Abstract
Background:
On March 11, 2020, World Health Organization announced that severe acute respiratory syndrome coronavirus 2 caused COVID-19 was a global pandemic. COVID-19 is associated with venous thromboembolism including deep vein thrombosis and pulmonary embolism. To further identify the current role of antiplatelet/anticoagulant therapy in the prophylaxis and treatment of COVID-19 patients is important.
Methods:
We will conduct a systematic review based on searches of major databases (eg, Pubmed, Web of Science, EMBASE, CENTRAL, MEDLINE, SCI-EXPANDED, CPCI-S, CBM, CNKI, and Wanfang Database) and clinical trial registries from inception to present without limitations of language and publication status. All published randomized control trials, quasi-randomized trials, retrospective and observational studies related to prophylactic antiplatelet/anticoagulant for severe COVID-19 will be included. Primary outcome includes incident acute thrombosis events. Second outcome is the incidence and severity of adverse effects. Full-text screening, data extraction and quality assessment will be conducted by 2 reviewers independently. The reporting quality, risk of bias, sensitivity analysis and subgroup analysis will be performed to ensure the reliability of our findings by other 2 researchers. The statistical analysis will be performed by RevMan V.5.3 software and Stata V.12.0 software.
Results:
The result of this systematic review will provide valid advice and consultation for clinicians on the management of prophylactic antiplatelet/anticoagulant for severe COVID-19 patients.
Conclusion:
This systematic review will provide evidence for prophylactic antiplatelet/anticoagulant of severe COVID-19 patients.
PROSPERO registration:
CRD42020186928.
Keywords: antiplatelet/anticoagulant, COVID-19, prophylaxis, thromboembolism
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused COVID-19 has been officially announced as a global pandemic by the World Health Organization since March 11, 2020. As of May 26, a total of 5495061 cases have been confirmed worldwide and 346232 deaths have been reported across 188 countries or regions. Currently, with over 1.3 million confirmed cases and over 82,000 deaths, the United States leads all countries.[1] Most patients with COVID-19 presented mild illness, others requiring hospitalization may presented fatal critical illness. Of these severe patients, a high prevalence of acute cardiovascular events has been observed.[2–7]
SARS-CoV-2 and SARS-CoV have shared cellular target and some clinical manifestations, such as thrombocytopenia, prolonged thrombin time and elevated D-dimer levels.[6,8] It means that similar with SARS-CoV, SARS-CoV-2 is likely to be complicated with coagulopathy namely disseminated intravascular coagulation (DIC) or pre-DIC secondary to an increased inflammatory state. Patient with DIC has a rather prothrombotic character with high risk of venous thromboembolism (VTE).[8–10]
Previous study reported that COVID-19 is associated with venous thrombotic events including deep vein thrombosis and pulmonary embolism (PE).[11] Emerging evidence shows that with a profound hypercoagulable state, complicating VTE in patients with COVID-19 are common.[12–14] The incidence of VTE in ICU patients without thromboprophylaxis was 25% with a mortality rate of 40%.[15] The prevalence of VTE in the study of Cui et al seems to be higher than other studies including patients admitted in ICUs for other disease conditions.[15,16] A meta-analysis indicated that patients with VTE in ICU had a marginally increased risk of in-hospital mortality (relative risk 1.31; 95% CI: 0.99–1.74).[17] In a study of 449 patients with severe COVID-19, patients with markedly elevated D-dimer or sepsis-induced coagulopathy were benefit from anticoagulant therapy, especially low molecular weight heparin.[18] Meanwhile, VTE is associated with infection. COVID-19, as a special infection, may has an increased VTE risk due to endothelial damage, microvascular thrombosis and occlusion, or even autoimmune mechanisms.[19] Thus, it appears that severe COVID-19 patients are in the high risk of VTE and high rate of mortality, and prognosis may be improved by anticoagulant therapy.
