Abstract
Objective
Maternal embryonic leucine zipper kinase (MELK) is receiving an attention as a therapeutic target in various types of cancers. In this study, we aimed to evaluate the prognostic significance of MELK expression in ovarian cancer using clinical samples, and assessed the efficacy of a small molecule MELK inhibitor, OTS167, using patient-derived ovarian cancer cells as well as cell lines.
Methods
Expression levels of MELK in 11 ovarian cancer cell lines were confirmed by western blotting. Inhibitory concentration of OTS167 was determined by colorimetric assay. MELK messenger RNA (mRNA) expression was evaluated in 228 ovarian cancer patients by quantitative polymerase chain reaction. Growth inhibition of OTS167 was also evaluated using freshly-isolated primary ovarian cancer cells including spheroid formation condition.
Results
MELK mRNA expression was significantly higher in ovarian cancer than in normal ovaries (p<0.001), and high MELK mRNA expression was observed in patients with advanced stage, positive ascites cytology and residual tumor size. Patients with high MELK mRNA expression showed shorter progression-free survival (p=0.001). Expression of MELK was also confirmed in 10 of 11 ovarian cancer cell lines tested, and the half maximal inhibitory concentration of MELK inhibitor, OTS167, ranged from 9.3 to 60 nM. Additionally, OTS167 showed significant growth inhibitory effect against patient-derived ovarian cancer cells, regardless of their tumor locations, histologic subtypes and stages.
Conclusions
We demonstrated MELK as both a prognostic marker and a therapeutic target for ovarian cancer using clinical ovarian cancer samples. MELK inhibition by OTS167 may be an effective approach to treat ovarian cancer patients.
Keywords: Ovarian Cancer, MELK, Prognostic Marker, Therapeutic Target
INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer-related deaths in women [1]. Diagnosis of this malignancy tends to be delayed because its symptoms are vague and often similar to those of other conditions. In fact, more than 60% of patients with ovarian cancer are diagnosed at advanced stages with a poor prognosis [2].
Maternal embryonic leucine zipper kinase (MELK) is a member of the AMPK serine/threonine kinase family and is involved in mammalian embryonic development [3]. MELK is initially implicated in the cell cycle and its messenger RNA (mRNA) levels are elevated during mitosis [4]. Moreover, MELK is not expressed in normal vital organs [5], implying the potential of MELK to be a target for anticancer therapeutics. Through high-throughput compound library screening and subsequent extensive structure-activity relationship studies, we previously developed a small molecule named OTS167 that selectively inhibits MELK kinase activity [6]. The growth suppressive effect of OTS167 was demonstrated in specific cancer cell lines originated from breast cancer, small cell lung cancer, kidney cancers and acute myeloid leukemia [6,7,8,9]. Regarding ovarian cancer, MELK expression was found in 13% of ovarian cancer tissue samples [10]. Since MELK works as markers for tumor aggressiveness, progression and prognostic predictor in various types of cancers [10,11,12,13,14,15,16,17,18], we thought MELK must have an essential role in tumorigenesis and can be a potential target for ovarian cancer treatments. In fact, previously, Kohler et al. [11] focused on the prognostic significance of mRNA expression of MELK in ovarian cancer by bioinformatics analysis using publicly available databases. They also confirmed the cytotoxic effect of OTS167 on ovarian cancer cell lines. Although their results lack the validation in clinical samples, the study strongly implicated the potential of MELK as a therapeutic target against ovarian cancer.
In this study, we first aimed to describe the clinical significance of MELK using surgical samples, then explored the therapeutic efficacy of MELK inhibitor, OTS167, using patient-derived ovarian cancer cells.
MATERIALS AND METHODS
1. Cell lines and western blotting
Human ovarian cancer cell lines, ES-2, OVCAR3, OV-90, PA-1, SKOV-3, SW626, and CaOV3 were purchased from American Type Culture Collection (Rockville, MD, USA). RMG-I, OVSAHO, OVTOKO were purchased from JCRB cell bank (Osaka, Japan) and A2780 was obtained from the European Collection of Authenticated Cell Cultures. Cell lines were cultured according to the recommendations of their respective depositors. Western blotting was performed as described previously [6,19]. Anti-MELK antibody (previously developed [20]) and anti-β-actin antibody (Cell Signaling) were used as primary antibodies. Knockdown and evaluation of MELK using small interfering RNA (siRNA) was examined following to previous study [21].
