Abstract
Background:
An increasing number of studies in recent years have identified mean platelet volume (MPV) as a predictive marker for neonatal sepsis. However, most of these studies focused on single regions, and therefore, the findings remain inconclusive. We, in this study, aimed to evaluate the potential of MPV as a biological indicator of neonatal sepsis through a systematic review and meta-analysis.
Methods:
We searched PubMed, the Cochrane Library, Embase, and WanFang database for articles on MPV and neonatal sepsis, published from January 1, 1990 to December 31, 2018. We included 11 studies on 932 neonates with sepsis in this meta-analysis.
Results:
The overall meta-analysis showed that MPV was significantly higher in patients with neonatal sepsis compared with healthy controls. Subgroup analysis revealed that the type of diagnostic criteria, analyzer, analyte, and controls used in the studies affected the difference in MPV between patients and healthy controls.
Conclusion:
MPV was significantly higher in the neonatal sepsis group compared to the control group. Therefore, in clinical practice, MPV could be used as an indicator for the early diagnosis of neonatal sepsis.
Keywords: diagnose, mean platelet volume, meta-analysis, neonatal sepsis
1. Introduction
Sepsis is defined as a host reaction disorder that leads to life-threatening organ dysfunction with infectious factors.[1] It is a common complication associated with severe trauma, burns, shock, infections, and major surgery. Approximately 19 million adults are diagnosed worldwide, with sepsis each year, and about 1.5 million cases in the United States alone.[2] The incidence of sepsis is growing by 1.5% every year. With the definition, diagnostic criteria, and management methods for sepsis being continuously updated, although the associated mortality has declined in recent years, the risk of mortality and morbidity remains very high.[3] Neonatal sepsis, also known as septicemia newborn, is a serious infection in newborns wherein pathogens (including bacterial, viral, or fungal (yeast)) enter the blood and produce toxins that result in a systemic inflammatory response. Clinical signs and symptoms, conventional diagnostics (pathogens isolated from sterile body fluids), culture-independent diagnostics, and non-culture-based diagnostic tests (including CPR and WBC count) are the main ways to diagnose neonatal sepsis.[4]
Sepsis is an important cause of neonatal morbidity and mortality.[5] The younger the gestational age, the lower is the birth weight, resulting in higher morbidity and mortality from sepsis. Statistical data show that the incidence of neonatal sepsis accounts for 0.1% to 0.5% of live births, with a mortality rate of 5% to 10%.[4,6] In the United States, 7% of all deaths in newborns are due to sepsis, costing approximately 1.97 billion dollars annually.
Platelets are one of the tangible components of blood. Upon activation, platelets release a large number of cytokines and inflammatory mediators, which play an important role in inflammation and immune response.[7,8] Release of cytokines, especially tissue factors, over-activate the coagulation system causing serious complications such as disseminated intravascular coagulation, ischemia, and hypoxia, ultimately resulting in multiple organ dysfunction syndrome.[9,10] The association between platelets and sepsis is well established. The mean platelet volume (MPV), which refers to the average volume of individual platelets, has been considered a marker of platelet size, function, and reactivity.[11] Higher the MPV, the more active are the platelets, which can increase the early formation of thrombus, leading to platelet adhesion, aggregation, and increased risk of severe complications and death.[12] Although several studies in recent years have recognized the relationship between neonatal sepsis and MPV, the exact relationship between them remains unclear.
This meta-analysis aims to summarize the findings of published studies, explore and clarify the role of MPV in the diagnosis of neonatal sepsis. We believe our findings will provide an early diagnostic marker for neonatal sepsis.
2. Materials and methods
2.1. Search strategy
This study was conducted strictly in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We systematically and comprehensively searched all relevant literature to reduce the impact of insufficient representativeness and improve comprehensive evaluation in this meta-analysis. The following keywords were used for the search: “mean platelet volume,” “platelet parameter,” “platelet index,” “platelet size,” “neonatal sepsis,” “newborn sepsis,” and “septicemia.” The following databases were searched: PubMed, Embase, the Cochrane Library, and WanFang database. We included publications from January 1, 1990 to December 31, 2018 with no restrictions on language and country. Meanwhile, we manually checked the reference lists of the included studies to ensure the research was authentic. This study adopted the secondary research method, wherein all data were obtained from published literature, and therefore, ethical approval was not necessary.
