Abstract
Many waterbird populations have become increasingly dependent on agricultural habitats for feeding. While habitat destruction has been proposed as a key reason forcing waterbirds to move from natural habitats to agricultural habitats, few have used long‐term data to test this hypothesis. The Siberian crane (Leucogeranus leucogeranus) is an IUCN Critically Endangered species. About 98% of its global population winters at Poyang Lake, China. Recently, many cranes shifted from feeding in natural wetlands to agricultural habitats. Here, we integrate bird surveys, Vallisneria tuber (the traditional food of cranes in natural wetlands) surveys, water level data, and remotely sensed images from 1999 to 2016 to explore the drivers of this habitat shift. Changes in Siberian crane numbers in natural wetlands and agricultural fields indicated that the habitat shift occurred in the winters of 2015–2016. Analyses using generalized linear mixed models suggested that crane numbers in natural wetlands were positively related to tuber density and the interaction between dry season (October–March) water level and tuber density. The changes in tuber density and dry season water level in 2015–2016 indicated that tuber disappearance may have been the primary driver of the habitat shift, with a smaller effect of high water level. Submerged plants at Poyang Lake have degraded seriously in the past two decades. The plant degradation at Shahu Lake, a sublake of Poyang Lake, may have been caused by high spring water, high winter temperature, and low summer temperature. However, the drivers of tuber disappearance at Poyang Lake may not be restricted to these variables. Because Poyang Lake is an important refuge for many waterbirds in the Yangtze River floodplain, it is urgent to take effective measures to restore its submerged plants and ecosystem health. Agricultural fields can be important refuges for Siberian cranes, mitigating the negative impacts of wetland deterioration.
Keywords: agricultural habitats, food shortage, Leucogeranus leucogeranus, submerged plants, Yangtze River
Our study, using field surveys and remotely sensed images from 18 years, suggests that habitat degradation affecting food shortage may have been the primary driver of the Siberian Crane, an IUCN Critically Endangered species, shifting from feeding in natural wetlands to agricultural habitats at its most important wintering ground, Poyang Lake, China, in the winters of 2015–2016.

1. INTRODUCTION
Many waterbird populations have increasingly become dependent on agricultural habitats for foraging during wintering and migration seasons (Amano, 2009; Bellio, Kingsford, & Kotagama, 2009; Czech & Parsons, 2002). While wetland loss and deterioration have been proposed drivers of these changes (Alonso, Alonso, & Bautista, 1994; Smart & Gill, 2003; Yasué, Patterson, & Dearden, 2007), few have used long‐term monitoring data to test this hypothesis. Alternatively, habitat shifts may be the result of the discovery of more profitable resources. For example, many goose (Fox & Abraham, 2017; Fox et al., 2005), crane (Amano, 2009; Nowald, 2012), heron (Fasola, Rubolini, Merli, Boncompagni, & Bressan, 2010), and shorebird (Toral & Figuerola, 2010) populations have obtained increased food quality and intake rate by shifting to agricultural habitats and have experienced improved breeding success and population increases as a result. Exploring the drivers of foraging habitat shifts is critical to understanding the response of waterbirds to land‐use changes and habitat destruction, and to developing protection measures.
The Siberian crane (Leucogeranus leucogeranus; Figure 1) is a Critically Endangered species (IUCN, 2018). All three subpopulations of this species breed in Russia, but they winter separately in Iran (western population), India (central population), and China (eastern population; Meine & Archibald, 1996). The populations that winter in Iran and India have declined to fewer than 10 birds (Kanai et al., 2002). The population that winters in China is estimated to comprise 3,800–4,000 individuals (Li, Wu, Harris, & Burnham, 2012). Cranes in the eastern population breed in Yakutia, Siberia, and migrate 5,000 km across eastern China to winter at Poyang Lake, China (Figure 2; Kanai et al., 2002).
FIGURE 1.

Siberian cranes fed in rice paddies. The photo was taken by Lanhua Wang at Chengxin Farmland, Nanchang City, on 12 February 2017
FIGURE 2.
Map of Poyang Lake, China, and the three tuber survey sublakes (Shahu Lake, Dahuchi Lake, and Meixihu Lake). The triangles indicate the Xingzi Hydrological Station and the Duchang Weather Station
The Siberian crane is the most aquatic of cranes, using wetlands for nesting, feeding and roosting (Harris & Mirande, 2013; Meine & Archibald, 1996). At Poyang Lake, the Siberian crane typically feeds in waters <45 cm deep (Wu, Li, & Burnham, 2013; Zhang et al., 2014), where they probe for tubers of submerged plants, primarily Vallisneria spp. (V. spinulosa and V. natans; Wu & Ji, 2002). However, in the winter of 2010 (2010/2011), large numbers of cranes were observed, for the first time, foraging in grasslands on the roots of Potentilla limprichtii and bulbs of Tulipa edulis (Burnham et al., 2017; Jia et al., 2013). A flood‐induced food collapse was proposed to be the main driver of the habitat and diet shift (Burnham et al., 2017; Jia et al., 2013). The diet shift may have influenced the Siberian crane behavior and energy stores, possibly resulting in lower breeding success in the next breeding season (Burnham et al., 2017). Siberian cranes at Poyang Lake largely returned to shallow waters in the next winter when tuber abundance rebounded (Burnham et al., 2017). However, in recent years, thousands of cranes were observed feed in agricultural habitats including rice paddies and lotus ponds (Lei, 2018; Li, 2018; Wang, Wang, & Hou, 2019). Their main foods changed from Vallisneria tubers to rice grains and lotus rhizomes (Hou, Wang, Jin, Wang, & Wang, 2019). Until now, the drivers of this habitat shift have not been explored.
Food abundance is a key factor determining foraging site selection of waterbirds, particularly during the winter (Newton, 1980; Tinkler, Montgomery, & Elwood, 2009; Wang, Fox, Cong, & Cao, 2013). In general, the distribution of animals can reveal information about food distribution, abundance, and availability (Fretwell, 1972). Water depth also is an important factor affecting the use of wetland habitats by waterbirds (Faragó & Hangya, 2012; Ma, Cai, Li, & Chen, 2010). It directly determines the food accessibility for waterbirds because of the restrictions of bird morphology, such as the lengths of tarsometatarsi and necks. Areas of suitable habitats also influence waterbird distribution and abundance, with large areas normally supporting more waterbirds than smaller areas (Li, Yang, et al., 2019; Roshier, Robertson, & Kingsford, 2002).
In this study, we integrated bird surveys, Vallisneria tuber surveys, water level data, and remotely sensed images from 1999 to 2016 to examine the drivers of the Siberian crane's shift to agricultural habitats. We first explored the numerical changes of Siberian cranes in natural wetlands to find out when the habitat shift occurred. Then, we examined factors that might have driven the habitat shift. We focused on four variables related to Siberian crane foraging habitat selection: tuber density, tuber biomass, dry season (October–March) water level, and seasonal water area. We defined seasonal water area as the area (km2) of Poyang Lake that was exposed during the dry season. It is the difference between the maximum area of the lake during the dry season and permanent water area. Water in seasonal water areas recedes slowly, providing large and dynamic areas of shallow water and mudflats suitable for Siberian cranes. Thus, seasonal water area is an index of foraging habitat available to cranes. We suggest that a variable may have been the driver of the habitat shift (a) if it is an important factor determining the abundance of Siberian cranes in natural wetlands, and (b) if it changed substantially when the habitat shift occurred in a way that might have negatively affected crane foraging (e.g., abnormally low tuber density or biomass). We used generalized linear mixed models (GLMM) to evaluate the effects of the four variables on the numerical change of crane numbers. Because the data were consistent with a tuber collapse being the primary driver of the habitat shift, we further explored whether the tuber collapse may have been driven by abnormal water levels or temperatures, important factors influencing the growth and production of Vallisneria (Cao, Li, & Jeppesen, 2014; Xu et al., 2016; Zhao et al., 2017).
2. METHODS
2.1. Study site
Poyang Lake (28°25′–29°45′N, 115°48′–116°44′E; Figure 2), the largest freshwater lake in China, is one of only two lakes that still connect freely with the Yangtze River. The northern portion of the lake is characterized by a narrow and deep outlet into the Yangtze River and the southern portion by a broader and shallower main extent of lake with delta from 5 tributary rivers (Ganjiang, Fuhe, Xiushui, Xinjiang, and Raohe). Poyang Lake is subject to substantial intra‐annual hydrological fluctuations (average 9.24 m between wet season high and dry season low water levels; Min, 1995). During the wet season (April–September), the inundated area is >2,500 km2 and sometimes exceeds 3,000 km2 (Feng et al., 2012). During the dry season, the inundated area can shrink to <1,000 km2 (Feng et al., 2012). Poyang Lake also is subject to substantial interannual hydrological fluctuations, with the monthly maximum/minimum inundation area ratios between 1.46 and 4.03 in 2000–2010 (Feng et al., 2012). The large hydrological fluctuations are driven primarily by local precipitation, which varies widely year‐to‐year, making Poyang Lake one of the most frequently flooded and also most frequently drought‐stricken areas in China (Min, 2007; Shankman, Keim, & Song, 2006). The water level fluctuation is a key reason that Poyang Lake is among the most productive lakes for waterbirds in the world (Barter, Cao, Chen, & Lei, 2005; Barzen, 2012; Wang, Fraser, & Chen, 2019). The lake contains 102 sublakes, which are inundated and integrated with the main body of Poyang Lake during the wet season, and become isolated during the dry season (Hu, Zhang, Liu, Ji, & Ge, 2015). When sublakes are connected with the main body of Poyang Lake, their water levels are the same as the main body. When sublakes are isolated, their water levels are primarily controlled by local fishermen who manage water levels with sluices (Wang, Fraser, et al., 2019). Fishermen release water when they want to catch fish. As water levels recede, extensive wet meadows, shallow waters and mudflats are exposed, providing a wide range of foraging and roosting habitat for hundreds of thousands of migratory waterbirds.
