Abstract
BACKGROUND
Post-traumatic stress disorder is a severe psychobiological disorder associated with hyperactivity of the amygdala, particularly on the right side. Highly selective laser ablation of the amygdalohippocampal complex is an effective neurosurgical treatment for medically refractory medial temporal lobe epilepsy that minimizes neurocognitive deficits relative to traditional open surgery.
OBJECTIVE
To examine the impact of amygdalohippocampotomy upon symptoms and biomarkers of post-traumatic stress disorder.
METHODS
Two patients with well-documented chronic post-traumatic stress disorder who subsequently developed late-onset epilepsy underwent unilateral laser amygdalohippocampotomy. Prospective clinical and neuropsychological measurements were collected in patient 1. Additional prospective measurements of symptoms and biomarkers were collected pre- and post-surgery in patient 2.
RESULTS
After laser ablation targeting the nondominant (right) amygdala, both patients experienced not only reduced seizures, but also profoundly abated post-traumatic stress symptoms. Prospective evaluation of biomarkers in patient 2 showed robust improvements in hyperarousal symptoms, fear potentiation of the startle reflex, brain functional magnetic resonance imaging responses to fear-inducing stimuli, and emotional declarative memory.
CONCLUSION
These observations support the emerging hypothesis that the right amygdala particularly perpetuates the signs and symptoms of post-traumatic stress disorder and suggests that focal unilateral amydalohippocampotomy can provide therapeutic benefit.
Keywords: Anxiety, Amygdala, Hippocampus, Resection, Lesion, Ablation, Epilepsy, Post-traumatic stress disorder
ABBREVIATIONS
- BAI
Beck Anxiety Inventory
- BDI-II
Beck Depression Inventory II
- CAPS-5
Clinician-Administered PTSD Scale of the DSM-5
- CS+
Conditioned Stimulus (+) paired with noxious stimulus
- CS-
Conditioned Stimulus (-) unpaired with noxious stimulus
- dACC
dorsal Anterior Cingulate Cortex
- fMRI
functional MRI
- IAPS
International Affective Picture System
- MRI
magnetic resonance imaging
- MTLE
Medial Temporal Lobe Epilepsy
- PTSD
Post-Traumatic Stress Disorder
Post-traumatic stress disorder (PTSD) is a severe psychobiological disorder that can develop after experiencing an event that causes or threatens serious harm. It is estimated to affect 6% to 8% of the general population,1 and 31% to 46% of military or highly traumatized civilian populations.2 Symptoms include unwanted re-experiencing of the event, hyperarousal and emotional distress or physical reactivity when confronted with trauma reminders, avoidance of trauma-related stimuli, and negative alterations in cognition and mood. PTSD is typically treated with trauma-focused psychotherapy with or without medications.3 Unfortunately, 30% to 50% of patients do not respond,4 rendering them chronically affected by emotional symptoms and psychosocial burden.
Functional brain imaging studies consistently indicate increased amygdala activation in PTSD5 and, moreover, show that amygdala hyper-reactivity predicts the development and maintenance of future PTSD symptoms6 as well as PTSD treatment nonresponse.7,8 The amygdala is a region that is critical for the formation and expression of fear memories, and (in particular, right) amygdala hyperactivation has been associated with PTSD hyperarousal symptoms.5 Furthermore, decreased prefrontal inhibition of the amygdala has often been demonstrated in PTSD.5 One notable study of combat veterans suffering traumatic brain injury suggested that damage encompassing the amygdala protected against subsequently developing PTSD.9 Taken together, these findings predict that targeting the amygdala could improve PTSD symptoms. We specifically hypothesized that reducing amygdala activity via surgical intervention would ameliorate chronic PTSD symptoms. Here, we took advantage of a clinical circumstance requiring the removal of the amygdalohippocampal complex via highly selective laser ablation to treat medically refractory medial temporal lobe epilepsy (MTLE) in 2 patients with comorbid PTSD. This allowed a scientific examination of PTSD symptoms and related biomarkers before and after surgery.
METHODS
Ethical Approval
After complete description of the study, both participants provided their written informed consent. Approval of study procedures was provided by the Institutional Review Board of Emory University and the Research Oversight Committee of Grady Memorial Hospital (IRB# 00010651 and 00078593).
