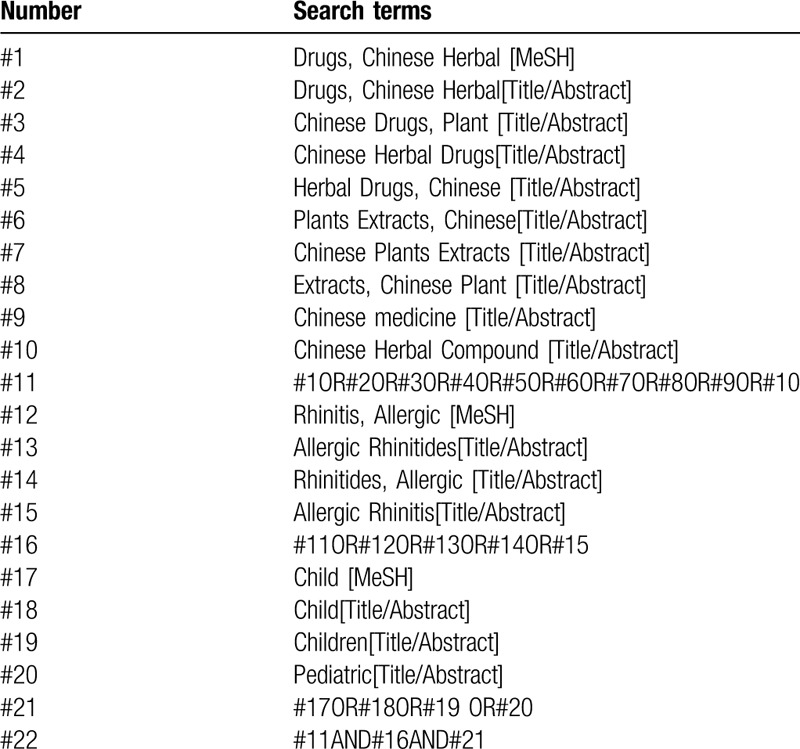
2.6. Data extraction
Two researchers independently screened and cross-checked the literature according to inclusion and exclusion criteria. In case of disagreement, it is resolved by discussion or with the assistance of a third investigator. Two researchers independently extracted the data, including: ① General information of the included study (title, first author, publication year, etc.); ② Baseline data of the subjects (gender, age, course of disease and degree of disease, etc.); ③ Intervention measures (name, composition, dosage, frequency and course of treatment of Chinese herbal compound; Name, dosage, frequency, and course of treatment of western medicine used in the control group); ④ The main factors in evaluating the risk of bias; ⑤ Outcome index. In case of disagreement, discuss and negotiate with the third researcher. The literature screening process is shown in Figure 1.
Figure 1.
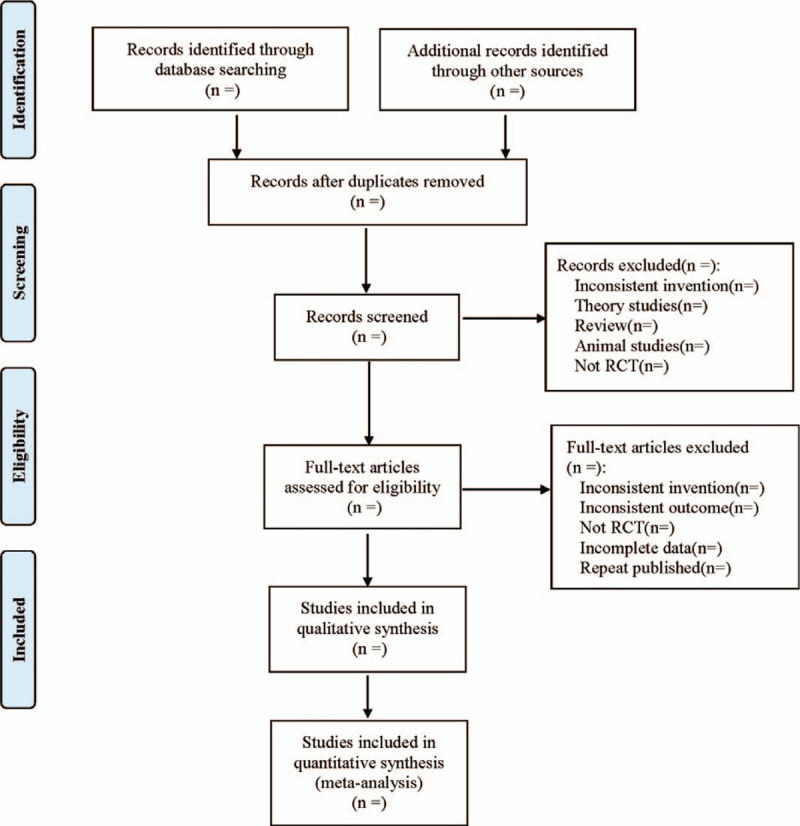
Flow diagram.
