Abstract
Objective
To compare survival outcomes between cervical adenocarcinoma (ADC) and squamous cell carcinoma (SCC) using a propensity score matching (PSM) analysis based on the Surveillance, Epidemiology, and End Results (SEER) Program.
Methods
Patients diagnosed with cervical cancer between 1998 and 2016 were identified from the SEER database. The Kaplan-Meier method and Cox regression analysis were used to analyze survival. A subgroup analysis of overall survival (OS) between patients with ADC and SCC was performed after the 1:1 PSM analysis.
Results
Of the 33,148 patients, 24,591 (79.19%) had SCC and 8,557 (25.81%) had ADC. In the unmatched cohort, after adjustment in multivariate analysis, patients with ADC had a worse prognosis than patients with SCC (hazard ratio [HR]=1.12; 95% confidence interval [CI]=1.07–1.18; p<0.001). In the propensity matched cohort, Kaplan-Meier analysis and subgroup analysis showed that ADC was associated with a worse prognosis than SCC (p=0.001). An analysis stratified by SEER stage revealed a worse prognosis for patients with ADC patients presenting with a regional disease than patients with SCC (HR=1.24; 95% CI=1.14–1.36 p<0.001), but no statistically significant differences were observed between the localized disease (HR=0.97; 95% CI=0.86–1.10; p=0.664) and distant disease (HR=1.09; 95% CI=0.97–1.22; p=0.162) subgroups.
Conclusion
The significant differences in survival outcomes between patients with cervical ADC and SCC were only observed in the regional disease subgroup, but not in the localized disease and distant disease subgroups.
Keywords: Cervical Cancer, Adenocarcinoma, Squamous Cell Carcinoma, Propensity Score, Survival
INTRODUCTION
Cervical cancer is the most common gynecological malignant tumor of the female genitals. Although both the incidence and mortality rates of cervical cancer have been decreasing over the past several decades [1,2], it remains an important public health problem worldwide. Approximately 569,847 new cases of cervical cancer are diagnosed and 311,365 patients die of the disease every year worldwide [3]. Squamous cell carcinoma (SCC) and adenocarcinoma (ADC) are the two most common histological subtypes of cervical cancer. With the increased detection of premalignant disease and wider implementation of cytological screening, the incidence of SCC has decreased substantially [4]. However, the absolute incidence of ADC and its proportion compared with SCC have increased over the same period, particularly among young women of reproductive age [5,6]. As a result, ADC currently accounts for approximately 20% of all cervical cancers [7].
According to many studies, the epidemiology, patterns of dissemination and recurrence, prognostic factors, and response to treatment of ADC differ from SCC [1]. Currently, the International Federation of Gynecology and Obstetrics (FIGO) staging system is the most accurate classification used to predict the prognosis and guide the treatment of cervical cancer. However, no difference in treatment strategies has been identified between SCC and ADC [8]. Many studies have attempted to determine whether these different histological subtypes have any effect on survival. However, previous studies produced inconsistent results [6,8,9,10,11,12]. Inconsistencies are not only due to small sample sizes but also to lack of randomized controlled trials. Therefore, studies investigating a large sample and using real-world data from multiple cancer registries might potentially provide more detailed results.
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) is a well-constructed database containing information from multiple institutions in the United States, which collects cancer incidence and survival data from population-based cancer registries covering approximately 34.6% of the U.S. population [13]. The present study aimed to compare survival outcomes between patients with cervical SCC and ADC using a propensity score matching (PSM) analysis based on the SEER database.
MATERIALS AND METHODS
This SEER analysis was approved by the Institutional Ethnic Committee of the Nanhai People's Hospital, the Second School of Clinical Medicine, Southern Medical University. Analysis was conducted using publicly available data files from the SEER database; the Institutional Ethnic Committee of the Nanhai People's Hospital therefore exempted this study from review. Data extracted from the SEER database do not require individual informed consent. However, a data use agreement submission was required to access the SEER Research Data File. The patient data in this study was anonymously managed in all stages, including stages of data cleaning and statistical analyses.