Procedures
Patients underwent standard neuropsychological testing (Table, Supplemental Digital Content 1), which included prospective clinical interviews at preoperative and postoperative time points. Patient 2 underwent prospective PTSD symptom assessment via the CAPS-5, a clinician-administered assessment, as well as prospective neuroimaging and psychophysiological testing before and after amygdalohippocampotomy to characterize changes in PTSD-related biomarkers as described in Methods, Supplemental Digital Content 2.
In the case of patient 1, direct PTSD symptom scales were not available from the preoperative time point because of long chronicity of PTSD and reluctance on the part of the patient to engage in any intervention to address his symptoms. Upon the resolution of his PTSD symptoms following amygdala ablation, he and his wife reported the change to our team, prompting documentation of the change. The study neuropsychologist had conducted clinical interviews with the patient at preoperative and postoperative time points, which were examined post hoc to reconstruct approximate scores on a PTSD symptom checklist (PCL-M), reported in the Results section. The clinical circumstances necessitated this approach, the limitations of which are described in the Discussion section.
Patients
Patient 1 was a 62-yr-old Caucasian male veteran of the Vietnam War with a >30-yr history of combat-related PTSD symptoms who presented for surgical evaluation of late-onset medically refractory right MTLE. His trauma exposure was a blast injury in 1972, in which fellow soldiers were killed. His PTSD symptoms had been refractory to medications, he had been unable to tolerate cognitive behavioral therapy, and he had undergone no other interventional procedures. His first seizure was 14 yrs prior to presentation. Video-electroencephalography documented right-anterior-temporal-onset dyscognitive seizures. Brain magnetic resonance imaging (MRI) demonstrated mild microvascular ischemic changes, functional MRI (fMRI) suggested left language dominance, and interictal fluorodeoxyglucose positron emission tomography demonstrated right medial temporal hypometabolism. He underwent uncomplicated right laser amygdalohippocampotomy for MTLE (Figure 1), and he was discharged on his home doses of antiepileptic medications.
FIGURE 1.
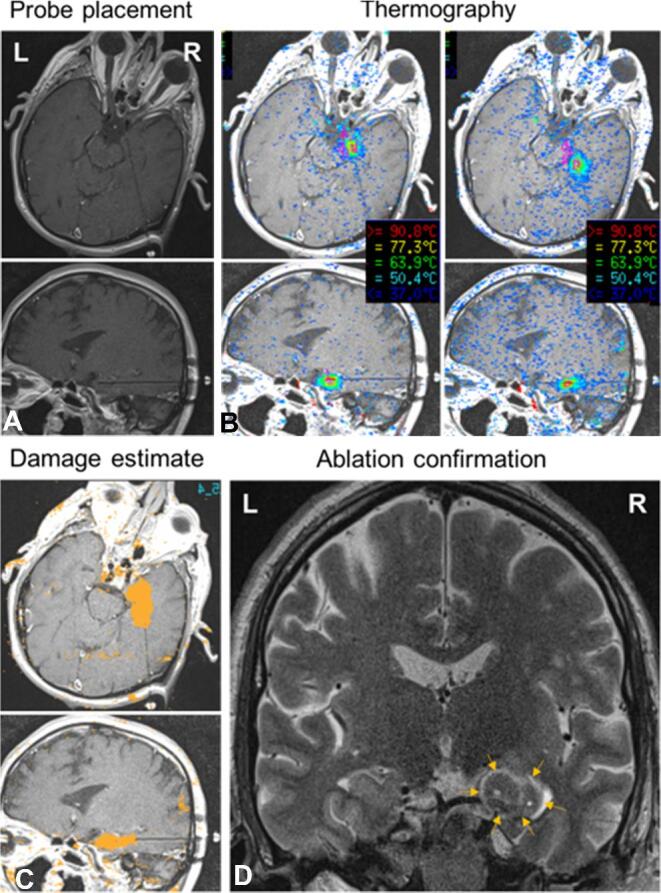
Stereotactic laser amygdalohippocampotomy in patient 1. A, Probe placement panels show initial laser probe location in approximately axial and sagittal trajectory views of T1-weighted sequence MRI. The probe is shown to pass from a right posterior approach through the anterior hippocampus to terminate in the amygdala. B, Thermography views show screenshots of real-time MR thermographic heat maps coregistered to anatomic images during ablation. Sequential steps of interstitial thermal ablation of the right amygdala, and anterior hippocampus, along a length of the laser probe are shown. C, Damage estimates demonstrate cumulative calculated irreversible damage zones (orange areas) in each trajectory plane. D, Ablation confirmation (T2-weighted inversion-recovery-sequence MRI) confirms the extent of final ablation in a standard coronal view. Arrows demarcate the rim of hyperintense edema surrounding the ablation zone.
