Abstract
Inflammatory bowel disease is associated with an increased risk of colorectal cancer. The study aims to identify the risk factors for ulcerative colitis-colorectal cancer and to perform a survival curve analysis of the outcome.
This retrospective cohort study included 254 patients from March 2016 to October 2017. Age, age at diagnosis, follow-up time, smoking status, and family history of colorectal cancer were analyzed as risk factors for colorectal cancer.
The mean patient age was 46.6 ± 16.9 years; 5.5% of the patients were smokers and 49.6% had pancolitis. Six patients (2.36%) had colorectal cancer, which was associated with age at diagnosis (odds/hazard ratio 1.059 [95% confidence interval: 1.001–1.121]; P = .04), family history of colorectal cancer (12.992 [1.611–104.7]; P = .02), and follow-up time (0.665 [0.513–0.864]; P = .002). Active smoking was the main identified risk factor, after both logistic (8.477 [1.350–53.232]; P = .02) and Cox proportional-hazards (32.484 [2.465–428.1]; P = .008) regression analysis. The risk of colorectal cancer was 3.17% at 10 years and 4.26% at 20 years of follow-up.
Active smoking and family history were identified as risk factors for colorectal cancer. These findings should aid the early identification of patients who require vigorous surveillance, and prevent exposure to risk factors.
Keywords: colorectal cancer, risk factors, smoking, ulcerative colitis
1. Introduction
Inflammatory bowel disease (IBD) is associated with an increased risk of colorectal cancer (CRC),[1] especially in patients with ulcerative colitis (UC). Studies have shown that 10% to 15% of all deaths in IBD patients are related to CRC, although this tumor accounts for only 1% to 2% of CRC cases in the general population.[2]
A population-based cohort study of 3117 UC patients reported a standardized CRC incidence ratio of 5.7 (95% confidence interval [CI]: 4.6–7.0) when compared with the expected CRC incidence in the general population.[3] A meta-analysis of 116 studies including 54,478 UC patients identified 1698 cases of IBD-CRC.[4] The risk of CRC has been suggested to markedly increase with cumulative probabilities of 2% by 10 years, 8% by 20 years, and 18% by 30 years.[4] However, recent cohort studies have suggested that the CRC risk in UC patients has decreased over time,[5,6] with 1 study reporting that the cumulative incidence of CRC was less than 1% at 10 years and only 1.1% to 2.5% at 20 years.[6] Yet, other studies have shown a continued high incidence[7] or even an increasing incidence.[8]
The main risk factors for IBD-CRC include certain disease characteristics such as age at onset,[9] extent and duration of disease,[10] as well as non-IBD characteristics such as family history of CRC[11] and concomitant diagnosis of primary sclerosing cholangitis.[12]
Despite the increased incidence of IBD in developing countries and the possible evolution to CRC, studies on the incidence of CRC and CRC-related risk factors in Latin America are lacking. Identifying patients at risk with respect to risk factors, and implementing appropriate surveillance is central to improving the early diagnosis and selecting the adequate treatment of the tumor. Therefore, the current study aimed to identify the risk factors for CRC in UC patients, and to conduct a survival curve analysis of the observed outcome.
2. Material and methods
2.1. Patients
A retrospective cohort study was performed on 284 eligible UC patients. Data collection was performed from March 2016 to October 2017. The inclusion criteria were age ≥15 years and confirmed UC diagnosis. Patients lacking data such as age, sex, and disease characteristics, or with unavailable UC-related CRC data were excluded from the study. In all, 30 patients were excluded because of the lack of essential data in the medical records. Data were captured from the patients’ electronic charts and all appointments were reviewed for collecting data.
2.2. Clinical assessment
The analyzed variables were current age, age at UC diagnosis, sex, ethnicity, follow-up time, and smoking status. Smoking status was evaluated at diagnosis and at the last visit recorded from the patient's chart. The patient was classified as an active smoker or non-smoker. Former smokers were considered as non-smokers. The presence of comorbidities such as hypertension, diabetes mellitus, irritable bowel syndrome, mood disorders such as anxiety or depression, dyslipidemia, coronary insufficiency or cardiac insufficiency, pulmonary diseases, osteoarthritis, psoriasis, and so on were evaluated at each visit. The presence of a family history of UC, Crohn's disease, or CRC was also evaluated.
