Abstract
Background
[177Lu]Lu-DOTA-ZOL has shown promising results from the dosimetry and preclinical aspects, but data on its role in the clinical efficacy are limited. The objective of this study is to evaluate the efficacy and safety of [177Lu]Lu-DOTA-ZOL as a bone pain palliation agent in patients experiencing pain due to skeletal metastases from various cancers.
Methods
In total, 40 patients experiencing bone pain due to skeletal metastases were enrolled in this study. The patients were treated with a mean cumulative dose of 2.1 ± 0.6 GBq (1.3–2.7 GBq) [177Lu]Lu-DOTA-ZOL in a median follow-up duration of 10 months (IQR 8–14 months). The primary outcome endpoint was response assessment according to the visual analogue score (VAS). Secondary endpoints included analgesic score (AS), global pain assessment score, Eastern Cooperative Oncology Group Assessment performance status (ECOG), Karnofsky performance status, overall survival, and safety assessment by the National Cancer Institute’s Common Toxicity Criteria V5.0.
Results
In total, 40 patients (15 males and 25 females) with a mean age of 46.6 ± 15.08 years (range 24–78 years) were treated with either 1 (N = 15) or 2 (N = 25) cycles of [177Lu]Lu-DOTA-ZOL. According to the VAS response assessment criteria, complete, partial, and minimal responses were observed in 11 (27.5%), 20 (50%), and 5 patients (12.5%), respectively with an overall response rate of 90%. Global pain assessment criteria revealed complete, partial, minimal, and no response in 2 (5%), 25 (62.5%), 9 (22.5%), and 4 (10%) patients, respectively. Twenty-eight patients died and the estimated median overall survival was 13 months (95% CI 10–14 months). A significant improvement was observed in the VAS, AS, and ECOG status when compared to baseline. None of the patients experienced grade III/IV haematological, kidney, or hepatotoxicity due to [177Lu]Lu-DOTA-ZOL therapy.
Conclusion
[177Lu]Lu-DOTA-ZOL shows promising results and is an effective radiopharmaceutical in the treatment of bone pain due to skeletal metastases from various cancers.
Keywords: [177Lu]Lu-DOTA-ZOL, Pain palliation, Skeletal metastases
Introduction
Bone is the most common site of metastases in the majority of the solid cancers. Skeletal metastases from prostate and breast cancer account for approximately 80% of all the bone metastases followed by lung and renal cancers that comprise 20–40% of all the patients [1]. The typical clinical symptom of skeletal metastases is bone pain. Apart from pain, other skeletal-related events (SREs), albeit to a lesser extent, are swelling, nerve compression, immobility, or pathological fractures. At the same time, few patients are even asymptomatic, particularly in the early part of the disease with marrow metastases.
Several algorithms have been evolved over the last 3 decades for the management of metastatic bone pain and SREs [2] in which a range of systemic to locoregional therapies are advocated. The most common approaches in clinical practice are chemotherapy, hormonal therapy, bisphosphonates, monoclonal antibody, namely denosumab and analgesics like non-steroidal anti-inflammatory drugs and opioids, molecules signalling growth factors, antidepressants, and endothelin receptor antagonists. Locoregional therapies are offered only for patients with oligo-metastases, namely, external beam radiotherapy (EBRT). Even though the list seems vast, none of them are curative in practice, with a majority of patients limited to palliative care, and involve a multidisciplinary approach.
Palliative external beam radiotherapy is the most effective way of bone pain management. Studies have revealed that 80–90% of patients receiving EBRT have demonstrated complete or partial pain relief within 10–14 days from initiation of treatment. However, EBRT, as mentioned above, is only limited to the treatment of oligo-metastases or in a worst-case scenario to hemi-body irradiation [3].
Radionuclide therapy is a systemic form of internal radiotherapy which constitutes an essential option as a routine part of the multidisciplinary treatment approach for decades. Several beta-emitting radiometals have been exploited for this purpose that includes 89Sr 186Re, 188Re, and 153Sm. All of them have been incorporated into bone-seeking phosphonates, except 89Sr. The 89Sr is a bivalent cation sharing properties similar to calcium. The same is true for 223Ra, the most recent radionuclide which has been approved for pain palliation in prostate cancer without visceral metastasis [4], except that it is an alpha emitter. However, the availability and the cost of 223Ra pose paramount restraints for most of the developing world. In this scenario, beta emitters are still affordable and an acceptable option. Owing to the suitable physical properties and decay characteristics of 177Lu [t1/2 = 6.73 days Eβmax = 497 keV, Eγ) = 113 keV (6.4%), 208 keV (11%)], it is now widely used in clinical practice. It is either produced via the 176Lu(n,γ) [5] or the 176Yb(n,γ) pathway [6].