1. Patients and data collection
All data from patients with cervical cancer were obtained from the SEER 18 Regs (with additional treatment fields, 1975–2016 varying), using the SEER*Stat software (version 8.3.6; Surveillance Research Program, NCI, Bethesda, MD). Additional selection criteria for the SEER*Stat software used to identify cervical cancer patients were as follows: 1) “Cervix Uteri” was limited to the site and morphology (TNM 7/CS v0204+ Schema) and 2) The histology was defined as SCC and ADC according to the International Agency for Research on Cancer (IARC) classification using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes [14]. The ICD-0-3 histology codes of histological subtypes were grouped as: SCC (8050–8078, 8083–8084), and ADC (8140–8141, 8190–8211, 8230–8231, 8260–8265, 8310, 8380, 8382–8384, 8440–8490, 8570–8574, 8576).
Study inclusion criteria were as follows: 1) Age ≥18 years and primary diagnosis of cervical cancer from 1998 to 2016; 2) Survival time ≥1 months; 3) Complete information on clinicopathological information (including demographic, clinical, treatment, and survival data). Exclusion criteria were defined as follows: 1) Patients who survived less than 1 month; 2) Cervical cancer was not the first or only malignant primary tumors; 3) An age less than 18 years; and 4) Incomplete information on any demographic or clinical characteristic. We limited our analysis to data collected from 1998 to 2016 because the SEER database started collecting the SEER summary stage (also called SEER stage) in 1998. Demographic, clinical, treatment, and survival data for each patient were also obtained.
2. Variables
The following clinicopathological information was obtained for each patient in SEER: histological subtypes, race, age at diagnosis, marital status, year of diagnosis, grade, SEER stage, surgery, radiation, chemotherapy, survival months, and vital status. The year of diagnosis was divided into 3 groups: 1998–2003, 2004–2009, and 2010–2016. All patients were staged according to the SEER stage (localized, regional, and distant). According to SEER rules, cervix uteri FIGO stage I; FIGO stages II, III; and FIGO stage IV are classified as localized, regional, and distant disease [15,16]. We also used X-tile 3.6.1 software (Yale University, New Haven, CT, USA) to transform the value of the age at diagnosis into a categorical variable (Supplementary Fig. 1).
3. PSM method
A 1:1 PSM method was used to match different patients with SCC and ADC and to minimize possible confounding effects and create well-matched cohorts. Variables used for matching were race, age at diagnosis, marital status, year of diagnosis, grade, SEER stage, surgery, radiation, and chemotherapy. The nearest neighbor matching algorithm without replacement was applied to ensure adequate matches.
4. Survival outcomes
The main endpoint of this study was overall survival (OS). OS was defined as the interval to the time of the last follow-up or the period from diagnosis to death. The last follow-up date was December 31, 2016.
5. Statistical analysis
The demographic, clinical, and treatment characteristics of patients with different histological subtypes of cervical cancer were compared using the χ2 test. Cumulative survival curves were generated using the Kaplan-Meier method, and differences were compared using the log-rank test. Univariate and multivariate survival analyses were performed using the Cox proportional hazard model, and the hazard ratios (HRs) with 95% confidence intervals (95% CI) were calculated. A subgroup analysis was also performed to determine the HRs of SCC and ADC in a matched population stratified according to covariates. The p-values were 2-sided, and values <0.05 were considered statistically significant. All statistical analysis were performed by using SPSS 24.0 software (IBM Corp., Armonk, NY, USA) and R software version 3.6.2 (http://www.R-project.org).