2.7. Risk of bias assessment
Two researchers independently evaluated the quality evaluation of randomized controlled trials according to the 7 dimensions of the Cochrane 5.1.0 Quality Evaluator's Manual, including: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The quality of studies is classified as being at of high, unclear, or low risk of bias. In case of disagreement, discuss and negotiate with the third investigator.
2.8. Statistical analysis
2.8.1. Data synthesis
The data were statistically analyzed using Cochrane Collaboration RevMan 5.3. The outcome index of dichotomized variables was expressed by relative risk (RR). For the outcome index of continuous variables, it was expressed by Weighted Mean Difference (WMD) if using the same measurement tools and units, and it was expressed by Standardized Mean Difference (SMD) for different measurement tools or units of data, all the above were represented by effect value and 95% confidence interval (CI). The heterogeneity between the included results was tested by χ2 test. If P ≥ .1 and I2 ≤ 50%, the heterogeneity was considered to be small, and the fixed-effect model was adopted. If P < .1 and I2 > 50%, inter-group heterogeneity was considered to be large, and subgroup analysis or sensitivity analysis was used to explore the source of heterogeneity. After excluding the effects of significant clinical heterogeneity, a random-effects model was used. If the inter-group heterogeneity was too large, descriptive analysis was conducted on the included literature without data merging.
2.8.2. Dealing with missing data
If the data in the included study is incomplete or missing, the author will be contacted to obtain the missing data. If the data cannot be obtained, the study will be excluded from the meta-analysis.
2.8.3. Subgroup analysis
In order to reduce inter-study heterogeneity and properly deal with research problems, the subjects were divided into infants and children for subgroup analysis according to their age. Subgroup analysis was conducted according to the degree of illness, course of treatment and efficacy of Chinese herbal compound.
2.8.4. Sensitivity analysis
To determine the robustness of the study results, the following 3 methods were used to analyze the sensitivity of the sources of clinical heterogeneity:
-
1.
Change the combined effect size model;
-
2.
The study with the maximum weight removed;
-
3.
Exclude and include the study one by one, carry out re-analysis, and observe the change of effect size and P value.
2.8.5. Reporting bias
When more than 10 studies were included in a certain outcome indicator, funnel plot was used to determine whether publication bias existed qualitatively. Also use Egger and Begg test to quantify publication bias.
2.8.6. Evidence quality evaluation
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE)[15] was used to evaluate the quality of evidence. Randomized controlled trials were set as the highest level of evidence, and the evaluation considers whether the quality of evidence is reduced from the following 5 factors: bias risk, consistency, directness, precision, and publication bias. And the quality of evidence will be rated as high, moderate, low, and very low.
