Abstract
The purpose of this study was to investigate novel biomarkers and potential mechanisms in nasopharyngeal carcinoma (NPC) patients with metastasis.
Two microarray datasets (GSE103611 and GSE36682) were obtained from GEO database, differentially expressed genes (DEGs) and differentially expressed miRNA (DEMs) were identified, Gene ontology (GO) as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were conducted with DEGs and DEMs targeted genes. Protein–protein interactions (PPI) network of the DEGs and DEMs targeted genes were constructed, furthermore, Connectivity Map (CMap) database was applied to select the potential drugs with therapeutic effects.
Overall, we identified 396 upregulated and 19 downregulated DEGs. Additionally, we identified 1 upregulated DEM, miR-135b, and a downregulated DEM, miR-574-5p. Functional enrichment analysis indicated that both DEGs and DEMs targeted genes participated in biological process (BP) of regulation of transcription from RNA polymerase II promoter, DNA-templated positive regulation of transcription, and Epstein-Barr virus infection signaling pathway. Besides, upregulated EP300 gene was a hub node both in DEGs and DEMs target genes. CMap database analysis indicated that sanguinarine, verteporfin, and chrysin are potential drugs for prevention and treatment of NPC metastasis.
In summary, the common hub gene, biological process and pathway identified in the study provided a novel insight into the potential mechanism of NPC metastasis. Furthermore, we identified several possible small molecule compounds for treatment of NPC metastasis.
Keywords: biomarkers, metastasis, microarray technology, nasopharyngeal carcinoma, small-molecule compounds
1. Introduction
Differ from other head and neck cancer, nasopharyngeal carcinoma (NPC) has a special geographical distribution, with a peak incidence approaching 50 cases per 100,000 in Southern China and Southeast Asia.[1] Approximately 80% of NPC patients were diagnosed with locoregionally advanced disease at presentation.[2] Due to its deep-seated location, radiation in combine with chemotherapy were considered the standard treatment for NPC patients and leads to excellent 5-year survival rate of 85%.[3] Nevertheless, the development of distant metastasis is the major cause of treatment failure and the overall survival of metastatic NPC is poor.[4] Clinically, an anatomy-based staging system was found to be insufficient in evaluating treatment efficiency and predict prognosis in metastatic NPC patients. Although some molecular biomarkers, such as Epstein–Barr virus DNA (EBV DNA) and lactate dehydrogenase (LDH), were found be linked with NPC metastasis, the molecular mechanisms of NPC metastasis have not yet been fully clarified.[5] Novel biomarkers and its underlying molecular mechanisms that affect tumor metastasis still need to be explored to guide treatment for NPC patients.
Over the past few decades, microarray technology established using high-throughput platforms serves as a promising and efficient tool to detect phenotypic characteristics of cancer.[6] For example, Zhang et al[7] revealed that 8 genes and immune-related pathways associated with the development of osteosarcoma metastasis by weighted gene co-expression network analysis (WGCNA). Cai et al[8] and Lu et al[9] identified 5 hub genes as prognostic biomarkers for breast cancer metastasis by bioinformatics analysis. However, studies integrating microarray datasets for investigation of key genes and regulatory networks of metastatic NPC are lacking. Thus, the goal of the present study was to systematically explore the molecular mechanisms underlying metastatic NPC.
In our study, we identified differentially expressed genes (DEGs) and differentially expressed microRNAs (DEMs) between metastatic NPC and non-metastatic NPC tissue based on information obtained from 2 GEO databases (GSE103611 and GSE36682). Next, we performed bioinformatic analyses of DEGs and DEMs targeted genes by utilizing Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and construction of protein–protein interaction (PPI) network. The common hub gene, biological process, and pathway were identified between DEGs and DEMs targeted genes, Diagram of data analysis process was showed in Fig. 1. Furthermore, DEGs were uploaded to CMap database to detect small active compounds with possible therapeutic effects on NPC metastasis.
Figure 1.
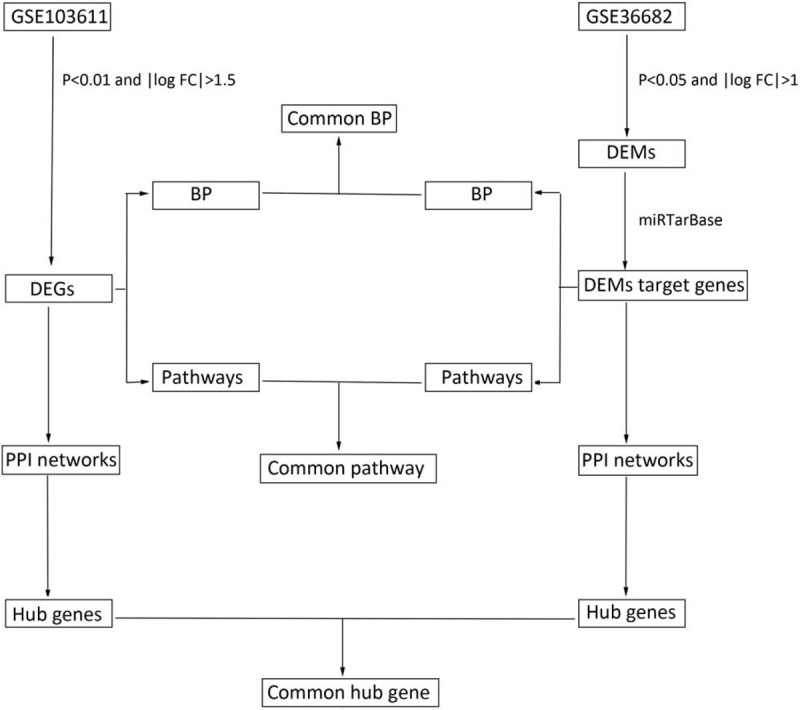
Flow chart of data analysis. BP = biological process, DEGs = differentially expressed genes, DEMs = differentially expressed miRNA, PPI = protein–protein interactions.