2. Cell viability assay using ovarian cancer cell lines
In vitro cell viability assay was performed as described previously [22]. Briefly, ovarian cancer cells were plated in 96-well plates (100 μL each) at a density expected to show linear growth (ES-2, 1,000 cells; OVCAR3, 5,000 cells; OV90, 4,000 cells; OVSAHO, 6,000 cells; OVTOKO, 2,000 cells; PA-1, 2,000 cells; RMG-1, 6,000 cells; SKOV3, 2,000 cells; SW626, 4,000 cells; A2780, 3,000 cells; and CaOV3, 5,000 cells). The cells were allowed to adhere overnight before exposure to OTS167, a MELK inhibitor provided by OncoTherapy Science, Inc. (Kawasaki, Japan) for 72 hours at 37°C. For the methyl thiazolyl tetrazolium assay, a cell counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added in the 96-well plate that was read at a wavelength of 450 nm using the SpectraMax 190 (Molecular Devices) after incubation for one hour. All assays were carried out in triplicates.
3. Clinical tumor samples
This study was reviewed and approved by the Institutional Review Board of Saitama Medical University International Medical Center (No.12-096), and all processes performed in accordance with the relevant guidelines and regulations. The clinical and histopathological information was obtained from clinical charts and pathological reports. Patients were basically operated within 6 weeks from the first visit to our hospital. Clinical stage of the tumor was reviewed based on the International Federation of Gynecology and Obstetrics staging system published in 2014 [23]. Clinical stage was decided by the surgical findings in cases with primary surgery. The stage of the cases without staging laparotomy including lymph node metastasis was categorized based on the computer tomography findings. Recurrence cases who had a disease progression within 6 months of last dose of platinum were considered as platinum-resistant. Tumor tissues for real-time polymerase chain reaction (PCR) were obtained by surgical resection from 228 patients with epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Patient-derived ovarian cancer cells were isolated as follows; surgical tissue samples were cut into pieces of less than 1 mm then gently pipetted with pre-warmed 0.25% trypsin-EDTA for 3 minutes. The tissues were resuspended in 10 mL of cold HBSS with 2% FBS. After centrifugation for 5 minutes at 1,500 rpm, the supernatant was removed as much as possible. Subsequently, samples were treated with 2 mL of pre-warmed dispase (STEMCELL Technologies, Vancouver, Canada) and 200 μL of DNase I (STEMCELL Technologies), followed by general pipetting for 2 minutes, resuspending in 10 mL of HBSS with 2% FBS, and filtration through 100 μm, 70 μm, and 40 μm cell strainers (BD Falcon, Bedford, MA, USA). Next, single suspension cells were centrifuged for 5 minutes at 1,500 rpm and counted. Finally, ovarian cancer cells were magnetically isolated from those cells using EasySep™ Human EpCAM Positive Selection Kit (STEMCELL Technologies) according to the manufacturer's instructions.
4. Spheroid cultures
Freshly-isolated primary ovarian cancer cells were cultured on ultra-low attachment culture dishes (Corning, New York, NY, USA) and grown in STEMPRO hESC SFM—Human Embryonic Stem Cell Culture Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 8.8 ng/mL of bFGF (Invitrogen), 20 μmol/L of Rho-associated coiled-coil forming kinase inhibitor Y-27632 (Wako, Osaka, Japan). Spheroid culture medium was changed every 2 or 3 days, and spheroid cells for serial passage were dispersed into single cells with Accumax (Innovative Cell Technologies, San Diego, CA, USA).
5. Growth inhibition assay using patient-derived cells and spheroids
Freshly-isolated primary ovarian cancer cells were seeded in 96-well collagen coated plates (5×104 cells/well) and incubated with OTS-167 at different concentrations (0, 1, 10, 40, and 100 nM) for 72 hours. The cell proliferation activity was measured using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. All assays were done in triplicates. Cells were monitored for viability with luminescence signals using RealTime-Glo™ MT Cell Viability Assay (Promega) according to the instructions (Supplementary Fig. 1). The spheroids were dispersed into single cells by Accumax, seeded in ultra-low attachment 96-well plates (2×104 cells/well) and incubated with OTS-167, then examined as above.
6. Real-time PCR using clinical samples
Total RNA (1 μg) was extracted from tumor tissue and converted into complementary DNA using ReverTra Ace (Toyobo, Osaka, Japan) with oligo (dT) 20 primer according to the manufacturer's instructions. Primers and fluorescent probes for MELK were designed using The Probe Finder software in the Universal ProbeLibrary (UPL) Assay Design Center (Roche Applied Science, Mannheim, Germany). The primers for MELK forward: 5′-caggtgtcattagccctgaga-3′, reverse: 5′-ggctccctttctttttgg-3′, and UPL Probe #25 were used. The Pre-Developed TaqMan® Assay Regents Human GAPDH (Applied Biosystems, Foster City, CA, USA) was used as an internal control. Each reaction was carried out in triplicates using LightCycler® 480 System II (Roche Diagnostics, Mannheim, Germany). The copies of MELK and GAPDH mRNA were calculated based on each calibration curve, and the expression of MELK was normalized to 1,000 copies of GAPDH.