2.2. Data collection
We collected data on Siberian Crane numbers in natural wetlands of Poyang Lake for the winters of 1999 to 2016 from the literature (Table S1). Crane surveys were conducted by the Poyang Lake National Nature Reserve (PLNNR) and the Jiangxi Wildlife Management Bureau (JWMB). The PLNNR and the JWMB usually conducted synchronous ground surveys, where field staff observed and counted Siberian cranes at approximately the same time on the same date, to avoid double counting or missing birds. Depending on lake size, 1–3 fixed observation points were established at each sublake. The observation sites were located on high ground so that most birds could be observed and counted. Although there were some differences in survey areas between the censuses conducted by the PLNNR and the JWMB, both censuses included more than 68 sublakes, covering most of the areas used by Siberian cranes (Li et al., 2012; Li, Qian, Silbernagel, & Larson, 2019). Therefore, these data are comparable and could be integrated to reflect population dynamics of Siberian cranes. Most surveys were conducted in December and January, when crane numbers were stable. The PLNNR conducted 1–2 census, and the JWMB conducted one census each winter. We used the average number counted each winter in our analyses.
In collaboration with the International Crane Foundation, the PLNNR has monitored Vallisneria tuber density and biomass at three sublakes of Poyang (Dahuchi Lake, Shahu Lake, and Meixihu Lake; Figure 2) since the winter of 1999 (Wu, Li, Liu, & Zeng, 2012). We obtained the tuber survey results for the winters of 1999–2016 from the literature (Table S2). The three sublakes were selected based on size, human disturbance, and environmental conditions (Wu et al., 2012). Dahuchi Lake and Shahu Lake are extensively used by Siberian cranes (Li et al., 2012; Wu et al., 2013). As tubers do not grow after October, tuber surveys usually were conducted in November, rarely in October and December (Wu et al., 2013). For each sublake, two crossed sampling transects were selected, one located along the long side of the sublake, and the other located on the short side. Plots were established every 50 m, with four quadrats in each plot. The plots were in the same place each year as determined with GPS locations. Samples were excavated with a locally made steel grab sampler. The sampler has two long handles. At the end of the handles, there are two scoops facing each other. The sampler was inserted into the substrate and the scoops brought together to collect about 15 cm long × 13 cm wide × 20 cm high sample of substrate. The same sampler was used in every survey so that the sample volume was constant. Mud was washed away through a mesh (pore size 0.5 cm × 0.5 cm), and tubers were cleaned and counted. The dry weight of tubers in each quadrat was weighed after oven‐drying at 80 ℃ until constant weight. The tuber density (ind/m2) and biomass (g/m2) in each plot were calculated by summing the total tuber number or tuber mass across four quadrats and dividing by the sum of the area of the four quadrats.
To explore the impacts of water level on Siberian crane numbers, we obtained daily water levels (Wu Song elevation datum) recorded at the Xingzi Hydrological Station (Figure 2). The hydrological station is located at the deep outlet of Poyang Lake. Its water level data have frequently been used to represent the water level of Poyang Lake (Wang et al., 2013; Xia, Yu, & Fan, 2010). Water level data of Dahuchi Lake, Shahu Lake, and Meixihu Lake (Wu Song elevation datum) also were collected to analyze the influences of water level on tuber abundance. Water level data of sublakes were used because tuber surveys were conducted at sublakes. During the dry season, water levels of most sublakes are controlled by local fishermen through sluices. Therefore, their water levels are independent from the water level of the main body of Poyang Lake. We also collected monthly air temperature data recorded at the Duchang Weather Station (Figure 2) to explore the influences of temperature on tuber abundance. The temperature data were obtained from the China Meteorological Administration (http://www.cma.gov.cn/).
2.3. Seasonal water area delineation
Due to the strong intra‐annual dynamics of water surface areas, we integrated all the available Landsat 5/7/8 surface reflectance images (30 m spatial resolution) from the Google Earth Engine (GEE) platform during the dry seasons of 1999 to 2016 to capture water body variability (Zou et al., 2017). We removed poor‐quality observations caused by clouds, shadows, and scan‐line gaps, using the Landsat quality assurance (QA) band. This left us with 1,022 usable images (Table S3). We employed three widely used indices to identify the surface water: the Normalized Difference Vegetation Index (NDVI; Tucker, 1979), Enhanced Vegetation Index (EVI; Huete et al., 2002), and modified Normalized Difference Water Index (mNDWI; Xu, 2006). NDVI and EVI are closely related to the greenness of vegetation, and mNDWI is one of the most commonly used indices for mapping surface water bodies (Wang et al., 2020). The three indices were calculated based on reflectance of different wavelengths measured in the Landsat images on a per pixel basis. We used a mNDWI/VIs algorithm combining NDVI, EVI, and mNDWI to reduce commission error in water detection (Xiao, Yu, & Wu, 2007; Xiao et al., 2005; Zou et al., 2017). Pixels whose water signal were stronger than their vegetation signal (mNDWI > EVI or mNDWI > NDVI) were classified as water pixels. To further remove the vegetation noise, EVI < 0.1 was used to remove the mixed pixels of water and vegetation. Therefore, only those pixels that met the criteria ((mNDWI > EVI or mNDWI > NDVI) and (EVI < 0.1)) were classified as surface water body pixels while the rest were classified as nonwater pixels (Zou et al., 2017, 2018). For each pixel, we calculated the water frequency, which was the ratio of the number of observations identified as water to the total number of useable observations within a dry season (Zou et al., 2017). Pixels with a water frequency ≥ 0.25 were classified as water pixels (Zou et al., 2017). Water pixels with a water frequency ≥ 0.75 were classified as permanent water pixels since they have water most of the dry season (Zou et al., 2017). For each winter, we generated maps of maximum water bodies (water frequency ≥ 0.25) and permanent water bodies (water frequency ≥ 0.75), respectively, and then calculated their areas. Area of annual seasonal water bodies was calculated as the area of maximum water bodies minus the area of permanent water bodies.
2.4. Statistical analyses
To explore the drivers of the cranes’ habitat shift, four variables were used: tuber density, tuber biomass, dry season water level, and seasonal water area. Tuber density and biomass were the average values of Dahuchi Lake, Shahu Lake, and Meixihu Lake. Dry season water level was the average water level recorded at the Xingzi Hydrological Station between October and March. Annual data of the four variables and crane numbers in the winters of 1999–2016 were used. GLMMs were used to evaluate the effects of the four explanatory variables on crane numbers. Before building the GLMMs, we assessed multicollinearity among explanatory variables by examining the variance inflation factors (VIFs). All VIF values were less than 2.5, indicating little evidence for multicollinearity (O'brien, 2007). All explanatory variables were standardized to a mean of 0 and standard deviation of 1 prior to analysis to facilitate comparison of coefficients among models. We used the four explanatory variables and two interactions (dry season water level × tuber density, dry season water level × tuber biomass) as fixed effects. Year was included as a random effect. The GLMM analysis generated a complete set of models with all possible combinations of all explanatory variables (i.e., 64 models) and graded the models according to their Akaike's information criterion corrected for small sample sizes (AICc) and Akaike weights (Burnham & Anderson, 2002). Following Burnham and Anderson (2002), we selected models with ΔAICc < 2 (the difference between each model's AICc and the lowest AICc) as candidates for top models. Model averaging was subsequently applied to estimate the correlation coefficient (β) and their 95% confidence intervals (CI) for each variable in the top models (Burnham & Anderson, 2002). The model averaging calculation was done on the top models. Model averaging incorporates the uncertainty associated with model selection by combining estimates over a set of models (Schomaker & Heumann, 2014). The relative importance of each variable was estimated by summing the Akaike weights across all models where a variable was included (Burnham & Anderson, 2002). We used relative importance and 95% CI of coefficient to determine the relative effect of each variable (Arnold, 2010; Catlin, Fraser, & Felio, 2015). All statistical analyses were conducted in R 3.6.0 with the packages lme4, MuMIn, vegan, and car.
As the graphs of crane number, tuber density, tuber biomass, dry season water level, and seasonal water area look cyclical (Figure 3), we used Fourier analysis, which has been previously used to detect population cycles (Blomqvist, Holmgren, Åkesson, Hedenström, & Pettersson, 2002; Fraser, Karpanty, Cohen, & Truitt, 2013) to examine the cycles in these variables. The Fourier analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). To explore whether submerged plants decreased in density and biomass over the study period, we calculated Spearman's rho correction coefficients between tuber density and year, and between tuber biomass and year.
FIGURE 3.