For bone pain palliation it has been labelled with several bisphosphonates such as ethylene diamine tetramethylene phosphonic acid (EDTMP) [7–9], (4-{[(bis(phosphonomethyl)) carbamoyl]methyl}-7,10-bi (carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl) acetic acid (BPAMD) [10–15] Zoledronate (DOTA-ZOL) [16–22], and DOTMP [23, 24]. Zoledronic acid, compared to EDTMP and DOTMP, has significantly higher hydroxyapatite binding and better internalisation by osteoclasts. These superior antiresorptive properties lead to increased apoptosis [25]. DOTA-ZOL can be labelled with both 68Ga and 177Lu to form a theranostic pair [19]. However, only two studies have reported its safety from the dosimetric aspects [26, 27]. Recently, in a pilot study, Nikzad et al. [26] labelled DOTA-zoledronate with 177Lu and have shown promising results and comparable pharmacokinetics to [177Lu]Lu-EDTMP. While dosimetry data revealed a higher absorbed dose for [177Lu]Lu-DOTA-ZOL compared to [177Lu]Lu-EDTMP (12.17 vs. 10.02 mSv/MBq) in the trabecular bone surface, the absorbed dose to the critical organs and the muscle from [177Lu]Lu-DOTA-ZOL was much lower compared to that of [177Lu]Lu-EDTMP [27] consistent with the results of the above study. Khawar et al. [21] revealed a similar biodistribution and normal organ absorbed doses of [177Lu]Lu-DOTAZOL. However, to the best of our knowledge, investigation on the efficacy and safety of [177Lu]Lu-DOTA-ZOL for pain palliation in a clinical setting has not been reported to date. Hence, in the present study, we aim to report the efficacy and toxicity data of the patients, treated with [177Lu]Lu-DOTA-ZOL, for the bone pain palliation of skeletal metastases from various cancers.
Materials and methods
The study was conducted at the Department of Nuclear Medicine, AIIMS, New Delhi, India. Skeletal metastases patients suffering from pain were referred from the Pain Clinic, Medical Oncology, and Radiation Oncology departments for [177Lu]Lu-DOTA-ZOL pain palliation treatment. This cohort study involved patients who were treated with [177Lu]Lu-DOTA-ZOL for pain palliation between January 2017 and February 2020.
Eligibility criteria
Eligibility criteria for the [177Lu]Lu-DOTA-ZOL pain palliation treatment included: histologically confirmed breast, prostate, or lung cancers, progressive pain or pain requiring escalation of analgesics, patients with more than one site of pain corresponding to the avid uptake on [68Ga]Ga-DOTA-ZOL PET/CT scan, patients with no prior history of radionuclide pain palliation therapy, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 4, KPS ≥ 50, patient on or with history of prior bisphosphonates, patients with haematological, kidney, and liver function parameters within normal limits which included baseline haemoglobin of < 9 g/dL, platelet counts: < 75,000/μL, leukocyte counts: ≥ 4 × 109/L, serum creatinine: > 1.4 mg/dL, serum bilirubin > 3 mg%, glomerular filtration rate (GFR): < 50 mL/min per 1.73 m2 body surface area (BSA). Patients with skeletal-related events involving pathological fractures and cord compression were not included.
[177Lu]Lu-DOTA-ZOL synthesis
The stock solution consisted of 1 mg DOTA-ZOL dissolved in 1 mL ultrapure water to give a concentration of 60 µg/60 µL. The 60 µL of DOTA-ZOL was radiolabelled with [177Lu]LuCl3 which was obtained from BRIT, India, in sodium ascorbate buffer, pH 4, in 0.01 M supra pure HCl with a specific activity ranging between 370 and 740 GBq/mg. The radiolabelled solution was heated at 95 °C for 30 min. Radiochemical quality control was carried out using the instant thin-layer chromatography method with sodium citrate buffer as the solvent.