7. Statistical analysis
The correlation between MELK mRNA expression levels and clinicopathological characteristics was analyzed using the Fisher's exact test, and prognostic values were analyzed by the Kaplan-Meier method and log rank test. Comparison in growth inhibitory effects/cytotoxicity of MELK inhibitors on patients-derived ovarian cancer cells were analyzed by the t-test (1-way). Statistical analyses were performed using JMP v11 (SAS, Cary, NC, USA) and GraphPad Prism 6 (La Jolla, CA, USA). In all tests, differences were considered to significant at p<0.05.
RESULTS
1. Expression levels of MELK in ovarian cancer cell lines, cytotoxicity of a MELK inhibitor OTS167 and knockdown of MELK using siRNA
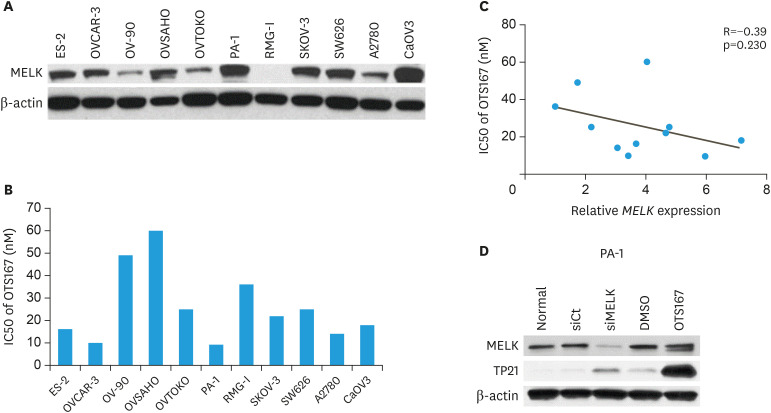
The relationship between expression levels of MELK in ovarian cancer cell lines and the cytotoxic effect of a MELK inhibitor, OTS167, was evaluated. As shown in Fig. 1A, MELK expression was observed in all ovarian cancer cell lines except for RMG-1. The cytotoxic effect of OTS167 was evaluated in all 11 cell lines using a sensitive colorimetric assay. The half maximal inhibitory concentration (IC50) of each cell line for OTS167 ranged from 9.3 to 60 nM (median 22 nM) (Fig. 2B). We observed a weak negative correlation between the expression levels of MELK and the IC50 value of OTS167 (R=−0.39, p=0.230) (Fig. 1C), suggesting that cells with a higher MELK expression are more sensitive to treatment with OTS167. Additionally, MELK expression in PA-1 cell line was silenced using siRNA. As shown in Fig. 1D, siRNA maternal embryonic leucine zipper kinase (siMELK) induced TP21 expression. Similarly, MELK inhibition by OTS167 induced TP21.
Fig. 1. Expression levels of MELK in ovarian cancer cell lines, cytotoxicity of a MELK inhibitor OTS167 and knockdown of MELK using small interfering RNA. (A) Expression level of MELK explored by western blotting. (B) IC50 values of OTS167. (C) Relationship between MELK expression and IC50 values of OTS167 in each cell line. (D) Expression level of MELK, TP21 in siMELK and OTS167.
IC50, half maximal inhibitory concentration; MELK, maternal embryonic leucine zipper kinase; siMELK, siRNA maternal embryonic leucine zipper kinase.
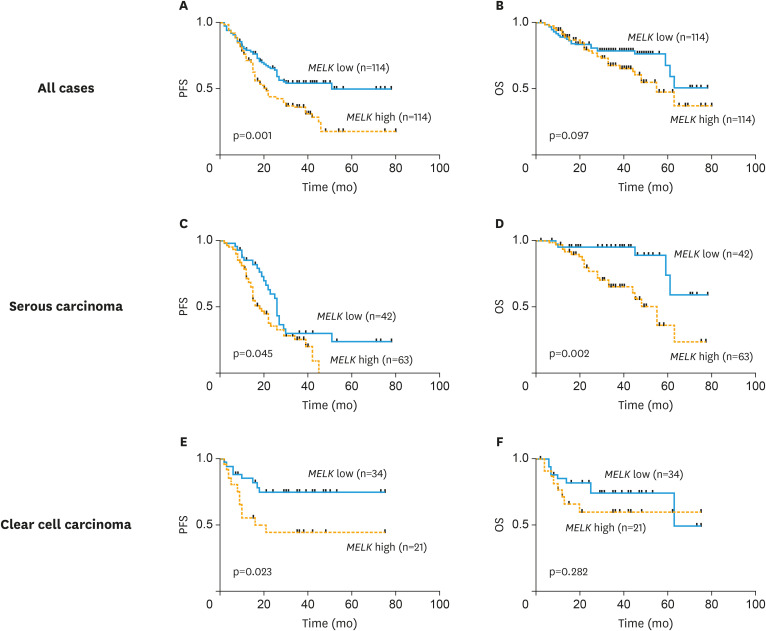
Fig. 2. Clinical significance of MELK mRNA expression in ovarian cancer. mRNA expression of MELK was explored by Kaplan-Meier methods and log rank test in (A) PFS and (B) OS in all cases, (C) PFS and (D) OS in serous carcinoma, and (E) PFS and (F) OS in clear cell carcinoma.