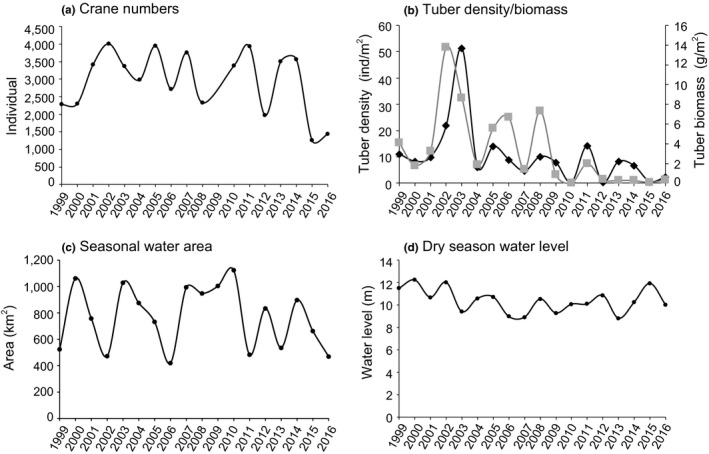
Annual data for (a) Siberian crane numbers in natural wetlands of Poyang Lake. (b) Average tuber density (ind/m2; black line) and biomass (g/m2; gray line) at Dahuchi Lake, Shahu Lake, and Meixihu Lake. (c) Seasonal water area (km2) during the dry season. (d) Dry season (October–March) water level (m) at the Xingzi Hydrological Station
To analyze the effects of water level and air temperature on tuber density and biomass, data from Dahuchi Lake and Shahu Lake were considered separately. Meixihu Lake was not included in this analysis because its water level was not recorded after 2010. Daily water level of Dahuchi Lake and Shahu Lake were averaged to derive yearly seasonal measures (December–February: winter; March–May: spring; June–August: summer; September–November: autumn) to avoid multicollinearity between variables. Daily air temperatures recorded at the Duchang Weather Station also were averaged to derive yearly seasonal measures. As Vallisneria overwinter in the forms of seeds and tubers which are produced before November (Li, Lan, Chen, & Song, 2018; Yuan, Li, & Li, 2013), winter water level and temperature would most likely affect tuber abundance in the following year. Therefore, eight explanatory variables were used, including winter water levels and temperatures in the preceding year, spring water levels and temperatures, summer water levels and temperatures, and autumn water levels and temperatures. Annual data spanning the winters of 1999–2016 were used. Linear regression models were used to evaluate the effects of the eight variables on tuber density and biomass. The eight variables were entered as independent variables, and tuber density and tuber biomass were entered as response variables. All explanatory variables had VIF values less than 4, suggesting multicollinearity did not affect the models. The linear regression analyses generated a total of 256 models, and models with ΔAICc < 2 were selected as candidates for the top models. Similar to the crane number analyses, the correlation coefficients and their 95% CI’s for each variable in the top models was calculated by model averaging, and the relative importance of each variable was estimated by summing the Akaike weights of the models containing the variable. The relative importance and 95% CI’s of coefficients were used to determine the relative effect of each variable.
3. RESULTS
Siberian crane numbers in natural wetlands fluctuated over our study period (mean = 2,942 ± 855 SD; Figure 3a and Figure S1). Several large year‐to‐year declines have occurred (e.g., 2012, 2015), with the most severe decline happening in the winters of 2015–2016, from 3,552 individuals in 2014 to 1,247 individuals in 2015 and 1,437 individuals in 2016.
Mean tuber density and tuber biomass were 10.33 ind/m2 ± 11.27 SD and 3.26 g/m2 ± 3.68 SD, respectively (Figure 3b). Tuber density (Spearman's ρ = −0.59, p = .01) and tuber biomass (Spearman's ρ = −0.71, p = .00) declined significantly over the study period. Tuber density declined to nearly zero in the winters of 2010, 2012, and 2015–2016, and tuber biomass declined to nearly zero in the winters of 2010 and 2012–2016. The average seasonal water area was 767.62 km2 ± 230.76 SD (Figure 3c and Figure S2), and the average dry season water level was 10.37 m ± 1.03 SD (Figure 3d). As the habitat shift of Siberian cranes occurred in the winters of 2015–2016 (see Section 4 for details), we focused on the values in the two winters. The seasonal water area in winter 2015 was similar to the historical average level, while the area in winter 2016 was the second lowest over the study period. The dry season water level in winter 2015 was the third highest over our study period, and the water level in winter 2016 was similar to the historical average water level.
The p‐values for the Kolmogorov–Smirnov statistic of crane numbers, tuber density, tuber biomass, seasonal water area, and dry season water level were 0.52, 0.36, 0.07, 0.23, and 0.47, respectively. Thus, there was no evidence of cyclic patterns based on Fourier analysis.
The top two models for crane numbers contained dry season water level, tuber density, seasonal water area, and the interaction between dry season water level and tuber density (Table 1). The 95% CI’s of coefficients for tuber density and the interaction between dry season water level and tuber density did not overlap 0, and the relative importance of the two variables were >0.70 (Table 2). Thus, the two parameters might be closely related to crane numbers in natural wetlands. Both variables were positively correlated with crane numbers (Table 2). Although dry season water level and seasonal water area also were included in the top two models, the 95% CI of coefficients for dry season water level overlapped with 0 and the relative importance value of seasonal water area was only 0.43. Therefore, their effects on crane numbers might be limited.
TABLE 1.
Top linear regression models (ΔAICc < 2) for Siberian crane numbers in natural wetlands of Poyang Lake, China
| ID | Models | df | AICc | ΔAICc | ωi |
|---|---|---|---|---|---|
| 1 | Dry season water level + tuber density + seasonal water area + dry season water level × tuber density | 6 | 282.67 | 0 | 0.30 |
| 2 | Dry season water level + tuber density + dry season water level × tuber density | 5 | 283.13 | 0.46 | 0.24 |
Abbreviations: AICc, Akaike information criterion for small sample size; df, degrees of freedom; ΔAICc, the difference between each model's AICc and the lowest AICc; ωi, Akaike weights.
TABLE 2.
Correlation coefficient (β), 95% confidence intervals (CI) of coefficient, and relative importance values for the effects of variables on Siberian crane numbers in natural wetlands of Poyang Lake, China
| Variable | β | 95% CI | Relative importance |
|---|---|---|---|
| Dry season water level | –0.11 | –0.23 to 0.00 | 0.86 |
| Seasonal water area | 0.07 | 0.02 to 0.25 | 0.43 |
| Tuber density | 0.30 | 0.15 to 0.45 | 0.82 |
| Dry season water level × tuber density | 0.30 | 0.13 to 0.46 | 0.73 |
The top models for tuber density at Shahu Lake contained last winter temperature, spring water level, autumn temperature, and summer water level (Table 3). The 95% CI’s of coefficients for last winter temperature and spring water level did not overlap with 0, and their relative importance values were about 0.60 (Table 4). Thus, the two variables might be closely related to tuber density at Shahu Lake. Both variables were negatively related to tuber density (Table 4). The 95% CI’s of coefficients for autumn temperature and summer water level included 0, and their relative importance values were only about 0.30. Thus, the two variables had little effect on tuber density.
TABLE 3.
Top linear regression models (ΔAICc < 2) for explaining tuber density and tuber biomass at Shahu Lake, a sublake of Poyang Lake, China
| ID | Models | df | AICc | ΔAICc | ωi |
|---|---|---|---|---|---|
| Tuber density | |||||
| 1 | Last winter temperature + spring water level | 4 | 126.12 | 0.00 | 0.13 |
| 2 | Last winter temperature + autumn temperature + spring water level | 5 | 127.19 | 1.07 | 0.08 |
| 3 | Last winter temperature + summer water level | 4 | 127.67 | 1.55 | 0.06 |
| 4 | Spring water level | 3 | 127.88 | 1.76 | 0.06 |
| Tuber biomass | |||||
| 1 | Last winter temperature + autumn temperature | 4 | 107.16 | 0.00 | 0.14 |
| 2 | Last winter temperature + autumn temperature + summer water level | 5 | 108.90 | 1.74 | 0.06 |
| 3 | Last winter temperature + autumn temperature + spring temperature | 5 | 108.94 | 1.78 | 0.06 |
Abbreviations: AICc, Akaike information criterion for small sample size; df, degrees of freedom; ΔAICc, the difference between each model's AICc and the lowest AICc; ωi, Akaike weights.
TABLE 4.
Correlation coefficient (β), 95% confidence intervals (CI) of coefficient, and relative importance values for the effects of variables on tuber density and biomass of Shahu Lake, a sublake of Poyang Lake, China
| Variable | β | 95% CI | Relative importance |
|---|---|---|---|
| Tuber density | |||
| Last winter temperature | –4.26 | –8.36 to –0.16 | 0.61 |
| Autumn temperature | 5.66 | –1.99 to 13.30 | 0.30 |
| Spring water level | –10.26 | –19.65 to –1.20 | 0.59 |
| Summer water level | –4.57 | –9.25 to 0.12 | 0.28 |
| Tuber biomass | |||
| Last winter temperature | –2.56 | –4.89 to –0.23 | 0.67 |
| Spring temperature | 2.42 | –1.35 to 6.18 | 0.22 |
| Autumn temperature | 5.05 | 0.45 to 9.66 | 0.70 |
| Summer water level | –1.66 | –4.21 to 0.90 | 0.26 |
The top models for tuber biomass at Shahu Lake included last winter temperature, autumn temperature, summer water level, and spring temperature (Table 3). The 95% CI’s of coefficients for last winter temperature and autumn temperature did not overlap with 0, and their relative importance values were >0.65 (Table 4). Last winter temperature was negatively related to tuber biomass, and autumn temperature was positively related to tuber biomass (Table 4). Although summer water level and spring temperature also were included in the top models, their 95% CI of coefficients included 0, and their relative importance values were <0.30.
The top models for tuber density and tuber biomass of Dahuchi Lake contained two variables, spring temperature and summer temperature (Table 5). The effects of the two variables on tuber density and tuber biomass of Dahuchi Lake was subtle because their 95% CI of coefficients included 0, and their relative importance values were <0.50 (Table 6).
TABLE 5.