Treatment protocol and follow-up
[177Lu]Lu-DOTA-ZOL infusion
The patients who fulfilled the eligibility criteria were administered with a fixed dose of 1295 MBq (35 mCi) [177Lu]Lu-DOTA-ZOL. The fixed activity of 1295 MBq of [177Lu]Lu-DOTA-ZOL was extrapolated from our previous [177Lu]Lu-EDTMP phase II data [8]. The infusion involved a dilution of [177Lu]Lu-DOTA-ZOL in 10 mL normal saline (0.9%), which was administered intravenously over 5 min, with subsequent flushing of 20 mL normal saline. The entire process was performed on an in-patient basis, and patients were discharged in a few hours of observation if they do not show any adverse reaction to [177Lu]Lu-DOTA-ZOL. Figure 1 shows biodistribution and uptake of [177Lu]Lu-DOTA-ZOL therapy in a patient with skeletal metastases from prostate cancer, while Fig. 2 gives similar data of [177Lu]Lu-DOTA-ZOL therapy in a patient with skeletal metastases from breast cancer.
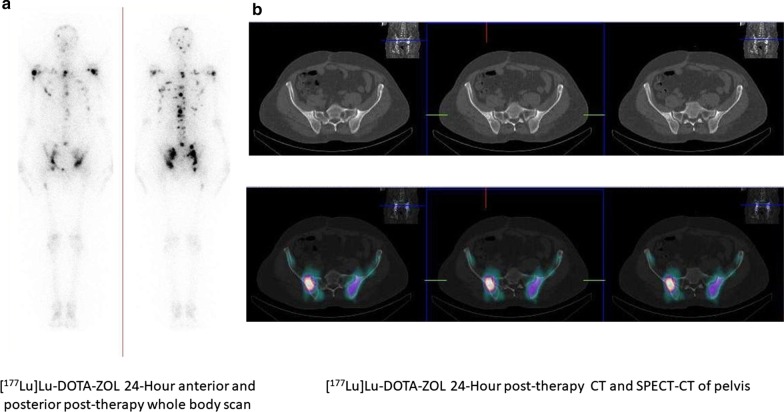
Fig. 1.
A 55-year-old male diagnosed with prostatic adenocarcinoma underwent radical prostatectomy in April 2017, and his Gleason score was 3 + 4 = 7. He underwent hormonal therapy until April 2018 and received 1# of radiotherapy to B/L hip and D5-vertebra. He was referred to us for symptomatic pain relief. His baseline pain scoring (VASmax) was 9/10, with maximum pain in the B/L hips. We administered 1.3 GBq of [177Lu]Lu-DOTA-ZOL and 24-h post-administration, a the 24-h anterior and posterior post-therapy whole-body scans demonstrating avid [177Lu]Lu-DOTA-ZOL uptake in multiple skeletal sites. b The uptake of [177Lu]Lu-DOTA-ZOL in the pelvic bone metastases on post-therapy SPECT/CT
Fig. 2.