MELK, maternal embryonic leucine zipper kinase; mRNA, messenger RNA; OS, overall survival; PFS, progression-free survival.
2. MELK as a prognostic factor in patients with ovarian cancer
We investigated an association between MELK mRNA expression and clinicopathological features in a total of 228 ovarian cancer patients. As shown in Table 1, high MELK mRNA expression based on the median value was observed in patients with advanced stage, positive ascites cytology and residual tumor size greater than 1 cm at the time of primary surgery. We next assessed the prognostic value of MELK mRNA expression in ovarian cancer patients. Of the 228 ovarian cancer patients included in this analysis, the median follow-up duration for patients who were still alive after the initial diagnosis was 31.5 months. Patients with lower MELK mRNA expression (based on the median value of all patients) demonstrated a significantly longer progression-free survival (PFS) than those with higher MELK mRNA expression (p=0.001, Fig. 2A). A similar trend was observed in the overall survival (OS) (p=0.097, Fig. 2B). We next performed survival analyses on the subgroups by histological subtypes. As shown in Fig. 2C and D, prognostic significance was found between MELK expression and both PFS (p=0.045) and OS (p=0.002) in serous carcinoma. Similarly, significance was found for PFS (p=0.002; Fig. 2E) in clear cell carcinoma, but not for OS (p=0.282; Fig. 2F).
Table 1. Association of clinicopathological factors with mRNA expression of MELK.
| Characteristics | No. (%) | mRNA expression of MELK | ||
|---|---|---|---|---|
| Mean±SD | p-value | |||
| Age | 0.568 | |||
| ≤60 | 124 (54.4) | 17.8±25.3 | ||
| >60 | 104 (45.6) | 16.2±14.7 | ||
| Menopause | 0.635 | |||
| Yes | 157 (68.9) | 16.6±20.3 | ||
| No | 71 (31.1) | 18.1±23.0 | ||
| FIGO stage | 0.008 | |||
| I, II | 90 (39.5) | 12.5±11.2 | ||
| III, IV | 138 (60.5) | 20.0±25.2 | ||
| Primary site | 0.833 | |||
| Ovary | 198 (86.8) | 17.1±22.1 | ||
| Fallopian tube | 12 (5.3) | 13.8±5.7 | ||
| Peritoneum | 18 (7.9) | 18.4±16.7 | ||
| Histology | 0.011 | |||
| Serous | 105 (46.1) | 17.9±18.9 | ||
| Endometrioid | 40 (17.5) | 13.6±13.3 | ||
| Clear cell | 56 (24.6) | 14.2±17.1 | ||
| Mucinous | 6 (2.6) | 5.2±2.2 | ||
| Others | 21 (9.2) | 30.6±42.3 | ||
| Ascites cytology | 0.033 | |||
| Positive | 159 (69.7) | 19.0±24.3 | ||
| Negative | 69 (30.3) | 12.6±9.5 | ||
| Lymph node metastasis | 0.282 | |||
| Positive | 38 (16.7) | 20.4±26.9 | ||
| Negative | 190 (83.3) | 16.4±19.8 | ||
| Residual tumor size | 0.007 | |||
| 0 | 93 (40.8) | 12.1±10.9 | ||
| <1 cm | 13 (5.7) | 14.8±16.4 | ||
| ≥1 cm | 122 (53.5) | 21.1±26.1 | ||
| Recurrence cases | 117 | 0.873 | ||
| Platinum sensitive (PFS ≥6 mo) | 64 (54.7) | 19.1±24.5 | ||
| Platinum resistance (PFS ≤6 mo) | 53 (45.3) | 19.7±18.7 | ||
FIGO, International Federation of Gynecology and Obstetrics; MELK, maternal embryonic leucine zipper kinase; mRNA, messenger RNA; PFS, progression-free survival; SD, standard deviation.