Clinical and endoscopic disease activity were assessed according to the Mayo score.[13] The extent of intestinal damage (proctitis, left-sided colitis, or extensive/pancolitis)[14] was determined by reviewing the report of the first colonoscopy.
The presence of extra-intestinal manifestations such as arthralgia, peripheral arthritis, ankylosing spondylitis, sacroiliitis, osteopenia, osteoporosis, erythema nodosum, pyoderma gangrenosis, Sweet syndrome, episcleritis, uveitis, primary sclerosing cholangitis, and venous thrombosis was recorded at any moment of follow-up.
2.3. Clinical treatment
The medical treatment in use at the last recorded visit was evaluated. The following medications were considered: mesalazine tablet, suppository, or enema, sulfasalazine, azathioprine, prednisone, and biological therapy such as infliximab, adalimumab, vedolizumab, or any investigational drug study in clinical trial.
2.4. CRC record
The presence of CRC and the date of cancer diagnosis were evaluated. CRC was confirmed by histological analysis.
2.5. Ethics statement
The study was approved by the Botucatu Medical School Research Ethics Committee (protocol number 50640915.2.0000.5411). The authors confirm that all research was performed in accordance with relevant regulations, and clarify that informed consent was not obtained from the participants because the study was based on data collection. The Ethics Committee waived the need of the application of the informed consent from the participants based on the fact that many patients lost follow-up and some died.
2.6. Statistical analysis
Data are expressed as mean ± standard deviation or median (range) for continuous variables, and as frequency (proportion) for qualitative variables. In order to study the associations between the categorical variables, the Chi-square test and Fisher exact test were used, when appropriate. Univariate logistic regression model was performed to identify variables associated with the outcome. Multivariate logistic regression model was performed using significant clinical variables identified in the univariate study or clinical variables relevant to the model. For each independent factor, the odds ratio (OR) and 95% CI were calculated. Survival analysis was performed using Kaplan–Meier curves and the log-rank test; the initial event was considered the UC diagnosis date and the final event was considered the CRC diagnosis. Cox proportional-hazards regression exploring the association between the variables and the outcome was performed. A P-value of <.05 was considered statistically significant. The statistical analyses were performed using SAS version 9.3 for Windows (SAS Institute Inc., Cary, NC). All authors had access to the study data.
3. Results
3.1. Clinical characteristics
The study included 254 subjects. Their mean age was 46.6 ± 16.9 years, 93.3% were Caucasian, and 63.0% were women. The mean age at diagnosis was 37.6 ± 15.8 years. Regarding smoking status, 11.9% of patients were smokers at diagnosis and 5.5% were smokers at the last recorded appointment (Table 1). The median follow-up time was 4.94 (1.77–11.4) years and the median time between onset of symptoms and UC diagnosis was 153.3 (51.1–390.5) days.
Table 1.
Clinical and endoscopic characteristics of patients with ulcerative colitis.
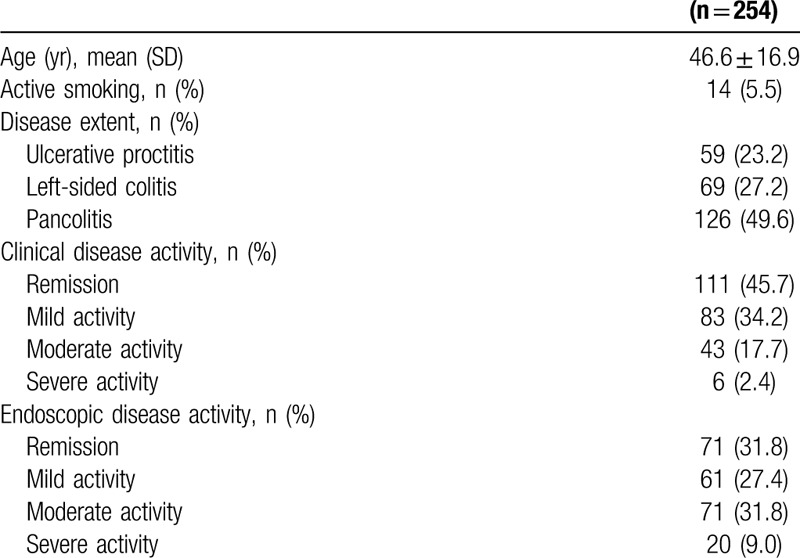
Comorbidities were reported by 51.6% of patients. The most prevalent were hypertension (24.8%), mood disorders (17.8%), diabetes mellitus (15.0%), and dyslipidemia (12.3%). Family history of UC was reported by 7.09% of the patients, family history of Crohn's disease by 0.79%, and a history of CRC by 6.69%.