2. Materials and methods
2.1. Data source
Microarray data (GSE103611 and GSE36682) was downloaded from NCBI Gene Expression Omnibus (GEO) database. The GSE103611 dataset contained gene expression from 48 samples, including 24 NPC tumor tissues with distant metastasis and 24 NPC tumor tissues without distant metastasis. The GSE36682 dataset contained the miRNA expression profiles of 68 samples, which included 17 NPC tumor tissues with distant metastasis and 45 NPC tumor tissues without distant metastasis, and were utilized for further analysis in our study. All relevant datasets were publicly available through open access. Therefore, an ethics committee approval was not necessary.
2.2. Screening results of DEGs and DEMs
The interactive online tool GEO2R was utilized to compare between 2 datasets from GEO.[10] In our study, GEO2R was used to analyze and screen DEGs and DEMs between the metastatic and non-metastatic NPC samples using the GSE103611 and GSE36682 data sets. Then, microarray data of DEGs and DEMs were obtained in text format. The gene probes were transformed into gene names by referencing the annotation file of probes in the platform. DEGs were screened due to the criteria of P < .01 and |log 2 fold–change| > 1.5. DEMs were screened due to the criteria of P < .05 and |log 2 fold–change| > 1.
2.3. DEMs related genes were predicted using miRTarBase
DEMs target genes were screened by the miRTarBase online databases (http://mirtarbase.mbc.nctu.edu.tw/php/index.php).[11] This database contains extensive information of experimentally validated miRNA-target interactions. The DEMs target genes were supported by strong experimental evidence, such as Western blot, quantitative polymerase chain reaction (qPCR), or reporter assay. If the DEMs target genes were not observed in the strong experimental evidence, which would screened by weak experimental evidence, such as NGS, pSILAC, or microarray. In our study, the intersections of target genes of DEMs were screened for further analysis.
2.4. Analyses of GO and KEGG pathway enrichment
GO database was applied to conduct functional studies based on the 2 gene datasets. The gene annotation terms were represented as biological process (BP), cellular component (CC), and molecular function (MF). KEGG was applied to identify functional pathways and practical application of genes.[12] Analyses were assessed using Database for Annotation, Visualization and Integrated Discovery (DAVID). P-value of <.05 indicated statistical significance with regards to GO terms and KEGG pathway. Venn Diagram online tool was utilized to identified the common biological process and pathways between the 2 gene datasets.
2.5. Construction of the PPI network
The STRING database is a web tool that is used for studying target protein interactions.[13] Cytoscape, an open source tool, was applied to visualize biological pathways and molecular interaction networks by integrating networks with gene expression profiles and annotations.[14] We downloaded the DEGs and DEMs from the GSE103611 and GSE36682 dataset, respectively, and we uploaded them onto STRING's official website. Then, the PPI interaction network of DEGs and DEMs target genes was visualized using the Cytoscape software (v3.6.1). Molecular Complex Detection (MCODE) of Cytoscape was utilized to established modules of DEGs and DEMs according to the following criteria: the highest MCODE score and node number. In addition, we applied Cytohubba of Cytoscape to screen hub nodes that are commonly linked to proteins.
2.6. Screening of small-molecules compounds
Connectivity Map (CMap) is a unique gene-expression-based drug development platform that is focused on finding associations between genes, drugs, and diseases. In this study, we transformed DEGs into probes and compared these probe sets with those that participate in small active molecular interference using the CMap website and obtained the corresponding small-molecule compounds.[15] Enrichment score representing similarity and P-value were calculated. Negatively related small-molecule compounds (P < .01 and enrichment <0) were suggested to be therapeutically effective for treatment of NPC metastasis.
3. Results
3.1. Identification of DEGs and DEMs
In our study, expression profiles from 2 datasets (GSE103611 and GSE36682) were obtained. Based on criteria of P < .01 and |log FC| > 1.5, we identified 415 DEGs, including 396 upregulated mRNAs and 19 downregulated mRNAs, volcano plot of DEGs were identified and showed in Fig. 2. Additionally, based on P < .05 and |log FC| > 1, we identified 1 upregulated microRNA (miR-135b) and 1 downregulated microRNA (miR-574-5p). Furthermore, we identified microRNA related genes using the miRTarBase online tool. One hundred eighty nine mRNAs were found to be miR-135b target genes while 415 mRNAs were found to be miR-574-5p target genes. All the DEGs and DEMs were identified by comparing expression profiles between the NPC tumor tissues with distant metastasis and NPC tumor tissues without distant metastasis.
