Abstract
For the treatment of huge unresectable hepatocellular carcinoma (HCC), transcatheter arterial chemoembolization (TACE) or transcatheter arterial embolization (TAE) generally had poor effects and high complication rates. Our previous study found that Hepatic arterial infusion chemotherapy (HAIC) is a safe procedure and provides better survival than symptomatic treatment for the patients with huge unresectable HCC. The aim of the study is to compare the effect of HAIC vs TAE in patients with huge unresectable HCC.
Since 2000 to 2005, patients with huge (size > 8 cm) unresectable HCC were enrolled. Twenty-six patients received HAIC and 25 patients received TAE. Each patient in the HAIC group received 2.5 + 1.4 (range: 1–6) courses of HAIC and in the TAE group received 1.8 + 1.2 (range: 1–5) courses of TAE. Baseline characteristics and survival were compared between the HAIC and TAE group.
The HAIC group and the TAE group were similar in baseline characteristics and tumor stages. The overall survival rates at 1 and 2 years were 42% and 31% in the HAIC group and 28% and 24% in the TAE group. The patients in the HAIC group had higher overall survival than the TAE group (P = .077). Cox-regression multivariate analysis revealed that HAIC is the significant factor associated with overall survival (relative risk: 0.461, 95% confidence interval: 0.218–0.852, P = .027). No patients died of the complications of HAIC but three patients (12%) died of the complications of TAE.
In conclusion, HAIC is a safe procedure and provides better survival than TAE for patients with huge unresectable HCCs.
Keywords: chemotherapy, hepatocellular carcinoma, huge, unresectable
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and ranked the 2nd cause of cancer death in Taiwan.[1,2] Although routine screening for high risk patients, huge HCCs with size of more than 8 cm are occasionally seen.[3] Surgical resection is considered to be the standard curative therapy for huge HCC in patients with good liver reserve.[2,4–8] According to the study from Kaohsiung Veterans General Hospital, Mok et al found that the advantage of hepatic resection in patients with huge HCC is marginal as compared with multimodality treatment including transcatheter arterial embolizatoin (TAE) or hepatic arterial infusion chemotherapy (HAIC).[9] However huge HCC often presented with poor liver reserve, with increased frequency of intrahepatic metastasis and vascular invasion, which made surgical resection not suitable. So transcatheter arterial embolization/chemoembolization (TAE/TACE) has been considered as the choice for the palliative treatment of huge unresectable HCC.10 However previous studies found that TACE for huge HCC had poor effect, and TACE related mortality rate of 6.5% to 20% has been reported.[10,11] HAIC is another option for the palliative treatment for inoperable advanced HCC.[12–15] In our previous study, HAIC with cisplatin, mitomycin C, leucovorin and 5-FU for advanced unresectable HCC had tumor response rate of 28.3% and only one patient died due to the complication of HAIC during 211 courses of treatments.[16] From another recent study from our hospital, HAIC for advanced HCC had overall response rate of 20%.[17] Our recent study also found that HAIC provided survival benefit over symptomatic treatment in patients with huge unresectable HCC and no patients died of the immediate complications of HAIC.[18] So HAIC seemed to be an effective and safe method for the treatment of huge unresectable HCC. But the effect of HAIC vs TAE for the treatment of huge unresectable HCC remained unclear. The aim of the study is to investigate the effect of HAIC vs TAE for the treatment of huge unresectable HCC.
2. Materials and methods
2.1. Patients
From January 2000 to December 2005, consecutive eligible patients with hepatocellular carcinoma (HCC) were enrolled in this study. HCC was diagnosed by pathology or elevation of alpha-fetoprotein (AFP) level above 400 ng/ml along with at least two different imaging techniques including computed tomography (CT) or magnetic resonance imaging (MRI). All patients met the following criteria:
-
(A)
tumor of 8 cm or more in diameter,
-
(B)
patients who were not suitable for operation,
-
(C)
portal vein is patent
-
(D)
platelet counts > 50000/cumm,
-
(E)
prothrombin time INR < 1.5.