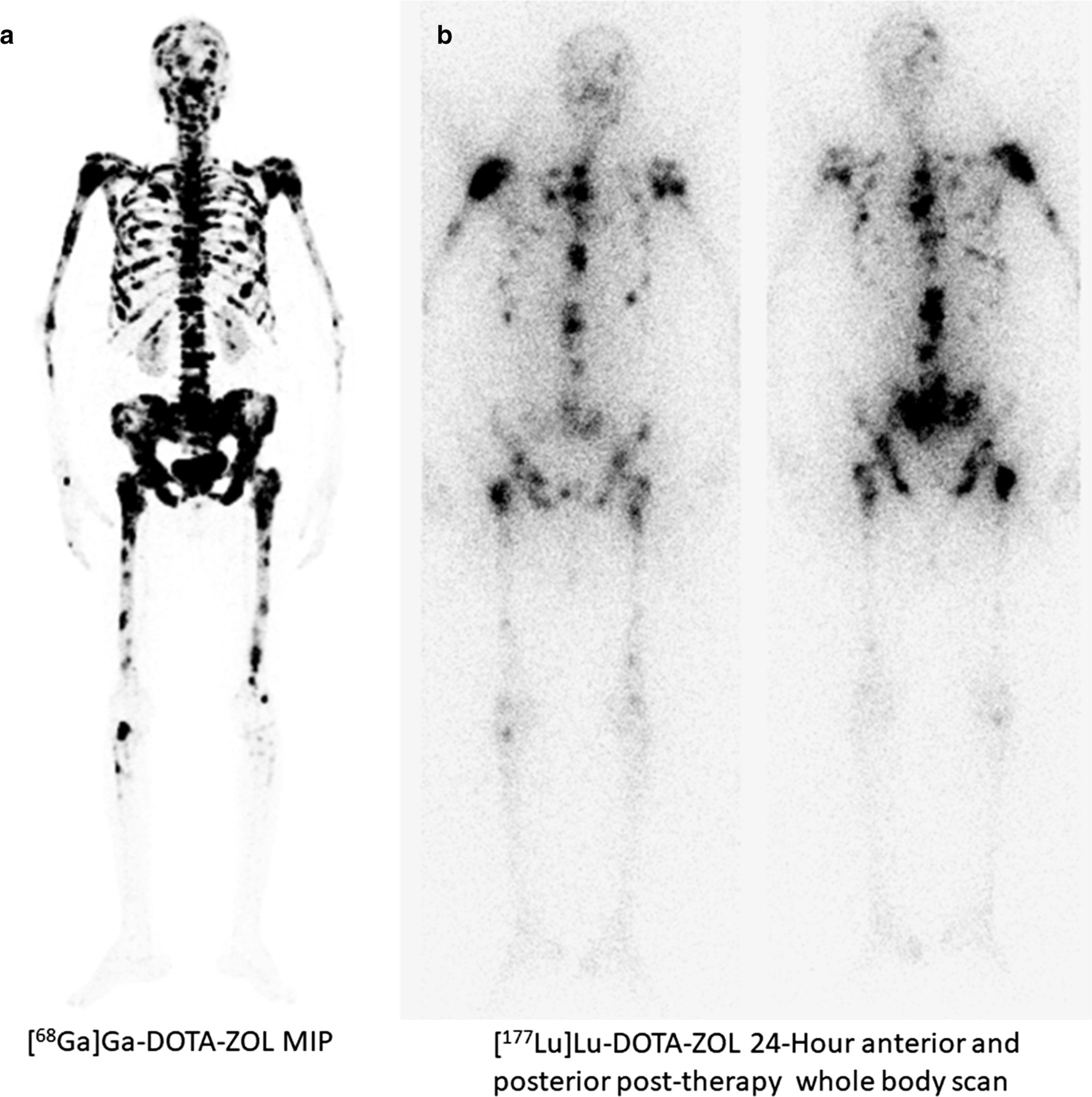
A 45-year-old female, diagnosed with triple-negative breast cancer was on opioid medications, but did not experience any relief in pain. Her baseline pain scoring (VASmax) was 8/10. Baseline [68Ga]Ga-DOTA-ZOL PET/CT (a) was conducted followed by administration of 1.3 GBq of [177Lu]Lu-DOTA-ZOL. Twenty-four-hour post-administration, post-therapy 24-h anterior and posterior whole body scan (b) demonstrate avid [177Lu]Lu-DOTA-ZOL uptake in multiple skeletal sites
Follow-up
The treatment was repeated if necessary at 3-monthly intervals. Post-[177Lu]Lu-DOTA-ZOL administration, patients were assessed at 2, 4, 8, and 12 weeks. Patients were assessed for laboratory parameters, and the adverse events were recorded according to the National Cancer Institute for Common Toxicity Criteria version (CTC) 5.0 [28]. The visual analogue score (VAS) [29], global pain assessment score, analgesic score (AS), Karnofsky performance status (KPS) [30, 31], and assessment of pain relief were recorded in the patient case files on each visit. Patients were instructed to maintain a diary and document the pain relief parameters such as initiation of pain relief, duration of pain relief, time of increase and/or recurrence of pain, decrease or increase in the consumption of pain killers.
Treatment response assessment
Primary outcome endpoint
The primary outcome endpoint was response assessment by VAS. According to this criteria, the complete response (CR), partial response (PR), minimal response(MR), and no response (NR) were categorised as > 70% reduction, 40–70% reduction, 20–40% reduction, and < 20% decrease in VAS or increase in pain, respectively [29].