Patient 2 was a 42-yr-old African American woman with a 19-yr history of civilian-related PTSD symptoms refractory to psychotherapy and antidepressant medications who presented with medically refractory right MTLE. Trauma exposures included multiple instances of fatal violence against family members. Her first seizure occurred 3 yrs prior to presentation. Video-electroencephalography documented right-anterior-temporal-lobe-onset dyscognitive seizures with frequent secondary generalization. Brain MRI was unremarkable, fMRI suggested left language dominance, and interictal FDG-PET demonstrated right medial temporal hypometabolism. At the time of presentation for epilepsy surgery, she had remained symptomatic with PTSD despite having been treated with antidepressants, anxiolytics, and cognitive behavioral therapy under the care of a psychologist. She had undergone no other interventions. She underwent uncomplicated right laser amygdalohippocampotomy for MTLE (Figure 2) and was discharged on stable medications.
FIGURE 2.
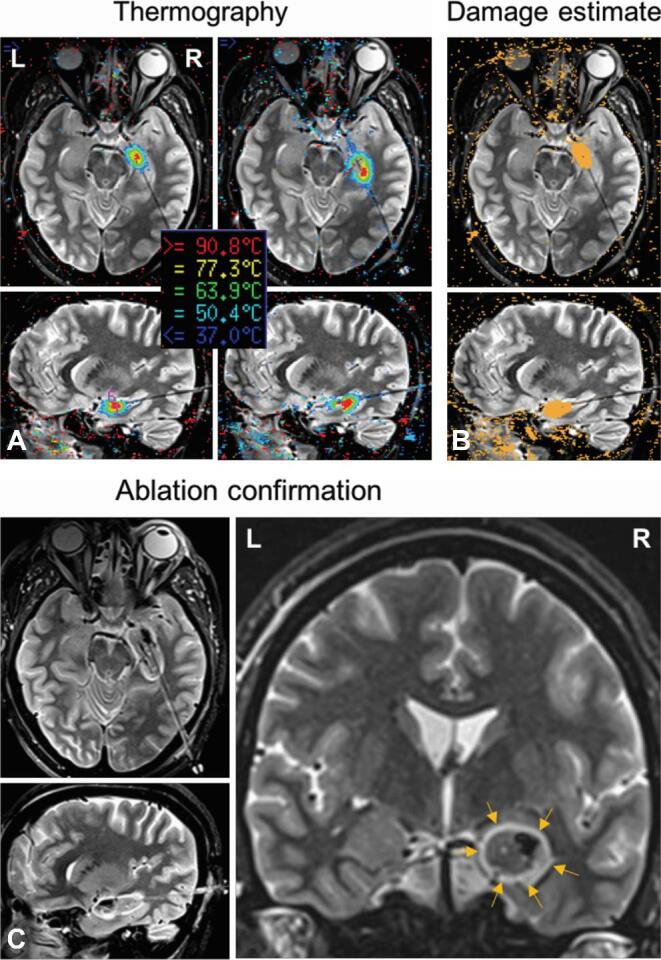
Stereotactic laser amygdalohippocampotomy in patient 2. A, Thermography views demonstrate probe location in approximately axial and sagittal trajectory views of T2-weighted inversion-recovery-sequence MRI. Coregistered thermographic heat maps display sequential real-time ablation of right amygdala and anterior hippocampus along a length of the laser probe. B, Damage estimates demonstrate cumulative calculated irreversible damage zones (orange areas) for individual ablation locations in each trajectory plane. C, Ablation confirmation (T2-weighted inversion-recovery) shows the extent of final ablation in trajectory and standard coronal views. Arrows demarcate the rim of hyperintense edema surrounding the ablation zone.