Regarding disease extent, 49.6% of the patients presented with pancolitis and most were classified both with clinical (45.7%) and endoscopic (31.8%) remission (Table 1). Mesalazine was the most used medication, as mesalazine tablets (40.6%), mesalazine suppository (24.4%), and mesalazine enema (1.57%). Azathioprine use was reported by 19.7%, prednisone by 15.4%, and biological therapy was noted in 10.6% of patients, as infliximab (5.12%), adalimumab (4.33%), and investigational drug in a clinical trial protocol (1.18%).
Extra-intestinal manifestations were reported in 52.4% of the patients. The most prevalent were arthralgia or peripheral arthritis (26.0%), osteopenia or osteoporosis (14.2%), and anemia (12.2%), followed by primary sclerosing cholangitis (4.72%), ankylosing spondylitis (3.94%), and sacroiliitis (3.15%).
3.2. CRC
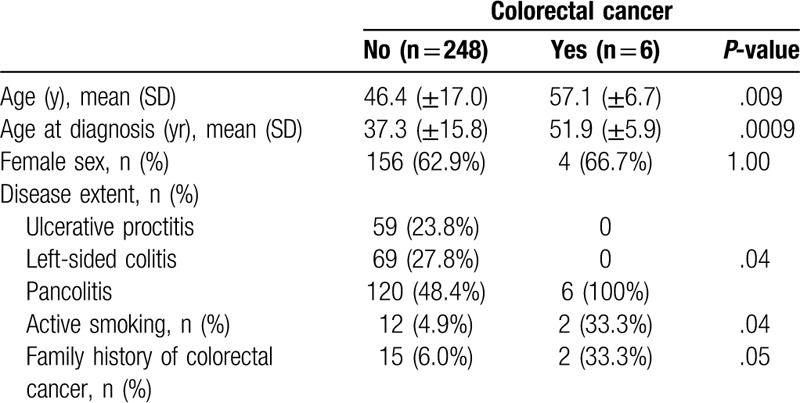
Six patients (2.36%) presented with CRC. Patients who developed CRC had advanced age (P = .009) and a higher age at diagnosis (P = .0009). Other associated factors were disease extent/pancolitis (P = .04) and active smoking at the last recorded appointment (P = .04) (Table 2). Presence of primary sclerosing cholangitis was not associated with CRC (P = .99).
Table 2.
Clinical variables according to colorectal cancer development in patients with ulcerative colitis.
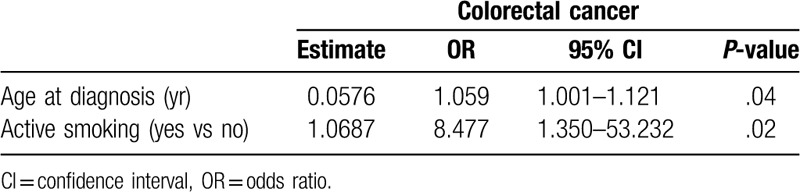
In the univariate logistic regression analysis, the presence of CRC was associated with age at diagnosis (OR: 1.058; 95% CI: 1.005–1.115; P = .03), active smoking (OR: 9.167; 95% CI: 1.395–60.237; P = .02), and hypertension (OR: 6.407; 95% CI: 1.145–35.865; P = 0.03). In the multivariate logistic regression analysis, the presence of CRC was associated with age at diagnosis (OR: 1.059; 95% CI: 1.001–1.121; P = .04) and active smoking (OR: 8.477; 95% CI: 1.350–53.232; P = .02); (Table 3). The interaction between the variables was not significant (P = .99) (data not shown).