However, patients with COVID-19 may have a prolonged activated partial-thromboplastin time (aPTT), which may indicate a clotting factor deficiency or the presence of an inhibitor.[20] In the study of Bowles, 216 patients with severe SARS-CoV-2 were received for coagulation screening, and 44 (20%) presented with a prolonged aPTT, and most aPTT prolonged patients were positive for lupus anticoagulant (91%). No bleeding tendency has been observed.[21] Thus, prolonged aPTT should not be a barrier to the use of thromboprophylaxis therapies with COVID-19. All these observations raise a challenging question that is what the current role of antiplatelet/anticoagulant therapy in the prophylaxis and treatment of COVID-19 patients is.[22]
2. Methods
2.1. Registration
The protocol will be performed following the recommendations from the Cochrane Handbook of Systematic Review of Intervention and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement (Fig. 1).[23] It is registered in the PROSPERO database (CRD42020186928).
Figure 1.
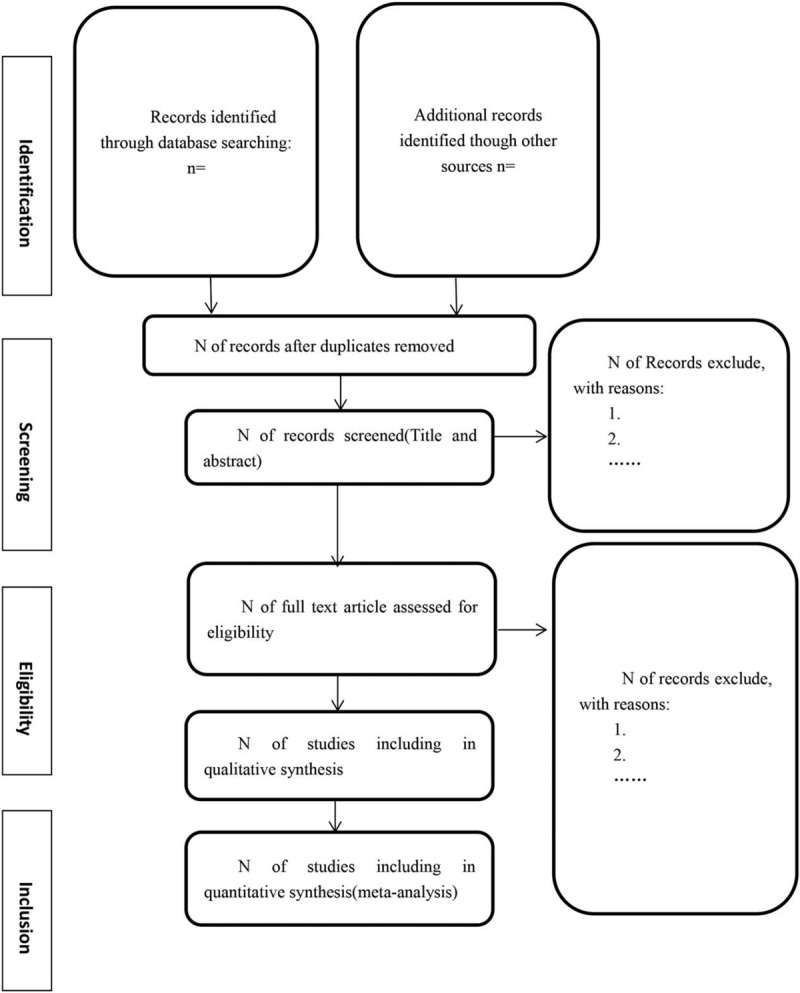
Flow diagram of literature search.
2.2. Eligibility criteria
Inclusion criteria followed the PICOS principles: patients, intervention, comparisons, outcome, and study design type.
2.2.1. Type of participants
Gender, ethnicity, disease duration or ethnicity are not restricted for all participants diagnosed with severe pneumonia caused by COVID-19 according to the laboratory conformation (such as real-time PCR) and chest CT findings and required advanced life support.
2.2.2. Type of comparisons and interventions
The aim of study is to assess the role of antiplatelet or anticoagulant as preventive agents for thrombosis events in patients with severe COVID-19, especially presenting with respiratory deterioration and/or hemodynamic instability. Therefore, patients will be assigned to intervention or control group. The intervention groups will include but not be limited to: antiplatelets (including aspirin, P2Y12 antagonists, αIIbβ3 antagonists), anticoagulants (heparin, vitamin K antagonists, direct thrombin inhibitors and direct factor Xa inhibitors), antiplatelets plus anticoagulants. The control group will include placebo or no thromboprophylaxis therapies at all. All therapeutic doses, the time of dosing, duration and means of administration (intravenous, oral) are no restricted.