3. Expression levels of MELK in tumor sites, pre/post-chemotherapy and primary/recurrent tumors
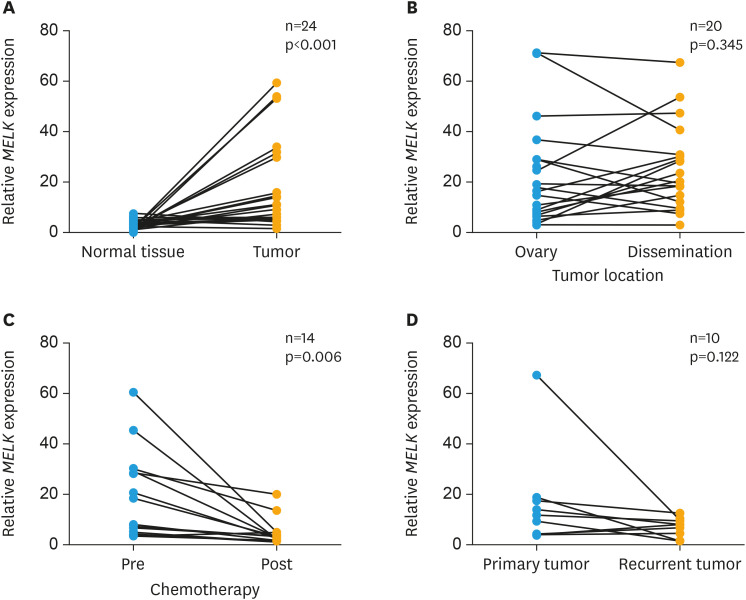
The levels of MELK mRNA expression were compared in various clinical contexts. First, the difference in MELK mRNA expression between normal ovaries and ovarian cancers was compared. As shown in Fig. 3A, level of MELK mRNA was significantly higher in ovarian cancer (p<0.001, n=24) than in normal ovaries. Next, MELK expression between primary ovarian tumor and peritoneal dissemination was compared, and no significant difference was observed (p=0.345, n=20; Fig. 3B). Then, the relationship between pre- and post-chemotherapy was examined. Since advanced ovarian cancer patients are often treated with neoadjuvant chemotherapy followed by debulking surgery, tissue samples were collected at the time of exploratory laparotomy as well as at the time of interval debulking surgery from the same patients. As shown in Fig. 3C, MELK mRNA was significantly higher in the tumors obtained pre-chemotherapy than in those post-chemotherapy (p=0.006, n=14). We evaluated MELK mRNA expression levels between the tumors at the time of primary surgery and those at recurrence from the same patients. As shown in Fig. 3D, no significant differences of MELK mRNA were observed between primary and recurrent tumors. (p=0.122, n=10).
Fig. 3. mRNA expression of MELK in different contexts in patients with ovarian cancer. mRNA expression levels of MELK were compared between (A) normal ovary and primary ovarian tumor, (B) primary ovarian tumor and dissemination, (C) pre- and post- chemotherapeutics, and (D) primary and recurrent tumors. Statistical analyses; paired t-test.
mRNA, messenger RNA.
4. Cytotoxicity of OTS167 in patient-derived primary ovarian cancer cells
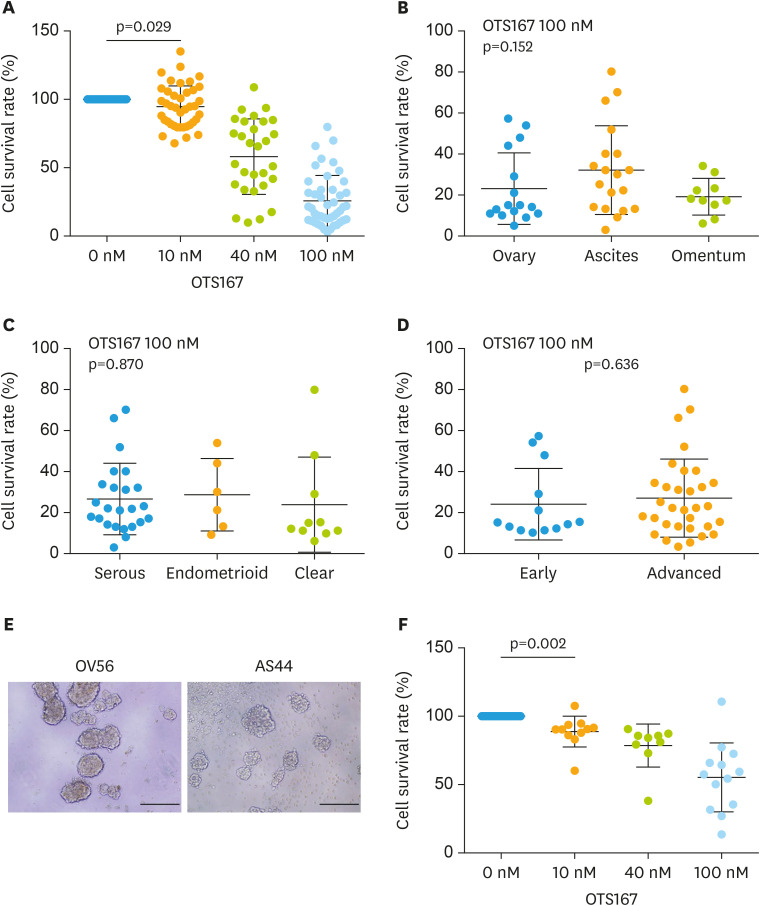
Next, we evaluated the growth inhibitory effect of OTS167 using ovarian cancer cells freshly isolated from a total of 45 patients with ovarian cancer. As shown in Fig. 4A, a strong growth inhibitory effect was induced by 10, 40, and 100 nM of OTS167 (p=0.029). Growth inhibitory effect of OTS167 was observed regardless of location, such as the ovary, ascites and omentum (p=0.152; Fig. 4B). In addition, OTS167 showed cytotoxic effect regardless of histological subtype, such as serous, endometrioid and clear cell carcinoma (p=0.870; Fig. 4C), or stage (p=0.636; Fig. 4D). To assess anchorage independent growth inhibition of OTS167, we used spheroid formation models of patient-derived ovarian cancer cells (Fig. 4E) and treated these with OTS167. A significant growth inhibition was observed even in cells able to form spheroids (Fig. 4F). Finally, we examined the correlation between MELK expression and survival rate of patient-derived primary ovarian cancer cells under the condition of 100 nM of OTS167; however, no significant correlation was observed (Supplementary Fig. 2).