2.2. Study selection
Studies that met the following criteria were included in this meta-analysis: evaluated newborns, assessed the association between MPV and sepsis or septic shock, included a control group, mean and standard deviation data on MPV was available. Studies that were expert consensus, case reports, conference literature, and reviews were excluded from the analysis.
2.3. Data extraction
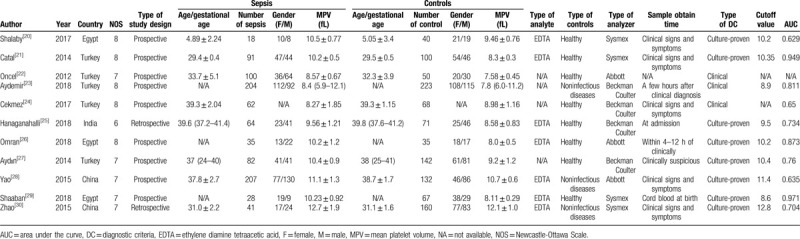
Based on the above inclusion and exclusion criteria, 2 researchers (JJW and ZSL) independently collected the literature and extracted data by cross-checking. Controversial studies were analyzed and evaluated by a third researcher (QL) to determine whether or not to include them in this study. The 3 researchers (JJW, ZSL, and QL) then rechecked the literature and data to ensure everything was correct. The baseline characteristics of the extracted data included: first author's name, publication year, country, type of study design, number and gender of patients in each group, diagnostic criteria, the mean and standard deviation of MPV in each group, cutoff values, and gestational age (Table 1).
Table 1.
Baseline characteristics of all studies included in meta-analysis.
2.4. Assessment of quality
The Newcastle-Ottawa scale (NOS) was used to evaluate the quality of the included studies. The NOS evaluation covers 3 major areas (a total of 8 items): study population selection (4 points), comparability between groups (2 points), and result measurement (3 points), adding up to a total score of 9. Based on the total scores, study quality was categorized as follows [13]: Scores of 7–9 indicated high-quality studies with low risk of bias, while scores of 4–6 indicated medium-quality studies suggestive of risk of bias. Scores of 0–3 represented low-quality studies with a high risk of bias.
2.5. Statistical analysis
The main objective of this study was to investigate the significance of MPV in the diagnosis of sepsis. The impact of MPV on the diagnosis and prognosis of sepsis was estimated by assessing the standardized mean difference (SMD) and its 95% confidence interval (CI). Statistical heterogeneity was quantified using the Cochrane's Q test and Higgins I-squared statistic (level of a test is α = 0.1 and I2 = 50%). Tests with α < 0.1 or I2 > 50% indicated significant heterogeneity.[14,15] If there was obvious heterogeneity among the studies, a fixed-effect model was applied for analysis. If not, a random-effect model was used.[16,17] To determine changes in heterogeneity and synthesis results, we implemented a sensitivity analysis by calculating both the fixed-effect and random-effect models for SMD values and 95% CI. Publication bias was evaluated by the Egger test and funnel plot.[18] All statistical analyses were performed using software stata12.0SE (Stata Corporation, College Station, TX).
3. Results
3.1. Study characteristics
The flowchart used for screening articles is shown in Figure 1. A total of 496 articles were retrieved from 4 databases (136 from PubMed, 320 from Embase, 8 from the Cochrane library, and 32 from the WanFang database). All articles were last retrieved on December 31, 2018. Duplicates articles (n = 344) were removed. Of the remaining 152 articles, 125 were excluded based on their titles and abstracts since they were not relevant to our study. The full text of 27 articles was reviewed, of which 15 were excluded due to insufficient data or no controls. One article was excluded since we could not contact the corresponding author.[19] Finally, 11 articles from 4 nations were included in our meta-analysis.[20–30] The included studies were all on neonatal sepsis and MPV. Four studies did not report the type of analyte. In 8 studies, the diagnosis of sepsis was confirmed by culture, while in 3 of them, it was based on clinical features. Table 1 summarizes the baseline characteristics of each study. The NOS was used to evaluate the quality of the included studies, and the details are shown in Table 1.
Figure 1.