Secondary outcome endpoints
Other clinical response assessment parameters involved analgesic score (AS), Karnofsky performance status (KPS), Eastern Cooperative Oncology Group (ECOG) performance status, global pain assessment, adverse event profile, and overall survival.
Analgesic scoring was conducted as per the Urological Group of the European Organization of Research and Treatment of Cancer (EORTC, Protocol 30921). As per EORTC protocol, the analgesic score is the product of two five-point scales (the type of analgesic and the frequency of its administration). A decline in the analgesic score was documented as a response to treatment.
Additionally, global pain response assessment was analysed according to the criteria adopted by Thapa et al. [32] that considered changes in both VAS and analgesic scores (rather than a single parameter). According to the criteria, the present study design considered post-therapy changes in both VAS and analgesic scores on a sliding scale. The global pain assessment criteria are accordingly complete (75% decrease in analgesic score with change in pain score), partial (50–75% decrease in analgesic score with a change in pain score), minimal (25–50% decrease in analgesic score with a change in pain score), or none (no change in pain score or, 25% decrease in the analgesic score). The KPS was scaled from 100 to 0. The ECOG status ranged from 0 to 5. All adverse events were assessed as per the National Cancer Institute’s Common Toxicity Criteria (NCI-CTC) version 5.0. The overall survival (OS) was defined as the time from the initiation of [177Lu]Lu-DOTA-ZOL treatment to the time of death. The death could be attributed to any cause or the last telephonic contact.
Statistical analysis
The normality of the data was examined by the D’Agostino–Pearson test. The data were presented as mean standard deviation (SD), median, and/or interquartile range (IQR). Unpaired samples t test (parametric test) or Mann–Whitney U test (nonparametric test) was performed for two independent patient groups. The paired t test (parametric test) or Wilcoxon signed-rank test (nonparametric test) was executed to compare parameters at pre- and post-treatment time points. Kaplan–Meier curves analysis was conducted to calculate the overall survival. MedCalc software was used for statistical analyses. P values ≤ 0.05 were considered significant.
Results
Patients
Forty documented skeletal metastases patients including 15 males and 25 females with a mean age of 46.6 ± 15.08 years (range 24–78 years) were enrolled and treated with [177Lu]Lu-DOTA-ZOL for bone pain palliation therapy. The patients were treated between January 2017 and February 2020 with the median follow-up duration of 10 (IQR 8–14) months.
The baseline demographic profile of the patients, tumour characteristics, previous and ongoing cancer-related treatments, and the analgesics consumed are outlined in Tables 1 and 2. Among the patients treated, breast cancer (23/40, 57.5%) accounted for the maximum number of cases followed by prostate cancer (11/40, 27.5%). The remaining 6 patients had lung cancer (Table 1). Except eight patients with prostate cancer who were on concomitant hormonal therapy, no other patients were on any anti-cancer treatment during the treatment. While 15 (37.5%) patients were on morphine medications at the baseline, the remaining patients (62.5%) were either on atypical opioids, non-morphine opioids, or other NSAIDs. Before being referred to our department for bone pain palliation, all the patients had undergone a minimum of two lines of prior treatment.
Table 1.
Patient clinical characteristics
| Variables | Number (%) |
|---|---|
| Age in years (mean ± SD, range) | 46.6 ± 15.08 (24–78) |
| Gender | |
| Male | 15 (37.5%) |
| Female | 25 (62.5%) |
| Primary disease | |
| Prostate | 11 (27.5%) |
| Breast | 23 (57.5%) |
| Lung | 6 (15%) |
| Baseline VAS pre-therapy (median, IQR) | 9 (8–10) |
| Baseline KPS pre-therapy (median, IQR) | 60 (50–70) |
| Baseline analgesic score (median) | 6 (6–8) |
| The extent of skeletal metastases | |
| < 6 | 2 (5%) |
| 6–20 | 17 (42.5%) |
| > 20 | 7 (17.5%) |
| Diffuse/superscan | 14 (35%) |
| ECOG performance status | |
| 2 | 12 (30%) |
| 3 | 15 (37.5%) |
| 4 | 13 (32.5%) |
| Mean cumulative activity (GBq) | 2.1 ± 0.6 GBq (1.3–2.7 GBq) |
IQR inter-quartile range, VAS visual analogue score, KPS Karnofsky performance status, ECOG Eastern Cooperative Oncology Group, GBq gigabecquerel
Table 2.