Top linear regression models (ΔAICc < 2) for tuber density and tuber biomass at Dahuchi Lake, a sublake of Poyang Lake, China
| ID | Models | df | AICc | ΔAICc | ωi |
|---|---|---|---|---|---|
| Tuber density | |||||
| 1 | Summer temperature | 3 | 169.35 | 0.00 | 0.13 |
| 2 | Intercept | 2 | 169.65 | 0.30 | 0.11 |
| 3 | Summer temperature + spring temperature | 4 | 170.81 | 1.45 | 0.06 |
| 4 | Spring temperature | 3 | 170.96 | 1.61 | 0.06 |
| Tuber biomass | |||||
| 1 | Intercept | 2 | 99.02 | 0.00 | 0.14 |
| 2 | Summer temperature | 3 | 99.54 | 0.52 | 0.10 |
| 3 | Spring temperature | 3 | 100.34 | 1.32 | 0.07 |
Abbreviations: AICc, Akaike information criterion for small sample size; df, degrees of freedom; ΔAICc, the difference between each model's AICc and the lowest AICc; ωi, Akaike weights.
TABLE 6.
Correlation coefficient (β), 95% confidence intervals (CI) of coefficient, and relative importance values for the effects of variables on tuber density and biomass of Dahuchi Lake, a sublake of Poyang Lake
| Variable | β | 95% CI | Relative importance |
|---|---|---|---|
| Tuber density | |||
| Summer temperature | 20.57 | –3.81 to 44.95 | 0.47 |
| Spring temperature | –15.40 | –40.92 to 10.13 | 0.29 |
| Tuber biomass | |||
| Summer temperature | 2.26 | –0.89 to 5.41 | 0.37 |
| Spring temperature | –1.93 | –5.23 to 1.38 | 0.29 |
4. DISCUSSION
4.1. Habitat shift of Siberian cranes
Long‐term monitoring data indicate that Siberian crane numbers in natural wetlands fluctuated in the winters of 1999–2016. This could have been driven by breeding success variation (Germogenov et al., 2013), weather variation on wintering ground (Li et al., 2014), or bird being missed on surveys due to habitat shifts (Li et al., 2019). The crane numbers underwent several large year‐to‐year declines (e.g., 2012, 2015); however, the decline in the winters of 2015–2016 was more severe than other declines, and concurrently, Siberian cranes at rice paddies and lotus ponds increased. For example, Wuxing Reclamation Farm (Nanchang City), a reclamation area composed of rice paddies and lotus ponds near Poyang Lake, supported an average of 47 (range: 0–170) cranes in the winters of 2010–2014, but the numbers increased to 297 in winter 2015 and 1,300 in winter 2016 (Wang et al., 2019). Lei (2018) indicated that Siberian cranes began to appear at farmlands of Yugan County (Shangrao City) in winter 2015, and more than 1,300 individuals were recorded in winter 2016. Li (2018) conducted waterbird surveys on seven transects at rice paddies adjacent to Poyang Lake, recoding 20 Siberian cranes in winter 2015 and 1,113 cranes in winter 2016. Although crane numbers in natural wetlands declined in other winters (e.g., 2012), no literature reported crane number increase at agricultural fields in these winters, and the local wildlife managers were unaware of any such movements. Cranes moving out of the survey areas might contribute to the decline of counting number in winter 2012 (Liu et al., 2013).
4.2. Drivers of the habitat shift
Our GLMM analyses indicate that crane numbers in natural wetlands were positively related to tuber density. Tuber density collapsed in the winters of 2010, 2012, and 2015–2016 at Poyang Lake. Waterbirds are expected to give up a foraging site when food density drops below a threshold value, and this giving‐up density threshold has been reported in many waterbirds (Jonzen, Nolet, Santamaria, & Svensson, 2002; Nolet et al., 2001; Sponberg & Lodge, 2005). The tuber collapse in the winter of 2010 was suggested to be a major driver for Siberian cranes moving from shallow waters to grasslands at Poyang Lake in that winter (Burnham et al., 2017; Jia et al., 2013). The decline of Siberian cranes in natural wetlands in winter 2012 was coincident with a tuber decline. It is likely that the tuber collapse in the winters of 2015–2016 was a major driver for Siberian cranes moving from natural wetlands to agricultural habitats. Similarly, food shortage was thought to be the main driver of other crane (Zheng, Zhou, Zhao, & Xu, 2015), goose (Clausen, Clausen, Fælled, & Mouritsen, 2012), and swan (Nolet, Bevan, Klaassen, Langevoord, & der Heijden, 2002) populations moving from natural wetlands to agricultural habitats.
The interaction between tuber density and dry season water level was an important factor influencing crane numbers in natural wetlands. A flood occurred at Poyang Lake in the winter of 2015 (Zeng, Schmitt, Li, & Zhu, 2017), and water reached the third highest level during our study period. Even when food is abundant, high water can reduce its availability if cranes cannot reach it (Ma et al., 2010), and cranes may leave in search of food elsewhere (Chen et al., 2014; Zhang et al., 2014). The 2015 winter flood at Poyang Lake led to the decline of total waterbird abundance (Wang, Wang, Hou, & Ouyang, 2019), and to diet and foraging habitat shifts of two goose species (Aharon‐Rotman et al., 2017). It likely reduced Siberian crane food availability as they primarily feed in shallow waters and mudflats (Wu et al., 2013; Zhang et al., 2014). Thus, the combination of low tuber density and high water levels probably drove cranes to move to agricultural habitats in winter 2015. The dry season water levels in winter 2016 were similar to the historical average water level, while many Siberian cranes still foraged in agricultural fields. Thus, high water levels may have contributed to the habitat shift, but played a less important role than low tuber density.
Tuber biomass did not closely relate to crane numbers in natural wetlands. Tuber biomass collapsed in the winters of 2012–2016, while Siberian cranes left natural wetlands in the winters of 2015–2016. Thus, many Siberian cranes still searched for tubers in natural wetlands before the winters of 2015, even though there only were small tubers available. Only when tuber density also collapsed, did Siberian cranes give up natural wetlands.
The effect of seasonal water area on Siberian crane numbers in natural wetlands was relatively weak. The seasonal water area in the winter of 2015 was similar to the historical average level, while the area in the winter of 2016 was the second smallest over the study period. There were similar small seasonal water areas in the winters of 2002 and 2006, while most cranes still foraged in natural wetlands, and numerous cranes were not recorded in agricultural fields. Thus, although the foraging habitat may have been diminished in winter 2016, its effect on habitat shift was less than the effect of the tuber collapse and high water level.
4.3. Reasons for the tuber collapse
The tuber density and biomass declined significantly at the three sublakes (Dahuchi Lake, Shahu Lake, and Meixihu Lake) of Poyang Lake over the study period, indicating severe degradation of the submerged plants and the wetland ecosystem. Similarly, Hu and Lin (2019) indicated that area of submerged plants declined between 1983 and 2013, from about 1,124 to 700 km2 (37.7% decline) throughout Poyang Lake. Submerged plants were widely distributed over Poyang Lake in 1983, but restricted to few sublakes by 2013 (Hu & Lin, 2019). The number of plant species also decreased with the disappearance of some plant species sensitive to water pollution (Hu & Lin, 2019; Jian et al., 2015).
Submerged plant growth is controlled by numerous factors, including water depth, temperature, light, nutrients, substrate, and water movements (Bornette & Puijalon, 2011). Water depth, which substantially affects underwater light availability, is a key factor controlling growth of submerged macrophytes (Søndergaard et al., 2013; Xu et al., 2016). Optimal water depth can promote Vallisneria growth (Li et al., 2018; Wei et al., 2018), while excessively high water level inhibits its growth (Xiao et al., 2007; Xu et al., 2016). Our results indicated that tuber density at Shahu Lake was negatively related to spring water level. Spring is the time of the initial growth of Vallisneria (Li et al., 2018) and is regarded as a key stage during the annual life history of many aquatic plants (Jia, Cao, Yésou, Huber, & Fox, 2017; Nishihiro & Washitani, 2009; Paillisson & Marion, 2011). High spring water level likely negatively affects tuber germination and shoot development of Vallisneria (Xu et al., 2016) and thus negatively affects tuber density in autumn.
Winter temperature influences seed and tuber germination of Vallisneria (Kauth & Biber, 2014; Xiao, Xing, & Liu, 2010; Zhao et al., 2017) and other aquatic plants (Schütz, 2000; van Wijk, 1989; Yin et al., 2009) as cold stratification is necessary for dormancy release and spring germination. Seeds and tubers normally have a higher germination rate after a cold storage than after a warm storage (Imanishi & Imanishi, 2014; Moravcová, Zákravský, & Hroudová, 2001). The negative relationships between last winter temperature and Vallisneria tuber density and biomass at Shahu Lake are consistent with previous studies. Higher winter temperature in the preceding year may lead to lower germination of Vallisneria seeds and tubers and thus lower plant density, which may further lead to lower tuber density and biomass.
In contrast to last winter temperature, summer temperature was positively correlated with tuber biomass at Shahu Lake. Summer is the active growing season of Vallisneria plants (Cao et al., 2014). Higher summer temperature can enhance the growth of Vallisneria (Bartleson, Hunt, & Doering, 2014; Cao et al., 2014) and other aquatic plants (Barko & Smart, 1981; Rooney & Kalff, 2000), while low summer temperature can impede plant growth.