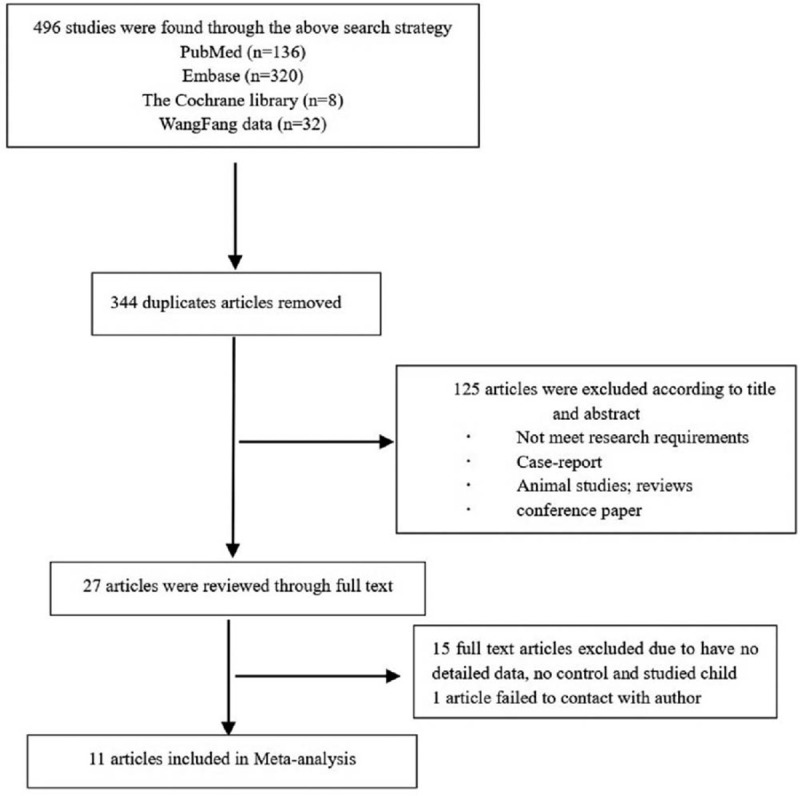
Flowchart for screening articles.
3.2. Overall meta-analysis of MPV
A total of 2020 participants from 11 studies, including 932 neonate sepsis patients and 1088 control patients, were analyzed for MPV. All the included studies showed a higher MPV in neonatal sepsis patients compared to the controls. The fixed-effect model analysis for MPV demonstrated significant heterogeneity (heterogeneity chi-squared = 389.70; df = 10; P = .000; I2 = 97.4%). We, therefore, used the random-effects model. The Forest plot in Figure 2 shows that the MPV was significantly higher in patients with neonate sepsis compared to controls (SMD = 1.49, 95% CI = 0.84–2.14, P < .0001).
Figure 2.
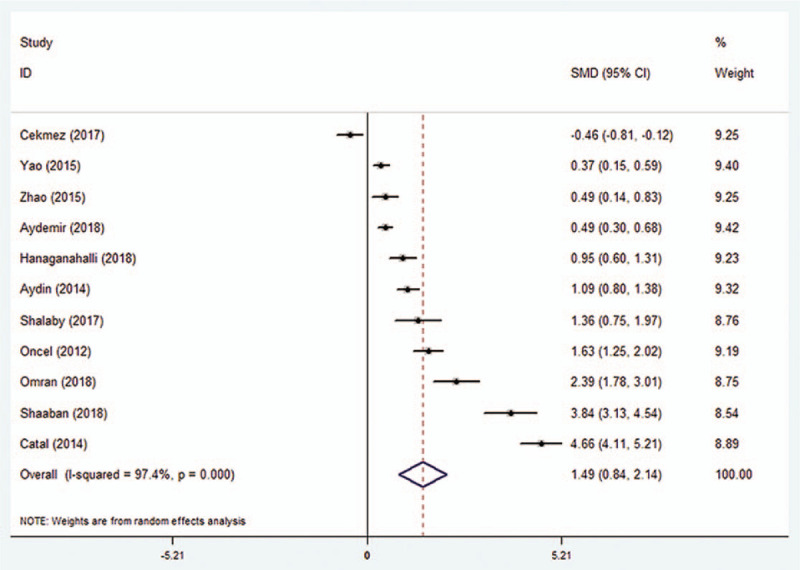
Standard mean difference (SMD) for the association between MPV and neonatal sepsis. MPV = mean platelet volume.