Figure 2.
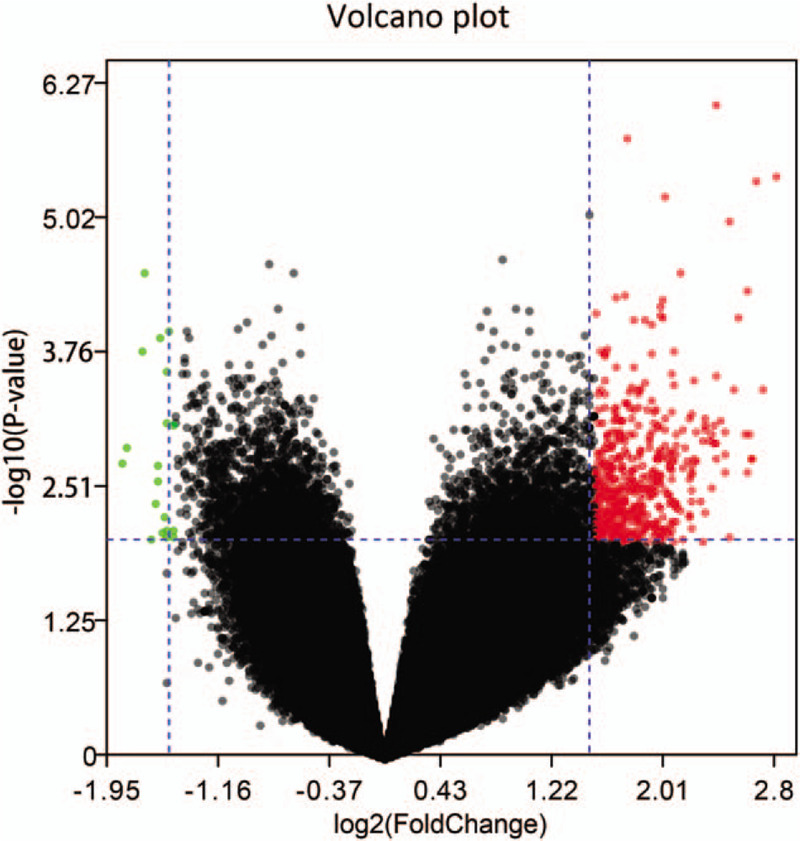
Volcano plot of differentially expressed genes (DEGs). The x-axis shows the gene expression difference by the fold change (log scaled) while the y-axis shows the significance P-value (log scaled). Red dot represents the up-regulated genes and green dot represents the down-regulated genes in metastasis NPC samples compared with no-metastasis NPC samples. The expression of gene is considered significantly differentially if its |log(FC)| > 1.5 and P-value < .01. NPC = nasopharyngeal carcinoma.
3.2. Gene ontology analysis of DEGs and DEMs
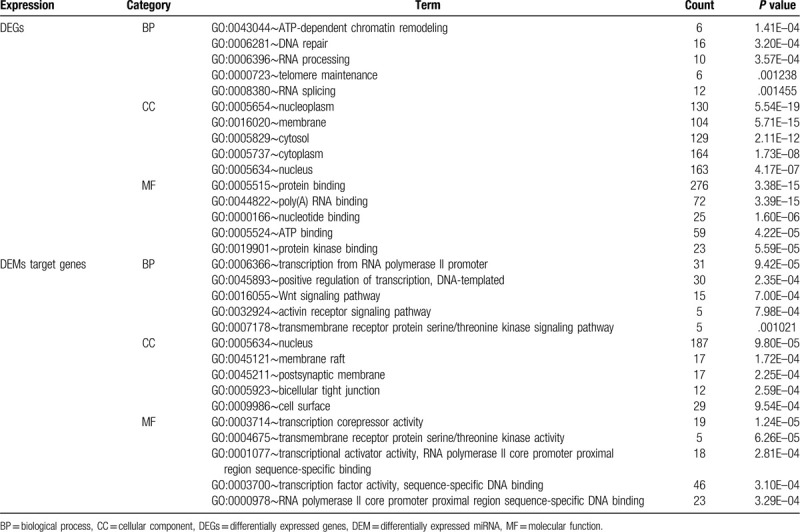
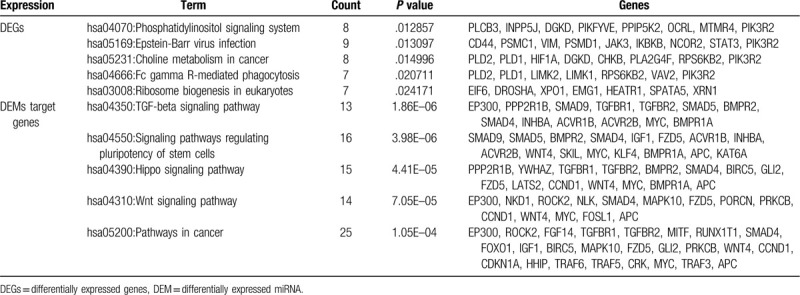
In order to further explore the potential molecular mechanism of NPC metastasis. GO analyses of the DEGs and DEMs target genes were conducted by using the DAVID web tool. With regards to BP, DEGs were mainly enriched in ATP-dependent chromatin remodeling, DNA repair, and RNA processing. DEMs target genes were mainly involved in regulation of transcription from RNA polymerase II promoter, DNA-templated positive regulation of transcription and Wnt signaling pathway. For the CC category, DEGs were significantly highly expressed in nucleoplasm, membrane, and cytosol. DEMs were mainly associated with nucleus, membrane raft and postsynaptic membrane. Additionally, for the MF category, DEGs were found to be largely involved in protein binding, poly (A) RNA binding, and nucleotide binding, DEMs target genes, on the other hand, were mainly involved in transcription corepressor activity, transmembrane receptor protein serine/threonine kinase activity and transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding. Result of Venn Diagram indicated that regulation of transcription from RNA polymerase II promoter and DNA-templated positive regulation of transcription were the common significant biological process across both DEGs and DEMs target genes (Fig. 3A). The top 5 most significant enriched GO terms (P value < .05) are summarized in Table 1.