2.2.3. Type of outcome measures
The primary objective is the efficacy of antithrombotic treatment in preventing the first thrombosis. The primary outcomes are incident acute thrombosis (arterial or venous) events confirmed by appropriate imaging studies or death. The secondary outcomes are security index, which will be measured by the incidence and severity of adverse effects, including:
-
(1)
bleeding events (significant bleeding events, clinically relevant insignificant bleeding events, or minor events).
-
(2)
changes in aPTT, D-dimer and platelet levels.
-
(3)
survival assays (main conclusions, mortality, survival time). Each adverse event will be separately assessed.
Thrombosis events were defined as 1 of the following events:
-
(1)
deep vein thrombosis.
-
(2)
PE.
-
(3)
sudden death with no obvious cause (possible fatal PE).
-
(4)
brain ischemia.
-
(5)
acute myocardial infarction.
2.2.4. Type of study
We will include randomized controlled trials, quasi-randomized trials, retrospective and observational studies, clinical trials and case series, irrespective of blinding, language, or publication status.
2.3. Exclusion criteria for study selection
We defined the following exclusion criteria:
-
(1)
Studies in which the same patients have been enrolled.
-
(2)
Commentaries, editorials, case reports, letters, editorials, and expert opinions.
-
(3)
Missing or insufficient data that cannot be obtained after contacting original authors.
-
(4)
Unpublished records such as conference papers, theses, and patent.
-
(5)
Studies in which the patients are under 18 years old.
2.4. Methods for searching
2.4.1. Electronic searched
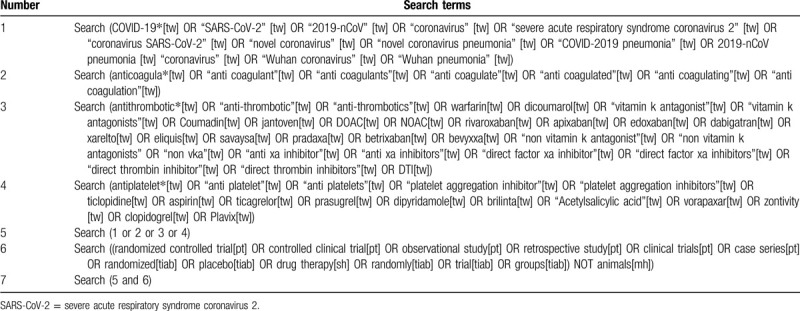
2 reviewers will research Pubmed, Web of Science, Cochrane Central Register of Controlled Trials, Medical Literature Analysis and Retrieval System Online, Excerpa Medica database, Science Citation Index Expanded (SCI-EXPANDED), Conference Proceedings Citation Index-Science, Chinese Biomedical Literature Database, China Network Knowledge Information and Chinese Science Journal Database, Wanfang database (Wanfang Data) from their inception to the present. The full search strategy for Pubmed is provided in Table 1.
Table 1.
Search strategy for the PubMed database.
2.4.2. Searching other resources
In addition, Reference lists of relevant trials and reviews will also be searched. For any unidentified clinical trials, we will search clinical trial registries, such as the International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, websites of US Food and Drug Administration (FDA), and European Medicines Agency (EMA) and contact the authors to receive the relevant data. We will also seek the COVID-19 Study Registry (https://covid-19.cochrane.org/ ) and COVID-evidence (https://covid-evidence.org/).
2.5. Study selection and data extraction
2.5.1. Selection of data
The review will be analyzed by using Stata version 16 (StataCorp LLC, College Station, TX) and illustrated with the PRISMA statement.[23] 2 reviewers will independently scan the title and abstract to remove obvious irrelevant studies. Where a study is potentially relevant, the full text will be retrieved and evaluate by 2 same reviewers to exclude the ineligible studies. Any discrepancy will be resolved through discussion or judged by a third reviewer.
2.5.2. Data extraction and management
Relevant data will be extracted independently from included trials in a predefined form by 2 independent reviewers, which include the following information:
-
(1)
The basic information of each study: first author, title, journal, time of publication, country, funding source, study design, and so on.
-
(2)
characteristics of patients: average age, gender, past medical history, severity of COVID-19 pneumonia, comorbidity, and so on.