3.3. Subgroup meta-analysis of MPV
The subgroup analyses were performed based on the type of diagnostic criteria (culture-proven vs. clinical), analyzer, study design (prospective or retrospective), analyte, and control. MPV was significantly higher in 566 cases from 8 studies where sepsis was confirmed based on culture findings compared to 747 control cases (SMD = 1.87; 95% CI: 0.98–2.75, P < .0001) (Fig. 3). On the other hand, in 366 cases from 3 studies, where sepsis was confirmed based on clinical findings, MPV showed no significant difference compared to 341 controls (SMD = 0.55; 95% CI: −0.43–1.53, P = .271) (Fig. 3).
Figure 3.
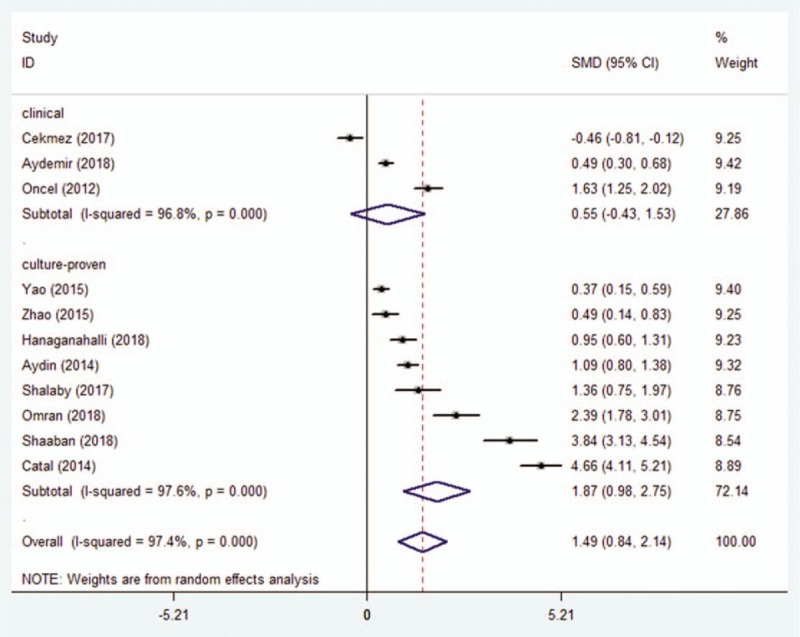
Association between MPV and neonatal sepsis confirmed by culture versus clinical methods.
MPV measured using a Coulter blood analyzer showed no difference between 412 patients and 504 controls (SMD = 0.521; 95% CI: −0.071 to 1.112, P = .085) (Fig. 4). On the other hand, in 3 studies that used the Abbott analyzer, MPV was significantly higher in 342 patients compared to 217 controls (SMD = 1.44; 95% CI: 0.258–2.622, P < .05) (Fig. 4). Similarly in another 4 studies that used the Sysmex analyzer, 178 patients had significantly higher MPV compared to 367 controls (SMD = 2.579; 95% CI: 0.455–4.702, P < .05) (Fig. 4).
Figure 4.
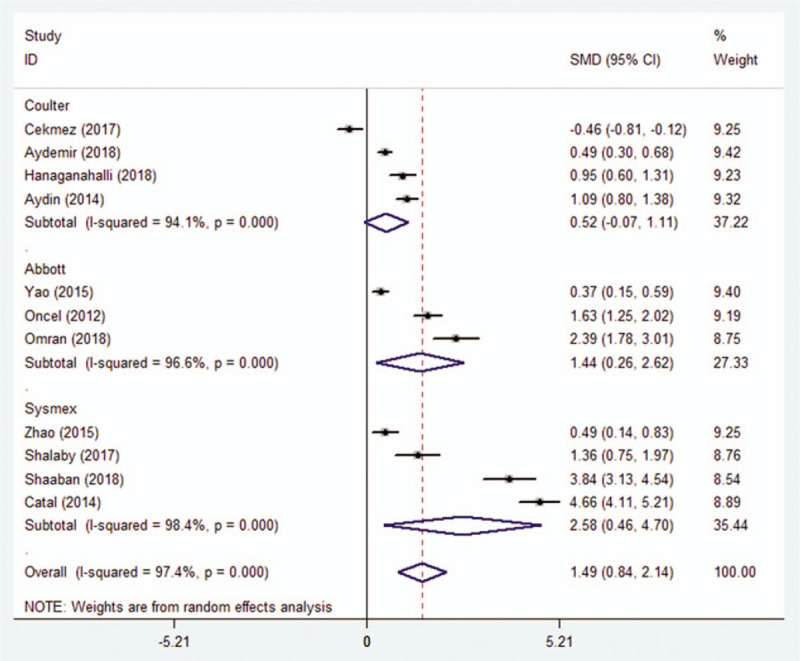
Association between MPV and neonatal sepsis based on subgroup analysis by the analyzer.