3. Discussion
In traditional Chinese medicine theory, allergic rhinitis in children can be classified as the area of “BiQiu”, and the incidence is mostly related to congenital deficiencies of vital qi, dysfunction of the lungs, spleen and kidney, exogenous cold, inordinate diet, and other factors. Qi deficiency of the spleen and lung is the pathogenesis, and the treatment principles are reinforcing the spleen and lung, reducing long-standing phlegm, strengthening vital qi, and eliminating the wind.[16] Huang Qi (Radix Astragali), Fang Feng (Radix Saposhnikoviae), Bai Zhu (Rhizoma Atractylodis Macrocephalae), Xin Yi (Flos Magnoliae), Cang Er Zi (Fructus Xanthii), Bai Zhi (Radix Angelicae Dahuricae), Chen Pi (Pericarpium Citri Reticulatae), Fu Ling (Poria), Jie Geng (Radix Platycodonis), Su Ye (Folium Perillae), and other traditional Chinese medicines in Chinese herbal compound are commonly used to treat allergic rhinitis in children; Xin Yi (Flos Magnoliae) and Cang Er Zi (Fructus Xanthii) can warm lung and unblock the orifices, dispel wind and cold; Chen Pi (Pericarpium Citri Reticulatae), Jie Geng (Radix Platycodonis), Su Ye (Folium Perillae) can warm lung, ventilate the lung qi and the middle and upper qi; Bai Zhu (Rhizoma Atractylodis Macrocephalae), Chen Pi (Pericarpium Citri Reticulatae), Fu Ling (Poria) can invigorate spleen and supplement qi and tonify the middle jiao, replenish body fluid of spleen; Huang Qi (Radix Astragali), Fang Feng (Radix Saposhnikoviae), Bai Zhu (Rhizoma Atractylodis Macrocephalae) can solidify guard qi, dispel wind without injuring vital qi.[17] Experimental results [18] showed that Biminkang mixture can significantly inhibit the ileum contraction and nasal secretion exudation caused by histamine in guinea pigs. Studies have found [19] that Bishu Dropping Pills can reduce the content of serum IgE, inhibit the release of histamine in whole blood and nasal mucosa, and thus play an anti-allergic role. Clinical trials found that,[20] Kebimin Spray can significantly decrease the serum IgE high values of different level in the patients with allergic rhinitis, mainly because that Fu Zi (Radix Aconiti Lateralis Preparata) in Kebimin Spray has adrenocorticoid-like effect, to reduce serum IgE produce, prevent being combined with target cells, to make eosinophils and mast cells in “sleep” state, to cause the loss of internal conditions for the formation of allergic rhinitis.
However, no systematic review and meta-analysis is performed to test the efficacy and safety of Chinese herbal compound in pediatric patients with allergic rhinitis. It is necessary to assess the efficacy and safety of Chinese herbal compound in pediatric patients with allergic rhinitis, to provide scientific evidence for clinician and further studies. However, the study has some limitations.
(1) The language of the literature is only Chinese and English, and there may be publication bias;
(2) Traditional Chinese medicine compound dosage form and composition are not unified, which may cause certain bias.
Author contributions
Data collection: Mengni Zhang and Yihua Fan.
Funding support: Qinxiu Zhang.
Literature retrieval: Yue Xie and Chunying Tian.
Software operating: Yao Huang and Shasha Yang.
Supervision: Chunying Tian and Qinxiu Zhang.
Writing – original draft: Mengni Zhang and Yihua Fan.
Writing – review & editing: Mengni Zhang and Qinxiu Zhang.
Footnotes
Abbreviations: CBM = China biomedical literature database, ChiCTR = Chinese Clinical Trials Registry, CI = Confidence interval, CNKI = China National Knowledge Infrastructure, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, ICTRP = International Clinical Trials Registry Platform, IgE = immunoglobulin E, RR = relative risk, SMD = Standardized Mean Difference, WMD = Weighted Mean Difference.
How to cite this article: Zhang M, Fan Y, Tian C, Xie Y, Huang Y, Yang S, Zhang Q. The efficacy and safety of Chinese herbal compound in pediatric patients with allergic rhinitis: a protocol for systematic review and meta-analysis. Medicine. 2020;99:32(e21643).