RESULTS
1. Demographic and clinical characteristics of the study cohort
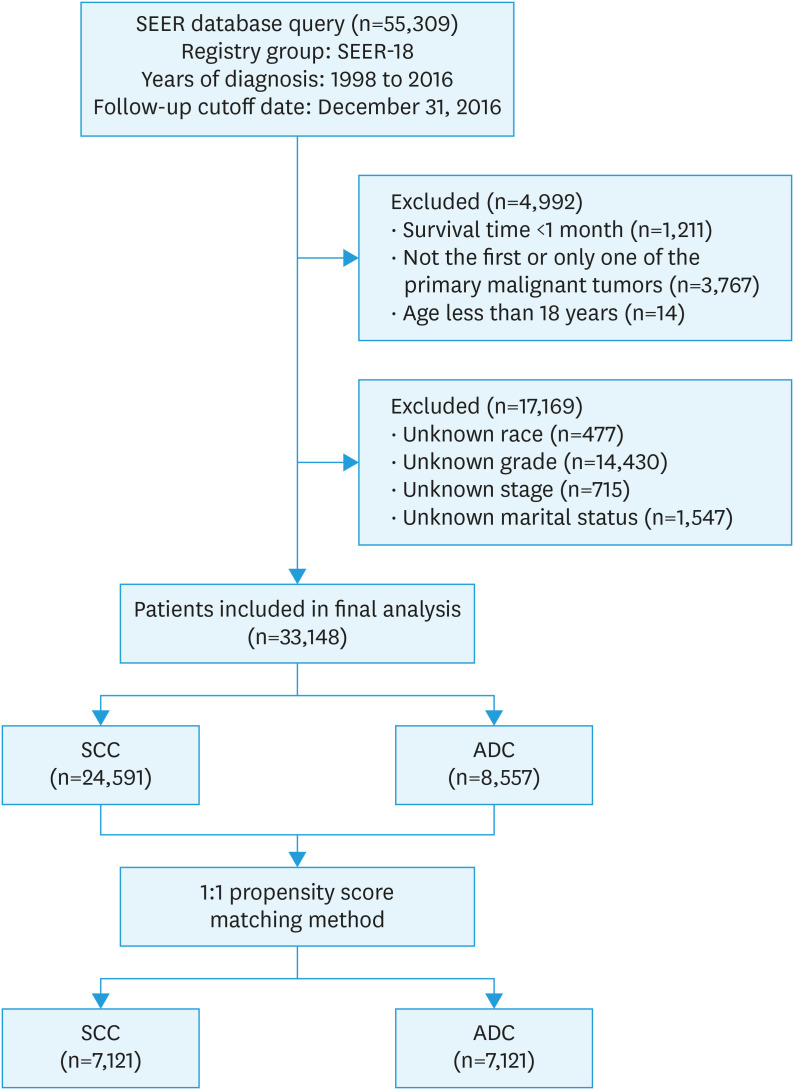
A total of 55,309 patients with cervical cancer were eligible for inclusion from the SEER database from 1998–2016 in this study. Among these, 33,148 patients were selected for the final data analysis; 22,161 patients were excluded according to the predefined exclusion criteria (see Fig. 1 for a flow chart). The demographic, clinical, and treatment characteristics of these selected patients are shown in Table 1. Of the 33,148 patients, 24,591 (79.19%) had SCC and 8,557 (25.81%) had ADC. Significant differences were detected in all demographic, clinical, and treatment characteristics between patients with SCC and ADC (all p-values <0.001) (Table 1). Compared with patients with SCC, patients with ADC had more well-differentiated tumors (grade I) and were more likely to have a localized disease. In addition, a higher percentage of patients with ADC underwent surgery, while fewer received chemotherapy and radiation treatment.
Fig. 1. Flow diagram showing the patient selection process.
ADC, adenocarcinoma; SEER, Surveillance, Epidemiology, and End Results; SCC, squamous cell carcinoma.
Table 1. Baseline characteristics of the unmatched cohort.
| Histological subtype | SCC (n=24,591) | ADC (n=8,557) | p-value | |
|---|---|---|---|---|
| Race | <0.001 | |||
| White | 18,242 (74.18) | 7,015 (81.98) | ||
| Black | 3,903 (15.87) | 612 (7.15) | ||
| Other* | 2,446 (9.95) | 930 (10.87) | ||
| Age at diagnosis | <0.001 | |||
| ≤45 | 10,634 (43.24) | 4,144 (48.43) | ||
| 46–70 | 11,379 (46.27) | 3,722 (43.50) | ||
| >70 | 2,578 (10.48) | 691 (8.08) | ||
| Marital status | <0.001 | |||
| Yes | 10,392 (42.26) | 4,870 (56.91) | ||
| No | 14,199 (57.74) | 3,687 (43.09) | ||
| Year of diagnosis | <0.001 | |||
| 1998–2003 | 7,132 (29.00) | 2,197 (25.67) | ||
| 2004–2009 | 8,254 (33.57) | 2,786 (32.56) | ||
| 2010–2016 | 9,205 (37.43) | 3,574 (41.77) | ||
| Grade | <0.001 | |||
| I | 1,966 (7.99) | 2,811 (32.85) | ||
| II | 11,317 (46.02) | 3,408 (39.83) | ||
| III+IV | 11,308 (45.98) | 2,338 (27.32) | ||
| SEER stage | <0.001 | |||
| Localized | 10,277 (41.79) | 5,344 (62.45) | ||
| Regional | 11,174 (45.44) | 2,346 (27.42) | ||
| Distant | 3,140 (12.77) | 867 (10.13) | ||
| Surgery | <0.001 | |||
| Yes | 13,597 (55.29) | 6,782 (79.26) | ||
| No | 10,994 (44.71) | 1,775 (20.74) | ||
| Radiation | <0.001 | |||
| Yes | 16,005 (65.08) | 3,736 (43.66) | ||
| No/unknown | 8,586 (34.92) | 4,821 (56.34) | ||
| Chemotherapy | <0.001 | |||
| Yes | 13,075 (53.17) | 2,965 (34.65) | ||
| No/unknown | 11,516 (46.83) | 5,592 (65.35) | ||
Values are presented as number (%).