The studies included in this meta-analysis were categorized as prospective or retrospective based on the study design. In both types of studies, compared to controls, MPV was significantly higher in patients (SMD = 1.677; 95% CI: 0.877–2.476, P < .0001 and SMD = 0.718; 95% CI: 0.259–1.176, P = .002, respectively) (Fig. 5).
Figure 5.
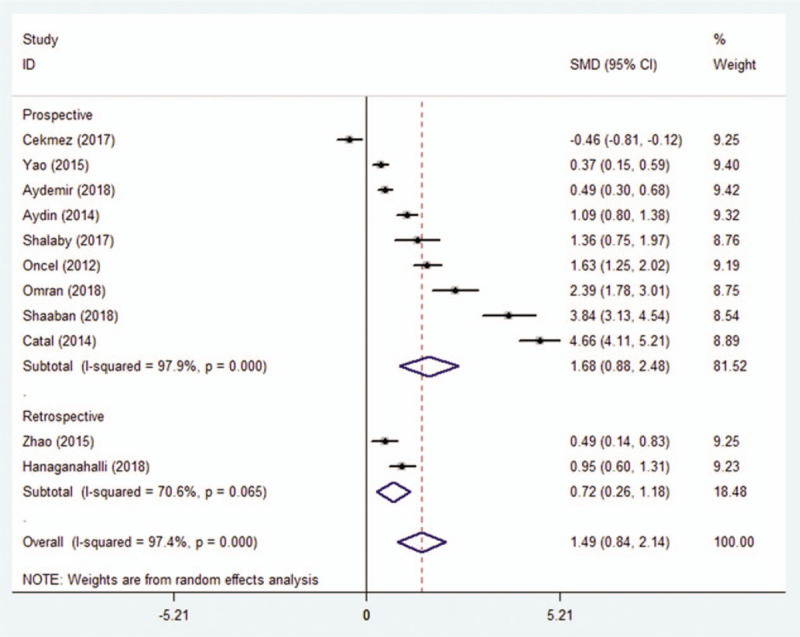
Association between MPV and neonatal sepsis based on subgroup analysis by study design.
In 8 studies, MPV was significantly higher in 480 patients compared to 573 healthy controls (SMD = 1.915; 95% CI: 0.893–2.937, P < .0001). It was also significantly higher in 452 patients with sepsis compared to those with noninfectious diseases (SMD = 0.445; 95% CI: 0.311–0.579, P < .0001) (Fig. 6).
Figure 6.