Data Availability
Data underlying the conclusions of this manuscript are presented in Methods, Supplemental Digital Content 2, and further deidentified data are available upon reasonable request.
RESULTS
Patient 1 remained seizure-free throughout 12 mo of follow-up. At 6-wk and 3-mo follow-ups, he and his wife reported profound amelioration of PTSD symptoms, including reduced aggression in response to triggers, increased emotional range, and improved mood. At 6 mo and 12 mo, the patient reported much less hypervigilance, anger, and irritability. He felt able to “reason through things rather than emote,” and reported increased energy and physical stamina. He had volitionally reentered group cognitive behavioral therapy, of which he was previously intolerant. Patient 1 completed prospective neuropsychometric evaluations including a clinical interview, which compared preoperative baseline to postoperative status (Table, Supplemental Digital Content 1). Neuropsychological clinical interview data were evaluated retrospectively against the standard PTSD scale (PCL-M; Weathers et al10) to provide clinician-estimated PCL scores from pre-surgical and post-surgical time points. The estimated baseline PCL total symptom severity score for patient 1 was 66 (maximum score 85), which was reduced to 34 at the postsurgical time point. Analysis using the PCL total symptom severity score has demonstrated a clinically meaningful change when a reduction of greater than 10 points is present,10 and other studies11,12 have considered a 30% decrease to be a positive treatment response, so the estimated reduction of 32 points (or 48%) is likely to be highly clinically meaningful. With regards to individual DSM-IV criteria for PTSD, the reduction was greatest in the area of hyperarousal (cluster D; 21 to 9), but reductions were also seen in re-experiencing (cluster B; 19 to 9) and avoidance and numbing (cluster C; 26 to 19) symptom clusters. As well, Beck Depression and Anxiety Inventories (BDI-II; BAI) showed significant improvements following surgery, with depressive symptoms declining from moderate to mild (BDI-II: pre = 26, 6 mo-post = 12, and 12 mo-post = 16) and anxiety declining from moderate/severe to minimal or mild (BAI: pre = 21, 6 mo-post = 2, and 12 mo-post = 12). At 12 mo, he had experienced improvements in complex attention (eg, sequencing, shifting between conceptual sets), famous-person naming, and stable verbal memory. By contrast, he exhibited 1 to 2 standard deviation declines in some aspects of visual memory and spatial learning and recall, domains considered at risk with nondominant medial temporal lobe surgery. The patient's ability to recognize emotional state from facial expressions showed no postoperative change, remaining low average.
Over 22 mo of follow-up, patient 2 experienced greatly reduced frequency and severity of seizures, which only recurred when she missed taking her antiseizure medicine lamotrigine. She reported decreased use of a sedative (trazadone) by 5 mo, and her lamotrigine prescription was increased slightly at 7 mo. At the 12-mo follow-up, she reported perceived improvements in memory and quality of life. She underwent neuropsychological testing at 22 mo after surgery and exhibited mild improvements in language, verbal memory, and motor speed (Table, Supplemental Digital Content 1). She exhibited mild declines in aspects of nonverbal intellectual function (eg, complex visual reasoning/pattern recognition).
