Abstract
To investigate the effects of probiotics on liver function, glucose and lipids metabolism, and hepatic fatty deposition in patients with non-alcoholic fatty liver disease (NAFLD).
Totally 140 NAFLD cases diagnosed in our hospital from March 2017 to March 2019 were randomly divided into the observation group and control group, 70 cases in each. The control group received the diet and exercise therapy, while the observation group received oral probiotics based on the control group, and the intervention in 2 groups lasted for 3 months. The indexes of liver function, glucose and lipids metabolism, NAFLD activity score (NAS), and conditions of fecal flora in 2 groups were compared before and after the treatment.
Before the treatment, there were no significant differences on alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamine transferase (GGT), total bilirubin (TBIL), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), insulin resistance index (HOMA-IR), NAFLD activity score (NAS), and conditions of fecal flora in 2 groups (P > .05). After the treatment, ALT, AST, GGT, TC, TG, HOMA-IR, NAS, and conditions of fecal flora in the observation group were better than those in the control group, and the observation group was better after treatment than before. All these above differences were statistically significant (P < .05).
Probiotics can improve some liver functions, glucose and lipids metabolism, hepatic fatty deposition in patients with NAFLD, which will enhance the therapeutic effects of NAFLD.
Keywords: hepatic fatty deposition, insulin resistance, liver function, non-alcoholic fatty liver disease, probiotics
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to clinical syndrome of hepatocellular lipodystrophy caused by pathogenic factors other than alcohol or definite etiology. It is closely related to the living environment, genetic susceptibility, and nutrition metabolic.[1,2] With the improvement of living conditions and obesity widely prevalent in the population, the NAFLD have gradually become a common liver disease of obesity, severe cases can lead to liver cirrhosis, liver cancer. Obesity is also a factor for cardiovascular disease, diabetes, and other metabolic syndrome.[3,4]
At present, no specific drugs are available for treating NAFLD. In clinical practice, the diet and life intervention, liver protection, and regulating drugs of glucose and lipids metabolism were regularly applied in the treatment.[5] According to relevant reports in recent years, intestinal flora imbalance, enterogenous endotoxin, microbe-gut-brain axis played an important role in the occurrence and development of NAFLD,[6] which suggesting that the probiotics to improve intestinal micro-ecology may be a therapeutic method for NAFLD.[7,8] The intestinal flora regulating drugs such as probiotics were widely used in diarrhea, malnutrition, and imbalance of intestinal flora with good safe profiles in clinical application. However, there is still lack of clinical reports on the efficacy of probiotics in patients with NAFLD. In this study, a number of 140 NAFLD patients in our hospital were collected to investigate the feasibility and effectiveness of probiotics for treating NAFLD.
2. Materials and methods
2.1. Patients
Patients diagnosed with NAFLD in our hospital from March 2017 to March 2019 were selected as the observational objects. A total of 152 patients were voluntarily recruited. This study was approved by the ethics committee of Beijing Shijitan Hospital (No: BSJ16019). A number of 12 patients were dropped out during the observation, and 140 were finally included and divided into the control group and observation group by using the random number table. Inclusion criteria: with or without abnormal indexes of liver function, diffuse fatty liver was detected on ultrasound, and NAFLD was confirmed by ultrasound-guided biopsy[9]; aged between 18 and 59; the patients were informed of the study and willing to be observed during the follow-up. Exclusion criteria: patients with other liver diseases with definite etiology, such as viral hepatitis; patients with liver fibrosis or cirrhosis; patients who had used these drugs for weight-loss, regulating glucose, and lipids metabolism, intestinal flora, and antibiotics in the past 3 months; patients with autoimmune diseases or other severely chronic comorbidities. Shedding criteria: patients who could not tolerate diet and exercise therapy; patients who failed to cooperate during the intervention treatment as required; other diseases occurred during the intervention of follow-up. These data of shedding cases were excluded in this study.