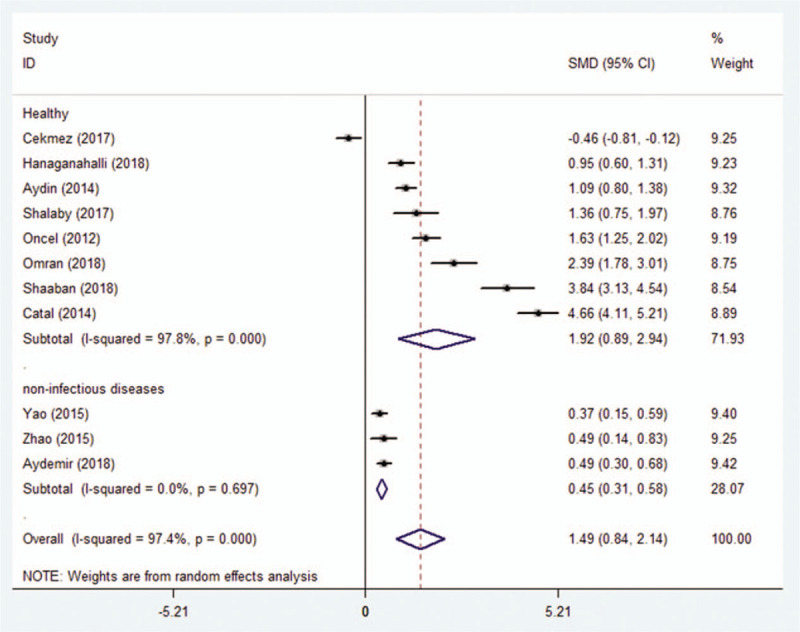
Association between MPV and neonatal sepsis based on subgroup analysis by types of control.
Seven studies reported the type of analytes used, while 4 did not. Ethylene diamine tetraacetic acid (EDTA) samples from 484 patients showed higher MPV compared to 605 controls (SMD = 1.98; 95% CI: 0.89–3.08, P < .0001).
3.4. Publication bias
In this meta-analysis, funnel plots and Egger regression asymmetry tests were used to evaluate publication bias. Figure 7 shows that the funnel plot was not completely symmetric, and some studies appear outside the 95% CI, suggesting that there was some bias. Publication bias could be due to several factors, including a small number of studies and nonsignificant results, both of which can result in an article not getting published.[31] Therefore, using the trim-and-fill method, we found 4 negative and unpublished studies missing from the initial analysis (Fig. 7). Adding these 4 studies did not change the effect size significantly, indicating that the publication bias had little effect on the relatively stable results.
Figure 7.
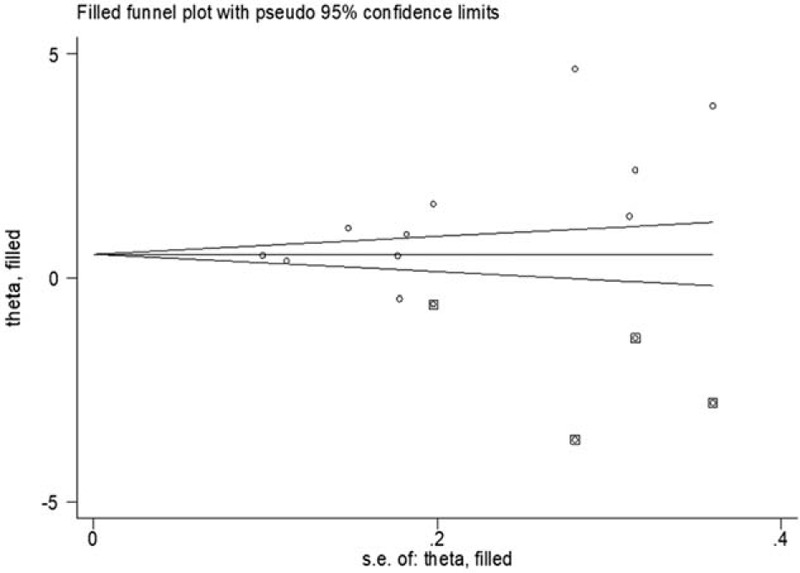
Evaluation of publication bias in studies on the association between MPV and neonatal sepsis.
4. Discussion
Various biomarkers are being used for the diagnosis of neonatal sepsis, which includes white blood cell count, c-reactive protein, procalcitonin, serum amyloid A, and lipopolysaccharide-binding protein.[6] In recent years, studies are starting to take notice of an association between MPV and neonatal sepsis. Studies by Shalaby[20] and Shaaban[29] showed a significant increase in MPV in neonates with sepsis. These findings suggested that a simple routine blood test for MPV can help with both the diagnosis and prognosis of sepsis in neonates. Ours is the first meta-analysis and systematic review to evaluate the diagnostic value of MPV for neonatal sepsis.