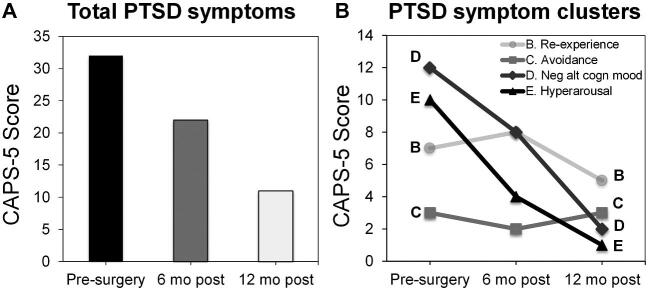
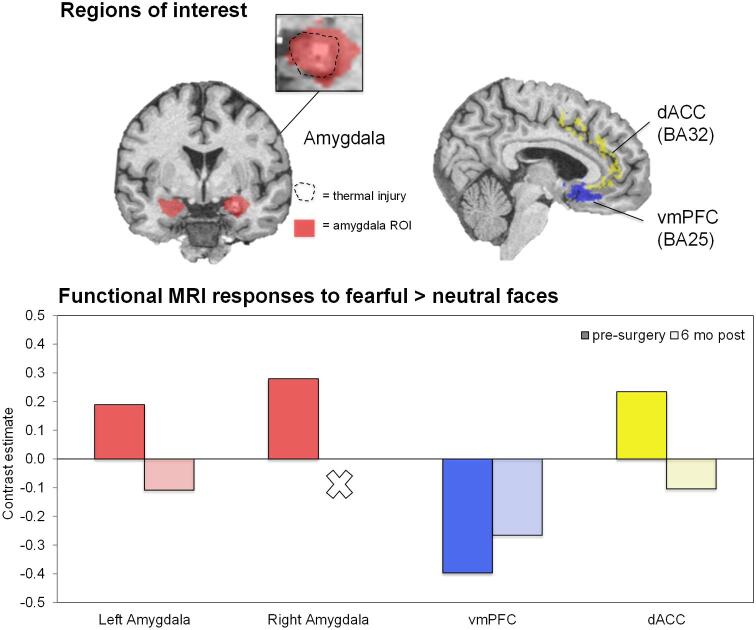
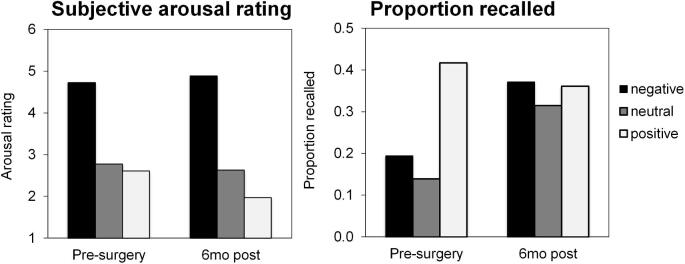
Patient 2 completed prospective measures of PTSD symptoms and biomarkers, which compared preoperative baseline to postoperative status (details in methodological supplement). She endorsed a high preoperative PTSD clinical symptom burden (Clinician-Administered PTSD Scale-5 = 33), which improved (-31% at 6 mo and -68% by 12 mo) such that she no longer met clinical diagnostic criteria for PTSD (Figure 3). This improvement was driven by greatest reductions in domains of hyperarousal (-90%) and negative alterations in cognition and mood (-83%) by 12 mo following surgery. BDI-II decreased from severe depression (pre = 31) to mild depression (6 mo-post = 15 and 12 mo-post = 19). Pre- and post-ablation fMRI scans were obtained during passive viewing of neutral and fearful faces (Figure 4), a task preferentially activating the amygdalae and prefrontal regulatory regions, including the ventromedial prefrontal cortex and dorsal anterior cingulate cortex (dACC).5 The patient preoperatively showed elevated bilateral amygdala and dACC activation to fearful faces vs neutral faces, similar to that reported in PTSD patients.5 These activations were reduced postablation, a pattern previously observed in PTSD patients experiencing treatment response.13 During a psychophysiological fear-potentiated startle task, she preoperatively showed high fear-conditioned responses and failure to appropriately discriminate danger and safety cues (Figure 5), consistent with severe PTSD. Postoperatively, fear-conditioned responses were clearly diminished with a greater reduction in response to the safety cue (improved fear inhibition), consistent with treatment response.14 Finally, patient 2 completed an emotional memory task pre- and post-operatively, in which her memory for photographs with emotional content underwent global improvements (Figure 6).
FIGURE 3.
Clinical PTSD outcome following ablation. A, Total PTSD symptoms as measured by the Clinician-Administered PTSD Scale (CAPS-5) in patient 2. B, PTSD symptom clusters breaks down the total PTSD scores at each time point to symptom domains measured by the CAPS-5, referred to as criteria B, C, D, and E. C, D, and E are postablation changes in PTSD-related markers.
FIGURE 4.
Postablation changes in PTSD-related neural activations. Changes in activation at 4 regions of interest (left amygdala, right amygdala, ventromedial prefrontal cortex, and dorsal anterior cingulate cortex) from presurgery to 6 mo postsurgery in patient 2. Region of interest boundaries are demonstrated on coronal and sagittal slices, as well as the ablation extent overlaid on the right amygdala region of interest.