ADC, adenocarcinoma; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
*Other was defined as American Indian/Alaska Native or Asian/Pacific Islander.
2. Survival analysis of unmatched patients
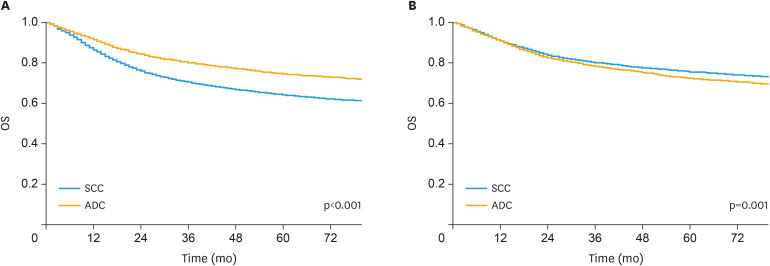
A Kaplan-Meier survival analysis was performed to estimate the OS in unmatched patients (Fig. 2A). The 3-year and 5-year OS rates were 79.91% and 74.37% for patients with ADC, 70.22% and 64.07% for patients with SCC, respectively. According to the Kaplan-Meier analysis, patients with ADC had a significantly better prognosis than patients with SCC (p<0.001).
Fig. 2.
Kaplan-Meier plot and log-rank test of OS in the (A) unmatched cohort and (B) matched cohort.
ADC, adenocarcinoma; OS, overall survival; SCC, squamous cell carcinoma.
Supplementary Table 1 shows the results of the univariate and multivariate analyses of potential predictors of OS. In the univariate analysis, all variables were identified as significant predictive factors for OS, with the exception of the year of diagnosis. In the multivariate analysis, all of these variables retained independent significance for OS, except for radiation treatment. After adjustment in the multivariate analysis, patients with ADC had a worse prognosis than patients with SCC. Compared to patients with SCC, the hazard ratio for patients with ADC was 1.12 (95% CI=1.07–1.18; p<0.001).
3. Analysis of patients stratified according to SEER stage
We stratified the research population by SEER stage to further evaluate the relationship between SCC and ADC. Univariate and multivariate analyses were performed to evaluate OS according to SEER stage (Table 2). In the regional disease subgroup, patients with ADC were predicted to have a worse prognosis than patients with SCC (HR=1.24; 95% CI=1.16–1.33; p<0.001). However, no statistically significant differences were detected between patients with SCC and ADC in the localized disease (HR=0.96; 95% CI=0.87–1.07; p=0.466) and distant disease (HR=1.05; 95% CI=0.96–1.15; p=0.294) subgroups.
Table 2. Univariate and multivariate analyses according to SEER stage of the unmatched cohort.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| SEER stage | ||||||
| Localized | ||||||
| SCC | 1 | 1 | ||||
| ADC | 0.60 (0.55–0.66) | <0.001 | 0.96 (0.87–1.07) | 0.466 | ||
| Regional | ||||||
| SCC | 1 | 1 | ||||
| ADC | 0.99 (0.93–1.06) | 0.812 | 1.24 (1.16–1.33) | <0.001 | ||
| Distant | ||||||
| SCC | 1 | 1 | ||||
| ADC | 0.92 (0.85–1.01) | 0.066 | 1.05 (0.96–1.15) | 0.2942 | ||
ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
4. Survival analysis of matched groups
The 1:1 PSM method was used to match patients with SCC with patients with ADC during the study and to control for potential confounding effects from an imbalance in baseline characteristics and ensure that our observations were reliable and stable. After matching, a group of 14,242 patients with cervical cancer, including 7,121 with SCC and 7,121 patients with ADC, were obtained for our subsequent analysis. The distribution of the demographic and clinical characteristics was well-balanced in the propensity-matched cohort (Supplementary Table 2). Based on the Kaplan-Meier analysis, ADC was associated with a worse prognosis than SCC in matched groups (p=0.001) (Fig. 2B). The 3-year and 5-year OS rates were 78.06% and 72.04% for patients with ADC, 79.96% and 75.33% for patients with SCC, respectively. The multivariate analyses also produced similar results for matched groups (Table 3).