In physiopathology, platelet activation has a pivotal role in thrombosis as well as inflammation. It is also involved in maintaining the integrity of blood vessel walls. The role of platelets in inflammation, anti-infection, and immune responses is being increasingly recognized.[32] MPV is the platelet index that indicates the average size of platelets in the peripheral blood and is associated with the maturity of platelets.[33] MPV is significantly lower in patients with high-grade inflammatory diseases such as systemic lupus erythematosus or inflammatory bowel disease compared to healthy controls.[34] On the other hand, patients with low-grade inflammatory diseases such as psoriasis or ankylosing spondylitis have higher MPV compared to healthy controls.
Eleven studies on MPV and neonatal sepsis published from 2012 to 2018 were included in our systematic review. The overall meta-analysis showed that MPV was significantly higher in patients with neonatal sepsis compared to healthy controls (SMD = 1.49, 95% CI = 0.84–2.14, P < .0001). One study concluded that an increase in MPV within 72 hours of admission could be an independent risk factor for adverse clinical outcomes in patients with severe sepsis or septic shock.[35] Thrombocytopenia is a good predictor of sepsis, and the platelet count is inversely related to MPV. Additionally, MPV can be influenced by various factors, such as smoking, diabetes mellitus, hypertension, and obesity. However, since our study focused on neonates, we eliminated the interference of the above factors and explored the potential of MPV as an accurate biological indicator of sepsis.
Our study has some limitations that need to be acknowledged. First, the results of our analysis showed heterogeneity, and therefore, should be interpreted with caution. Clinical, methodological, and statistical heterogeneity are the 3 main sources of variations between different studies. We believe the heterogeneity in our results could be from the different types of diagnostic criteria, analyzers, study designs, and anticoagulants used in the studies. While these factors and characteristics can influence the research findings, we could not pinpoint the exact source of heterogeneity. Furthermore, we used a sensitivity analysis to assess the pooled SMDs, which showed no change after replacing individual studies, indicating the stability of the results. Second, the sample size in most studies was small. Large-scale studies with multiple research centers were missing. Third, the age of the newborns was not clearly defined in the original studies, which could have been one of the factors contributing to heterogeneity. Finally, there are no details on mortality (in both the patient and control groups) in each included study. Despite these shortcomings, our findings help objectively evaluate the association between neonatal sepsis and MPV. Further studies are required to determine the sensitivity and specificity of pooled MPV as a diagnostic marker for neonatal sepsis.
In conclusion, the available evidence confirms significantly higher MPV in newborns with sepsis compared to healthy controls. Therefore, in clinical practice, MPV could be used as an indicator for the early diagnosis of neonatal sepsis. Studies on the dynamic changes in MPV will help further validate our findings.
Author contributions
Formal analysis: Jingjing Wang, Min Zhang, Jiaxiang Deng, Zhenshuai Lou.
Methodology: Jingjing Wang, Jiaxiang Deng, Min Zhang, Zhenshuai Lou.
Resources: Jingjing Wang, Qian Li.
Software: Jingjing Wang, Zhen Wang.
Data curation: Jingjing Wang, Qian Li, Zhen Wang.
Conceptualization: Jingjing Wang, Qian Li.
Project administration: Jingjing Wang, Qian Li.
Supervision: Jingjing Wang, Qian Li, Zhen Wang.
Writing – original draft: Jingjing Wang.
Writing – review & editing: Qian Li, Zhen Wang.
Footnotes
Abbreviations: CI = confidence interval, EDTA = ethylene diamine tetraacetic acid, F = female, I2 = I-squared, M = male, MPV = mean platelet volume, NOS = Newcastle-Ottawa scale, SMD = standardized mean difference.
How to cite this article: Wang J, Wang Z, Zhang M, Lou Z, Deng J, Li Q. Diagnostic value of mean platelet volume for neonatal sepsis: A systematic review and meta-analysis. Medicine. 2020;99:32(e21649).
This work was supported by Wuhu City, Anhui Provincial Science and Technology Planning project (2018cg29).