-
(3)
interventions and comparators: types, doses, frequencies and lengths of thromboprophylaxis therapies and comparators where applicable, and so on.
-
(4)
outcomes: measures, main conclusions, mortality, length of stay, rates and types of incident acute thrombosis (including arterial or venous) and adverse events, and so on.
In order to assume the integrity of extracted items, we will contact original authors via email to request for missing data or clarification necessary. If there is no response, with repeated contacts, the data will be reconsidered, or deleted if necessary. 2 reviewers will cross-check the received data and transfer into the Stata file. Disagreements will be judged by a third reviewer. Finally, the data will be recorded in a unified form.
2.6. Risk of bias assessment
As with the previous process, pilot risk of bias of the included studies will be assessed by 2 independent reviewers at study level in accordance with the guidance in the latest version of Cochrane Handbook for systematic reviews of interventions, which covers: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome reporting, selective reporting and other sources of bias.[24] Each of the domains will be scored as “low risk” or “high risk” depending on the extracted information from each study. “Unclear” record will be made if there is insufficient detailed in the publication and will contact to authors of primary studies for missed or unpublished data. Disagreements will be judged by a third reviewer. The RevMan V5.5.3 software will be used to evaluate each study.
2.7. Data synthesis and statistical analysis
2.7.1. Assessment of heterogeneity and data synthesis
We will analyze heterogeneity by considering the variability in the population characteristics, interventions and outcomes among included trials using RevMan V.5.3 software and Stata V.12.0 software. Dichotomous outcomes such as occurrence of thrombosis events will be determined by using risk ratios with a confidence interval of 95%. Continuous variables will be recorded as the mean differences with 95% confidence interval. When identical method or unit of measure of the intervention effect in all studies is observed, weighted average difference is preferred. On the other hand, standard mean difference will be used to express the size of the same intervention effect in each study related to the variability observed in that study.
To explore the impact of the statistical heterogeneity on the meta-analysis, we will primarily use forest plots to assess any sign of potential heterogeneity visually. Then I2 statistic and Chi-squared test will be used to assess the presence of statistical heterogeneity and calculate the heterogeneity severity. When P > .1, I2 < 50%, it is considered that there is acceptable heterogeneity between the trials, and the fixed effect model will be chosen;[25,26] otherwise, the random effect model will be considered and we will try to explain the underlying cause of heterogeneity by subgroup or sensitivity analysis (see “Subgroup analysis and sensitivity analysis” section below).[27] If heterogeneity is high, the meta-analysis should be avoided.
2.7.2. Subgroup analysis
When heterogeneity is identified, we will apply subgroup analysis to explore the source of heterogeneity. As the first step in the analysis of subgroup, make sure that the number of studies is adequate. Then we will stratify the subgroup by different subdomains. The criteria are as follows: population characteristics, complications, research quality, type of control interventions, dose and duration of intervention, follow-up period.
Moreover, we will also perform analysis for any other subgroups as reported in the included studies. It should be noted that selective reporting bias should be paid attention and minimized.
2.7.3. Sensitivity analysis
To check the robustness of pooled data of the review process, we will perform sensitivity analysis. The principle decision nodes included methodological quality, the effect of missing data, type of study, sample size. We will delete every eligible study 1 by 1 and a second meta-analysis will be performed. The result will be compared and discussed in order to test whether the results could have been influenced by a single study.
2.8. Reporting bias
As most publications process tends to favor positive results or large-scale studies, the meta-analysis that aims to pool and analyze prepublished studies should be examined for the presence of any publication bias. We will use funnel plots, Egger test and Begg tests to assess the publication bias if more than 10 of the studies are included.[28,29] Egger bias indicator test will be used to plot a regression line, which shows the symmetry of the plotted studies and examine for the presence of any publication bias. If insufficient number of articles are included, the test for funnel plot asymmetry will be inappropriate.[30] If publication bias is significant, trim and fill method will be constructed for correcting the probable publication bias.
2.9. Confidence in cumulative evidence
The strength of evidence will be assessed on the Grading of recommendations, assessment, development, and evaluation system according to eight criteria: indirectness, inconsistency (heterogeneity), imprecision, and/or publication bias in addition to 4 criteria of risk of bias assessment tool.[31] Quality of evidence for each outcome will be graded as high, moderate, low, or very low.