Thus, high spring water level, high winter temperature, and low summer temperature probably contributed jointly to the tuber disappearance at Shahu Lake. Due to global climate change and human activities, spring water level (Li, Tao, Yao, & Zhang, 2016) and winter temperature (Li et al., 2016; Ye, Zhang, Liu, Li, & Xu, 2013) have increased at Poyang Lake in recent decades. The operation of the Three Gorges Dam, located at the upper reaches of the Yangtze River, has also led to increase of spring water level at Poyang Lake (Wu et al., 2009). In addition, the frequency of extreme precipitation (Sun et al., 2014) and temperature (Wang, Zhang, Wang, Ma, & Sun, 2014) has increased. These climate changes are consistent with the large fluctuation of tuber abundance over our study period and frequent occurrences of tuber disappearances in recent years. Under future climate change, precipitation and temperature extremes are expected to increase in frequency and intensity (Xu, Xu, Gao, & Luo, 2009; Ye, Zhang, Bai, & Hu, 2011). Therefore, tuber disappearance may occur more frequently in the future, potentially threatening the survival of Siberian cranes.
Unlike at Shahu Lake, a close relationship between tuber density/biomass and water level/temperature was not detected at Dahuchi Lake. These contrasting results indicate that the drivers of tuber collapse might be different among different sublakes. Besides water level and temperature, other factors might also have contributed to the tuber collapse at Poyang Lake. Some potential drivers include declining water quality (Li et al., 2015; Wang, Liu, Fang, & Feng, 2014), extensive aquaculture of fish and crabs (Wang, Fraser, & Chen, 2017), and herbivory by the invasive crayfish (Procambarus clarkia; Carreira, Dias, & Rebelo, 2014; Zou et al., 2014). In addition, the operation of the Three Gorges Dam and widespread sand dredging, leading to inundation shrinkage of Poyang Lake during the dry season, have compressed the living space of submerged plants (Han, Feng, Hu, & Chen, 2018; Lai, Jiang, Yang, & Lu, 2014).
4.4. The role of agricultural fields in Siberian crane protection
Siberian cranes foraged in agricultural fields when tubers collapsed in natural wetlands in the winters of 2015–2016. This indicates that agricultural fields can be important refuges for Siberian cranes, mitigating the negative impacts of wetland deterioration. Human activity in agricultural fields in eastern China is considerably higher than in many countries (Yu et al., 2017; Zhao, Wang, Cao, & Fox, 2018). Approximately 10 million people live around Poyang Lake, and more than 14 million free‐ranging domestic fowl are fed on spilled grain in rice paddies (Choi et al., 2016; Xing & Wang, 2016). Such intensive free‐range poultry is extremely rare in other countries (Zhao et al., 2018). Intensive gleaning by domestic poultry and associated human presence may affect foraging efficiency of Siberian cranes (Zhao et al., 2018). High disturbance may be why Chinese wintering geese continue to use natural wetlands and fail to exploit the riches of the modern agricultural habitats (Yu et al., 2017; Zhao et al., 2018). Because of high human disturbance, Siberian cranes spent twice as much time alert in agricultural fields (25.02%) than in natural wetlands (11.94%; Shao et al., 2018). As foraging habitat quality greatly influences waterbird behavior, energy accumulation, breeding success, and population dynamics (Burnham et al., 2017; Newton, 2004; Tinkler et al., 2009), research should focus on the impacts of agricultural feeding on Siberian Crane fitness to provide a scientific underpinning for conservation.
5. CONCLUDING REMARKS
In conclusion, our study, using long‐term data, suggests that Siberian cranes shifted foraging habitats from natural wetlands to agricultural fields in the winters of 2015–2016. Tuber disappearance was likely a key driver of this shift. High dry season water levels might also have contributed to the habitat shift, but to a less extent than tuber disappearance. The submerged plants at Poyang Lake have degraded seriously in the past two decades. The plant degradation at Shahu Lake was likely driven by high spring water level, high winter temperature, and low summer temperature. The subtle effects of water level and temperature on tuber abundance at Dahuchi Lake indicated that, besides water level and temperature, other factors might also have contributed to the submerged plant degradation at Poyang Lake. Because Poyang Lake is an important refuge for many waterbirds in the Yangtze River floodplain (Wang et al., 2017), effective conservation measures are needed to restore its ecosystem health. Agricultural fields are important refuges of Siberian cranes, buffering the negative impacts of wetland degradation. However, because the human disturbance in agricultural fields is high, further research is needed to evaluate the impacts of agricultural feeding on Siberian Crane fitness.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Jinjin Hou: Data curation (equal); Writing‐original draft (equal). Yifei Liu: Formal analysis (equal); Writing‐original draft (equal). James D. Fraser: Conceptualization (equal); Writing‐review & editing (equal). Lei Li: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Bin Zhao: Writing‐review & editing (equal). Zhichun Lan: Formal analysis (equal); Writing‐review & editing (equal). Jiefeng Jin: Investigation (equal). Guanhua Liu: Investigation (equal). Nianhua Dai: Writing‐review & editing (equal). Wenjuan Wang: Conceptualization (equal); Funding acquisition (equal); Visualization (equal); Writing‐original draft (equal).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the Poyang Lake National Nature Reserve, the International Crane Foundation, the Xingzi Hydrological Station, and the Duchang Weather Station for permitting the use of data, Tianlong Cai, Chunlan Zhang, Pengpeng Dou, Dunmei Lin, Dan Gibson, and Daniel H. Catlin for assisting in statistical analyses, and Jeb Barzen and James Burnham for their comments on the manuscript. This work was supported by the National Natural Science Foundation of China (No. 31772480, 31401969), the Natural Science Foundation of Jiangxi Province (No. 20181BAB214007), and Funding Project of Jiangxi Academy of Sciences (No. 2020‐YGY‐01).
Hou J, Liu Y, Fraser JD, et al. Drivers of a habitat shift by critically endangered Siberian cranes: Evidence from long‐term data. Ecol Evol. 2020;10:11055–11068. 10.1002/ece3.6720
DATA AVAILABILITY STATEMENT
All data used in this paper are included in the manuscript.
REFERENCES
- Aharon‐Rotman, Y. , McEvoy, J. , Zhaoju, Z. , Yu, H. , Wang, X. , Si, Y. , … Fox, A. D. (2017). Water level affects availability of optimal feeding habitats for threatened migratory waterbirds. Ecology and Evolution, 7, 10440–10450. 10.1002/ece3.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J. C. , Alonso, J. A. , & Bautista, L. M. (1994). Carrying capacity of staging areas and facultative migration extension in common cranes. Journal of Applied Ecology, 31, 212–222. 10.2307/2404537 [DOI] [Google Scholar]
- Amano, T. (2009). Conserving bird species in Japanese farmland: Past achievements and future challenges. Biological Conservation, 142, 1913–1921. 10.1016/j.biocon.2008.12.025 [DOI] [Google Scholar]
- Arnold, T. W. (2010). Uninformative parameters and model selection using Akaike's Information Criterion. Journal of Wildlife Management, 74, 1175–1178. 10.2193/2009-367 [DOI] [Google Scholar]
- Barko, J. W. , & Smart, R. M. (1981). Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecological Monographs, 51, 219–236. 10.2307/2937264 [DOI] [Google Scholar]
- Barter, M. , Cao, L. , Chen, L. , & Lei, G. (2005). Results of a survey for waterbirds in the lower Yangtze floodplain, China, in January–February 2004. Forktail, 21, 1–7. [Google Scholar]
- Bartleson, R. D. , Hunt, M. J. , & Doering, P. H. (2014). Effects of temperature on growth of Vallisneria americana in a sub‐tropical estuarine environment. Wetlands Ecology and Management, 22, 571–583. 10.1007/s11273-014-9354-6 [DOI] [Google Scholar]
- Barzen, J. (2012). Studying and understanding wetland dynamics In Li F., Liu G., Wu J., Zeng N., Harris J., & Jin J. (Eds.), Ecological study of wetlands and waterbirds at Poyang Lake (pp. 256–266). Beijing, China: Popular Science Press. [Google Scholar]