Table 3. Univariate and multivariate analyses of the 1:1 matched cohort.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Histological subtype | |||||
| SCC | 1 | 1 | |||
| ADC | 1.11 (1.04–1.18) | 0.001 | 1.19 (1.11–1.26) | <0.001 | |
| Race | |||||
| White | 1 | 1 | |||
| Black | 1.93 (1.75–2.12) | <0.001 | 1.25 (1.13–1.37) | <0.001 | |
| Other* | 1.10 (0.99–1.22) | 0.063 | 0.99 (0.89–1.09) | 0.794 | |
| Age at diagnosis | |||||
| ≤45 | 1 | 1 | |||
| 46–70 | 2.69 (2.49–2.90) | <0.001 | 1.60 (1.48–1.73) | <0.001 | |
| >70 | 8.10 (7.39–8.88) | <0.001 | 3.31 (3.00–3.66) | <0.001 | |
| Marital status | |||||
| Yes | 1 | 1 | |||
| No | 1.75 (1.64–1.86) | <0.001 | 1.24 (1.16–1.33) | <0.001 | |
| Year of diagnosis | |||||
| 1998–2003 | 1 | 1 | |||
| 2004–2009 | 0.92 (0.86–1.00) | 0.037 | 0.94 (0.87–1.01) | 0.092 | |
| 2010–2016 | 0.86 (0.79–0.94) | 0.001 | 0.83 (0.76–0.90) | <0.001 | |
| Grade | |||||
| I | 1 | 1 | |||
| II | 1.80 (1.61–2.00) | <0.001 | 1.31 (1.17–1.46) | <0.001 | |
| III+IV | 3.86 (3.47–4.29) | <0.001 | 1.74 (1.55–1.94) | <0.001 | |
| SEER stage | |||||
| Localized | 1 | 1 | |||
| Regional | 4.71 (4.36–5.09) | <0.001 | 2.77 (2.52–3.05) | <0.001 | |
| Distant | 15.78 (14.46–17.22) | <0.001 | 8.05 (7.23–8.97) | <0.001 | |
| Surgery | |||||
| Yes | 1 | 1 | |||
| No | 5.43 (5.09–5.78) | <0.001 | 2.29 (2.13–2.47) | <0.001 | |
| Radiation | |||||
| Yes | 1 | 1 | |||
| No/unknown | 0.29 (0.27–0.31) | <0.001 | 0.92 (0.84–1.01) | 0.077 | |
| Chemotherapy | |||||
| Yes | 1 | 1 | |||
| No/unknown | 0.34 (0.31–0.36) | <0.001 | 1.19 (1.10–1.30) | <0.001 | |
ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
*Other was defined as American Indian/Alaska Native or Asian/Pacific Islander.
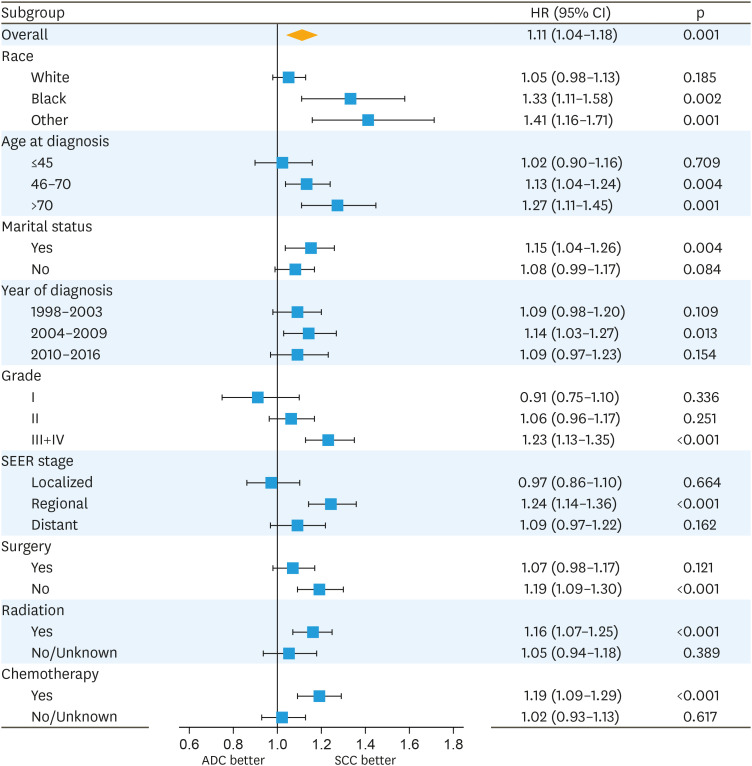
5. Subgroup analysis of matched groups
We performed a subgroup analysis of matched groups to evaluate the differences between patients with SCC and ADC stratified according to various characteristics. As shown in Fig. 3, matched patients with ADC also exhibited a worse survival than matched patients with SCC. However, after stratification according to SEER stage, a significant difference in HRs was not observed for the localized disease (HR=0.97; 95% CI=0.86–1.10; p=0.664) and distant disease (HR=1.09; 95% CI=0.97–1.22; p=0.162) subgroups.
Fig. 3. Forest plot of HRs for patients with ADC compared with patients with SCC in the subgroup analysis of the matched cohort.
ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
DISCUSSION
Controversy persists regarding differences in survival outcomes between ADC and SCC in patients with cervical cancer [6,8,9,10,11,12]. In the present study, we performed a PSM analysis to compare survival outcomes between patients with cervical ADC and SCC from the SEER database. The initial results of this study indicate that patients with ADC presented worse survival outcomes than patients with SCC. However, when study participants were stratified by SEER stage, only patients with ADC presenting with a regional disease exhibited a worse prognosis than patients with SCC, but a difference was not observed between the localized disease and distant disease subgroups. The PSM analysis and subgroup analysis also yielded similar survival outcomes in matched groups of patients with SCC and patients with ADC.
According to many studies, patients with ADC exhibit worse survival outcomes than patients with SCC [17,18]. The Japan Society of Obstetrics and Gynecology collected and analyzed data from 15,698 patients with cervical cancer, and found that patients with SCC had a significantly better prognosis than patients with ADC (p=0.004); the 5-year OS rates were 80.4% and 75.7% for patients with SCC and ADC, respectively [19]. Similar results have been reported by Lee et al. [20] and Zhou et al. [21]. However, other studies have reported inconsistent results. Wu et al. [22] and Wang et al. [23] reported an equivalent survival for patients with ADC and patients with SCC. In a retrospective competing-risks regression analysis of 2,108 patients with cervical cancer, Intaraphet et al. [12] observed an association of ADC with poorer survival compared with SCC in advanced stages, while no difference was observed at early stages. These findings were partially consistent with our results. We speculated that several possible factors may explain these inconsistent results. First, the study population varies from study to study. Our study population was composed of participants with all stages of cervical cancer. Some studies only enrolled patients with early-stage cervical cancer, while others enrolled patients with locally advanced cervical cancer. Second, due to the rarity of ADC, the number of patients with ADC in some studies was considerably low, which affected the statistical significance. Finally, large-sample, multicenter studies are currently lacking.
To further evaluate the relationship between SCC and ADC, we stratified analyses by SEER stage. Surgical resection remains the primary treatment for patients with localized disease which corresponds to FIGO stage I. Because of the excellent results after surgical therapy, survival was not significantly influenced by histologic subtype in this stage [24]. Few studies have reported to examine whether histologic subtype has an influence on outcome for patients with distant disease (FIGO stage IV). Despite significant advancements in cervical cancer treatment, the prognosis for patients with distant disease remains poor. Consequently, no difference in outcome for ADC and SCC was observed in distant disease in our study. This factor may expound why ADC with SCC had similar survival for patients with distant disease. For patients with regional disease which includes FIGO stages II and III, the standard treatment is radiotherapy with concurrent chemotherapy. The primary causes of treatment failure are local recurrence and distant metastases. Differences in the pattern of metastatic recurrence between SCC and ADC have been reported [1]. Of note, patients with SCC were found to have more locoregional and lymph node metastasis, whereas those with ADC seem to exhibit greater hematogenous spread [6]. Distant recurrence had poorer survival compared with patients with locoregional or lymphatic recurrence. Some investigators have suggested that ADC is a distinct clinical entity from SCC and may be different at the molecular level [17,20]. This situation, taken as a whole, our results suggest that ADC associated with worse survival than SCC even after PSM.
Compared with previous studies of similar populations, the present study has some strengths and unique features. Notably, the sample size of patients with ADC was sufficiently large to provide good statistical power. This large population-based analysis, which contains real-world data from multiple cancer registries, may reflect the differences in clinical conditions between SCC and ADC. As this study employed a retrospective design, bias and confounding were inevitable. Thus, we used the PSM method to minimize the effects of possible confounding factors and create well-matched cohorts. The stratified analysis uses data better and produces stable conclusions for the different subgroups analyzed in this study.
However, the present study has limitations that should also be acknowledged. First, the SEER database, a large population-based retrospective database, has some inevitable limitations that are common to all retrospective database analyses. For instance, the SEER database does not record recurrence data and other prognostic factors. Additionally, detailed descriptions of the chemotherapy regimens and the dosage of radiation were also unavailable. Second, FIGO staging and TNM staging systems for cervical cancer are the most widely used clinical staging systems. Therefore, these staging systems differ in different periods, and we used the SEER stage instead of these systems in the present study. However, the SEER stage is not commonly used in clinical practice. Third, we excluded 22,161 (40.07%) patients based on the exclusion criteria reported in this study, which might result in selection bias. In addition, the PSM analysis per se may also introduce potential selection bias [25]. Finally, although the PSM method eliminated the effects of confounders, the level of evidence was still lower than a randomized controlled clinical trial. After considering these limitations, additional multi-center, prospective, randomized controlled trials are needed to minimize confusion and confirm these findings.
In conclusion, based on the results of the present study, patients with cervical ADC have a significantly worse overall survival than patients with SCC. However, the significant differences in survival outcomes between patients with ADC and SCC were only observed in the regional disease subgroup, but not in the localized disease and distant disease subgroups. Therefore, these results must be confirmed in prospective studies with large sample sizes of patients.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Y.W., T.W.
- Data curation: P.X., W.Z., T.L., T.W.
- Formal analysis: Y.W.
- Methodology: Y.W., W.Z., T.W.
- Resources: L.F.
- Software: P.X., W.Z., L.F.
- Writing - original draft: P.X., W.Z., T.L.
- Writing - review & editing: Y.W., T.W.
SUPPLEMENTARY MATERIALS
Univariate and multivariate analyses of the unmatched cohort
Baseline characteristics of the 1:1 matched cohort
Identification of optimal cutoff values for the age of diagnosis using the X-tile analysis. Optimal cutoff values of age were identified as 45 and 69 years based on OS.
References
- 1.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol. 2010;116:140–146. doi: 10.1016/j.ygyno.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard CS, El-Zein M, Ramanakumar AV, Ferenczy A, Franco EL. Assessment of mediators of racial disparities in cervical cancer survival in the United States. Int J Cancer. 2016;138:2622–2630. doi: 10.1002/ijc.29996. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Sun H, Wang S, Yan Y, Sun F, Li Y, et al. Trend in relative survival in squamous cervical cancer by decade from 1983 to 2012: a period analysis. Cancer Manag Res. 2018;10:3177–3191. doi: 10.2147/CMAR.S167442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Shimada M, Nakamura K, Takei Y, Ushijima K, Sumi T, et al. Predictors for pathological parametrial invasion in clinical stage IIB cervical cancer. Eur J Surg Oncol. 2019;45:1417–1424. doi: 10.1016/j.ejso.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung EJ, Byun JM, Kim YN, Lee KB, Sung MS, Kim KT, et al. Cervical adenocarcinoma has a poorer prognosis and a higher propensity for distant recurrence than squamous cell carcinoma. Int J Gynecol Cancer. 2017;27:1228–1236. doi: 10.1097/IGC.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 7.Yokoi E, Mabuchi S, Takahashi R, Matsumoto Y, Kuroda H, Kozasa K, et al. Impact of histological subtype on survival in patients with locally advanced cervical cancer that were treated with definitive radiotherapy: adenocarcinoma/adenosquamous carcinoma versus squamous cell carcinoma. J Gynecol Oncol. 2017;28:e19. doi: 10.3802/jgo.2017.28.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie X, Song K, Cui B, Jiang J, Yang X, Kong B. A comparison of the prognosis between adenocarcinoma and squamous cell carcinoma in stage IB–IIA cervical cancer. Int J Clin Oncol. 2018;23:522–531. doi: 10.1007/s10147-017-1225-8. [DOI] [PubMed] [Google Scholar]
- 9.Rose PG, Java JJ, Whitney CW, Stehman FB, Lanciano R, Thomas GM. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol. 2014;135:208–212. doi: 10.1016/j.ygyno.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teke F, Yoney A, Teke M, Inal A, Urakci Z, Eren B, et al. Lack of any impact of histopathology type on prognosis in patients with early-stage adenocarcinoma and squamous cell carcinoma of the uterine cervix. Asian Pac J Cancer Prev. 2014;15:2815–2819. doi: 10.7314/apjcp.2014.15.6.2815. [DOI] [PubMed] [Google Scholar]
- 11.Winer I, Alvarado-Cabrero I, Hassan O, Ahmed QF, Alosh B, Bandyopadhyay S, et al. The prognostic significance of histologic type in early stage cervical cancer - A multi-institutional study. Gynecol Oncol. 2015;137:474–478. doi: 10.1016/j.ygyno.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Intaraphet S, Kasatpibal N, Siriaunkgul S, Sogaard M, Patumanond J, Khunamornpong S, et al. Prognostic impact of histology in patients with cervical squamous cell carcinoma, adenocarcinoma and small cell neuroendocrine carcinoma. Asian Pac J Cancer Prev. 2013;14:5355–5360. doi: 10.7314/apjcp.2013.14.9.5355. [DOI] [PubMed] [Google Scholar]
- 13.Pan X, Yang W, Chen Y, Tong L, Li C, Li H. Nomogram for predicting the overall survival of patients with inflammatory breast cancer: a SEER-based study. Breast. 2019;47:56–61. doi: 10.1016/j.breast.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, et al. Cancer incidence in five continents, Vol. XI. Lyon: IARC Publication; 2014. [Google Scholar]
- 15.Gultekin M, Dundar S, Kucukyildiz I, Karaca MZ, Boztas G, Turan SH, et al. Survival of gynecological cancers in Turkey: where are we at? J Gynecol Oncol. 2017;28:e85. doi: 10.3802/jgo.2017.28.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhl JL, Callaghan C, Hurlbut A, Ries LA, Adamo P, Dickie L, et al. Summary stage 2018: codes and coding instructions [Internet] Bethesda, MD: National Cancer Institute; 2018. [cited 2020 Apr 19]. Available from: https://seer.cancer.gov/tools/ssm. [Google Scholar]
- 17.Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, Kowalska M, Kotowicz B. Adenocarcinoma histology is a poor prognostic factor in locally advanced cervical cancer. Curr Med Res Opin. 2019;35:595–601. doi: 10.1080/03007995.2018.1502166. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Chen Y, Xu X, Yan D, Lou H. Postoperative clinicopathological factors affecting cervical adenocarcinoma: stages I–IIB. Medicine (Baltimore) 2018;97:e9323. doi: 10.1097/MD.0000000000009323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J Obstet Gynaecol Res. 2014;40:338–348. doi: 10.1111/jog.12360. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Kim YT, Kim S, Lee B, Lim MC, Kim JW, et al. Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS One. 2015;10:e0144887. doi: 10.1371/journal.pone.0144887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Wu SG, Sun JY, Li FY, Lin HX, Chen QH, et al. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol. 2017;143:115–122. doi: 10.1007/s00432-016-2246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu SG, Sun JY, He ZY, Chen QH, Zhou J. Early-stage node negative cervical adenocarcinoma and squamous cell carcinoma show similar survival outcomes after hysterectomy: a population-based study. J Gynecol Oncol. 2017;28:e81. doi: 10.3802/jgo.2017.28.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Yang C, Wang W, Xia B, Li K, Sun F, et al. A prognostic nomogram for cervical cancer after surgery from SEER database. J Cancer. 2018;9:3923–3928. doi: 10.7150/jca.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara H, Yokota H, Monk B, Treilleux I, Devouassoux-Shisheboran M, Davis A, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for cervical adenocarcinoma. Int J Gynecol Cancer. 2014;24:S96–101. doi: 10.1097/IGC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 25.Sumida H, Sugino S, Kuratani N, Konno D, Hasegawa JI, Yamauchi M. Effect of forced-air warming by an underbody blanket on end-of-surgery hypothermia: a propensity score-matched analysis of 5063 patients. BMC Anesthesiol. 2019;19:50. doi: 10.1186/s12871-019-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate and multivariate analyses of the unmatched cohort
Baseline characteristics of the 1:1 matched cohort
Identification of optimal cutoff values for the age of diagnosis using the X-tile analysis. Optimal cutoff values of age were identified as 45 and 69 years based on OS.