-
(F)
white cell counts > 2500/cumm, and
-
(G)
Child A or B liver reserve. Patients with a previous history of treatment for HCC, or distant metastasis were excluded.
From 2000 to 2005, 365 consecutive patients first diagnosed with huge HCC defined as tumor size greater than or equal to 8 cm were admitted to Kaohsiung Veterans General Hospital. Among the 365 patients, 272 were excluded (48 received surgical resection, 64 had Child C liver reserve and 50 had distant metastasis, and 110 refused aggressive treatment). Thirty-two patients who had portal vein invasion were excluded. Among the 28 patients who received HAIC, 2 were lost to follow up and 26 patients were enrolled in the HAIC group. Among the 33 patients who received TAE, 8 patients were lost to follow up, so 25 patients were enrolled in the TAE group (Fig. 1). The choice of either HAIC or TAE was determined in a somewhat random manner by the operator and was not influenced by the clinical condition of the patients.
Figure 1.
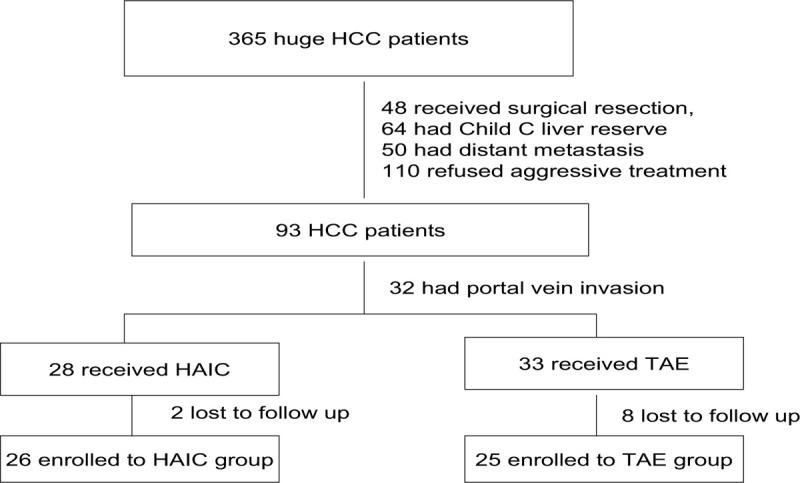
Flowchart summarizes patient inclusion.
2.2. Hepatic arterial infusion chemotherapy (HAIC)
The left subclavian artery was cannulated with a catheter and the tip of the catheter was placed in the proper hepatic artery under fluoroscopic guidance before each course of chemotherapy.[13] The main trunk of the gastroduodenal artery was occluded by metallic coil routinely. Continuous infusion of 5000 units (5cc) heparin solution daily was filled in the catheter for prevention of occlusion by thrombosis. Each course of treatment was 5 days. Cisplatin (10 mg/m2) and mitomycin-C (2 mg/m2) were dissolved in 50 ml isotonic sodium chloride solution which was infused for 20 to 30 minutes each time and continued for 5 days. In addition, 100 mg/ m2 of 5-fluorouracil (5-FU), dissolved in 250 ml of isotonic sodium chloride solution was administered for 24 hours by infusion pump for 5 days. Leucovorin (15 mg/m2) was given daily to improve the efficacy of 5-FU during HAIC. The interval between 2 courses of treatment was 3 to 4 weeks. Each patient received at least one session of treatment. Three-phase computed tomography (CT) scan of liver was done after every 2 courses of treatment. Termination of treatment when patients received 6 courses of treatment or until clinical conditions of the patients were not suitable for another course of HAIC.
2.3. Transcatheter arterial embolization (TAE)
TAE was performed through selective hepatic arterial catheterization. Whenever possible, the arteries that supply the tumor were catheterized superselectively and 5 to 15 ml of lipiodol was injected, followed by embolization with small gelfoam pellets of 1x1 mm in size. CT scan of liver was performed 2 to 3 months after TAE and further TAE was performed every 2 to 3 months if viable or recurrent tumors were found and patient had suitable liver reserve and no contraindication for TAE. All patients were followed by CT or MRI of liver and AFP every 3 months.