FIGURE 5.
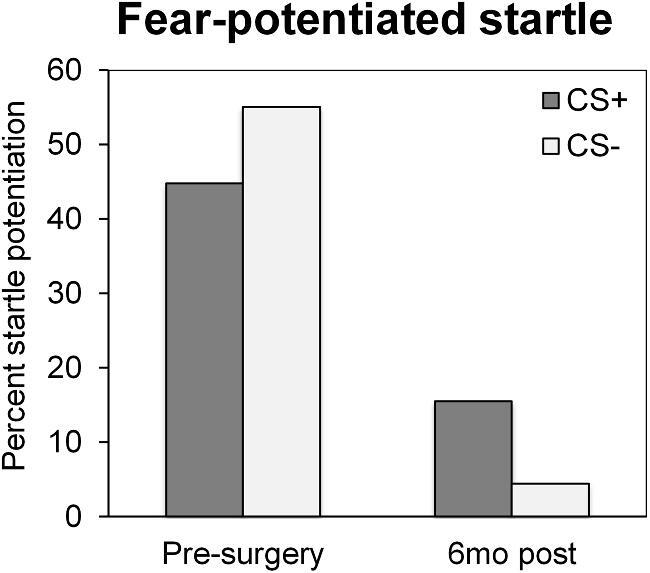
Change in fear-potentiated startle responses following amygdala ablation. Change is demonstrated from presurgery to 6 mo postsurgery in response to the conditioned stimulus paired with noxious stimulus (CS+) and to the conditioned stimulus not paired with noxious stimulus (CS-).
FIGURE 6.
Postablation change in emotional memory. Change in emotional memory from presurgery to 6 mo postsurgery in terms of the ratings subjective arousal and proportion of images recalled for negative, neutral, and positive images presented from the International Affective Picture System (IAPS).
DISCUSSION
To our knowledge, this is the first prospective investigation of the effects of amygdala ablation on PTSD. We describe 2 patients in whom highly selective right amygdalohippocampotomy for epilepsy was associated with profound improvements of symptoms and biological markers of established PTSD. Although a previous retrospective lesion study showed that traumatic injury encompassing either amygdala was protective against the development of PTSD,9 we found that ablating the right amygdala can ameliorate established PTSD. Thus, although intact bilateral amygdalae are necessary to develop PTSD, the nondominant amygdala may maintain PTSD. If our study is replicated in PTSD patients without epilepsy, it would support the notion that refractory PTSD could be treated with unilateral amygdalotomy. Given the high nonresponder rate with current therapies, novel interventions for PTSD should be considered.
Bilateral amygdalotomy is reported sporadically in the historical medical literature, falling out of favor by the 1970s because of emerging pharmacologic alternatives and growing public skepticism related to indiscriminate use, poor patient selection, and potentially serious side effects. Recent technical and neuroscientific advances, however, support reconsideration of focal interventions for intractable psychiatric and cognitive conditions. Compared to traditional open temporal lobe surgery, stereotactic laser amygdalohippocampotomy provides a minimally invasive, highly selective approach that minimizes collateral injury and yields a more positive impact on cognition.15 By targeting the persistent amygdala hyperactivation that is observed in treatment nonresponders,8,16 amygdalotomy likely reduces hyperarousal, making trauma-focused therapy more tolerable and effective.