Figure 3.
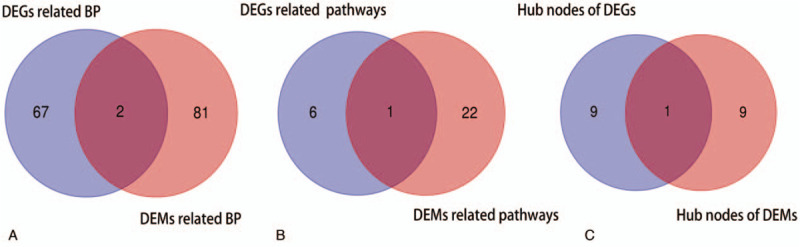
Venn diagrams indicated the common biological process between differentially expressed genes (DEGs) and differentially expressed miRNAs (DEMs) target genes (A). Venn diagrams indicated the common pathway between DEGs and DEMs target genes (B). Venn diagrams indicated the common hub gene between DEGs and DEMs target genes (C).
Table 1.
Go function annotation for the DEGs and DEMs targeted genes (top 5).
3.3. Pathway enrichment analyses of DEGs and DEMs
We analyzed cell signaling pathway enrichment of DEGs and DEMs target gene by using KEGG. A total of 30 significant pathways were selected. DEGs were found to be involved in phosphatidylinositol signaling system, Epstein-Barr virus infection, and choline metabolism in cancer. DEMs target genes were enriched in TGF-beta signaling pathway, signaling pathways regulating pluripotency of stem cells, and Hippo signaling pathway. We further identified that the Epstein-Barr virus infection was common pathway both DEGs and DEMs target genes (Fig. 3B). The top 5 most significant KEGG pathways (P value of <.05) are summarized in Table 2.
Table 2.
Kyoto encyclopedia of genes and genomes pathway analysis for the DEGs and DEMs targeted genes (top 5).
3.4. PPI network construction
We input all DEGs and DEMs target genes into STRING database, as shown in Figs. 4A and 5A, red colored nodes represent up-regulation, while blue colored nodes represent down-regulation. The PPI network of DEGs contained 363 nodes and 1470 edges. The PPI network of DEMs target genes were composed of 379 nodes and 832 edges. We visualized the network using Cytoscape software. Next, we modularized the network using the plug-in MCODE and identified the highest MCODE score and node number. The data indicated that the DEGs module was mainly correlated with the spliceosome and RNA polymerase (Fig. 4B), and the seed gene was heterogeneous nuclear ribonucleoprotein D (HNRNPD). On the other hand, the DEMs target genes module mainly correlated with the cell adhesion molecules (CAMs), and the seed gene was ring finger protein 138 (RNF138) (Fig. 5B). We also used cytohubba, a plug-in, to select the hub nodes. The top 10 hub nodes with higher degrees of interaction in DEGs were identified, which included EP300, XPO1, SMARCA4, ATM, STAT3, YWHAZ, DICER1, POLR2B, GAPDH, and HNRNPD, all of them were up-regulated (Fig. 4C). The top 10 hub nodes with higher degrees of interaction in DEMs target genes were screened, including EP300, MAPK10, CCND1, CDKN1A, IGF1, TGFBR1, RUNX2, MYC, KLF4, FOXO1 (Fig. 5C). miR-574-5p target genes were EP300, MAPK10, CCND1, CDKN1A, and IGF1, while miR-135b-5p target genes were TGFBR1, RUNX2, MYC, KLF4, and FOXO1. Among the above genes, EP300 had a high degree in the protein–protein interaction network and was identified as a hub gene in both DEGs and DEMs target genes (Fig. 3C). Therefore, upregulated EP300 may play an important role in metastatic NPC.
Figure 4.