2.4. Follow-up
All patients in the HAIC group who completed total 6 courses of chemotherapy or not suitable for further chemotherapy or patients in the TAE group who were not suitable for further TAE received follow-up with liver function test, AFP, sonography, CT scan or MRI of liver every 3 months.
2.5. Statistical analysis
The data were expressed as mean + standard deviation. Categorical variables were compared with the X2 test or Fisher exact test when appropriate and continuous variables were compared with the Mann-Whitney test. Overall survival was estimated using the Kaplan–Meier method and the difference was determined by the log-rank test. Univariate and multivariate analysis were performed using Cox's regression model with proportional hazards. A P value of less than .05 was considered as statistically significant.
The study was approved by the Kaohsiung Veterans General Hospital Institutional Review Board. This was a retrospective study without intervention or obtaining clinical specimens and all the data were analyzed anonymously, so informed consent was waived. The waiving of informed consent was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital.
3. Results
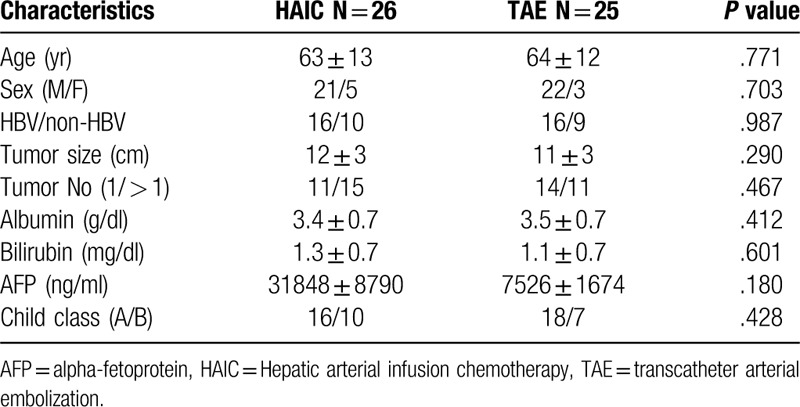
The baseline characteristics of patients in the HAIC group and the symptomatic treatment group were similar in age, sex, tumor size, tumor number, ALT level, albumin level, bilirubin level, presence of ascites, Child's classification, Okuda stage, CLIP stage, BCLC substage and AJCC stage (Table 1).[19,20]
Table 1.
Baseline characteristics of the patients in the HAIC or TAE group.
Total 64 courses of HAIC were performed for the 26 patients in the HAIC group. Each patient received 2.5 + 1.4 (range: 1–6) courses of HAIC. No patients died of the immediate complications of HAIC. One patients developed bacteremia during HAIC and were treated successfully by antibiotics. Total 45 courses of TAE were performed for the 25 patients in the TAE group. Each patient received 1.8 + 1.2 (range: 1–5) courses of TAE. Three patients (12%) died of the immediate complications of TAE (one died of tumor rupture and two died of liver failure). One patient developed liver abscess after TAE and resolved after pig-tail drainage and antibiotics treatment.
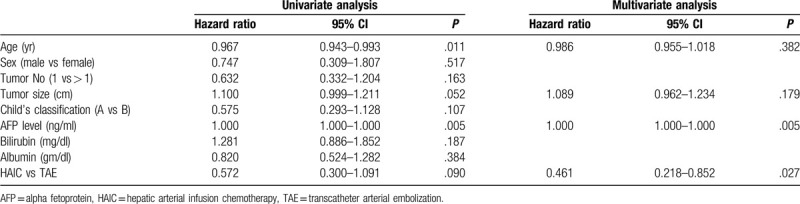
Mean follow-up time was 8.3 + 11 months (range: 1–45 months). The overall survival rates at one and two years were 42% and 31% in the HAIC group and 28% and 24% in the TAE group. The patients in the HAIC group had higher overall survival than the TAE group with borderline statistical significance (P = .077) (Fig. 2). Cox-regression multivariate analysis revealed the significant factor associated with overall survival were HAIC (relative risk: 0.461, 95% confidence interval: 0.218–0.852, P = .027) and AFP level (relative risk: 1.000, 95% confidence interval: 1.000–1.000, P = .005) (Table 2).