2.10. Ethics and dissemination
Research ethic approval will not be required because no individual patient data will be collected. We expect this systematic review will be published in a peer-reviewed journal.
3. Discussion
COVID-19 has been officially announced as a global pandemic, which are associated with a high rate of coagulopathy and high risk of VTE. Emerging evidence shows that anticoagulant therapy might improve the prognosis of severe COVID-19 patients. However, whether antiplatelet/anticoagulant therapy should be used in COVID-19 patients who required advanced life support until diagnostic assessment for VTE is still controversial. In addition to its safety and effectiveness, the current study also provides the evidence on the characteristics of therapy (eg, types, doses, frequencies and lengths) and participants. Therefore, we will summarize the current systematic analysis to figure out what the role of antiplatelet/anticoagulant therapy in prophylaxis of patients with severe COVID-19 is.
Currently, this is the first systematic review to evaluate the application of antiplatelet/anticoagulant therapy as preventive agents for patients with severe COVID-19. It will be reported following Cochrane Handbook for Systematic Reviews of Interventions and PRISMA statement guidelines. The potential limitations that inherent to systematic reviews and meta-analyses include publication bias, poor statically analyses and methodological quality, information bias, as well as inadequate reporting of methods and findings of the included studies. This review will provide valid advice and consultation for clinicians on the management of prophylactic antiplatelet/anticoagulant for severe COVID-19 patients.
Acknowledgments
Not applicable.
Author contributions
Conceptualization: Can Chen, Wei Hu.
Data curation: Yiwei Li, Ying Xu.
Formal analysis: Can Chen, Yiwei Li, Ying Xu.
Funding acquisition: Wei Hu.
Methodology: Can Chen, Yiwei Li.
Project administration: Can Chen, Wei Hu.
Software: Yiwei Li, Ying Xu.
Supervision: Can Chen, Wei Hu.
Validation: Yiwei Li, Ying Xu, Pengfei Shi, Ying Zhu, Can Chen, Wei Hu.
Writing – original draft: Yiwei Li, Ying Xu, Can Chen.
Writing – review and editing: Yiwei Li, Ying Xu, Pengfei Shi, Ying Zhu, Can Chen, Wei Hu.
Footnotes
Abbreviations: aPTT = activated partial-thromboplastin time, DIC = disseminated intravascular coagulation, PE = pulmonary embolism, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, VTE = venous thromboembolism.
How to cite this article: Li Y, Xu Y, Shi P, Zhu Y, Hu W, Chen C. Antiplatelet/anticoagulant agents for preventing thrombosis events in patients with severe COVID-19: a protocol for systematic review and meta-analysis. Medicine. 2020;99:32(e21380).
YL and YX Contributed equally.
This study was supported by the Zhejiang Technology Project of Medicine under Grant:20181KY567 and 2018KY577
This study is partly supported by Science and Technology Development Project of Hangzhou (Grant. 20142013A61).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].CORONAVIRUS RESOURCE CENTER of Johns Hopkins https://coronavirus.jhu.edu/map.html May 5, 2020. [Google Scholar]
- [2].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan. China JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [8].Yu X, Sun X, Cui P, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis 2020;67:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986–94. [DOI] [PubMed] [Google Scholar]
- [10].Lang ZW, Zhang LJ, Zhang SJ, et al. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology 2003;35:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Danzi GB, Loffi M, Galeazzi G, et al. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020;18:1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18:1421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care 2015;19:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Malato A, Dentali F, Siragusa S, et al. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus 2015;13:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 2020;383:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol 2020;189:846–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J. Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–364. [DOI] [PubMed] [Google Scholar]
- [24].Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [25].Hopp L. Risk of bias reporting in Cochrane systematic reviews. Int J Nurs Pract 2015;21:683–6. [DOI] [PubMed] [Google Scholar]
- [26].Weng H, Zeng XT, Li S, et al. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: a systematic review and meta-analysis. Sci Rep 2017;7:11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baicus C, Purcarea A, von Elm E, et al. Alpha-lipoic acid for diabetic peripheral neuropathy. Cochrane Database of Syst Rev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ 2015;351:h4718. [DOI] [PubMed] [Google Scholar]
- [30].Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- [31].Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]