This manuscript was funded by the open project of Key Laboratory of Non-coding RNA Transformation Research of Anhui Higher Education Institution (RNA201910).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017;376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51. [DOI] [PubMed] [Google Scholar]
- [4].Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet 2017;390:1770–80. [DOI] [PubMed] [Google Scholar]
- [5].Rajaratnam JK, Marcus JR, Flaxman AD, et al. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970-2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet 2010;375:1988–2008. [DOI] [PubMed] [Google Scholar]
- [6].Sharma D, Farahbakhsh N, Shastri S, et al. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med 2018;31:1646–59. [DOI] [PubMed] [Google Scholar]
- [7].Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol 2004;25:489–95. [DOI] [PubMed] [Google Scholar]
- [8].Zarbock A, Polanowskagrabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 2007;21:99–111. [DOI] [PubMed] [Google Scholar]
- [9].Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010;38:S26–34. [DOI] [PubMed] [Google Scholar]
- [10].Vincent JL. Emerging therapies for the treatment of sepsis. Curr Opin Anaesthesiol 2015;28:411–6. [DOI] [PubMed] [Google Scholar]
- [11].Kokacya MH, Copoglu USC, Kivrak Y, et al. Increased mean platelet volume in patients with panic disorder. Neuropsych Dis Treat 2015;11:2629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oh GH, Chung SP, Park YS, et al. Mean platelet volume to platelet count ratio as a promising predictor of early mortality in severe sepsis. Shock 2016;47:323–30. [DOI] [PubMed] [Google Scholar]
- [13].Lo KL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [16].Lau J, Ioannidis J, Schmid C, et al. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [17].Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lu Q, Duan H, Yu J, et al. Are global coagulation and platelet parameters useful markers for predicting late-onset neonatal sepsis? Clin Lab 2016;62:73–9. [DOI] [PubMed] [Google Scholar]
- [20].Shalaby MM, Sobeih AA, Abdulghany WE, et al. Mean platelet volume and serum uric acid in neonatal sepsis: a case-control study. Ann Med Surg (Lond) 2017;20:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Catal F, Tayman C, Tonbul A, et al. Mean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants. Clin Lab 2014;60:1193–200. [DOI] [PubMed] [Google Scholar]
- [22].Oncel MY, Ozdemir R, Yurttutan S, et al. Mean platelet volume in neonatal sepsis. J Clin Lab Anal 2012;26:493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aydemir C, Aydemir H, Kokturk F, et al. The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr 2018;18:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cekmez Y, Dizdar Gulecoglu M, Ozcan C, et al. The utility of maternal mean platelet volume levels for early onset neonatal sepsis prediction of term infants. Ginekol Pol 2017;88:312–4. [DOI] [PubMed] [Google Scholar]
- [25].Hanaganahalli SB, Sreeram S, Bompada M, et al. Is MPV a predictive marker for neonatal sepsis?. A pilot study. J Pediatr Hematol Oncol 2018;40:548–52. [DOI] [PubMed] [Google Scholar]
- [26].Omran A, Maaroof A, Mai HS, et al. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J) 2018;94:82–7. [DOI] [PubMed] [Google Scholar]
- [27].Aydin B, Dilli D, Zenciroglu A, et al. Mean platelet volume and uric acid levels in neonatal sepsis. Indian J Pediatr 2014;81:1342–6. [DOI] [PubMed] [Google Scholar]
- [28].Yao Y, Tu Y, Lu Q. Values of C-reactive protein, percentage of neutrophils and mean platelet volume in early diagnosis of neonatal sepsis. Zhongguo Dang Dai Er Ke Za Zhi 2015;17:425–9. [PubMed] [Google Scholar]
- [29].Shaaban HA, Safwat N. Mean platelet volume in preterm: a predictor of early onset neonatal sepsis. J Matern Fetal Neonatal Med V 22 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [30].Dongying Z, Gang Q, Zhongcheng L, et al. Platelet parameters and (1, 3)-β-D-glucan as a diagnostic and prognostic marker of invasive fungal disease in preterm infants. PLoS One 2015;10:e0123907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 2019;98:e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rondina MT, Garraud O. Emerging evidence for platelets as immune and inflammatory effector cells. Front Immunol 2014;5:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pattraporn T, Arintaya P, Atikun L, et al. The role of mean platelet volume as a predictor of mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Res Pract 2016;2016:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yuri Gasparyan A, Ayvazyan LP, Mikhailidis D, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47–58. [DOI] [PubMed] [Google Scholar]
- [35].Kim CH, Kim SJ, Lee MJ, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One 2015;10:e0119437. [DOI] [PMC free article] [PubMed] [Google Scholar]