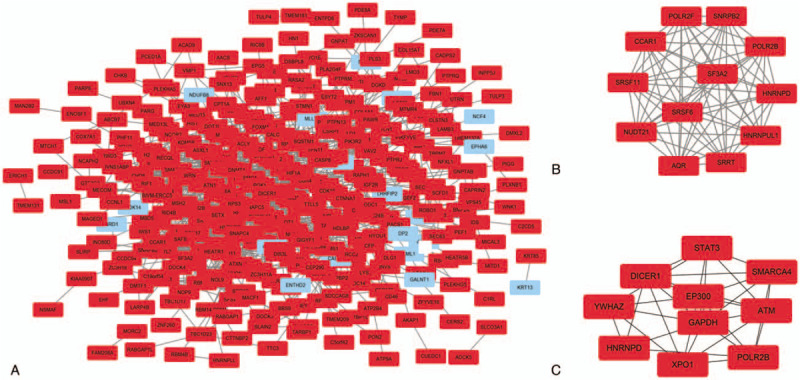
PPI network of the differentially expressed genes (DEGs) (A). A significant module of the DEGs (B). Top 10 hub nodes of DEGs (C). Red colored nodes represent up-regulated genes, blue colored nodes represent down-regulated genes. PPI = protein–protein interactions.
Figure 5.
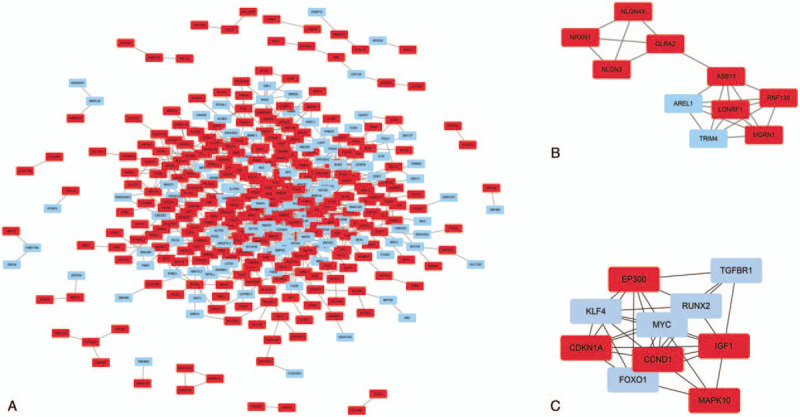
PPI network of the differentially expressed miRNAs (DEMs) target genes (A). A significant module of the DEMs target genes (B). Top 10 hub nodes of DEMs target genes (C). Red colored nodes represent down-regulated DEM target genes, blue colored nodes represent up-regulated DEM target genes. PPI = protein–protein interactions.
3.5. CMap analysis of DEGs
We screened the compounds with molecular features that have the potential to treat NPC metastasis. In order to do so, we uploaded previously selected DEGs into the CMap database, the potential small molecular compounds were ranked according to negative connectivity scores and P < .05. The top 10 small-molecule compounds that demonstrated high correlation with NPC metastasis are shown in Table 3. Among these molecules, sanguinarine, verteporfin, and chrysin were described to be significantly associated with NPC metastasis (Fig. 6).
Table 3.
The top 10 most significant small-molecule compounds that could reverse the tumoral metastasis status of NPC.
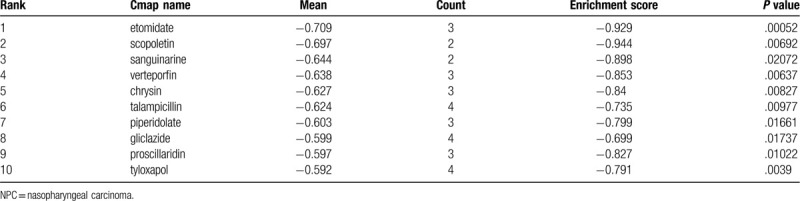
Figure 6.
The 2D structures of the 3 compounds that could reverse the changes of gene expression in NPC metastasis. Sanguinarine (A), vertipofen (B), chrysin (C). NPC = nasopharyngeal carcinoma.
4. Discussion
NPC is a malignant disease of the head and neck area and causes 34.1 million deaths annually.[16] The disease is characterized by unique geographic, etiologic, and biologic features. As the widely application of intensity modulated radiotherapy and chemotherapy, the survival rate and quality of NPC patients have increased largely. Nevertheless, distant metastases are still an obstacle to successful treatment. It is reported that approximately 30% to 40% of patients with locoregionally advanced NPC develop distant metastasis after receiving treatment.[17] In fact, distant metastasis accounts for cancer-specific mortality among approximately 70% of NPC patients, there is no obvious clinical evidence of metastases in the initial diagnosis and regular examinations is hard to detected subclinical micrometastases.[18] On the other hand, studies to date have indicated that some molecules, such as specific mRNAs, microRNAs, and proteins regulate metastasis through different biological process, including methylation, cell cycle, and adhesion.[19–21] Whereas, the interaction and molecular mechanisms between mRNAs and microRNAs in NPC metastasis remain unclear. Therefore, it is urgent to screen the key genes and explore the potential mechanisms for NPC patients with metastasis.