The current findings could imply a pathophysiological link between PTSD and the development of late-onset MTLE. Although differing in age, sex, race, and type of trauma, our patients only developed epilepsy decades after having chronic PTSD. Thus, PTSD may be a risk factor for developing late-onset MTLE through a shared substrate of pathological amygdala activity. Indeed, amygdala overactivation by repeated electrical stimulation is well recognized to kindle epilepsy in animal models. Notably, a recent demographic study found that having PTSD is associated with an elevated risk of subsequently developing epilepsy.17
Limitations
This study has important limitations. First, we ablated the right amygdala but did not directly evaluate the influence of the left amygdala upon PTSD. Previous functional imaging studies suggest right > left amygdala hyperactivation in PTSD.5 Two published cases report either onset or worsening of PTSD after open left temporal lobectomy,18,19 but the effects of a highly selective left amygdalotomy on PTSD remain unknown. Second, we cannot exclude the possibility that ipsilateral hippocampotomy contributed to amelioration of PTSD. Neuroimaging studies, however, suggest that intact hippocampal functioning contributes to resilience,7 at least in subjects without coexisting MTLE. Third, we cannot conclude that PTSD symptoms improved independently from amelioration of epilepsy. Seizure reduction, however, is unlikely to fully explain our findings given that patient 2 continued to experience seizures, whereas PTSD symptoms improved. Fourth, there are important limitations to the interpretation of the estimated PCL-M scores for patient 1, which were not provided directly by the patient and were instead estimated by the patient's clinical neuropsychologist on the basis of documentation from prospective clinical interviews at both preoperative and postoperative time points in which all relevant symptom domains were discussed. These ratings are likely to provide a gross snapshot of the patient's symptom burden but are also limited by the absence of rigorously validated methods for reconstruction from clinical data.
CONCLUSION
The current study examined the hypothesis that right amygdalohippocamptomy for MTLE may have previously unrecognized benefits for the symptoms of PTSD. Specifically, in 2 cases, selective laser ablation of the right (nondominant) amygdala and anterior hippocampus benefitted symptoms and biomarkers of comorbid PTSD. Thus, the right amygdala may particularly perpetuate PTSD. With further investigation, this procedure may prove effective for refractory PTSD.
Disclosures
The current study was supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation to Dr. van Rooij, from the NIH-NCATS (UL1TR002378, to Dr. Bijanki: KL2 TR002381), an Emory University Research Committee pilot grant to Dr. Bijanki, the NINDS (Dr. Bijanki and Dr. Willie: R21 NS104953; Dr. Drane: R01 NS088748), and by the NIMH (Dr. Bijanki: K01 MH116364; Dr. Jovanovic: R01 MH100122). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr. Willie serves as a consultant for Medtronic Inc., MRI Interventions Inc., and Neuropace Inc. Dr. Drane receives grant funding from Medtronic Inc. and honoraria from Neuropace Inc.
Supplementary Material
Acknowledgments
The authors would like to thank the patients for their willingness to participate in this research.
Notes
This work will be presented as a poster presentation at the Society of Biological Psychiatry Annual Scientific Meeting in New York, New York, on April 30, 2020; Bijanki, KR, van Rooij, SJH, Inman, C, Carter, SE, Ely, TD, Jovanovic, T, Willie, JT, (poster) Case series: ablating the non-dominant amygdala improves PTSD symptoms and biomarkers in human epilepsy patients. This work has been previously presented as a poster at the American College of Neuropsychopharmacology Annual Meeting in Hollywood, Florida, on December 12, 2018; van Rooij, SJH, Bijanki, KR, Inman, C, Carter, SE, Ely, TD, Jovanovic, T, Willie, JT, (poster) Amygdala ablation: a neurosurgical approach to PTSD treatment? Annual Meeting of the American College of Neuropsychopharmacology. This work has been presented as a poster presentation at the American Society of Clinical Psychopharmacology Annual Meeting in Scottsdale, Arizona, on May 29, 2019; van Rooij, SJH, Bijanki, KR, Ely, TD, Stevens, JS, Inman, C, Carter, SE, Jovanovic, T, Willie, JT, (poster, New Investigator Award) Effect of Amygdala Ablation on PTSD Symptoms and Biomarkers. Annual Meeting of the American Society of Clinical Psychopharmacology Annual Meeting.
Contributor Information
Kelly R Bijanki, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas; Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia; Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Sanne J H van Rooij, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Timothy D Ely, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Jennifer S Stevens, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Cory S Inman, Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia.
Rebecca E Fasano, Department of Neurology, Emory University School of Medicine, Atlanta, Georgia.
Sierra E Carter, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Sterling J Winters, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Justin R Baman, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia.
Daniel L Drane, Department of Neurology, Emory University School of Medicine, Atlanta, Georgia; Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia.
Tanja Jovanovic, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia; Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, Michigan.
Jon T Willie, Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia; Department of Neurology, Emory University School of Medicine, Atlanta, Georgia.