Fig. 4. Evaluation of OTS167 using patient-derived ovarian cancer cells. Cytotoxicity of OTS 167 in patient-derived ovarian cancer cells was assessed at a concentration of 10 nM, 40 nM, and 100 nM, (A) cancer cells from ovary, ascites and omentum, (B) serous, endometrial and clear cell subtypes, and (C) cancer cells from the patients with early and advanced stages. (D) Cytotoxic effect of OTS167 was also assessed via spheroid formation of primary ovarian cancer cells, at a concentration of 10 nM, 40 nM, and 100 nM. Statistical analysis; (A) t-test between 0 nM and 10 nM, (B) ANOVA, (C) ANOVA, (D) t-test, (F) t-test between 0 nM and 10 nM.
ANOVA, analysis of variance.
DISCUSSION
In this study, we found that 1) MELK mRNA expression is increased in ovarian cancer especially in advanced stages, 2) High MELK mRNA expression correlates with a poor prognostic in ovarian cancer, and 3) OTS167 exhibits cytotoxicity against patient-derived ovarian cancer cells as well as cell lines.
Recently, various novel molecular target agents have been developed and many clinical studies have been conducted. However, most of these agents failed to obtain approval from the Food and Drug Administration mainly due to their toxicities [24]. To reduce toxicity, selecting a target expressed only in cancer cells but not in normal cells is crucial [25]. We previously identified MELK as a therapeutic target for various types of cancers by comparing the expression between normal and cancer tissues [5]. In addition, we conducted a high-throughput screening of a compound library followed by structure-activity relationship studies, and successfully identified a highly potent MELK inhibitor OTS167 [6].
As no appropriate antibodies for immunohistochemistry have been identified, expression of MELK was evaluated by reverse transcription PCR in the current study. We observed that MELK mRNA was highly expressed in ovarian cancers in comparison to normal ovaries. Higher MELK mRNA expression was found in cases with advanced stages compared to those in early stages, which was associated with poorer clinical outcomes. Previous reports have showed prognostic utility of MELK in various types of cancers such as hepatocellular carcinoma [13], melanoma [14], renal clear cell carcinoma [15], breast cancer [16,17], and gastric cancer [18]. These reports support our findings and indicate that MELK mRNA expression can be a marker to prognosticate clinical outcomes, suggesting that the MELK targeting strategy is a reasonable cancer treatment.
Interestingly, we observed that levels of MELK mRNA significantly decreased after chemotherapy, suggesting an association between MELK expression and chemo-sensitivity or cell proliferation. The oncogenic function of MELK is attributed to disable critical cell-cycle checkpoints and reduce replication stress [26]. Our results also showing induction of TP21 expression by OTS167 in ovarian cancer. OTS167 was known to inhibit the expression of T-lymphokine–activated killer cell-originated protein kinase (TOPK), which is a Ser/Thr protein kinase and highly activated during cell mitosis [7]. TOPK inhibition was also known to induces the TP21 expression [27]. Therefore, this might be one the reasons that the induction of TP21 expression by OTS167 was more obvious than that of siMELK in this study. One strength in this study was the use of patient-derived ovarian cancer cells for the evaluation of OTS167. Previously, Kohler et al. [11] demonstrated the effect of OTS167 in ovarian cancer cell lines. Inhibition of MELK reduced cell proliferation in both anchorage dependent and independent growth conditions in various ovarian cancer cell lines [11]. However, cancer cell lines contain artificial factors due to the frequent passages [28]. In addition, it is estimated that between 18% and 36% of cell lines may be contaminated or misidentified [29]. Therefore, patient-derived cancer cells are more biologically relevant tools than cell lines for evaluating efficacy of drugs [28]. Moreover, the use of primary cancer cells enables us to assess drug efficacy with the patients' clinicopathological characteristics. We originally thought MELK expression could be a biomarker for the efficacy of OTS167. However, a limited correlation was shown based on the cell lines and patient's derived ovarian cancer cells in our study. We observed obvious potential of OTS167 in the treatment of ovarian cancer, regardless of tumor location, histological subtype, and stage was shown in our study. Therefore, we think MELK inhibition may be effective to various subtypes of ovarian cancer. Warranted further studies to investigate the relationship between MELK expression and its antitumor activity using in vivo models.