Microarray data sets as well as other omics data sets, such as proteomics, phosphoproteomics, and single cell RNA seq data have been increasingly used in cancer research.[22–25] This study is focused on DEGs and DEMs in metastatic NPC compared with non-metastatic NPC based on 2 expression datasets downloaded from the GEO database. Up-regulated gene EP300 was a core node between DEGs and DEMs target genes in the PPI network. In the study of Liao et al,[26] result of western blotting showed that EP300 protein was increased expression in NPC cell lines compared with normal nasopharyngeal cells. What's more, upregulation of EP300 promote invasion and metastasis of NPC cells by the induction of epithelial-mesenchymal transition (EMT). A previous study indicated that the expression levels of EP300 was significantly higher in NPC tissues than adjacent non-cancerous tissues, additionally, higher E300 expression is associated with poor overall survival and progression-free survival in NPC patients.[27] EP300 has been proved to be upregulated in several malignancies, such as non-small cell lung cancer, colorectal carcinomas, hepatocellular carcinoma.[28–30] A recent study demonstrated that mutation of EP300 is correlated with higher tumor mutation burden (TMB) and promotes antitumor immunity in bladder cancer patients.[31] Dou et al[32] showed that phosphorylation of EP300 associated with liver metastasis. Besides, our results also suggested that EP300 is involved in the TGF-beta signaling pathway, Wnt signaling pathway, and pathways in cancer. Currently, it is well recognized that TGF-beta contributes to the metastatic potential of tumor cells through promoting EMT, cell migration, and invasion.[33] A reported by Liao et al[26] proved that EP300 enhance EMT through acetylation of Smad2 and Smad3 via the TGF-β signaling pathway, and thus promoted the NPC metastasis, which is similar to the result of our study. In addition, several studies demonstrated an important role for aberrant Wnt signaling in NPC involving diverse cellular processes, including cell migration, hypermethylation, and stemness.[34] Taken together, our study reveals that high expression of EP300 is enriched in TGF-beta signaling pathway and Wnt signaling pathway in metastatic NPC. Up-regulation of EP300 is significantly correlated with metastasis in NPC patients and may promote the development of metastatic NPC.
MicroRNAs (miRNA) constitute a family of small noncoding RNAs and help control a wide range of biological pathways such as cell growth, differentiation, migration, and apoptosis.[35] Moreover, miRNAs play a potential role in cancer development and are often associated with cancer metastasis. In the present study, an upregulated DEM (miR-135b) and a downregulated DEM (miR-574–5p) were selected. The hub gene EP300 was one of the miR-574-5p upregulated genes. MiR-135b was first identified as having a role in differentiation of somatic stem cells.[36] Prior studies have indicated that miR-135b encourages cell migration and invasion by downregulation of LZTS1 in tumor tissue in the beginning stages of squamous cell carcinoma progression.[37] Similarly, results of our study indicated that MiR-135b was upregulated in NPC metastatic tissue. On the other hand, another study demonstrated that miR-574-5p suppresses colorectal cancer liver metastasis by negatively regulating MACC-1 expression.[38] Results of our study also demonstrated that miR-574-5p was downregulated in metastatic NPC tissue. Therefore, our results suggest that the above mentioned microRNAs, particularly miR-574-5p, may play an important role in the metastasis of NPC, and can potentially predict metastasis of NPC.
Our study showed that many pathways were detected in the GO enrichment analysis as related to NPC metastasis. Additionally, we identified 2 significant biological processes across both DEGs and DEMs target genes, such as regulation of transcription from RNA polymerase II promoter and DNA-templated positive regulation of transcription. Previous reports indicated that these processes were involved in metastasis of cancer, such as liver cancer, breast cancer, and osteosarcomas.[39–41] Furthermore, a KEGG pathway, the Epstein-Barr virus infection was enriched and regulated by DEGs and DEMs target genes together. Li et al[42] reported that the Epstein-Barr virus-encoded latent membrane protein-1 could induce MicroRNA-10b and promote the metastasis of human nasopharyngeal carcinoma cells. Therefore, we can infer that Epstein-Barr virus signaling process likely contributes to the mechanism of NPC metastasis and therefore, may be a possible therapeutic target.
Chemotherapy plays an important role in the treatment of cancer metastasis.[43,44] We identified a few small molecules that hold promise of therapeutic efficacy against NPC metastasis. These small molecules including sanguinarine, verteporfin, and chrysin, have been reported to display anti-metastatic activity. Sanguinarine (SNG) serves as quaternary benzophenanthridine alkaloid, which stimulates cytotoxicity across various human cancers and inhibits specific pro-tumorigenic processes including invasion, angiogenesis, and metastasis.[45] Vertipofen is a Yap1 inhibitor that blocks metastasis by effectively reducing the expression of Yap1 and FGFR1 in lung cancer.[46] Chrysin, 5,7-dihydroxyflavone, is a naturally-occurring flavonoid that is known to block angiogenesis and metastasis.[47] Lin et al[48] showed that chrysin inhibits IL-6-mediated angiogenesis by reducing the soluble IL-6 receptor/gp130/JAK1/ STAT3/VEGF. Hence, our results suggest that these small molecules can function to combat NPC metastasis.
5. Conclusion
In conclusion, we analyzed GEO data for metastatic NPC, the result indicated that an upregulated gene EP300, which was potentially targeted by downregulated miR-574-5p, may associated with the NPC metastasis. The DEGs and DEMs target genes might be involved in the biological process of regulation of transcription from RNA polymerase II promoter and DNA-templated positive regulation of transcription and ultimately affect the development of NPC metastasis through Epstein-Barr virus signaling pathway. Besides, our study identified small-molecule compounds including sanguinarine, verteporfin, and chrysin which may be efficacious in the treatment of NPC metastasis. Nonetheless, our study had some limitations. First, microarray datasets and platform obtained from the GEO were different. Second, the hub gene, biological process, and pathway should be validated using in vitro or in vivo experiments. Despite the limitations in our study, the findings in the study might contribute to advance our understanding of the development of NPC metastasis and provide reference information for the treatment of NPC metastasis.