Supplemental Digital Content 1. Table. Preoperative and postoperative neuropsychological scores for patients 1 and 2. Raw = raw score, SS = scaled score, %ile = percentile, WNL = within normal limits, Z = normalized z-score, * = postoperative measures collected at 6 mo (all other measures were collected at 12 mo), ↓ = 1-standard deviation (SD) decline, ↓↓ = 2-SD decline, ↑ = 1-SD improvement, ↑↑ = 2-SD improvement. Statistical changes are measured against normative data sets (Drane, 201814; Drane et al, 201514; Gross et al, 201815).
Supplemental Digital Content 2. Methods. Supplementary material: clinical and methodological addenda.
COMMENT
The authors of this study observed impressive improvements in post-traumatic stress disorder (PTSD) in two patients who underwent MRI-guided stereotactic laser amygdalohippocampectomy for treatment of medically intractable mesial temporal lobe epilepsy. Both seizures and PTSD improved after the procedure and remained improved over a 1–2 years of follow-up.
It is difficult to draw conclusions from a single-center experience with two clinical cases, particularly in light of limitations that are thoughtfully spelled out by the authors in their discussion. Nevertheless, I feel that this limited experience may serve as a basis for future investigations, perhaps focusing on metabolic activity of both operated and contralateral amygdalae before and after the intervention, checking effect of left sided surgery in similar clinical circumstances, separating amygdala and hippocampal lesioning and, eventually, considering similar or even more focused/less invasive interventions (with stimulation rather than ablation?) in patients with PTSD without epilepsy.
Konstantin Slavin
Chicago, Illinois
REFERENCES
- 1. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593-602. [DOI] [PubMed] [Google Scholar]
- 2. Gillespie CF, Bradley B, Mercer K et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Association AP. Diagnostic and statistical manual of mental disorders. 5th ed.Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 4. Bradley RG, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatr. 2005;162(2):214-227. [DOI] [PubMed] [Google Scholar]
- 5. Stevens JS, Jovanovic T, Fani N et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47(10):1469-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens JS, Reddy R, Kim YJ et al. Episodic memory after trauma exposure: medial temporal lobe function is positively related to re-experiencing and inversely related to negative affect symptoms. NeuroImage Clin. 2017;17:650-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Rooij SJH, Kennis M, Vink M, Geuze E. Predicting treatment outcome in PTSD: a longitudinal functional MRI study on trauma-unrelated emotional processing. Neuropsychopharmacology. 2016;41(4):1156-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fonzo GA, Goodkind MS, Oathes DJ et al. PTSD psychotherapy outcomes predicted by brain activation during emotional reactivity and regulation. Am J Psychiatry. 2017;174(12):1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koenigs M, Huey ED, Raymont V et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11(2):232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20(2):131-138. [DOI] [PubMed] [Google Scholar]
- 11. Brady K, Pearlstein T, Asnis GM et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844. [DOI] [PubMed] [Google Scholar]
- 12. Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58(5):485-492. [DOI] [PubMed] [Google Scholar]
- 13. Roy MJ, Francis J, Friedlander J et al. Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:142-149. [DOI] [PubMed] [Google Scholar]
- 14. Jovanovic T, Norrholm SD, Fennell JE et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167(1-2):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gross RE, Stern MA, Willie JT et al. Stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Ann Neurol. 2018;83(3):575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Rooij SJH, Stevens JS, Ely TD et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen YH, Wei HT, Bai YM et al. Risk of epilepsy in individuals with posttraumatic stress disorder: a nationwide longitudinal study. Psychosom Med. 2017;79(6):664-669. [DOI] [PubMed] [Google Scholar]
- 18. Smith SD, Abou-Khalil B, Zald DH. Posttraumatic stress disorder in a patient with no left amygdala. J Abnorm Psychol. 2008;117(2):479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adami P, Konig P, Vetter Z, Hausmann A, Conca A. Post-traumatic stress disorder and amygdala-hippocampectomy. Acta Psychiatr Scand. 2006;113(4):360-363; discussion 363-364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the conclusions of this manuscript are presented in Methods, Supplemental Digital Content 2, and further deidentified data are available upon reasonable request.