2.2. Treatment methods
The control group was received the diet and exercise schemes, while the observation group was received the oral probiotics combined with the therapy. The patients and an authorized member from patients’ family in 2 groups were interviewed for about 60 minutes, and the guidelines of the diet and exercise schemes were taught in the interview. The diet and exercise therapy was as follows[10,11]: the diet consisted of grains, vegetables, fruits, meat and so on, a reasonable collocation of a balanced low-calorie diet with rich nutrition, in which: calorie intake was 40 to 55 cal/(kg d) and carbohydrates accounted for 50% to 60%, proteins 25% to 35%, and lipids 5% to 15%. The food choice should consider the patients’ individual characteristic and the nutritional balance. At the same time, the patients should do aerobic exercises, including jogging, ball games, swimming, etc, at least 3 times a week and 30 to 60 minutes each time.[12] The exercises should increase progressively according to the patients’ tolerance, and the above exercise requirements should be reached within 1 month. Probiotics treatment: Live Combined Bifidobacterium, Lactobacillus and Enterococcus Powder (product name: Bifid Lriple Viable, approval number: S10970105, Shanghai xinyi pharmaceutical co., LTD) was given orally, 1 g/time, 2 times/d. The intervention treatment in the 2 groups lasted for 3 months, and liver protection treatment were given according to the principle of clinical treatment when necessary. Follow-up was regularly carried out to check the practice of treatment scheme.
2.3. Observation indexes
The following indexes were recorded in both groups at 3 months before and after the treatment: liver function indexes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamine transferase (GGT), and total bilirubin (TBIL). Glucolipid metabolic indexes, including total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), fasting insulin (Fins), fasting plasma glucose (FPG), and insulin resistance index (HOMA-IR). All the above blood biochemical indexes required morning fasting blood samples which were sent to the hospital clinical laboratory for tests. Non-alcoholic fatty liver disease activity score (NAS), ultrasound guided needle biopsy, obtained liver tissues which were fixed with 10% neutral formaldehyde, paraffin-embedded sections, hematoxylin-eosin staining, and reticular fiber staining to determine the NAS score (0–8 points), which was the sum of three parts: hepatocyte fatty change: calculated according to the score 0, 1, 2, and 3, respectively representing the hepatocyte fatty change <5%, 5% to 33%, 34% to 66%, and >66%; inflammation in the lobule: necrotic foci were counted at 20× magnification, and 0, 1, 2, and 3 points respectively represented no, <2, 2 to 4, and >4; balloon-like changes of liver cells: 0, 1, and 2 points respectively represented no, rare, common. Fecal flora condition determination[13]: fecal samples were collected for testing; classification criteria: the ratio of gram negative bacilli to gram positive cocci by gram staining in fecal smear was >90%, 10% to 90%, and <10%, respectively representing normal, imbalance, and serious imbalance.
2.4. Statistical analysis
The data were analyzed by using SPSS25.0 software (SPSS Inc., Chicago, Ill., USA), and the measurement data were expressed as mean ± standard deviation (¯x ± s). t test was used for comparisons in the 2 groups. The counting data was expressed as frequency or percentage (n/%) by using chi-squared test, and the ranked data were analyzed by using the Kruskal-Wallis test. P < .05 was considered as statistically significant.
3. Results
3.1. Comparisons of general data between the 2 groups
There were no significant differences between the 2 groups in sex, age, waist circumference, body mass index (BMI), and course of disease (P > .05), as shown in Table 1.
Table 1.
Comparisons of general data between the 2 groups [n,  ].
].

3.2. Comparisons of liver function indexes in the 2 groups
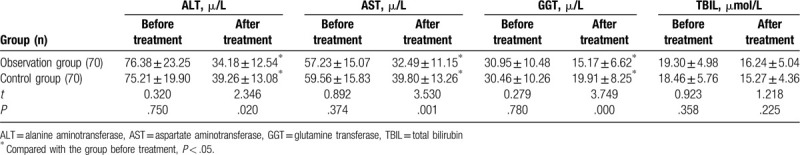
Before the treatment, AST, ALT, GGT, and TBIL were compared between the 2 groups, and no significant differences were observed (P > .05). After the treatment, AST, ALT, and GGT in both groups were significantly lower than those before the treatment, and AST, ALT, GGT in the observation group were lower than those in the control group, with statistically significant differences (P < .05). Before and after the treatment, there was no significant difference in TBIL in the 2 groups (P > .05). As shown in Table 2.
Table 2.
Comparisons of liver function indexes in the 2 groups  .
.