In conclusion, we have demonstrated that MELK is a viable prognostic marker and therapeutic target in ovarian cancer. In addition, cytotoxicity of OTS167 was demonstrated using patient-derived ovarian cancer cells. As the clinical trial of OTS167 is currently ongoing in triple negative breast cancer in the U.S. (NCT02926690), the evaluation of the efficacy of OTS167 in patients with ovarian cancer is warranted.
ACKNOWLEDGMENTS
We thank Drs. Yuichi Imai and Yuri Yano for their support in sample collection during our study. We also thank Satoko Shimoyokkaichi, Akiko Iwasa and Shuhei Okabe for their excellent technical assistance, and Yo Matsuo (OncoTherapy Science, Inc.) for providing materials.
Footnotes
Funding: The work was supported in part by a Grant-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan (KH) under grant numbers 16K11152.
Conflict of Interest: N.Y. is a stock holder and a scientific advisor of OncoTherapy Science, Inc. T.M. is an employee of OncoTherapy Science, Inc.
- Conceptualization: I.Y., H.K.
- Data curation: I.Y., S.S., Y.A., S.D., O.A., M.M., Z.M., M.T., F.K., N.Y., H.K.
- Formal analysis: I.Y., S.S., H.K.
- Funding acquisition: N.Y., H.K.
- Investigation: I.Y., H.K.
- Methodology: I.Y., S.S., Y.A., S.D., O.A., M.M., Z.M., M.T., F.K., N.Y., H.K.
- Project administration: I.Y., S.S.,H.K.
- Resources: Y.A., S.D., O.A., M.M., Z.M., M.T., F.K., N.Y., H.K.
- Software: I.Y., N.Y., H.K.
- Supervision: N.Y., H.K.
- Validation: I.Y., S.S., H.K.
- Visualization: Y.A., S.D., O.A., M.M., Z.M., M.T., F.K.
- Writing - original draft: I.Y., S.S., H.K.
- Writing - review & editing: I.Y., S.S., Y.A., S.D., O.A., M.M., Z.M., M.T., F.K., N.Y., H.K.
SUPPLEMENTARY MATERIALS
A representative case of monitoring cell viability for patients-derived primary ovarian cancer cells by a luminescence-based assay.
Correlation between MELK expression and survival rate of patient derived primary ovarian cancer cells under 100 nM of OTS167.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Heyer BS, Warsowe J, Solter D, Knowles BB, Ackerman SL. New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol Reprod Dev. 1997;47:148–156. doi: 10.1002/(SICI)1098-2795(199706)47:2<148::AID-MRD4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J Cell Biol. 2005;170:413–427. doi: 10.1083/jcb.200412115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin ML, Park JH, Nishidate T, Nakamura Y, Katagiri T. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res. 2007;9:R17. doi: 10.1186/bcr1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung S, Suzuki H, Miyamoto T, Takamatsu N, Tatsuguchi A, Ueda K, et al. Development of an orally-administrative MELK-targeting inhibitor that suppresses the growth of various types of human cancer. Oncotarget. 2012;3:1629–1640. doi: 10.18632/oncotarget.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H, Kato T, Olugbile S, Tamura K, Chung S, Miyamoto T, et al. Effective growth-suppressive activity of maternal embryonic leucine-zipper kinase (MELK) inhibitor against small cell lung cancer. Oncotarget. 2016;7:13621–13633. doi: 10.18632/oncotarget.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato T, Inoue H, Imoto S, Tamada Y, Miyamoto T, Matsuo Y, et al. Oncogenic roles of TOPK and MELK, and effective growth suppression by small molecular inhibitors in kidney cancer cells. Oncotarget. 2016;7:17652–17664. doi: 10.18632/oncotarget.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alachkar H, Mutonga MB, Metzeler KH, Fulton N, Malnassy G, Herold T, et al. Preclinical efficacy of maternal embryonic leucine-zipper kinase (MELK) inhibition in acute myeloid leukemia. Oncotarget. 2014;5:12371–12382. doi: 10.18632/oncotarget.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, Yi S, et al. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 2005;65:9751–9761. doi: 10.1158/0008-5472.CAN-04-4531. [DOI] [PubMed] [Google Scholar]