Table 3.
Multivariate logistic regression between evolution of colorectal cancer and clinical variables in patients with ulcerative colitis.
The risk of developing CRC was 1.48% at 5 years of follow-up, 3.17% at 10 years, and 4.26% at 20 years. The risk was higher in smokers (P = .006) (Fig. 1), and did not differ with UC extent (P = .05) (Fig. 1).
Figure 1.
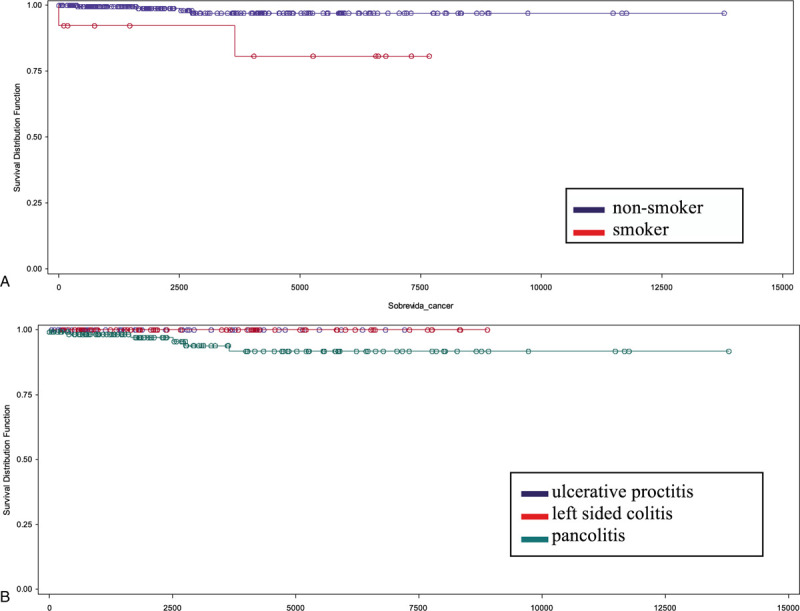
Kaplan–Meier survival curve showing the relationship between follow-up time (days) and colorectal cancer development in patients with ulcerative colitis. (A) According to smoking status (P = .02). (B) According to disease extent (P = .05).
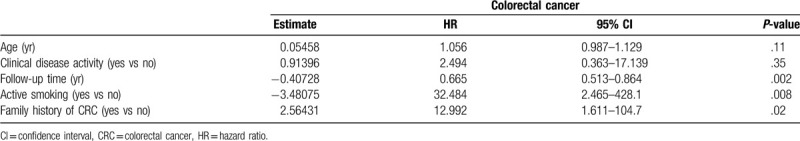
According to Cox proportional-hazards regression, the risk factors for CRC were active smoking (HR: 32.484; 95% CI: 2.465–428.1; P = .008), follow-up time (HR: 0.665; 95% CI: 0.513–0.864; P = .002), and family history of CRC (HR: 12.992; 95% CI: 1.611–104.7; P = .02) (Table 4).
Table 4.
Cox proportional-hazards regression analysis of the relationship between colorectal cancer development and clinical variables in patients with ulcerative colitis.
4. Discussion
CRC is a severe complication of IBD and, in the case of UC, the CRC risk varies according to the family history of CRC, extent and duration of the disease, and presence/absence of primary sclerosing cholangitis.[15] Notably, we found that the incidence of CRC was relatively low (2.36%) and active smoking was the main CRC risk factor identified in UC patients.