- Bellio, M. G. , Kingsford, R. T. , & Kotagama, S. W. (2009). Natural versus artificial‐ wetlands and their waterbirds in Sri Lanka. Biological Conservation, 142, 3076–3085. 10.1016/j.biocon.2009.08.007 [DOI] [Google Scholar]
- Blomqvist, S. , Holmgren, N. , Åkesson, S. , Hedenström, A. , & Pettersson, J. (2002). Indirect effects of lemming cycles on sandpiper dynamics: 50 years of counts from southern Sweden. Oecologia, 133, 146–158. 10.1007/s00442-002-1017-2 [DOI] [PubMed] [Google Scholar]
- Bornette, G. , & Puijalon, S. (2011). Response of aquatic plants to abiotic factors: A review. Aquatic Sciences, 73, 1–14. 10.1007/s00027-010-0162-7 [DOI] [Google Scholar]
- Burnham, J. , Barzen, J. , Pidgeon, A. M. , Sun, B. , Wu, J. , Liu, G. , & Jiang, H. (2017). Novel foraging by wintering Siberian cranes Leucogeranus leucogeranus at China’s Poyang Lake indicates broader changes in the ecosystem and raises new challenges for a critically endangered species. Bird Conservation International, 27, 204–223. 10.1017/S0959270916000150 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). New York, NY: Springer. [Google Scholar]
- Cao, Y. , Li, W. , & Jeppesen, E. (2014). The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: A microcosm approach. Hydrobiologia, 738, 49–59. 10.1007/s10750-014-1914-5 [DOI] [Google Scholar]
- Carreira, B. M. , Dias, M. P. , & Rebelo, R. (2014). How consumption and fragmentation of macrophytes by the invasive crayfish Procambarus clarkii shape the macrophyte communities of temporary ponds. Hydrobiologia, 721, 89–98. 10.1007/s10750-013-1651-1 [DOI] [Google Scholar]
- Catlin, D. H. , Fraser, J. D. , & Felio, J. H. (2015). Demographic responses of piping plovers to habitat creation on the Missouri River. Wildlife Monographs, 192, 1–42. 10.1002/wmon.1016 [DOI] [Google Scholar]
- Chen, B. , Cui, P. , Liu, G. , Li, F. , Wu, X. , Wu, J. , … Xu, H. (2014). Relationships between changing water levels and numbers of wintering tuber‐eating birds in Poyang Lake National Nature Reserve. Journal of Lake Science, 26, 243–252 (In Chinese). [Google Scholar]
- Choi, C.‐Y. , Takekawa, J. Y. , Xiong, Y. , Liu, Y. , Wikelski, M. , Heine, G. , … Xiao, X. (2016). Tracking domestic ducks: A novel approach for documenting poultry market chains in the context of avian influenza transmission. Journal of Integrative Agriculture, 15, 1584–1594. 10.1016/S2095-3119(15)61292-8 [DOI] [Google Scholar]
- Clausen, K. K. , Clausen, P. , Fælled, C. C. , & Mouritsen, K. N. (2012). Energetic consequences of a major change in habitat use: Endangered Brent Geese Branta bernicla hrota losing their main food resource. Ibis, 154, 803–814. 10.1111/j.1474-919X.2012.01265.x [DOI] [Google Scholar]
- Czech, H. A. , & Parsons, K. C. (2002). Agricultural wetlands and waterbirds: A review. Waterbirds, 25, 56–65. [Google Scholar]
- Faragó, S. , & Hangya, K. (2012). Effects of water level on waterbird abundance and diversity along the middle section of the Danube River. Hydrobiologia, 697, 15–21. 10.1007/s10750-012-1166-1 [DOI] [Google Scholar]
- Fasola, M. , Rubolini, D. , Merli, E. , Boncompagni, E. , & Bressan, U. (2010). Long‐term trends of heron and egret populations in Italy, and the effects of climate, human‐induced mortality, and habitat on population dynamics. Population Ecology, 52, 59–72. 10.1007/s10144-009-0165-1 [DOI] [Google Scholar]
- Feng, L. , Hu, C. , Chen, X. , Cai, X. , Tian, L. , & Gan, W. (2012). Assessment of inundation changes of Poyang Lake using MODIS observations between 2000 and 2010. Remote Sensing of Environment, 121, 80–92. 10.1016/j.rse.2012.01.014 [DOI] [Google Scholar]
- Fox, A. D. , & Abraham, K. F. (2017). Why geese benefit from the transition from natural vegetation to agriculture. Ambio, 46, 188–197. 10.1007/s13280-016-0879-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. D. , Madsen, J. , Boyd, H. , Kuijken, E. , Norriss, D. W. , Tombre, I. M. , & Stroud, D. A. (2005). Effects of agricultural change on abundance, fitness components and distribution of two arctic‐nesting goose populations. Global Change Biology, 11, 881–893. 10.1111/j.1365-2486.2005.00941.x [DOI] [Google Scholar]
- Fraser, J. D. , Karpanty, S. M. , Cohen, J. B. , & Truitt, B. R. (2013). The Red Knot (Calidris canutus rufa) decline in the western hemisphere: Is there a lemming connection? Canadian Journal of Zoology, 91, 13–16. 10.1139/cjz-2012-0233 [DOI] [Google Scholar]
- Fretwell, S. D. (1972). Populations in a seasonal environment. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- Germogenov, N. I. , Solomonov, N. G. , Pshennikov, A. E. , Degtyarev, A. G. , Sleptsov, S. M. , Egorov, N. N. , … Okoneshnikov, V. V. (2013). The ecology of the habitats, nesting, and migration of the eastern population of the Siberian crane (Grus leucogeranus Pallas, 1773). Contemporary Problems of Ecology, 6, 65–76. 10.1134/S1995425513010071 [DOI] [Google Scholar]
- Han, X. , Feng, L. , Hu, C. , & Chen, X. (2018). Wetland changes of China's largest freshwater lake and their linkage with the Three Gorges Dam. Remote Sensing of Environment, 204, 799–811. 10.1016/j.rse.2017.09.023 [DOI] [Google Scholar]
- Harris, J. , & Mirande, C. (2013). A global overview of cranes: Status, threats and conservation priorities. Chinese Birds, 4, 189–209. 10.5122/cbirds.2013.0025 [DOI] [Google Scholar]
- Hou, J. , Wang, Y. , Jin, B. , Wang, L. , & Wang, W. (2019). Food composition of Siberian cranes in agricultural fields in the Poyang Lake, China. Chinese Journal of Zoology, 32, 15–21 (In Chinese). [Google Scholar]
- Hu, Z. , & Lin, Y. (2019). Analysis of evolution process and driving factors for aquatic vegetations of Poyang Lake in 30 years. Resources and Environment in the Yangtze Basin, 28, 1947–1955 (In Chinese). [Google Scholar]
- Hu, Z. , Zhang, Z. , Liu, Y. , Ji, W. , & Ge, G. (2015). The function and significance of the shallow‐lakes in the Poyang Lake wetland ecosystem. Jiangxi Hydraulic Science & Technology, 41, 317–323 (In Chinese). 10.3969/j.issn.1004-4701.2015.05.01 [DOI] [Google Scholar]
- Huete, A. , Didan, K. , Miura, T. , Rodriguez, E. O. , Gao, X. , & Ferrieira, L. G. (2002). Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sensing of Environment, 83, 195–213. 10.1016/S0034-4257(02)00096-2 [DOI] [Google Scholar]
- Imanishi, A. , & Imanishi, J. (2014). Seed dormancy and germination traits of an endangered aquatic plantspecies, Euryale ferox Salisb. (Nymphaeaceae). Aquatic Botany, 119, 80–83. 10.1016/j.aquabot.2014.08.001 [DOI] [Google Scholar]
- IUCN . (2018). The IUCN red list of threatened species, v. 2018.1. Retrieved from http://www.iucnredlist.org [Google Scholar]
- Jia, Q. , Cao, L. , Yésou, H. , Huber, C. , & Fox, A. D. (2017). Combating aggressive macrophyte encroachment on a typical Yangtze River lake: Lessons from a long‐term remote sensing study of vegetation. Aquatic Ecology, 51, 177–189. 10.1007/s10452-016-9609-9 [DOI] [Google Scholar]
- Jia, Y. , Jiao, S. , Zhang, Y. , Zhou, Y. , Lei, G. , & Liu, G. (2013). Diet shift and its impact on foraging behavior of Siberian crane (Grus Leucogeranus) in Poyang Lake. PLoS One, 8, e65843 10.1371/journal.pone.0065843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian, M. , Jian, M. , Li, L. , Wang, S. , Yu, H. , & Yu, G. (2015). Distribution pattern of submerged pants in typical wetlands of Poyang Lake and its influencing factors of water environment. Resources and Environment in the Yangtze Basin, 24, 765–772 (In Chinese). [Google Scholar]
- Jonzen, N. , Nolet, B. A. , Santamaria, L. , & Svensson, M. G. E. (2002). Seasonal herbivory and mortality compensation in a swan‐pondweed system. Ecological Modelling, 147, 209–219. 10.1016/S0304-3800(01)00426-4 [DOI] [Google Scholar]
- Kanai, Y. , Ueta, M. , Germogenov, N. , Nagendran, M. , Mita, N. , & Higuchi, H. (2002). Migration routes and important resting areas of Siberian cranes (Grus leucogeranus) between northeastern Siberia and China as revealed by satellite tracking. Biological Conservation, 106, 339–346. 10.1016/S0006-3207(01)00259-2 [DOI] [Google Scholar]
- Kauth, P. J. , & Biber, P. D. (2014). Moisture content, temperature, and relative humidity influence seedstorage and subsequent survival and germination of Vallisneria americana seeds. Aquatic Botany, 120, 297–303. 10.1016/j.aquabot.2014.09.009 [DOI] [Google Scholar]
- Lai, X. , Jiang, J. , Yang, G. , & Lu, X. X. (2014). Should the Three Gorges Dam be blamed for the extremely low water levels in the middle‐lower Yangtze River? Hydrological Process, 28, 150–160. 10.1002/hyp.10077 [DOI] [Google Scholar]