- 11.Kohler RS, Kettelhack H, Knipprath-Mészaros AM, Fedier A, Schoetzau A, Jacob F, et al. MELK expression in ovarian cancer correlates with poor outcome and its inhibition by OTSSP167 abrogates proliferation and viability of ovarian cancer cells. Gynecol Oncol. 2017;145:159–166. doi: 10.1016/j.ygyno.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Xia H, Kong SN, Chen J, Shi M, Sekar K, Seshachalam VP, et al. MELK is an oncogenic kinase essential for early hepatocellular carcinoma recurrence. Cancer Lett. 2016;383:85–93. doi: 10.1016/j.canlet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Chen X, Hu C, Wang J, Shen Y, Zhong Z. Up-regulated maternal embryonic leucine zipper kinase predicts poor prognosis of hepatocellular carcinoma patients in a Chinese Han population. Med Sci Monit. 2017;23:5705–5713. doi: 10.12659/MSM.907600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolomsky A, Heusschen R, Schlangen K, Stangelberger K, Muller J, Schreiner W, et al. Maternal embryonic leucine zipper kinase is a novel target for proliferation-associated high-risk myeloma. Haematologica. 2018;103:325–335. doi: 10.3324/haematol.2017.172973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Lu L, Wu L, Chen H, Zhu W, He Y. Identification of prognostic genes in kidney renal clear cell carcinoma by RNA‑seq data analysis. Mol Med Rep. 2017;15:1661–1667. doi: 10.3892/mmr.2017.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speers C, Zhao SG, Kothari V, Santola A, Liu M, Wilder-Romans K, et al. Maternal embryonic leucine zipper kinase (MELK) as a novel mediator and biomarker of radioresistance in human breast cancer. Clin Cancer Res. 2016;22:5864–5875. doi: 10.1158/1078-0432.CCR-15-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickard MR, Green AR, Ellis IO, Caldas C, Hedge VL, Mourtada-Maarabouni M, et al. Dysregulated expression of Fau and MELK is associated with poor prognosis in breast cancer. Breast Cancer Res. 2009;11:R60. doi: 10.1186/bcr2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Li Z, Guo T, Xing XF, Cheng X, Du H, et al. Maternal embryonic leucine zipper kinase serves as a poor prognosis marker and therapeutic target in gastric cancer. Oncotarget. 2016;7:6266–6280. doi: 10.18632/oncotarget.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y, Park JH, Miyamoto T, Takamatsu N, Kato T, Iwasa A, et al. T-LAK cell-originated protein kinase (TOPK) as a prognostic factor and a potential therapeutic target in ovarian cancer. Clin Cancer Res. 2016;22:6110–6117. doi: 10.1158/1078-0432.CCR-16-0207. [DOI] [PubMed] [Google Scholar]
- 20.Chung S, Kijima K, Kudo A, Fujisawa Y, Harada Y, Taira A, et al. Preclinical evaluation of biomarkers associated with antitumor activity of MELK inhibitor. Oncotarget. 2016;7:18171–18182. doi: 10.18632/oncotarget.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda T, Kato T, Kiyotani K, Tarhan YE, Saloura V, Chung S, et al. p53-independent p21 induction by MELK inhibition. Oncotarget. 2017;8:57938–57947. doi: 10.18632/oncotarget.18488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo Y, Park JH, Miyamoto T, Yamamoto S, Hisada S, Alachkar H, et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Transl Med. 2014;6:259ra145. doi: 10.1126/scitranslmed.3010277. [DOI] [PubMed] [Google Scholar]
- 23.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96:990–997. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- 25.Kamel HFM, Al-Amodi HSAB. Exploitation of gene expression and cancer biomarkers in paving the path to era of personalized medicine. Genomics Proteomics Bioinformatics. 2017;15:220–235. doi: 10.1016/j.gpb.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beke L, Kig C, Linders JT, Boens S, Boeckx A, van Heerde E, et al. MELK-T1, a small-molecule inhibitor of protein kinase MELK, decreases DNA-damage tolerance in proliferating cancer cells. Biosci Rep. 2015;35:e00267. doi: 10.1042/BSR20150194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefka AT, Johnson D, Rosebeck S, Park JH, Nakamura Y, Jakubowiak AJ. Potent anti-myeloma activity of the TOPK inhibitor OTS514 in pre-clinical models. Cancer Med. 2020;9:324–334. doi: 10.1002/cam4.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra A, Mishra L, Li S. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol. 2013;31:347–354. doi: 10.1016/j.tibtech.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes P, Marshall D, Reid Y, Parkes H, Gelber C. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques. 2007;43:575–586. doi: 10.2144/000112598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative case of monitoring cell viability for patients-derived primary ovarian cancer cells by a luminescence-based assay.
Correlation between MELK expression and survival rate of patient derived primary ovarian cancer cells under 100 nM of OTS167.