In UC, smoking is considered a protective factor both for disease onset and relapse.[16,17] Moreover, it is associated with a decrease in hospitalization rates, relief of disease symptoms, and improvement in clinical remission rates.[16] This protective ability is probably caused by the modulation of the immune response, change in inflammatory cytokine levels, and in mucus composition, in addition to vascular and prothrombotic effects and changes in intestinal permeability.[18]
In non-IBD patients, smoking has been associated with an increased risk of CRC,[19] more than that associated with a family history of CRC.[20] This mechanism could be related to angiogenesis induction and/or suppression of cell-mediated immunity facilitating tumor growth.[21] Tobacco smoke contains carcinogens such as aromatic amines, nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons,[22] which can lead to the production of abnormal DNA and mutations in CRC-suppressor genes and CRC-oncogenes, resulting in tumor development.[23] Furthermore, smoking may modify the relationship between susceptibility to single-nucleotide polymorphism and CRC risk, supporting the existence of gene-smoking interactions.[24]
However, it is important to emphasize that UC-CRC is distinct from sporadic CRC, both in disease mechanism and presentation,[25] and does not display the classic adenoma-carcinoma sequence. Chronic inflammation repeatedly destroys the mucosa epithelia promoting increased epithelial proliferation as a repair mechanism which may be uncontrolled leading to CRC.[26] The inflammatory infiltrate generates reactive oxygen species, which causes oxidative stress and damage to DNA, proteins and lipids, and contributes to the generation of dysplastic lesions.[25–27] Furthermore, cytokines released from chronic inflammation participate in all stages of cancer development, including tumor initiation, tumor promotion, angiogenesis, and metastasis. Activated NF-κB upregulates the expression of many pro-inflammatory mediators, such as cytokines, TNF-α, and IL-6, in addition to adhesion molecules, which play a critical role in tumor development.[28]
Only a few studies have identified smoking behavior as a CRC risk factor in UC patients and have evaluated the mechanisms by which smoking leads to CRC. IBD and cancer are complex diseases resulting from an interaction between genetic and environmental factors, such as smoking behavior. The chronic inflammation and the compounds produced by cigarettes have deleterious effects on intestinal health and the added effects may increase the chances of tumor onset. Furthermore, some studies point to an intestinal microbiome alteration in IBD and in smokers, which, in addition to an impaired mucosal barrier, may play a role in UC-CRC development.[29–31]
We also observed that CRC risk increased according to age at diagnosis; further, older age at IBD onset may be related to a more aggressive development of IBD-CRC and early inclusion in screening programs might be considered for this group of patients.[9] A recent multicenter study conducted in Hong Kong revealed that 5.8% of older-onset patients developed flat dysplasia, with similar rates between those with <8 and ≥ 8 years of disease, suggesting that older age at IBD onset, rather than disease duration, is a leading risk factor for IBD-CRC.[32] Similar findings were noted in a population study conducted in 93 general hospitals in the Netherlands.[33] The authors reported that in 251 IBD-related CRC cases, the median time from IBD diagnosis to CRC diagnosis was 12 years (interquartile range 4–20) and IBD diagnosis at older age (HR for age 10 years older 2.25; 95% CI: 1.92–2.63) was related to early CRC.[33] Previous studies have shown that colonocyte telomeres shorten with age almost twice as rapidly as in normal controls, which may explain this faster evolution of CRC in older patients.[34]
In addition to smoking and age, other factors increase the risk of CRC, such as long duration of the disease, presence of pancolitis, and moderate/severe or persistent inflammatory activity.[10,15,35] The presence of persistent inflammation increases by 2-fold the CRC risk for approximately 10, 5, or 3.3 years for continuously mild, moderate, or severe active microscopic inflammation, respectively.[36] Despite the high moderate to severe activity rate, both clinical (20.1%) and endoscopic (40.8%), and the high prevalence of extensive disease (49.6%), these factors were not associated with the presence of CRC in the present study. We would like to emphasize that we did not study the accumulative inflammatory burden as a risk factor for CRC and this could have interfered with the results.
We also identified that a family history of CRC increased CRC risk. A population-based cohort study including 19,876 IBD patients[11] observed that a family history of CRC was associated with more than a 2-fold risk of IBD-CRC (adjusted risk ratio 2.5; 95% CI: 1.4–4.4) and the individuals with a first-degree relative diagnosed with CRC before 50 years of age had a higher risk (risk ratio 9.2; 95% CI: 3.7–23). Other risk factors not related to IBD such as the male sex and the presence of primary sclerosing cholangitis[12,15–17,35] are also associated with CRC development. In our study, we did not identify sex or primary sclerosing cholangitis as CRC risk factors, probably due to the low prevalence of primary sclerosing cholangitis (4.72%) in our patients.