- Lei, X. (2018). Large flock of Siberian crane recorded at farmland of Ganquanzhou, Yugan County in Jiangxi Province. China Crane News, 22, 31–32. [Google Scholar]
- Li, C. , Yang, Y. , Wang, Z. , Yang, L. , Zhang, D. , & Zhou, L. (2019). The relationship between seasonal water level fluctuation and habitat availability for wintering waterbirds at Shengjin Lake, China. Bird Conservation International, 29, 100–114. 10.1017/S0959270918000035 [DOI] [Google Scholar]
- Li, F. , Wu, J. , Harris, J. , & Burnham, J. (2012). Number and distribution of cranes wintering at Poyang Lake, China during 2011–2012. Chinese Birds, 3, 180–190. 10.5122/cbirds.2012.0027 [DOI] [Google Scholar]
- Li, L. , Lan, Z. , Chen, J. , & Song, Z. (2018). Allocation to clonal and sexual reproduction and its plasticity in Vallisneria spinulosa along a water‐depth gradient. Ecosphere, 9, e02070 10.1002/ecs2.2070 [DOI] [Google Scholar]
- Li, X. , Zhang, L. , Yang, G. , Li, H. , He, B. , Chen, Y. , & Tang, X. (2015). Impacts of human activities and climate change on the water environment of Lake Poyang Basin, China. Geoenvironmental Disasters, 2, 22 10.1186/s40677-015-0029-2 [DOI] [Google Scholar]
- Li, Y. (2018). Rice paddy use by waterbirds at Poyang Lake. China Crane News, 22, 25–26. [Google Scholar]
- Li, Y. , Qian, F. , Shan, J. , Li, J. , Yuan, F. , Miao, L. , & Xie, G. (2014). The effect of climate change on the population fluctuation of the Siberian crane in Poyang Lake. Acta Ecologica Sinica, 34, 2645–2653. 10.5846/stxb201304150715 [DOI] [Google Scholar]
- Li, Y. , Qian, F. , Silbernagel, J. , & Larson, H. (2019). Community structure, abundance variation and population trends of waterbirds in relation to water level fluctuation in Poyang Lake. Journal of Great Lakes Research., 45, 976–985. 10.1016/j.jglr.2019.08.002 [DOI] [Google Scholar]
- Li, Y. , Tao, H. , Yao, J. , & Zhang, Q. (2016). Application of a distributed catchment model to investigate hydrological impacts of climate change within Poyang Lake catchment (China). Hydrology Research, 47, 120–135. 10.2166/nh.2016.234 [DOI] [Google Scholar]
- Liu, G. , Wu, J. , Jin, J. , Wen, S. , He, S. , & Cao, R. (2013). Number and distribution of wintering waterbirds at Poyang Lake In Liu G., & Jin J. (Eds.), 2012–2013 Survey reports of natural resources at the Poyang Lake National Nature Reserve (pp. 110–118). Shanghai, China: Fudan University Press. [Google Scholar]
- Ma, Z. , Cai, Y. , Li, B. , & Chen, J. (2010). Managing wetland habitats for waterbirds: An international perspective. Wetlands, 30, 15–27. 10.1007/s13157-009-0001-6 [DOI] [Google Scholar]
- Meine, C. , & Archibald, G. (1996). The cranes: Status survey and conservation action plan. Gland, Switzerland: IUCN. [Google Scholar]
- Min, Q. (1995). On the regularites of water level fluctuations in Poyang Lake. Journal of Lake Science, 7, 281–288 (In Chinese). [Google Scholar]
- Min, Q. (2007). Quantitative distinguish and variation characteristic of droughts in Poyang Lake region. Water Resources Research, 28, 5–7 (In Chinese). [Google Scholar]
- Moravcová, L. , Zákravský, P. , & Hroudová, Z. (2001). Germination and seedling establishment in Alisma gramineum, A. plantago‐aquatica and A. lanceolatum under different environmental conditions. Folia Geobotanica, 36, 131–146. 10.1007/BF02803158 [DOI] [Google Scholar]
- Newton, I. (1980). The role of food in limiting bird numbers. Ardea, 68, 11–30. 10.5253/arde.v68.p11 [DOI] [Google Scholar]
- Newton, I. (2004). Population limitation in migrants. Ibis, 146, 197–226. 10.1111/j.1474-919X.2004.00293.x [DOI] [Google Scholar]
- Nishihiro, J. , & Washitani, I. (2009). Quantitative evaluation of water‐level effects on “regeneration safe‐sites” for lakeshore plants in Lake Kasumigaura, Japan. Lake and Reservior Management, 25, 217–223. 10.1080/07438140902938332 [DOI] [Google Scholar]
- Nolet, B. A. , Bevan, R. M. , Klaassen, M. , Langevoord, O. , & der Heijden, Y. G. J. T. V. (2002). Habitat switching by Bewick’s swans: Maximization of average long‐term energy gain? Journal of Animal Ecology, 71, 979–993. 10.1046/j.1365-2656.2002.00662.x [DOI] [Google Scholar]
- Nolet, B. A. , Langevoord, O. , Bevan, R. M. , Engelaar, K. R. , Klaassen, M. , Mulder, J. W. , & Dijk, S. V. (2001). Spatial variation in tuber depletion by swans explained by differences in net intake rates. Ecology, 82, 1655–1667. [Google Scholar]
- Nowald, G. (2012). Cranes and people: Agriculture and tourism In Harris J. (Ed.), Cranes, agriculture and climate change (pp. 60–64). Baraboo, WI: International Crane Foundation. [Google Scholar]
- O’brien, R. M. (2007). A caution regarding rules of thumb for variance inflation factors. Quality & Quantity, 41, 673–690. 10.1007/s11135-006-9018-6 [DOI] [Google Scholar]
- Paillisson, J. M. , & Marion, L. (2011). Water level fluctuations for managing excessive plant biomass in shallow lakes. Ecological Engineering, 37, 241–247. 10.1016/j.ecoleng.2010.11.017 [DOI] [Google Scholar]
- Rooney, N. , & Kalff, J. (2000). Inter‐annual variation in submerged macrophyte community biomass and distribution: The influence of temperature and lake morphometry. Aquatic Botany, 68, 321–335. 10.1016/S0304-3770(00)00126-1 [DOI] [Google Scholar]
- Roshier, D. A. , Robertson, A. I. , & Kingsford, R. T. (2002). Responses of waterbirds to flooding in an arid region of Australia and implications for conservation. Biological Conservation, 106, 399–411. 10.1016/S0006-3207(01)00268-3 [DOI] [Google Scholar]
- Schomaker, M. , & Heumann, C. (2014). Model selection and model averaging after multiple imputation. Computational Statistics and Data Analysis, 71, 758–770. 10.1016/j.csda.2013.02.017 [DOI] [Google Scholar]
- Schütz, W. (2000). Ecology of seed dormancy and germination in sedges (Carex). Perspectives in Plant Ecology, Evolution and Systematic, 3, 67–89. 10.1078/1433-8319-00005 [DOI] [Google Scholar]
- Shankman, D. , Keim, B. D. , & Song, J. (2006). Flood frequency in China’s Poyang Lake region: Trends and teleconnections. International Journal of Climatology, 26, 1255–1266. 10.1002/joc.1307 [DOI] [Google Scholar]
- Shao, M. , Gong, H. , Dai, N. , Zhi, Y. , Xu, N. , & Lu, P. (2018). Study on time budgets and behavioral rhythm of wintering Siberian cranes in a lotus pond reclamation area in Poyang Lake. Acta Ecologica Sinica, 38, 5206–5212 (In Chinese). [Google Scholar]
- Smart, J. , & Gill, J. A. (2003). Non‐intertidal habitat use by shorebirds: A reflection of inadequate intertidal resources? Biological Conservation, 111, 359–369. 10.1016/S0006-3207(02)00304-X [DOI] [Google Scholar]
- Søndergaard, M. , Phillips, G. , Hellsten, S. , Kolada, A. , Ecke, F. , Mäemets, H. , … Oggioni, A. (2013). Maximum growing depth of submerged macrophytes in European lakes. Hydrobiologia, 704, 165–177. 10.1007/s10750-012-1389-1 [DOI] [Google Scholar]
- Sponberg, A. F. , & Lodge, D. M. (2005). Seasonal belowground herbivory and a density refuge from waterfowl herbivory for Vallisneria americana . Ecology, 86, 2127–2134. 10.1890/04-1335 [DOI] [Google Scholar]
- Sun, S. , Chen, H. , Ju, W. , Yu, M. , Hua, W. , & Yin, Y. (2014). On the attribution of the changing hydrological cycle in Poyang Lake Basin, China. Journal of Hydrology, 514, 214–225. 10.1016/j.jhydrol.2014.04.013 [DOI] [Google Scholar]
- Tinkler, E. , Montgomery, W. I. , & Elwood, R. W. (2009). Foraging ecology, fluctuating food availability and energetics of wintering Brent geese. Journal of Zoology, 278, 313–323. 10.1111/j.1469-7998.2009.00578.x [DOI] [Google Scholar]
- Toral, G. M. , & Figuerola, J. (2010). Unraveling the importance of rice fields for waterbird populations in Europe. Biodiversity and Conservertion, 19, 3459–3469. 10.1007/s10531-010-9907-9 [DOI] [Google Scholar]
- Tucker, C. J. (1979). Red and photographic infrared linear combinations for monitoring vegetation. Remote Sensing of Environment, 8, 127–150. 10.1016/0034-4257(79)90013-0 [DOI] [Google Scholar]
- Van Wijk, R. J. (1989). Ecological studies on Potamogeton pectinatus L. III. Reproductive strategies and germination ecology. Aquatic Botany, 33, 271–299. 10.1016/0304-3770(89)90042-9 [DOI] [Google Scholar]
- Wang, Q. , Zhang, M. , Wang, S. , Ma, Q. , & Sun, M. (2014). Changes in temperature extremes in the Yangtze River Basin, 1962–2011. Journal of Geographical Sciences, 24, 59–75. 10.1007/s11442-014-1073-7 [DOI] [Google Scholar]
- Wang, S. , Liu, Z. , Fang, H. , & Feng, M. (2014). The ecological safety of Poyang Lake (pp. 109–124). Beijing, China: Science Press. [Google Scholar]