Author contributions
Conceptualization: Ren-Sheng Wang.
Data curation: Jing-Lin Mi.
Formal analysis: Meng Xu.
Funding acquisition: Ren-Sheng Wang.
Project administration: Chang Liu.
Software: Jing-Lin Mi.
Supervision: Ren-Sheng Wang.
Validation: Jing-Lin Mi, Chang Liu.
Visualization: Meng Xu.
Writing – original draft: Jing-Lin Mi.
Writing – review & editing: Rensheng Wang.
Footnotes
Abbreviations: BP = biological process, Camp = Connectivity Map CMap, DEGs = differentially expressed genes, DEM = differentially expressed miRNA, EP300 = E1A binding protein p300, GEO = Gene Expression Omnibus, GO = Gene Ontology, KEGG = Kyoto Encyclopedia of Genes and Genomes, NPC = nasopharyngeal carcinoma.
How to cite this article: Mi JL, Xu M, Liu C, Wang RS. Identification of novel biomarkers and small-molecule compounds for nasopharyngeal carcinoma with metastasis. Medicine. 2020;99:32(e21505).
This study was supported by the Basic Ability Enhancement Project of Young Teachers in Guangxi Zhuang Autonomous Region (No. 2018KY0134), Guangxi Science and Technology Cooperation and Exchange Project (GKH 159905-2-11), Central Guided Local Science and Technology Development Project (GK ZY18076006), Guangxi Science and Technology Program Project (GK AD17129013).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet 2016;387:1012–24. [DOI] [PubMed] [Google Scholar]
- [2].Xia WX, Zhang HB, Shi JL, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. Eur J Cancer 2013;49:2152–60. [DOI] [PubMed] [Google Scholar]
- [3].Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883–92. [DOI] [PubMed] [Google Scholar]
- [4].Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 2015;33:3356–64. [DOI] [PubMed] [Google Scholar]
- [5].Tang XR, Li YQ, Liang SB, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol 2018;19:382–93. [DOI] [PubMed] [Google Scholar]
- [6].Liu K, Kang M, Zhou Z, et al. Bioinformatics analysis identifies hub genes and pathways in nasopharyngeal carcinoma. Oncol Lett 2019;18:3637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang H, Guo L, Zhang Z, et al. Co-expression network analysis identified gene signatures in osteosarcoma as a predictive tool for lung metastasis and survival. J Cancer 2019;10:3706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cai Y, Mei J, Xiao Z, et al. Identification of five hub genes as monitoring biomarkers for breast cancer metastasis in silico. Hereditas 2019;156:20.doi: 10.1186/s41065-019-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu X, Gao C, Liu C, et al. Identification of the key pathways and genes involved in HER2-positive breast cancer with brain metastasis. Pathol Res Pract 2019;215:152475.doi: 10.1016/j.prp.2019.152475. [DOI] [PubMed] [Google Scholar]
- [10].Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res 2013;41:D991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 2018;46:D296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kanehisa M, Sato Y, Kawashima M, et al. The KEGG Pathway Maps. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 2016;44:D457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015;43:D447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Su G, Morris JH, Demchak B, et al. Biological network exploration with Cytoscape 3. Curr Protoc Bioinformatics 2014;47:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006;313:1929–35. [DOI] [PubMed] [Google Scholar]
- [16].Sun PY, Chen YH, Feng XB, et al. High-dose static and dynamic intensity-modulated radiotherapy combined with chemotherapy for patients with locally advanced nasopharyngeal carcinoma improves survival and reduces brainstem toxicity. Med Sci Monit 2018;24:8849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–20. [DOI] [PubMed] [Google Scholar]
- [18].Mikhail O, Andrey V, Anna K, et al. PET-CT and occupational exposure in oncological patients. SciMeJ 2020;2:63–9. [Google Scholar]
- [19].Ruco LP, Stoppacciaro A, Uccini S, et al. Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in undifferentiated nasopharyngeal carcinoma (lymphoepithelioma) and in malignant epithelial tumors. Hum Pathol 1994;25:924–8. [DOI] [PubMed] [Google Scholar]
- [20].Yi B, Tan SX, Tang CE, et al. Inactivation of 14-3-3 sigma by promoter methylation correlates with metastasis in nasopharyngeal carcinoma. J Cell Biochem 2009;106:858–66. [DOI] [PubMed] [Google Scholar]
- [21].Chen L, Wang L, Zhu L, et al. Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol 2002;283:C1313–23. [DOI] [PubMed] [Google Scholar]