Similar to the UC-CRC incidence rate, which decreased from 4.29/1000 patients/yr in studies published in the 1950s to 1.21/1000 patients/yr in the last decade,[37] the cumulative risk of CRC in UC patients has been decreasing over the most recent decades, as shown by a review published by Yashiro in 2014.[35] The cumulative CRC risk was of 2% at 10 years,[4] 5% to 7% at 20 years, 7% to 14% at 25 years, and 18% at 30 years[35] in studies published from the 1980s to 2001. Otherwise, recent studies have shown a cumulative risk of 0.4% to 0.5% at 10 years, 1.1% to 4.1% at 20 years, and 2.3% to 7.6% at 30 years,[35] which may reflect better disease control, high rates of surgery, and chemoprevention use for UC, in addition to more frequent surveillance colonoscopy.[35]
Although our patients have difficulty accessing surveillance colonoscopy and drug treatment such as biological therapy, our CRC rates are low, comparable to the most recent studies. However, 30 patients were excluded from the analysis due to the lack of important data, which may have caused a selection bias in the study. These patients could compose the most severe group of the sample. Therefore, population studies are necessary to clarify the reasons for this low cumulative rate.
Accumulating studies suggest that long-term inflammation control is vital for CRC prevention; thus, anti-inflammatory therapy might be useful for chemoprevention in UC patients. A recent review suggested mesalamine use for all UC patients but the current level of evidence is too low for thiopurines and anti-TNF agents.[38] The first screening colonoscopy should be offered over 8 years following the onset of symptoms,[38] with the interval for further surveillance guided by the presence of related risk factors, in addition to factors such as post-inflammatory polyps, personal history of colonic dysplasia, and colonic strictures.[39]
Regarding the present study, it should be emphasized the small simple size limits data extrapolation to the UC population. Longitudinal studies including a greater number of patients are necessary to confirm the causal factors in the association between risk factors such as active smoking and older age at onset and IBD-CRC development, in addition to evaluating the duration of tobacco exposure and the intensity of this exposure. Furthermore, dietary factors were not available for inclusion in our analysis, and other risk factors such as thiopurine use and the presence of premalignant lesions evaluation were not studied.
5. Conclusions
The present study showed that CRC development in UC patients was relatively low and that the main risk factor for CRC was the presence of active smoking. Despite the outlined limitations, the results of this study contribute to the knowledge about the risk factors for IBD-CRC and highlights smoking as an important risk factor. This is important not only for the early identification of patients who require vigorous surveillance or tight control of the inflammatory process, thus, allowing better rates of early tumor detection with consequently higher cure rates, but also to achieve less exposure to risk factors and a decreased possibility of tumor onset.
Acknowledgment
The authors thank Eloisa Elena Pascoalinotte from the Botucatu Medical School at São Paulo State University (UNESP) for her valuable help in statistical analysis.
Author contributions
All authors contributed to this manuscript. Elen Farinelli de Campos Silva, Julio Pinheiro Baima, Jaqueline Ribeiro de Barros, Suzana Erico Tanni, Thomas Schreck, Rogerio Saad-Hossne, and Ligia Yukie Sassaki contributed to the conception and design of the study, acquisition, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content, and final approval of the version to be submitted.
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, HR = hazard ratio, IBD = inflammatory bowel disease, OR = odds ratio, UC = ulcerative colitis.