Figure 2.
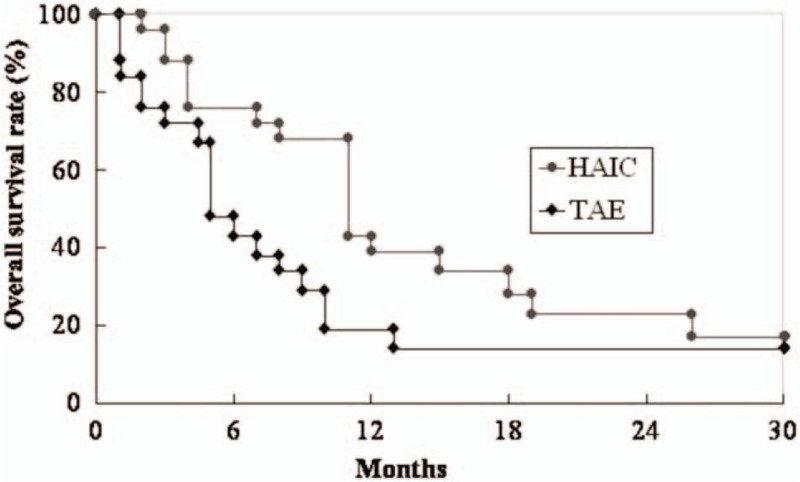
Comparison of the overall survival rate between the HAIC and TAE group. The patients in the HAIC group had higher overall survival than the TAE group (P = .077).
Table 2.
Factors associated with overall mortality in the HAIC or TAE group.
4. Discussion
Surgical resection is the treatment of choice for patients with huge HCC and well-preserved liver function.[5–8] However, only a small proportion of patients with huge HCC can fit the criteria for surgical resection. But patients with huge HCC often had a higher prevalence of extracapsular tumor invasion into liver parenchyma, more frequent intrahepatic metastasis and worse survival than those with smaller tumors.[21–23] Our recent study also found that HAIC provided survival benefit over symptomatic treatment in patients with huge unresectable HCC and no patients died of the immediate complications of HAIC.[18] There remained much controversies regarding the treatment for huge unresectable HCC.
Although TAE/TACE has been considered as the choice for the palliative treatment of huge unresectable HCC, severe liver injury after TAE/TACE was anticipated in patients with huge HCC and treatment related mortality rate as high as 20% has been reported.[11] Large tumor size was also found to be a poor prognostic factor in patients undergoing TACE.[11,24] In our hospital, HAIC has been found to be effective and safe for the treatment of advanced or huge unresectable HCC.[16–18] Besides, according to the study by Yamasaki et al, tumor size was not a prognostic factor that influenced the outcome of HAIC for patients with advanced HCC.[12] Studies to compare the treatment outcome of HAIC vs TAE for huge unresectable HCC have never been reported before. This is the first study that compared the treatment outcome of HAIC and TAE in patients with huge unresectable HCC and we found that HAIC is the independent factor associated with overall survival.
HAIC was performed every 3 to 4 weeks and treatment was terminated when patients received 6 courses of treatment or until clinical conditions of the patients were not suitable for another course of HAIC, but TAE was performed every 2 to 3 months if viable or recurrent tumors were found and patient had suitable liver reserve and no contraindication for TAE; Longer interval between each TAE and poor tumor response and deterioration of liver reserve may explain only 1.8 courses of TAE was performed.
During the 64 courses of HAIC, most patients tolerated the procedure well and no patients died of the immediate complications of HAIC. However, the mortality rate related to TAE in this study was 12%. So HAIC may be a more safe treatment procedure for the treatment of huge unresectable HCC.