Detailed clinical history of patients
| Patient | Age/gender | Type of cancer | Previous therapy | Ongoing treatment | Analgesic score (type) | Analgesic score (quantity) |
|---|---|---|---|---|---|---|
| 1 | 51/M | mCRPC | First- and second-generation anti-androgens, chemotherapy | Androgen synthesis inhibitor | Morphine | 2 |
| 2 | 25/F | Non-small cell right lung cancer | Lobectomy, chemotherapy, radiotherapy | – | Morphine | 2 |
| 3 | 60/M | Right breast cancer | Chemotherapy, radiotherapy | – | Morphine | 2 |
| 4 | 44/F | B/L breast cancer | B/L breast mastectomy, chemotherapy, radiotherapy, monoclonal antibody therapy | – | Morphine | 2 |
| 5 | 61/M | Right breast cancer | Hormonal therapy, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 3 |
| 6 | 70/M | mCRPC | Medical castration, first-generation anti-androgen therapy, chemotherapy, radiotherapy | Second-generation anti-androgens | Morphine | 4 |
| 7 | 63/M | mCRPC | B/L orchidectomy, first-generation anti-androgens, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 8 | 60/F | Small-cell lung cancer | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 3 |
| 9 | 44/F | Right breast cancer | Right breast mastectomy, hormonal therapy, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 10 | 65/F | Left breast cancer | Hormonal therapy, chemotherapy, | – | Atypical opioids and non-morphine opioids | 2 |
| 11 | 33/F | Right breast cancer | Chemotherapy, radiotherapy | – | Morphine | 2 |
| 12 | 74/M | mCRPC | Radical Prostatectomy, B/L orchidectomy, first-generation anti-androgens, chemotherapy, | - | Atypical opioids and non-morphine opioids | 2 |
| 13 | 45/F | Left breast cancer | Left breast modified radical mastectomy, hormonal therapy, chemotherapy, radiotherapy, | – | Atypical opioids and non-morphine opioids | 3 |
| 14 | 27/F | B/L breast cancer | Hormonal therapy, B/L mastectomy, chemotherapy, radiotherapy, | – | Morphine | 2 |
| 15 | 47/F | Left breast cancer | Hormonal therapy, chemotherapy, radiotherapy, antibody therapy | – | Other NSAIDs | 1 |
| 16 | 27/F | Left breast cancer | chemotherapy, radiotherapy, | – | Other NSAIDs | 2 |
| 17 | 55/M | mCRPC | B/L orchidectomy, first- and second-generation anti-androgens, chemotherapy, radiotherapy | Androgen synthesis inhibitors | Other NSAIDs | 3 |
| 18 | 44/F | Left breast cancer | Left modified radical mastectomy, chemotherapy, radiotherapy | – | Morphine | 2 |
| 19 | 58/F | Right breast cancer | Rt breast mastectomy chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 4 |
| 20 | 24/M | Squamous cell cancer of lung | Surgery, chemotherapy | – | Morphine | 2 |
| 21 | 55/F | Left breast cancer | Surgery, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 22 | 55/F | Squamous cell cancer of lung | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 23 | 54/F | Right breast cancer | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 3 |
| 24 | 40/F | Right breast cancer | Chemotherapy, radiotherapy | – | Morphine | 2 |
| 25 | 54/M | mCRPC | Medical castration, Chemotherapy, radiotherapy | Androgen-synthesis inhibitors | Morphine | 2 |
| 26 | 63/F | B/L breast cancer | Bilateral mastectomy, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 27 | 47/F | Right breast cancer | Right breast mastectomy, hormonal therapy, chemotherapy, radiotherapy | – | Morphine | 2 |
| 28 | 50/M | Right breast cancer | Right modified radical mastectomy, chemotherapy, radiotherapy | – | Morphine | 2 |
| 29 | 40/F | B/L breast cancer | Hormonal therapy, chemotherapy, radiotherapy | – | