Abstract
Introduction
Greater use of nicotine replacement therapy (NRT) is related to smoking cessation success, but the causal direction is unclear. This study characterized the relationship between NRT use and smoking lapse and relapse.
Methods
Participants (N = 416 smokers; 57% female, 85% White) were recruited from primary care for a smoking cessation factorial experiment and analyzed if abstaining ≥1 day in the first 2 weeks post-target quit day (TQD). Participants were randomized to counseling and 8 versus 26 weeks of nicotine patch plus nicotine gum post-TQD. Participants carried electronic dispensers that timestamped each gum use. Participants who lapsed (smoked after abstaining) within 6 weeks post-TQD were matched with nonlapsers (n = 146 pairs) on multiple variables. We compared lapsers’ versus matched nonlapsers’ gum use in the 5 days before and after the lapsers’ first lapse.
Results
By week 6 post-TQD, 63% of participants lapsed. Compared with nonlapsers, lapsers used less gum 1 and 2 days pre-“lapse” and on the 5 days post-lapse. Lapsers used less gum during the 5 days post-lapse than the 5 days pre-lapse. Univariate survival analyses with lapsers showed greater gum use during both pre- and post-lapse periods predicted longer latency to relapse in the first 6 weeks.
Conclusions
In a smoking cessation attempt using nicotine patch plus gum, lapsers versus matched nonlapsers used less gum immediately preceding and following their first lapse. Lower mean gum use before and after lapses predicted a more rapid escalation to relapse. Decreased nicotine gum use both precedes and follows returns to smoking during cessation attempts.
Implications
This research examined electronically monitored nicotine gum use collected in real time and found that among smokers engaged in a quit attempt, lapsers (vs. matched nonlapsers) tended to decrease their gum use 1–2 days prior to lapsing and to further decrease their gum use from pre- to post-lapse. Decreased gum use pre-lapse may signal heightened lapse risk in 1–2 days, with lower level of gum use predicting a more precipitous course of relapse. These results encourage further exploration of objective measures of smoking medication use patterns to examine their signaling properties and to inform understanding of cessation failure.
Clinical Trial Registration
ClinicalTrials.gov NCT01120704.
Introduction
When people attempt to quit smoking, it is common for them to either not use smoking medication at all or use it nonadherently.2–4 Smoking cessation trials have documented such nonadherence, and it may be even more common in real-world use.2,5–7 Nonadherence is of interest due to the strong association between adherence and smoking cessation success.4,8–11 Important questions remain, however, and the question of causal direction is paramount. That is, to what extent does medication disuse cause cessation failure and to what extent does cessation failure cause medication disuse? (The latter has been termed “reverse causation.” 9,10,12,13)
Credible accounts can be generated for either direction of causal influence; that is, people may decrease their use of smoking medication and this leads to smoking because their medication disuse reduces medication benefits such as craving suppression (e.g., 14,15). Conversely, people who have begun to smoke during their quit attempt may stop using smoking medication because they assume the medication is not working or because they have given up on their quit attempt. Indeed, a high proportion of smokers attribute their nonadherence to resuming smoking.16 It is also possible a third variable (e.g., an acute stressor) may cause people to both decrease their smoking medication use and return to smoking; that is, gum use and lapsing may not be directly causally related.
Researchers have attempted to clarify the directionality of the causal relationship between medication use and smoking outcomes. Raupach et al.10 reviewed five studies that attempted to isolate the influence of adherence on later tobacco abstinence by looking at medication use prior to relapse. While this approach contributes to our understanding of medication use–relapse relations, further research is needed. For instance, the studies Raupach reviewed did not examine post-lapse medication use and only one used real-time electronic medication monitoring to reduce memory and recording errors. These features may have reduced sensitivity for detecting medication use–smoking relations. In an effort to address such concerns, the current research used an electronic medication monitoring device and examined medication use both pre- and post-lapse.
Many studies of medication adherence have used retrospective self-report to assess medication use (e.g., 17). However, even with frequent data collection points, forgetting and inattention may introduce nontrivial error in self-reported medication use estimates, especially for medications designed to be taken as needed,18,19 and thus limit temporal precision. Furthermore, medication use data that are temporally remote from smoking events such as relapse may not sensitively capture their interrelations.10 In addition, some studies have used broad, categorical measures of adherence (e.g., 20), which may less sensitively index the risk of smoking lapse or relapse than do continuous measures.
A further gap in our understanding of medication use–smoking relations is that, to eliminate reverse causal effects (nonadherence due to smoking), studies have typically focused only on participants who established continuous abstinence over periods of 1–4 weeks.9,13,17,21 The representativeness of such samples could be questioned because many smokers lapse early in a quit attempt.22
The current research examined medication use among participants in a factorial smoking cessation experiment (see Schlam et al.1 for primary outcomes) where everyone was assigned to receive nicotine patch plus nicotine gum for ≥8 weeks. A previous report showed greater electronically monitored gum use over the first 6 weeks of treatment was strongly associated with abstinence through 1-year follow-up.11 In contrast to the current report, this previous report did not attempt to capture temporal ordering between lapses and medication use, and thus did not attempt to clarify the temporal relationship (i.e., precedence) between medication use and smoking outcomes. The current report focuses on electronically monitored gum use in the 5 days pre- and post-lapse during the first 6 weeks of a quit attempt. By more accurately characterizing the relationship between smoking medication use and smoking cessation outcomes, this study seeks to illuminate (1) the extent to which decreases in medication use signal increased risk for a first lapse after cessation, or progression from lapse to relapse, and (2) the extent to which lapses predict reduction of medication use.
Materials and Methods
Procedure
Participants were recruited from 2010 to 2013 in 11 primary care clinics from two southern Wisconsin healthcare systems. A medical assistant (prompted by the electronic health record) invited adult outpatients who smoked to be in a smoking cessation or reduction research study.23,24 Study staff called those interested in quitting within the next month and assessed them for eligibility including: literacy in English; smoking five or more cigarettes per day for the past 6 months; using neither varenicline nor bupropion; and having no NRT contraindications. Eligible patients were invited to their clinic to provide written informed consent and start treatment (see Schlam et al.1 for details).
Assessments
At 1 week pre-target quit day (TQD), we assessed demographic variables, age started smoking daily, motivation to quit (from 1 = not at all motivated to 10 = extremely motivated), Wisconsin Predicting Patients’ Relapse (WI-Prepare) score,25 current smoking, and the Heaviness of Smoking Index (made up of two items: time to first cigarette and cigarettes per day26). Timeline follow-back interviews in which participants reported whether they had smoked on each day since the last contact27 were used at visits (weeks 1, 4, and 8 post-TQD) to establish lapse (smoking at all after establishing abstinence) and relapse occurrence (smoking on the first of seven consecutive days of smoking) in the first 6 weeks post-TQD. Automated evening calls occurred on days 0, 1, 2, 3, and 5 post-TQD and then weekly or less frequently; these interactive voice response (IVR) calls asked participants to report whether they had smoked that day. Because IVR calls did not occur daily, we used timeline follow-back data to determine lapse and relapse occurrence.
Medication Adherence Measures
Timeline follow-back was used during visits to collect daily patch use data. All participants were asked to carry a medication dispenser,28 which electronically timestamped each removal of the nicotine gum blister pack from the dispenser. Staff downloaded these data at each study visit.
Study Design
This 25 factorial experiment evaluated five two-level factors described below. All participants were offered 8 weeks of nicotine patch plus nicotine gum to be used starting on the TQD, and counseling totaling 50 minutes. For treatment details and medication dosing, see Schlam et al.1.
Medication Adherence Counseling. Half of participants were randomized to two 10-minute Medication Adherence Counseling sessions designed to correct their misconceptions about smoking medication and half did not receive Medication Adherence Counseling.
Automated Adherence Calls. Half of participants received automated medication reminder calls encouraging them to use the medication as recommended and half did not.
Electronic Medication Monitoring with Feedback and Counseling (e-Monitoring Counseling). Half of participants received printouts of their electronic records of gum use, plus counseling discussing the printouts and encouraging adherence, while half did not.
Extended Medication. Half of participants were randomized to 8 weeks of patches plus gum post-TQD, while half were randomized to 26 weeks. Instructions included (1) use at least five pieces of gum per day for the first 6 weeks and (2) use the medication even if you are smoking. Participants with side effects were told to decrease or discontinue their gum use as needed.
Maintenance Counseling. Half of participants received three brief Maintenance Counseling calls in the first 6 weeks designed to prevent relapse while half did not.
Analytic Plan
This analytic plan was not pre-registered, and findings should be viewed as exploratory. We examined medication use in the first 6 weeks post-TQD (before participants randomized to 8 weeks of medication were instructed to taper their gum use). Gum use was analyzed only on days where it made sense for the participant to have medication use data (i.e., the participant had not been instructed to stop the medication due to side effects, had not withdrawn from the study, and had a functioning medication dispenser). Following the approach in Hollands et al.,9 analyzable days where gum use data were missing (i.e., no medication use data were recorded) were set to zero based on the assumption that missing gum use data likely meant the participant had returned to smoking, discontinued the gum, and stopped showing up for study visits.
Because we wanted to examine lapsing following abstinence, participants were only included in analyses if they had ≥1 day of abstinence in the first 2 weeks post-TQD. For each included participant, we calculated their (1) actual quit date (the first date from the TQD to Day 13 that they remained abstinent for 24 hours), (2) lapse date if they lapsed (the first day they smoked from the day after their actual quit date through Day 41 post-TQD), and (3) their relapse date if they relapsed (the first of seven consecutive days of smoking; this first day needed to occur between the day after their actual quit date and Day 41 post-TQD). These analyses assumed missing data equaled smoking.
We created matched samples of participants (matching lapsers who smoked in the first 6 weeks post-TQD with nonlapsers who did not) and compared the gum use of the lapsers with that of matched comparison nonlapsers. We used the SAS macro Gmatch, developed at the Mayo Clinic,29 to match lapsers with nonlapsers without replacement on five variables: (1) randomized to 26 versus 8 weeks of nicotine patch plus gum; (2) randomized to e-monitoring counseling versus no e-monitoring counseling; (3) gender (male or female); (4) age (range 18–84); and (5) Heaviness of Smoking Index (range 0–6). We matched on extended medication and e-monitoring counseling, but not the other three manipulated factors because only 26 versus 8 weeks of medication had a main effect increasing abstinence and only e-monitoring counseling had a main effect increasing gum adherence,1,11 and we wanted to limit the number of matching variables.
In the analyses of medication use, time for each matched pair was anchored around the lapser’s lapse day (e.g., if a lapser first smoked 8 days after their actual quit date, we assigned their matched nonlapser a “lapse” day 8 days after the nonlapser’s actual quit date). Throughout this article, when we refer to a nonlapser’s “lapse” day, we mean this assigned “lapse” day which was based on their paired lasper’s actual lapse day. We focused on medication use in the 5 days pre-lapse and the 5 days post-lapse (with the lapse day counting as the first day post-lapse). A 5-day period balanced the need to model pre- and post-lapse gum use trajectories while reducing the amount of missing data.
Patch use data are presented for the pre- and post-lapse periods but are not the focus of inferential tests because patch use was not electronically monitored. Gum use was analyzed in a series of t-tests comparing lapsers’ versus nonlapsers’ (1) mean gum use on the actual quit day (the first day participants were abstinent) and on the day after the actual quit day, (2) mean gum use for each of the five pre-lapse and post-lapse days, and (3) pre- and post-lapse gum use slopes. We also used logistic regression to examine the relationship of gum use in the 5 days pre-lapse with lapsing in the first 6 weeks post-TQD. In the lapsers only, Cox regression survival analyses were computed to predict latency to relapse (after the actual quit day and through 6 weeks post-TQD) using pre- and post-lapse gum use means and slopes, gum use on the actual quit day and the following day, and change in gum use from the day before the lapse to the lapse day. We selected these variables in an effort to capture gum use at theoretically key points in the quit attempt (immediately post-TQD and in the days surrounding the lapse).
Results
Participants were included in the analysis sample because they established abstinence for ≥1 day in the first 2 weeks post-TQD (n = 416; 76.5% of the parent sample of 544). Participants’ actual quit day was a median of 0.0 days and a mean of 1.04 days (SD = 2.22) after their target quit day. The analysis sample of 416 had a mean age of 46.0 years (SD = 12.8) and smoked a mean of 17.8 cigarettes/d (SD = 8.1). The sample was 57.0% female, 85.1% White, and 9.9% African American; 3.7% were Hispanic; and 14.0% had a college degree.
Of the 416 participants, 261 (62.7%) lapsed in the first 6 weeks post-TQD, a median of 4.0 days and a mean of 8.0 days (SD = 9.3) after their actual quit day. In the first 6 weeks post-TQD, 28.6% (119/416) relapsed; their first day of the relapse occurred a median of 0.0 days and a mean of 5.3 days (SD = 9.2) after they lapsed. There were 261 lapsers and 155 nonlapsers; therefore, the maximum number of matched pairs of lapsers and nonlapsers was 155. We set Gmatch to find exact matches for the binary matching variables of medication duration condition, e-monitoring counseling condition, and gender, and to match participants within ±10 years for age and within ±2 points for the Heaviness of Smoking Index. Matching on these five variables was successful and yielded 149 pairs of matched lapsers and nonlapsers.
We examined the gum use of these 149 pairs in the 5 days pre- and post-lapse and found 37.6% (112/298) had two or fewer days of gum use data pre-lapse due primarily to their “lapse day” occurring shortly after their actual quit day. Post-lapse, however, 97.7% (291/298) had gum use data for all 5 days. Based on inspection, the frequencies of missingness both pre-lapse and post-lapse were similar for nonlapsers and lapsers. Pre-lapse medication use was only analyzed on however many abstinent days participants had (up to 5 days) prior to their lapse day. Three people (one lapser and two nonlapsers) had zero valid days of gum use data for the 5 days pre- and post-lapse, so these three people and their corresponding matched participants were omitted from analyses, leaving 146 pairs.
To examine the effectiveness of the matching, we tested whether the 146 matched pairs of lapsers and nonlapsers differed on baseline variables we thought might affect their medication use. We found the lapsers versus nonlapsers did not differ significantly on any of the following baseline variables: education (some high school, high school graduate, some college, college graduate), race (White, African American, American Indian or Alaska Native, Mixed), the age they started smoking daily, motivation to quit, and WI-Prepare score (all p’s > .05; see Table 1).
Table 1.
Matched Lapsers’ Versus Nonlapsers’ Scores on Selected Baseline Variables
| Lapsers (n = 146) | Nonlapsers (n = 146) | t-statistic or χ 2-statistic | p | |
|---|---|---|---|---|
| Education | 3.02 | .39 | ||
| % with some high school | 7.5% | 10.3% | ||
| % graduated from high school | 29.5% | 30.8% | ||
| % with some college | 50.0% | 41.1% | ||
| % graduated from college | 13.0% | 17.8% | ||
| Racea | 6.68 | .08 | ||
| % White | 83.5% | 90.9% | ||
| % African American | 15.2% | 6.3% | ||
| % American Indian or Alaska Native | 0.7% | 0.7% | ||
| % Mixed | 0.7% | 2.1% | ||
| Mean age started smoking dailyb (SD) | 17.3 (4.5) | 17.8 (4.2) | 0.98 | .33 |
| Mean motivation to quit (SD) | 8.9 (1.2) | 8.9 (1.3) | −0.19 | .85 |
| Mean Wisconsin Predicting Patients’ Relapse (WI-Prepare) score (SD) | 5.2 (2.2) | 5.1 (2.4) | −0.20 | .84 |
aFive participants did not report their race; 145 lapsers and 142 nonlapsers did report their race and are included here.
bTwo participants did not report the age they started smoking daily; 146 lapsers and 144 nonlapsers did report this and are included here.
We also assessed the reliability of the timeline follow-back smoking calendar data, which we used to establish lapsers’ first lapse date, by examining the agreement between the 146 lapsers’ timeline follow-back data and their IVR data on smoking during the first week post-TQD when many lapses typically occur22 and when IVR completion rates are generally still high. On days when these participants reported their smoking status via both timeline follow-back and IVR reports, the two reports agreed on 80.3% of days (400/498 days).
Patch use both pre- and post-lapse was high, and group differences were small. As we found with gum use, a little over a third of the sample (111/298; 37.2%) had two or fewer days of patch use data pre-lapse due primarily to their lapse day occurring shortly after their actual quit day. In the up to 5 days pre-lapse when participants could have used the patch, nonlapsers used the patch a mean of 87.9% of days (SD = 31.2), whereas lapsers used the patch 90.3% of days (SD = 28.3). In the 5 days post-lapse starting on the lapse day, nonlapsers and lapsers both used the patch 85.2% of days (SDnonlapsers = 34.1; SDlapsers = 31.1).
Nonlapsers and lapsers did not differ in their gum use on the actual quit day: Mnonlapsers = 4.24 pieces/d (SD = 2.83) versus Mlapsers = 3.73 pieces/d (SD = 2.96); t(290) = 1.52, p = .13, d = 0.18. However, by 1 day after their actual quit day, lapsers were using less gum than nonlapsers: Mnonlapsers = 4.08 pieces/d (SD = 2.97) versus Mlapsers = 3.37 pieces/d (SD = 2.64); t(290) = 2.14, p = .033, d = 0.25.
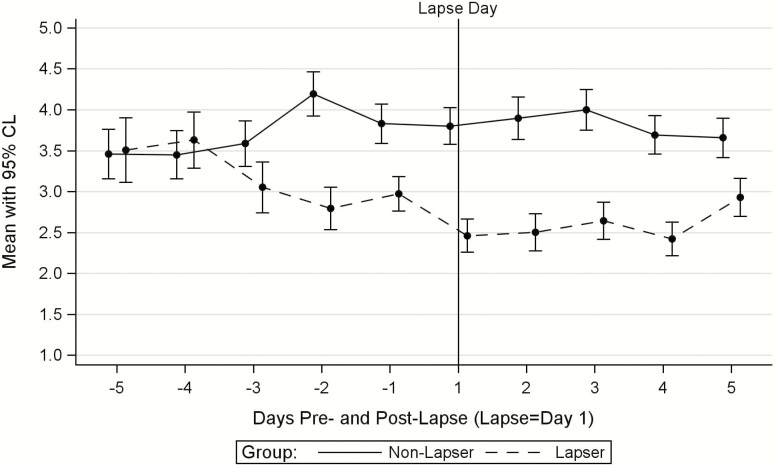
Compared with the nonlapsers, lapsers (1) did not differ in their gum use 5, 4, or 3 days before the lapse day, (2) used fewer pieces of gum 2 and 1 days before the lapse day (p’s = .01 and .0004, respectively), and (3) used fewer pieces of gum on the lapse day and on each of the four following days (p’s range from <.0001 on the lapse day to .04 on post-lapse day 5; see Figure 1; Figure 1 is based on the 146 matched pairs, some of whom had only 1 or 2 days of gum use data pre-lapse. The figure looked essentially identical, however, when based only on the 92 matched pairs with at least 3 days of gum use data in both the pre- and post-lapse periods).
Figure 1.
Lapsers’ and matched nonlapsers’ mean gum use with 95% confidence limits before and after the lapsers’ first lapse (N = 146 pairs).
Lapsers’ and nonlapsers’ gum use slopes differed from one another both pre-lapse and post-lapse. Lapsers’ gum use showed a decreasing trajectory (mean slope = −0.15, SD = 0.50) between day 5 and day 1 pre-lapse, whereas nonlapsers’ pre-lapse gum use showed a variable but nondecreasing trajectory (mean slope = 0.00, SD = 0.43; p-value for the difference between the pre-lapse slopes = .02). Lapsers’ post-lapse gum use slope was mostly flat, but a small uptick in gum use on day 5 resulted in a positive slope of 0.08 (SD = 0.60). Nonlapsers’ mean post-lapse slope (slope = −0.05, SD = 0.52) was similar to their pre-lapse slope (p-value for the difference between the lapsers’ and the nonlapsers’ post-lapse slopes = .048). Nonlapsers’ gum use grand mean did not differ in the 5 days pre- versus post-lapse (Mpre-lapse = 3.93, SD = 2.67; Mpost-lapse = 3.79, SD = 2.61; t(145) = 0.95, p = .35, d = 0.08); lapsers, however, decreased their mean gum use from pre- to post-lapse (Mpre-lapse = 3.16, SD = 2.46; Mpost-lapse = 2.63, SD = 2.23; t(144) = 3.48, p = .0007, d = 0.29).
In the full sample of lapsers and matched nonlapsers, we examined the relation of pre-lapse gum use with lapsing using logistic regression and found that the mean number of pieces of gum used a day in the 5 days pre-lapse was associated with lapsing in the first 6 weeks post-TQD (b = −0.12, p = .01; odds ratio = 0.89, 95% confidence interval [0.81, 0.98]). This implies that for each additional piece of gum used daily in the 5 days pre-lapse, the likelihood of lapse decreases by 11%.
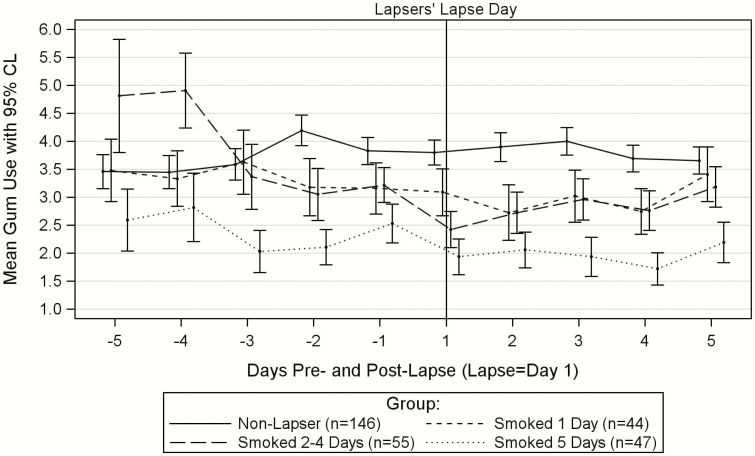
To examine gum use and post-lapse smoking in greater detail, in exploratory analyses, we divided those who lapsed into three groups by the number of days they smoked in the first 5 days post-lapse (Figure 2). Those who smoked on only 1 day of the first 5 days post-lapse (n = 44) did not decrease their gum use significantly from the 5 days pre-lapse to the 5 days post-lapse (Mpre-lapse = 3.52, SD = 2.85; Mpost-lapse = 3.00, SD = 2.52; t(43) = 1.78, p = .08, d = 0.27). Those who smoked 2–4 days post-lapse (n = 55) decreased their gum use from the 5 days pre-lapse to the 5 days post-lapse (Mpre-lapse = 3.53, SD = 2.45; Mpost-lapse = 2.81, SD = 2.21; t(53) = 2.68, p = .0098, d = 0.36). Finally, those who smoked all 5 days post-lapse (n = 47) did not decrease their gum use significantly from the 5 days pre-lapse to the 5 days post-lapse (Mpre-lapse = 2.39, SD = 1.88; Mpost-lapse = 2.07, SD = 1.87; t(46) = 1.39, p = .17, d = 0.20). This last group had very low rates of gum use both pre- and post-lapse.
Figure 2.
Lapsers’ and matched nonlapsers’ mean gum use with 95% confidence limits before and after the lapsers’ first lapse by group (groups based on number of days smoked out of the first 5 days post-lapse).
We examined whether the three exploratory groups of lapsers decreased their gum use pre-lapse by testing whether their pre-lapse gum use slopes differed from the nonlapsers’ pre-lapse gum use slopes described earlier. Lapsers who smoked on 1 day or on 2–4 of the first 5 days post-lapse showed a decreasing trajectory in gum use over the five pre-lapse days (mean slope = −0.16, SD = 0.45 and mean slope = −0.27, SD = 0.51, respectively). Both groups’ pre-lapse trajectories differed from the nonlapsers’ nondecreasing pre-lapse trajectory. For the smoked 1-day group versus the nonlapsers: t(148) = 2.00, p = .047. For the smoked 2- to 4-days group versus the nonlapsers: t(146) = 3.19, p = .002. Those who smoked all 5 days post-lapse showed a low and flat trajectory (mean slope = 0.004, SD = 0.51) that did not differ in slope from that of the nonlapsers: t(143) = −0.01, p = .99.
Finally, we used Cox regression survival analyses to examine which gum use variables predicted latency to relapse in the first 6 weeks post-TQD among the 146 who lapsed during that time period. Participants who lapsed but did not relapse by 6 weeks (n = 83) were treated as right censored. In univariate tests, survival analyses found greater gum use (as assessed by three different variables) predicted a longer latency to relapse in the first 6 weeks. The three variables were as follows: gum use on the day after the actual quit day (B = −0.12, SE = 0.05, p = .02), mean gum use over the 5 days pre-lapse (B = −0.11, SE = 0.06, p = .04), and mean gum use over the 5 days post-lapse (B = −0.16, SE = 0.06, p = .02). Four variables did not predict latency to relapse in the first 6 weeks: gum use on the actual quit day, gum use slopes pre-lapse or post-lapse, or change in gum use from the day before the lapse to the lapse day. The significant predictors from the univariate tests did not have significant, orthogonal relations with latency to relapse when entered together in multivariable survival analyses.
Discussion
This study examined the relationship of electronically monitored nicotine gum use with lapsing among participants in a smoking cessation trial who were all assigned to use nicotine patch plus nicotine gum. Those who lapsed showed lower gum use than nonlapsers at multiple time points: using fewer pieces of gum as early as the day after their actual quit day, tending to decrease their gum use 1–2 days prior to lapsing, and, compared with their pre-lapse use, tending to decrease their gum use still further after lapsing. Although the magnitude of this decrease in gum use after lapsing was modest, it appears clinically meaningful in that lapsers’ post-lapse gum use was associated with their frequency of post-lapse smoking and their relapse latency. These results suggest that decreased smoking medication use both precedes and reflects lapse occurrence. However, exploratory analyses showed that people who smoked all 5 days post-lapse used very little gum pre-lapse and remained at that level post-lapse. Thus, either low or decreasing gum use prior to any lapsing appears to increase the risk of lapse (strong inference is not possible).
These data suggest that decreased nicotine gum use pre-lapse can signal heightened lapse risk in 1–2 days, with lower gum use predicting a more precipitous course of relapse. Thus, it is possible that increasing pre-lapse medication use could forestall lapses. Of course, the data show that lapsing is related to prior gum use; they do not show conclusively the extent to which gum use accurately predicts or causes subsequent lapsing. Also, a third variable such as decreased motivation or an unanticipated stressor may cause both decreased gum use and lapsing. Or those who experience greater craving while using nicotine gum may conclude it is ineffective and use less gum as a result. This might explain why low gum use in the days immediately pre-lapse is related to the rapidity of relapse; both may reflect failing motivation, self-efficacy, or treatment efficacy.
General motivation to use NRT does not appear to be an underlying cause for these effects. For instance, lapsers and nonlapsers did not significantly differ in the amount of gum they used on the actual quit day. Also, patch use was high and appeared comparable for lapsers and matched nonlapsers in the days before and after the lapse; lapsing did not lead to an immediate steep decline in patch use (the decrease in patch use after lapsing was modest: from using the patch ~90% of days to ~85% of days). Thus, differential patch use by lapsers versus nonlapsers is probably not responsible for the two groups’ differential gum use.
It is unclear why gum use is a relatively sensitive index of lapse while patch use is not. It could be because binary (once-per-day) patch dosing makes patch use a less sensitive index for psychometric reasons or because using the patch requires less effort and therefore is less sensitive to fluctuations in quitting motivation. Further research is needed to understand the causal paths relating pre-lapse and post-lapse gum use with later smoking. It could be revealing, for example, to examine the effects of the amount of ad lib NRT use on later smoking in a cessation trial involving only an oral NRT monotherapy (i.e., nicotine gum or lozenge). One might expect the magnitude of the effects of ad lib NRT use on subsequent smoking to be larger in a context in which the nicotine patch is not also used. It might also be revealing to collect frequent qualitative data pre- and post-lapse to elucidate the reasons smokers report for decreasing their medication use. Researchers could also collect calendar data on stressors and smoking triggers (via either IVR or timeline follow-back) to examine the relationship of such variables with medication adherence and lapses. Finally, it might be fruitful to test interventions, including perhaps just-in-time adaptive mobile health interventions,30 to increase adherence if nicotine gum use is low or begins to decrease, suggesting a possible increased risk of lapsing.
One limitation of this research is the use of memory-dependent timeline follow-back to collect data on patch adherence and smoking lapses. Although the timeline follow-back data on days smoked agreed substantially with the IVR data, there was no doubt some error in estimating lapse timing. Additionally, although the lapsers and nonlapsers did not differ on the baseline variables examined, they may have differed on unmeasured variables. Importantly, they did not differ on baseline motivation to quit, perhaps due to the matching process or because willingness to quit was an inclusion criterion for the study. Another limitation is that this research examined a select sample of smokers (those able to quit for ≥1 day in the first 2 weeks post-TQD) and results may not generalize to other smokers. Finally, post-lapse smoking complicates the interpretation of the post-lapse gum use findings; that is, smoking may have caused lapsers to use less gum (e.g., because smoking provided them with nicotine) or using less gum may have caused lapsers to smoke more. By contrast, interpreting the pre-lapse gum use findings when no participants were smoking is more straightforward.
In conclusion, this research examined electronically monitored nicotine gum use collected in real time among people engaged in a quit attempt using nicotine gum plus nicotine patch. This research revealed that lapsers versus matched nonlapsers tended to decrease their gum use 1–2 days prior to lapsing and lapsers tended to further decrease their gum use after lapsing. There was also a subgroup of lapsers at high risk for relapse who already had low gum use rates pre-lapse and these low use rates persisted post-lapse. These results encourage further exploration of objective measures of smoking medication use patterns to examine their signaling properties and to inform understanding of cessation failure.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This research was supported by grants 9P50CA143188, 5P01CA180945, 2P01CA180945, and 1K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. Dr. Cook is also supported by Merit Review Award 101CX00056 from the United States Department of Veterans Affairs.
Acknowledgments
We acknowledge the staff at Aurora Health Care, Dean Medical Group, and Epic Systems Corporation for their collaboration in this research. We are very grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their help with this research. The primary smoking cessation outcomes from this experiment were reported previously in Schlam et al.1 A poster with a preliminary version of some of the findings in this article was presented at the Society for Research on Nicotine and Tobacco annual convention in 2019. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board, and all participants gave written informed consent.
Declaration of Interests
None declared.
References
- 1. Schlam TR, Fiore MC, Smith SS, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111(1):142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: Data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13(2):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacek LR, McClernon FJ, Bosworth HB. Adherence to pharmacological smoking cessation interventions: a literature review and synthesis of correlates and barriers. Nicotine Tob Res. 2018;20(10):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–1858. [DOI] [PubMed] [Google Scholar]
- 5. Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34(3):212–215. [DOI] [PubMed] [Google Scholar]
- 6. Lam TH, Abdullah AS, Chan SS, Hedley AJ; Hong Kong Council on Smoking and Health Smoking Cessation Health Centre (SCHC) Steering Group . Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacology (Berl). 2005;177(4):400–408. [DOI] [PubMed] [Google Scholar]
- 7. Liberman JN, Lichtenfeld MJ, Galaznik A, et al. Adherence to varenicline and associated smoking cessation in a community-based patient setting. J Manag Care Pharm. 2013;19(2):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catz SL, Jack LM, McClure JB, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;13(5):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hollands GJ, Sutton S, McDermott MS, Marteau TM, Aveyard P. Adherence to and consumption of nicotine replacement therapy and the relationship with abstinence within a smoking cessation trial in primary care. Nicotine Tob Res. 2013;15(9):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raupach T, Brown J, Herbec A, Brose L, West R. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction. 2014;109(1):35–43. [DOI] [PubMed] [Google Scholar]
- 11. Schlam TR, Cook JW, Baker TB, et al. Can we increase smokers’ adherence to nicotine replacement therapy and does this help them quit? Psychopharmacology (Berl). 2018;235(7):2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollands GJ, Naughton F, Farley A, Lindson N, Aveyard P. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database Syst Rev. 2019;8:CD009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102(5):809–814. [DOI] [PubMed] [Google Scholar]
- 14. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piper ME, Federmen EB, McCarthy DE, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117(1):94–105. [DOI] [PubMed] [Google Scholar]
- 16. Etter JF, Schneider NG. An internet survey of use, opinions and preferences for smoking cessation medications: nicotine, varenicline, and bupropion. Nicotine Tob Res. 2013;15(1):59–68. [DOI] [PubMed] [Google Scholar]
- 17. Cooney NL, Cooney JL, Perry BL, et al. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009;104(9):1588–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimmerling M, Wagner G, Ghosh-Dastidar B. Factors associated with accurate self-reported adherence to HIV antiretrovirals. Int J STD AIDS. 2003;14(4):281–284. [DOI] [PubMed] [Google Scholar]
- 19. Wagner GJ, Rabkin JG. Measuring medication adherence: are missed doses reported more accurately then perfect adherence? AIDS Care. 2000;12(4):405–408. [DOI] [PubMed] [Google Scholar]
- 20. Raupach T, Shahab L, Neubert K, Felten D, Hasenfuss G, Andreas S. Implementing a hospital-based smoking cessation programme: evidence for a learning effect. Patient Educ Couns. 2008;70(2):199–204. [DOI] [PubMed] [Google Scholar]
- 21. Schneider MP, van Melle G, Uldry C, et al. Electronic monitoring of long-term use of the nicotine nasal spray and predictors of success in a smoking cessation program. Nicotine Tob Res. 2003;5(5):719–727. [DOI] [PubMed] [Google Scholar]
- 22. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. [DOI] [PubMed] [Google Scholar]
- 23. Fraser D, Christiansen BA, Adsit R, Baker TB, Fiore MC. Electronic health records as a tool for recruitment of participants’ clinical effectiveness research: lessons learned from tobacco cessation. Transl Behav Med. 2013;3(3):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piper ME, Baker TB, Mermelstein R, et al. Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Transl Behav Med. 2013;3(3):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolt DM, Piper ME, McCarthy DE, et al. The Wisconsin predicting patients’ relapse questionnaire. Nicotine Tob Res. 2009;11(5):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 27. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. [DOI] [PubMed] [Google Scholar]
- 28. De Bleser L, Vincke B, Dobbels F, et al. A new electronic monitoring device to measure medication adherence: usability of the Helping Hand™. Sensors (Basel). 2010;10(3):1535–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergstralh EJ, Kosanke JL.. Computerized Matching of Cases to Controls. Technical Report Series No. 56. Rochester, MN: Department of Health Science Research, Mayo Clinic; 1995. [Google Scholar]
- 30. Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.