This work is supported by the National Natural Science Foundation of China(No. 81473523).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368:733–43. [DOI] [PubMed] [Google Scholar]
- [2].Sha JC, Zhu DD, Dong Z, et al. Analysis of clinical characteristics and investigation of related problems in children with allergic rhinitis. Chin J Otorhinolaryngol Head Neck Surg 2011;01:26–30. [PubMed] [Google Scholar]
- [3].Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology and Pediatrics, Dociety of Otprhinolaryngology Head and Neck Surgery, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and treatment of pediatric allergic rhinitis (2010, Chongqing). Zhonghua Er Ke Za Zhi 2011;49:116–7. [PubMed] [Google Scholar]
- [4].Kong WJ, Chen JJ, Zheng ZY, et al. Prevalence of allergic rhinitis in 3-6-year-old children in Wuhan of China. Clin Exp Allergy 2009;39:869–74. [DOI] [PubMed] [Google Scholar]
- [5].Dong Z, Cheng L. More understanding of pediatric allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012;47:617–8. [PubMed] [Google Scholar]
- [6].Fan QZ, Chen XN, Li SH, et al. Observation on the therapeutic effect of Yiqi Demin Decoction on allergic rhinitis of lung deficiency and cold type. Inner Mongolia Tradit Chin Med 2015;02:30–1. [Google Scholar]
- [7].Yang Q, Wu YJ, Yan DN. Clinical effect of Yiqi Wenyang Recipe on allergic rhinitis in children. Chin J Tradit Chin Med 2015;4:1337–9. [Google Scholar]
- [8].Bachert C, Maspero J. Efficacy of second-generation antihistamines in patients with allergic rhinitis and comorbid asthma. J Asthma 2011;48:965–73. [DOI] [PubMed] [Google Scholar]
- [9].Seidman MD, Gurgel RK, Lin SY, et al. AAO-HNSF. clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg 2015;152:S1–43. [DOI] [PubMed] [Google Scholar]
- [10].Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63:8–160. [DOI] [PubMed] [Google Scholar]
- [11].Qiu X, Wang HT. Opinion on children's rhinitis: recommended by European Society of allergy and clinical immunology. Int J Otorhinolaryngol Head Neck Surg 2015;39:116–21. [Google Scholar]
- [12].van de Rijn M, Mehlhop PD, Judkins A, et al. A murine model of allergic rhinitis: studies on the role of IgE in pathogenesis and analysis of the eosinophil influx elicited by allergen and eotaxin. J Allergy Clin Immunol 1998;102:65–74. [DOI] [PubMed] [Google Scholar]
- [13].Bao XL, Gao L, An Y. Mechanism of Chinese herbal compound preparation in the treatment of allergic rhinitis. J Xinjiang Med Univ 2005;05:485–7. [Google Scholar]
- [14].Wang CR. Efficacy assessment with allergic rhinitis treated by desloratadine citrate disodium combined with Tongqiao biyan granule. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016;30:1806–7. [DOI] [PubMed] [Google Scholar]
- [15].Phi L, Ajaj R, Ramchandani MH, et al. Expanding the Grading of Recommendations Assessment, Development, and Evaluation (Ex-GRADE) for evidence-based clinical recommendations: validation study. Open Dent J 2012;6:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun XL, Liu HJ, Fu L, et al. Effect of Compound Xinyi nasal drops combined with loratadine in the treatment of children with allergic rhinitis and its influence on immune function indexes of children. Progr Modern Biomed 2019;3:482–5. [Google Scholar]
- [17].Zhang BG. Clinical observation of Xincang Ezhi Decoction in the treatment of allergic rhinitis with lung qi deficiency type. Shanxi Univ Tradit Chin Med 2017. [Google Scholar]
- [18].Bao L, sun QW, Hu L, et al. Clinical and experimental study of Biminkang in the treatment of allergic rhinitis. Chin J Integr Tradit Western Med 1997;2:70–2. [PubMed] [Google Scholar]
- [19].Zhang M, Ren Y, Song CS, et al. Anti allergic effect and mechanism analysis of Bishu dropping pills. Chinese J Trad Chin Med 2003;6:63–7. [Google Scholar]
- [20].Qian YF, Li BW, Li JR, et al. Effect of Kebimin on symptoms and signs and IgE of allergic rhinitis. J Beijing Univ Tradit Chin Med 1998;6:49–51. [Google Scholar]