- [22].Dwivedi P, Chutipongtanate S, Muench DE, et al. SWATH-proteomics of Ibrutinib's action in myeloid leukemia initiating mutated G-CSFR signaling. Proteomics Clin Appl 2020;e1900144.doi: 10.1002/prca.201900144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dwivedi P, Muench DE, Wagner M, et al. Phospho serine and threonine analysis of normal and mutated granulocyte colony stimulating factor receptors. Sci Data 2019;6:21.doi: 10.1038/s41597-019-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dwivedi P, Muench DE, Wagner M, et al. Time resolved quantitative phospho-tyrosine analysis reveals Bruton's Tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors. Leukemia 2019;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].David EM, Andre O, Kyle F, et al. Mouse models of neutropenia reveal progenitor-stage-specific defects. Nature 2020;582:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liao ZW, Zhao L, Cai MY, et al. P300 promotes migration, invasion and epithelial-mesenchymal transition in a nasopharyngeal carcinoma cell line. Oncol Lett 2017;13:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liao ZW, Zhou TC, Tan XJ, et al. High expression of p300 is linked to aggressive features and poor prognosis of nasopharyngeal carcinoma. J Transl Med 2012;10:110.doi: 10.1186/1479-5876-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hou X, Gong R, Zhan J, et al. p300 promotes proliferation, migration, and invasion via inducing epithelial-mesenchymal transition in non-small cell lung cancer cells. BMC Cancer 2018;18:641.doi: 10.1186/1479-5876-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [29].Ishihama K, Yamakawa M, Semba S, et al. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol 2007;60:1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li M, Luo RZ, Chen JW, et al. High expression of transcriptional coactivator p300 correlates with aggressive features and poor prognosis of hepatocellular carcinoma. J Transl Med 2011;9:5.doi: 10.1186/1479-5876-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhu G, Pei L, Li Y, et al. EP300 mutation is associated with tumor mutation burden and promotes antitumor immunity in bladder cancer patients. Aging (Albany NY) 2020;12:2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dou C, Liu Z, Tu K, et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 2018;154:2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McEarchern JA, Kobie JJ, Mack V, et al. Invasion and metastasis of a mammary tumor involves TGF-beta signaling. Int J Cancer 2001;91:76–82. [DOI] [PubMed] [Google Scholar]
- [34].Zong D, Yin L, Zhong Q, et al. ZNF488 enhances the invasion and tumorigenesis in nasopharyngeal carcinoma via the wnt signaling pathway involving epithelial mesenchymal transition. Cancer Res Treat 2016;48:334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Farazi TA, Hoell JI, Morozov P, et al. MicroRNAs in human cancer. Adv Exp Med Biol 2013;774:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schaap-Oziemlak AM, Raymakers RA, Bergevoet SM, et al. MicroRNA hsa-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells Dev 2010;19:877–85. [DOI] [PubMed] [Google Scholar]
- [37].Olasz EB, Seline LN, Schock AM, et al. MicroRNA-135b regulates leucine zipper tumor suppressor 1 in cutaneous squamous cell carcinoma. PLoS ONE 2015;10:e125412.doi: 10.1371/journal.pone.0125412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cui Z, Tang J, Chen J, et al. Hsa-miR-574-5p negatively regulates MACC-1 expression to suppress colorectal cancer liver metastasis. Cancer Cell Int 2014;14:47.doi: 10.1186/1475-2867-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Matouk IJ, Abbasi I, Hochberg A, et al. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol 2009;21:688–92. [DOI] [PubMed] [Google Scholar]
- [40].Sloan DD, Nicholson B, Urquidi V, et al. Detection of differentially expressed genes in an isogenic breast metastasis model using RNA arbitrarily primed-polymerase chain reaction coupled with array hybridization (RAP-array). Am J Pathol 2004;164:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rupp B, Lorenz U, Schmidt J, et al. Discordant effects of activator protein-1 transcription factor on gene regulation, invasion, and metastasis in spontaneous, radiation-induced, and fos-induced osteosarcomas. Mol Carcinog 1998;23:69–75. [PubMed] [Google Scholar]
- [42].Li G, Wu Z, Peng Y, et al. MicroRNA-10b induced by Epstein-Barr virus-encoded latent membrane protein-1 promotes the metastasis of human nasopharyngeal carcinoma cells. Cancer Lett 2010;299:29–36. [DOI] [PubMed] [Google Scholar]
- [43].Yavari K. Anti-angiogenesis therapy of cancer cells using 153Sm-Bevasesomab. Emerg Sci J 2018;2:130–9. [Google Scholar]
- [44].Habibeh Z. Effects of salvia officinalis extract on the breast cancer cell line. SciMedJ 2019;1:25–9. [Google Scholar]
- [45].Rahman A, Pallichankandy S, Thayyullathil F, et al. Critical role of HO in mediating sanguinarine-induced apoptosis in prostate cancer cells via facilitating ceramide generation, ERK1/2 phosphorylation, and Par-4 cleavage. Free Radic Biol Med 2019;134:527–44. [DOI] [PubMed] [Google Scholar]
- [46].Lu T, Li Z, Yang Y, et al. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett 2018;423:36–46. [DOI] [PubMed] [Google Scholar]
- [47].Xia Y, Lian S, Khoi PN, et al. Chrysin inhibits tumor promoter-induced MMP-9 expression by blocking AP-1 via suppression of ERK and JNK pathways in gastric cancer cells. PLoS ONE 2015;10:e124007.doi: 10.1371/journal.pone.0124007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lin CM, Shyu KG, Wang BW, et al. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: an in vitro and in ovo approach. J Agric Food Chem 2010;58:7082–7. [DOI] [PubMed] [Google Scholar]