3.3. Comparisons of lipids metabolism indexes in the 2 groups
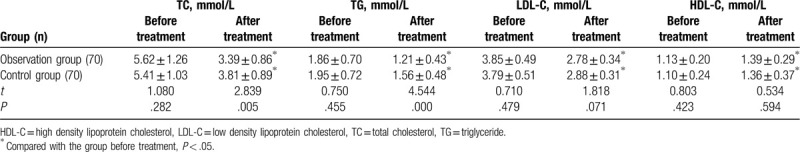
Before the treatment, there were no significant differences in TC, TG, LDL-C, and HDL-C between the 2 groups (P > .05). After the treatment, TC, TG, LDL-C, and HDL-C in the 2 groups were significantly improved. Among them, TC and TG in the observation group were lower than those in the control group, with statistically significant differences (P < .05). As shown in Table 3.
Table 3.
Comparisons of lipid metabolism indexes in the 2 groups  .
.
3.4. Comparisons of HOMA-IR and NAS score in the 2 groups
Before the treatment, HOMA-IR and NAS score of the 2 groups were compared, and the differences were not statistically significant (P > .05). After the treatment, HOMA-IR and NAS score of the 2 groups were significantly improved, and the above indexes in the observation group were lower than those in the control group, with statistically significant differences (P < .05). As shown in Table 4.
Table 4.
Comparisons of HOMA-IR and NAS score in the 2 groups  .
.

3.5. Comparisons of fecal flora conditions in the 2 groups
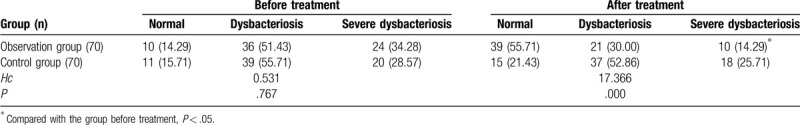
Before the treatment, there was no significant difference on the conditions of fecal flora between the 2 groups (P > .05). After the treatment, fecal flora conditions in the observation group were better than those in the control group (55.71% vs 21.43%), and the observation group was better after treatment than before treatment (55.71% vs 14.29%). All the above differences were statistically significant (P < .05). As shown in Table 5.
Table 5.
Comparisons of fecal flora imbalance in the 2 groups (n [%]).
4. Discussions
A major therapeutic purpose of NAFLD is to reduce liver function damage, hepatic cell fat deposition, and inflammatory response, as well as to prevent the occurrence of liver fibrosis, cirrhosis, liver cancer, and other late complications. Some reports showed that diet and exercise intervention could effectively reduce the liver fat deposition, improve some liver biochemical indexes, and benefit NAFLD patients.[14] In this study, both groups received the diet and exercise therapy for 3 months and achieved improvements of NAFLD, which demonstrated that the diet and exercise therapy was an effective and important option for NAFLD. Furthermore, intestinal microflora development had close correlation with the incidence of NAFLD, and the correction of intestinal microecological disorder could improve the condition of patients with NAFLD.[15–17] In this study, the observation group received oral probiotics drug (Bifid Lriple Viable) combined with the diet and exercise therapy continuously for 3 months, and the results showed that the improvement of some observation indexes in the observation group was better than that in the control group, which proved that probiotics was also effective to a certain extent in the treatment of NAFLD.[18,19] The results in our study were consistent with above views.
Previous studies showed that probiotic-assisted treatment for NAFLD could significantly improve some indexes of liver functions.[20] Kobyliak et al[21] reported that the modulation of gut microbiota by probiotic supplementation can significantly reduce liver fat accumulation and attenuate the levels of TNF-α, IL-6, AST, and GGT, which meant that this modulation might represent an optional treatment method for NAFLD patients. Moreover, Famouri et al[22] found that probiotics not only significantly reduced the levels of AST, ALT, and other index of liver functions, but also dramatically ameliorated patients’ body mass index. These viewpoints mentioned above were similar to the results in our study. The increasing of TBIL is more common in severe liver function injury diseases. However, the liver disease of the patients in this study did not progress to severe liver injury, so there was no significant change in TBIL before and after the treatment.
After the treatment, the relavant indexes of glucose and lipid metabolism of the patients in the 2 groups were significantly improved, which indicated that probiotics should be helpful to improve disorders of lipid and glucose metabolism in patients and reduce insulin resistance. Intestinal flora disorder can lead to the over-reproduction of intestinal bacterial and the increase intestinal permeability. Then, such as bacterial peptides, lipopolysaccharides, immune factors, and other immunogen inducing by intestinal flora enter the liver through the intestinal mucosa, which will result in activation of specific immune and nonspecific immune response between the intestinal tract and liver. This may lead to body blood lipid and glucose metabolism disorders as well as insulin resistance.[23] In addition, the metabolites of probiotics such as lactobacillus inhibit cholesterol synthase, which helps to inhibit the formation of cholesterol. Furthermore, the intestinal bacteria can combine with cholesterol synthase and subsequently inhibit the absorption of cholesterol; Afterwards, normal intestinal bacteria can affect the enterohepatic circulation of bile salts, increase the supplements of cholesterol in bile salts circulation, and promote cholesterol excretion. These mechanisms mentioned above should be related to the improvements of lipid metabolism disorder by probiotics intake.[24]
The observation group of this study showed that probiotics had a good effect on reducing the NAS score of patients, indicating that probiotics can help to inhibit liver fat deposition. Intestinal flora disorder resulted in activation of liver abnormal immune and nuclear factor-kappa B (NF-κB) or its related signaling pathways. Thus, some inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL) were produced, and affected the mediators of cholesterol adjustment protein-1c and serum adiponectin. After that, the liver fat deposition gradually formed in clinic.[8,25] Regulation in intestinal flora disorder will help to inhibit the above signaling pathways and the deposition of liver fat. So, the supplementation of probiotics could promote the growth of beneficial intestinal bacteria, inhibit the growth of pathogenic bacteria, and restore the normal intestinal flora ecology, so as to maintain the stability of intestinal mucosal biochemical barriers, reduce abnormal immune and inflammatory responses, and improve glucose and lipid metabolism disorders.[26] After the treatment, the dysbacteriosis of the observation group was significantly better than before treatment, which should be related to the above mechanism.
The glucose and lipid metabolism disorder and insulin resistance were closely related to NAFLD, suggesting that probiotics played a therapeutic role by improving glucose and lipid metabolism disorder, regulation of intestinal flora imbalance, and reducing insulin resistance.[8] Yan et al[27] carried out similar studies and found that probiotics drug (Bifid Lriple Viable) could reduce transaminase, inhibit inflammatory factors such as IL-6 and TNF-a, and slow down the process of liver fibrosis with good safety,[28] which was similar to the conclusion of this study. The limitations of this study were that the sample size was small, the observation time was a little short, and the cytokine changes and molecular pathogenesis related to NAFLD had not been explored. So, the study was still unable to fully explain the therapeutic mechanism of probiotics, which needed to be supplemented in future studies.
In conclusion, the probiotics can regulate imbalance of intestinal flora, improve glucose and lipid metabolism disorder, and liver fat deposition in NAFLD patients. Thus, the probiotics can be exploratory applied in the treatment of NAFLD.
Author contributions
C.G. and Z.J. conceived and designed the experiments. C.G. and S.H. performed the experiments and analyzed the data. C.G. wrote the paper. Z.J. reviewed and revised the paper. All authors approved the submission of this work.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, Fins = fasting insulin, FPG = fasting plasma glucose, GGT = glutamine transferase, HDL-C = high density lipoprotein cholesterol, IL = interleukin, LDL-C = low density lipoprotein cholesterol, NAFLD = Non-alcoholic fatty liver disease, NF-Bκ = activation of nuclear factor, TBIL = total bilirubin, TC = total cholesterol, TG = triglyceride, TNF = tumor necrosis factor.
How to cite this article: Cai Gs, Su H, Zhang J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease (NAFLD). Medicine. 2020;99:32(e21464).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].The Chinese National Workshop on Fatty Liver, Alcoholic Liver disease for the Chinese Liver Disease Association, Fatty liver disease expert committee of Chinese medical doctor association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: 2018 edition. Infectious Disease Inf 2018;31:393–402. [Google Scholar]
- [2].Soleimani D, Ranjbar G, Rezvani R, et al. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes 2019;12:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jang I, Kim JS. Risk of cardiovascular disease related to metabolic syndrome in college students: a cross-sectional secondary data analysis. Int J Environ Res Public Health 2019;16:3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li X-h, Lai W. Homogeneity and heterogeneity of nonalcoholic fatty liver disease. J Clin Hepat 2018;34:10–3. [Google Scholar]
- [5].Romero-Gomez M, Zelber-Sagis S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–46. [DOI] [PubMed] [Google Scholar]
- [6].Martin CR, Osadchiy V, Kalani A, et al. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 2018;6:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yuan Y, Sun Z-m, Zhang Y, et al. Influence of gut microecology on the pathogenesis and treatment of nonalcoholic fatty liver disease. J Chin Hepat 2016;24:375–9. [DOI] [PubMed] [Google Scholar]
- [8].Doulberis M, Kotronis G, Gialamprinou D, et al. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism 2017;71:182–97. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y-y, Zhang G-y, Zhang L-m, et al. Clinical significance of liver biopsy in diagnosis of non-viral liver diseases. Chin J Dig 2017;37:756–60. [Google Scholar]
- [10].Coughlin SS, Hatzigeorgiou C, Anglin J, et al. Healthy lifestyle intervention for adult clinic patients with type 2 diabetes mellitus. Diabetes Manag (Lond) 2017;7:197–204. [PMC free article] [PubMed] [Google Scholar]
- [11].Africa JA, Newton KP, Schwimmer JB. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig Dis Sci 2016;61:1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–46. [DOI] [PubMed] [Google Scholar]
- [13].Wang W, Shi L-p, Shi L, et al. Clinical study on the adjuvant treatment of nonalcoholic fatty liver disease with intestinal probiotics. Chin J Intern Med 2018;57:101–6. [Google Scholar]
- [14].Zhaowei Li, Qiao LIN, Xiang YAN, et al. Effect of aerobic exercise in non-alcoholic fatty liver disease patients: a Meta-analysis. Chin J Gastroenterol Hepatol 2016;25:398–404. [Google Scholar]
- [15].Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients 2018;10:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eshraghian A. Current and emerging pharmacological therapy for non-alcoholic fatty liver disease. World J Gastroenterol 2017;23:7495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gao X, Zhu Y, Wen Y, et al. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: a meta-analysis of randomized controlled trials. Hepatol Res 2016;46:1226–33. [DOI] [PubMed] [Google Scholar]
- [18].Barengolts E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endocr Pract 2016;22:1224–34. [DOI] [PubMed] [Google Scholar]
- [19].Xue L, He J, Gao N, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep 2017;7:45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang W, Shi LP, Shi L, et al. Efficacy of probiotics on the treatment of non- alcoholic fatty liver disease. Chin J Int Med 2018;57:101–6. [DOI] [PubMed] [Google Scholar]
- [21].Kobyliak N, Abenavoli L, Mykhalchyshyn G, et al. A Multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis 2018;27:41–9. [DOI] [PubMed] [Google Scholar]
- [22].Famouri F, Shariat Z, Hashemipour M, et al. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr 2017;64:413–7. [DOI] [PubMed] [Google Scholar]
- [23].Ding Z-y, Bo L, Lu H-y, et al. Correlation analysis of serum vitamin A, liver fat content and insulin resistance in patients with nonalcoholic fatty liver disease. J Clin Hepat 2017;33:2361–5. [Google Scholar]
- [24].Bai L-m, Zheng P-y, Zhang J, et al. Effects of lipid-lowering probiotics on bile acid metabolism in rats with nonalcoholic fatty liver disease and its mechanism. Chin J Microbiol Immunol 2016;36:110–6. [Google Scholar]
- [25].Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD)–pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 2017;49:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yilmaz Y, Fatih E. Nonalcoholic steatohepatitis and gut microbiota: future perspectives on probiotics in metabolic liver diseases. Turk J Gastroenterol 2017;28:327–8. [DOI] [PubMed] [Google Scholar]
- [27].Yan YH, Lei XY, Fu LG, et al. Effects of enteric soluble capsules of dimonium glycyrrhizinate combined with tetrafilm of bifidobacteria on inflamm- atory indexes and liver injury in NAFLD patients. Chin J Mod Appl Pharm 2018;35:121–6. [Google Scholar]
- [28].Hou J, Liu L-x. Effect of probiotics in treatment of nonalcoholic fatty liver disease: a meta-analysis. J Clin Hepatol 2018;34:2624–30. [Google Scholar]