From a previous randomized controlled study in our hospital, TAE compared with TACE had similar effect for the treatment of HCC.[25] Several other studies that directly compared TAE and TACE did not provide evidence of survival advantages favoring TACE.[26–29] From the results of these studies, TACE did not have significant survival benefit over TAE for the treatment of HCC. So TAE instead of TACE was performed in this study.
Sorafenib has been developed and is recommended for the treatment of advanced HCC.[30,31] But the effect of sorafanib for HCC is not satisfactory and actually the response rate of sorafenib is low.[32] Effects of sorafenib in patients with huge unresectable HCC is unclear. Besides, sorafenib is limited by a high cost and many patients cannot afford to receive the treatment, so HAIC provided a good treatment option for patients with huge unresectable HCC.
This study has several limitations. This is not a randomized controlled study, and selection bias may be possible in this study. But the baseline characteristics including age, sex, liver reserve, tumor stages are similar between the 2 groups of patients. Although the case numbers in this study are small, using Cox regression multivariate analysis, we found that the HAIC group has survival benefit over patients who received TAE. Further randomized controlled studies that enrolled more patients are required to compare the outcome of HAIC vs TAE/TACE for huge unresectable HCC.
In conclusion, HAIC is a safe procedure and provided better survival than TAE for patients with huge unresectable HCCs.
Author contributions
Conceptualization: Wei-Lun Tsai, Huei-Lung Liang, Jin-Shiung Cheng.
Data curation: Wei-Chi Sun, Chia-Ling Chiang, Huey-Shyan Lin, Huei-Lung Liang.
Formal analysis: Wei-Lun Tsai, Huey-Shyan Lin, Huei-Lung Liang, Jin-Shiung Cheng.
Investigation: Wei-Lun Tsai, Wei-Chi Sun, Chia-Ling Chiang, Huei-Lung Liang, Jin-Shiung Cheng.
Methodology: Wei-Lun Tsai, Huei-Lung Liang, Jin-Shiung Cheng.
Resources: Wei-Lun Tsai, Wei-Chi Sun, Chia-Ling Chiang.
Software: Huey-Shyan Lin.
Supervision: Wei-Lun Tsai.
Writing – original draft: Wei-Lun Tsai.
Writing – review & editing: Huei-Lung Liang, Jin-Shiung Cheng.
Footnotes
Abbreviations: AFP = alpha fetoprotein, BCLC = Barcellona Clinic Liver Cancer, CT = computed tomography, HAIC = Hepatic arterial infusion chemotherapy, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, MRI = magnetic resonance imaging, TAE = transcatheter arterial embolization.
How to cite this article: Tsai WL, Sun WC, Chen WC, Chiang CL, Lin HS, Liang HL, Cheng JS. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Medicine. 2020;99:32(e21489).
This study was supported by Kaohsiung Veterans General Hospital (VGHKS-104-059 and VGHKS-105-073).
The authors have no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the FarEast and Southeast Asia. Liver Int 2005;25:696–703. [DOI] [PubMed] [Google Scholar]
- [2].European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [3].Sheu JC, Sung JL, Chen DS, et al. Early detection of hepatocellular carcinoma by real-time ultrasonography. A prospective study. Cancer 1985;56:660–6. [DOI] [PubMed] [Google Scholar]
- [4].Hsu CY, Hsia CY, Huang YH, et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol 2012;19:842–9. [DOI] [PubMed] [Google Scholar]
- [5].Zhong JH, Wu FX, Li H. Hepatic resection associated with good survival for selected patients with multinodular hepatocellular carcinoma. Tumour Biol 2014;35:8355–8. [DOI] [PubMed] [Google Scholar]
- [6].Noguchi T, Kawarada Y, Kitagawa M, et al. Clinicopathologic factors influencing the long-term prognosis following hepatic resection for large hepatocellular carcinoma more than 10 cm in diameter. Semin Oncol 1997;24: Suppl. 6: 7–13. [PubMed] [Google Scholar]
- [7].Lee NH, Chau GY, Lui WY, et al. Surgical treatment and outcome in patients with a hepatocellular carcinoma greater than 10 cm in diameter. Br J Surg 1998;85:1654–7. [DOI] [PubMed] [Google Scholar]
- [8].Furuta T, Sonoda T, Matsumata T, et al. Hepatic resection for a hepatocellular carcinoma larger than 10 cm. J Surg Oncol 1992;51:114–7. [DOI] [PubMed] [Google Scholar]
- [9].Mok KT, Wang BW, Lo GH, et al. Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surg 2003;197:730–8. [DOI] [PubMed] [Google Scholar]
- [10].Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter artherial cheomoembolization in patients with Hepatocellular carcinoma larger than 10 cm in diameter. Alim Pharm Therp 2006;23:129–35. [DOI] [PubMed] [Google Scholar]
- [11].Poon RT, Ngan H, Lo CM, et al. Transarterial chemoembolization for inoperable Hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol 2000;73:109–14. [DOI] [PubMed] [Google Scholar]
- [12].Yamasaki T, Kimura T, Kurokawa F, et al. Prognostic factors in patients with advanced Hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy. J Gastroenterol 2005;40:70–8. [DOI] [PubMed] [Google Scholar]
- [13].Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95:588–95. [DOI] [PubMed] [Google Scholar]
- [14].Song MJ. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J Gastroenterol 2015;21:3843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nouso K, Miyahara K, Uchida D, et al. Liver Cancer Study Group of Japan. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Cancer 2013;109:1904–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin CP, Yu HC, Cheng JS, et al. Clinical effects of intra-arterial infusion chemotherapy with cisplatin, mitomycin C, leucovorin and 5-fluorouracil for unresectable advanced Hepatocellular carcinoma. J Chin Med Assoc 2004;67:602–10. [PubMed] [Google Scholar]
- [17].Liang HL, Huang JS, Lin YH, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma by placing a temporary catheter via the subclavian route. Acta Radiologica 2007;48:734–40. [DOI] [PubMed] [Google Scholar]
- [18].Tsai WL, Lai KH, Liang HL, et al. Hepatic arterial infusion chemotherapy for huge unresectasble hepatocellular carcinoma. Plos One 2014;9:e92784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maida M, Orlando E, Cammà C, et al. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol 2014;20:4141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weinmann A, Koch S, Sprinzl M, et al. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int 2015;35:591–600. [DOI] [PubMed] [Google Scholar]
- [21].Hsu HC, Sheu JC, Lin YH, et al. Prognostic histologic features of resected small hepatocellular carcinoma (HCC) in Taiwan- a comparison with resected large HCC. Cancer 1985;56:672–80. [DOI] [PubMed] [Google Scholar]
- [22].Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology 1992;16:1367–71. [DOI] [PubMed] [Google Scholar]
- [23].Adachi E, Maeda T, Kajiyama K, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivartiate analyses of 232 resected cases without preoperative treatment. Cancer 1996;77:2022–31. [DOI] [PubMed] [Google Scholar]
- [24].Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterol 2006;131:461–9. [DOI] [PubMed] [Google Scholar]
- [25].Chang JM, Tzeng WS, Pan HB, et al. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer 1994;74:2449–53. [DOI] [PubMed] [Google Scholar]
- [26].Kawai S, Okamura J, Ogawa M, et al. The Cooperative Study Groupo for Liver Cancer Treatment of Japan. Prospective and randomized trial for the treatment of hepatocellular carcinoma: a comparison of lipiodol-transcatheter arterial ebmolization with and without adriamycin (first cooperative study). Cancer Chemother Pharmacol 1992;31: Suppl: S1–6. [DOI] [PubMed] [Google Scholar]
- [27].Ikeda K, Saitoh S, Suzuki Y, et al. A prospective randomized administration of 5’-deoxy-5-fluorouridine as adjuvant chemotherapy for hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Am J Clin Oncol 1997;20:202–8. [DOI] [PubMed] [Google Scholar]
- [28].Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 2011;3:CD004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lanza E, Donadon M, Poretti D, et al. Transarterial therapies for hepatocellular carcinoma. Liver Cancer 2016;6:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2001;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [32].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]