- Wang, W. , Fraser, J. D. , & Chen, J. (2017). Wintering waterbirds in the middle and lower Yangtze River floodplain: Changes in abundance and distribution. Bird Conservation International, 27, 167–186. 10.1017/S0959270915000398 [DOI] [Google Scholar]
- Wang, W. , Fraser, J. D. , & Chen, J. (2019). Distribution and long‐term population trends of wintering waterbirds in Poyang Lake, China. Wetlands, 39, S125–S135. 10.1007/s13157-017-0981-6 [DOI] [Google Scholar]
- Wang, W. , Wang, L. , & Hou, J. (2019). Man‐made habitat have become important foraging areas of Siberian cranes. Chinese Journal of Wildlife, 40, 133–137 (In Chinese). [Google Scholar]
- Wang, W. , Wang, Y. , Hou, J. , & Ouyang, S. (2019). Flooding influences waterbird abundance at Poyang Lake, China. Waterbirds, 42, 30–38. 10.1675/063.042.0104 [DOI] [Google Scholar]
- Wang, X. , Fox, A. D. , Cong, P. , & Cao, L. (2013). Food constraints explain the restricted distribution of wintering Lesser White‐fronted Geese Anser erythropus in China. Ibis, 155, 576–592. 10.1111/ibi.12039 [DOI] [Google Scholar]
- Wang, X. , Xiao, X. , Zou, Z. , Hou, L. , Qin, Y. , Dong, J. , … Li, B. O. (2020). Mapping coastal wetlands of China using time series Landsat images in 2018 and Google Earth Engine. ISPRS Journal of Photogrammetry and Remote Sensing, 163, 312–326. 10.1016/j.isprsjprs.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. , He, F. , Xu, D. , Zhou, Q. , Xiao, E. , Zhang, L. , & Wu, Z. (2018). A comparison of the growth and photosynthetic response of Vallisneria natans (Lour.) Hara to a long‐term water depth gradient under flowing and static water. Journal of Freshwater Ecology, 33, 223–237. 10.1080/02705060.2018.1432509 [DOI] [Google Scholar]
- Wu, G. , De Leeuw, J. , Skidmore, A. K. , Prins, H. H. , Best, E. P. H. , & Liu, Y. (2009). Will the Three Goreges Dam affect the underwter light climate of Vallisneria spiralis L. and food habitat of Siberian crane in Poyang Lake? Hydrobiologia, 623, 213–222. 10.1007/s10750-008-9659-7 [DOI] [Google Scholar]
- Wu, J. , Li, F. , & Burnham, J. (2013). Numerical distribution of Siberian cranes and their relation to food and water depth in Sha Hu at Poyang Lake, China. Wetland Science, 11, 305–312 (In Chinese). [Google Scholar]
- Wu, J. , Li, F. , Liu, G. , & Zeng, N. (2012). Monitoring winter buds of Vallisneria In Li F., Liu G., & Wu J. (Eds.), Ecological study of wetlands and waterbirds at Poyang Lake (pp. 37–45). Beijing, China: Popular Science Press. [Google Scholar]
- Wu, Y. , & Ji, W. (2002). Study on Jiangxi Poyang Lake National Nature Reserve. Beijing, China: China Forestry Publishing House. [Google Scholar]
- Xia, S. , Yu, X. , & Fan, N. (2010). The wintering habitat of migrant birds and their relationship with water level in Poyang Lake, China. Resources Science, 32, 2072–2078 (In Chinese). [Google Scholar]
- Xiao, C. , Xing, W. , & Liu, G. (2010). Seed germination of 14 wetland species in response to duration of cold‐wet stratification and outdoor burial depth. Aquatic Biology, 11, 169–177. 10.3354/ab00300 [DOI] [Google Scholar]
- Xiao, K. , Yu, D. , & Wu, Z. (2007). Differential effects of water depth and sediment type on clonal growth of the submersed macrophyte Vallisneria natans . Hydrobiologia, 589, 265–272. 10.1007/s10750-007-0740-4 [DOI] [Google Scholar]
- Xiao, X. , Boles, S. , Liu, J. , Zhuang, D. , Frolking, S. , Li, C. , … Moore, B. (2005). Mapping paddy rice agriculture in southern China using multi‐temporal MODIS images. Remote Sensing of Environment, 95, 480–492. 10.1016/j.rse.2004.12.009 [DOI] [Google Scholar]
- Xing, Y. , & Wang, D. (2016). Research progress on surveillance related to avian influenza activity in the Poyang Lake region of China. Chinese Journal of Zoonoses, 32, 388–391 (In Chinese). 10.3969/j.issn.1002-2694.2016.04.014 [DOI] [Google Scholar]
- Xu, H. (2006). Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. International Journal of Remote Sensing, 27, 3025–3033. 10.1080/01431160600589179 [DOI] [Google Scholar]
- Xu, W. , Hu, W. , Deng, J. , Zhu, J. , Zhou, N. , & Liu, X. (2016). Impacts of water depth and substrate type on Vallisneria natans atwave‐exposed and sheltered sites in a eutrophic large lake. Ecological Engineering, 97, 344–354. 10.1016/j.ecoleng.2016.10.029 [DOI] [Google Scholar]
- Xu, Y. , Xu, C. , Gao, X. , & Luo, Y. (2009). Projected changes in temperature and precipitation extremes over the Yangtze River Basin of China in the 21st century. Quaternary International, 208, 44–52. 10.1016/j.quaint.2008.12.020 [DOI] [Google Scholar]
- Yasué, M. , Patterson, A. , & Dearden, P. (2007). Are saltflats suitable supplementary nesting habitats for Malaysian Plovers Charadrius peronii threatened by beach habitat loss in Thailand. Bird Conservation International, 17, 211–223. 10.1017/S0959270907000780 [DOI] [Google Scholar]
- Ye, X. , Zhang, Q. , Bai, L. , & Hu, Q. (2011). A modeling study of catchment discharge to Poyang Lake under future climate in China. Quaternary International, 244, 221–229. 10.1016/j.quaint.2010.07.004 [DOI] [Google Scholar]
- Ye, X. , Zhang, Q. , Liu, J. , Li, X. , & Xu, C. (2013). Distinguishing the relative impacts of climate change and human activities on variation of streamflow in the Poyang Lake catchment, China. Journal of Hydrology, 494, 83–95. 10.1016/j.jhydrol.2013.04.036 [DOI] [Google Scholar]
- Yin, L. , Wang, C. , Chen, Y. , Yu, C. , Cheng, Y. , & Li, W. (2009). Cold stratification, light and high seed density enhance the germination of Ottelia alismoides . Aquatic Botany, 90, 85–88. 10.1016/j.aquabot.2008.05.002 [DOI] [Google Scholar]
- Yu, H. , Wang, X. , Cao, L. , Zhang, L. U. , Jia, Q. , Lee, H. , … Fox, A. D. (2017). Are declining populations of wild geese in China 'prisoners' of their natural habitats? Current Biology, 27, R376–R377. 10.1016/j.cub.2017.04.037 [DOI] [PubMed] [Google Scholar]
- Yuan, L. , Li, S. , & Li, W. (2013). The effects of water level fluctuation on the winter bud formation of submerged macrophyte Vallisneria spinulosa . Journal of Jiangxi Normal University (Natural Science), 37, 355–358 (In Chinese). [Google Scholar]
- Zeng, L. , Schmitt, M. , Li, L. , & Zhu, X. (2017). Analysing changes of the Poyang Lake water area using Sentinel‐1 synthetic aperture radar imagery. International Journal of Remote Sensing, 38, 7041–7069. 10.1080/01431161.2017.1370151 [DOI] [Google Scholar]
- Zhang, X. , Jin, B. , Chen, J. , Wu, J. , Liu, G. , & Ma, Z. (2014). Relationship between habitat use of four waterbird species and water depth and food resource in Poyang Lake. Chinese Journal of Zoology, 49, 657–665 (In Chinese). 10.13859/j.cjz.201405004 [DOI] [Google Scholar]
- Zhao, Q. , Wang, X. , Cao, L. , & Fox, A. D. (2018). Why Chinese wintering geese hesitate to exploit farmland. Ibis, 160, 703–705. 10.1111/ibi.12605 [DOI] [Google Scholar]
- Zhao, S. , Zhang, R. , Liu, Y. , Yin, L. , Wang, C. , & Li, W. (2017). The effect of storage condition on seed germination of six Hydrocharitaceae and Potamogetonaceae species. Aquatic Botany, 143, 49–53. 10.1016/j.aquabot.2017.09.005 [DOI] [Google Scholar]
- Zheng, M. , Zhou, L. , Zhao, N. , & Xu, W. (2015). Effects of variation in food resources on foraging habitat use by wintering Hooded Cranes (Grus monacha). Avian Research, 6, 11 10.1186/s40657-015-0020-3 [DOI] [Google Scholar]
- Zou, J. , Wang, Y. , Zhong, Y. , Zhu, C. , Zhang, X. , Shi, L. , … Zhou, X. (2014). Current distribuiton of Procambarus clarkii (Crustacea, Decapoda, Cambaridae) in Poyang Lake and the preliminary morphological cluster analysis of different populaitons. Resources and Environment in the Yangtze Basin, 23, 415–421 (In Chinese). 10.11870/cjlyzyyhj201403016 [DOI] [Google Scholar]
- Zou, Z. , Dong, J. , Menarguez, M. A. , Xiao, X. , Qin, Y. , Doughty, R. B. , … David Hambright, K. (2017). Continued decrease of open surface water body area in Oklahoma during 1984–2015. Science of the Total Environment, 595, 451–460. 10.1016/j.scitotenv.2017.03.259 [DOI] [PubMed] [Google Scholar]
- Zou, Z. , Xiao, X. , Dong, J. , Qin, Y. , Doughty, R. B. , Menarguez, M. A. , … Wang, J. (2018). Divergent trends of open‐surface water body area in the contiguous United States from 1984 to 2016. Proceedings of the National Academy of Sciences of the United States of America, 115(15), 3810–3815. 10.1073/pnas.1719275115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data used in this paper are included in the manuscript.