How to cite this article: Silva EF, Baima JP, Barros JR, Tanni SE, Schreck T, Saad-Hossne R, Sassaki LY. Risk factors for ulcerative colitis-associated colorectal cancer: a retrospective cohort study. Medicine. 2020;99:32(e21686).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Botucatu Medical School Research Ethics Committee (Protocol Number: 50640915.2.0000.5411)) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population-based cohorts. Inflamm Bowel Dis 2010;17:471–8. [DOI] [PubMed] [Google Scholar]
- [2].Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 2003;18: Suppl 2: 1–5. [DOI] [PubMed] [Google Scholar]
- [3].Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990;323:1228–33. [DOI] [PubMed] [Google Scholar]
- [4].Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Söderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 2009;136:1561–7. [DOI] [PubMed] [Google Scholar]
- [6].Jess T, Simonsen J, Jørgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375–81. [DOI] [PubMed] [Google Scholar]
- [7].Desai D, Shah S, Deshmukh A, et al. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J Gastroenterol 2015;21:3644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Watanabe T, Konishi T, Kishimoto J, et al. Japanese Society for Cancer of the Colon and Rectum. Ulcerative colitis associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis 2011;17:802–8. [DOI] [PubMed] [Google Scholar]
- [9].Brackmann S, Andersen SN, Aamodt G, et al. Relationship between clinical parameters and the colitis-colorectal cancer interval in a cohort of patients with colorectal cancer in inflammatory bowel disease. Scand J Gastroenterol 2009;44:46–55. [DOI] [PubMed] [Google Scholar]
- [10].Magro F, Gionchetti P, Eliakim R, et al. European Crohn's and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- [11].Askling J, Dickman PW, Karlén P, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 2001;120:1356–62. [DOI] [PubMed] [Google Scholar]
- [12].Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol 2016;28:383–90. [DOI] [PubMed] [Google Scholar]
- [13].Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- [14].Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Annese V, Beaugerie L, Egan L, et al. ECCO. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis 2015;9:945–65. [DOI] [PubMed] [Google Scholar]
- [16].Blonski W, Buchner AM, Lichtenstein GR. Clinical predictors of aggressive/disabling disease: ulcerative colitis and Crohn disease. Gastroenterol Clin N Am 2012;41:443–62. [DOI] [PubMed] [Google Scholar]
- [17].Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16:343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol 2004;18:481–96. [DOI] [PubMed] [Google Scholar]
- [19].Fagunwa IO, Loughrey MB, Coleman HG. Alcohol, smoking and the risk of premalignant and malignant colorectal neoplasms. Best Pract Res Clin Gastroenterol 2017;31:561–8. [DOI] [PubMed] [Google Scholar]
- [20].Hoffmeister M, Schmitz S, Karmrodt E, et al. Male sex and smoking have a larger impact on the prevalence of colorectal neoplasia than family history of colorectal cancer. Clin Gastroenterol Hepatol 2010;8:870–6. [DOI] [PubMed] [Google Scholar]
- [21].O’Byrne KJ, Dalgleish AG, Browning MJ, et al. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer 2000;36:151–69. [DOI] [PubMed] [Google Scholar]
- [22].Durko L, Malecka-Panas E. Lifestyle modifications and colorectal cancer. Curr Colorectal Canc Rep 2014;10:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 2014;12:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song N, Shin A, Jung HS, et al. Effects of interactions between common genetic variants and smoking on colorectal cancer. BMC Cancer 2017;17:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen R, Lai LA, Brentnall TA, et al. Biomarkers for colitis-associated colorectal cancer. World J Gastroenterol 2016;22:7882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett 2014;345:235–41. [DOI] [PubMed] [Google Scholar]
- [27].Roessner A, Kuester D, Malfertheiner P, et al. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract 2008;204:511–24. [DOI] [PubMed] [Google Scholar]
- [28].O’Connor PM, Lapointe TK, Beck PL, et al. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis 2010;16:1411–20. [DOI] [PubMed] [Google Scholar]
- [29].Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012;338:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012;91:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol 2017;31:579–88. [DOI] [PubMed] [Google Scholar]
- [32].Shi HY, Chan FK, Leung WK, et al. Natural history of elderly-onset ulcerative col-itis: results from a territory-wide inflammatory bowel disease registry. J Crohns Colitis 2016;10:176–85. [DOI] [PubMed] [Google Scholar]
- [33].Baars JE, Kuipers EJ, van Haastert M, et al. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol 2012;47:1308–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Risques RA, Lai LA, Brentnall TA, et al. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology 2008;135:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol 2014;20:16389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut 2017;68:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Castano-Milla C, Chaparro M, Gisbert JP. Systematic review with metaanalysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther 2014;39:645–59. [DOI] [PubMed] [Google Scholar]
- [38].Lopez A, Pouillon L, Beaugerie L, et al. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract Res Clin Gastroenterol 2018;32–33:103–9. [DOI] [PubMed] [Google Scholar]
- [39].Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639–51. [DOI] [PubMed] [Google Scholar]


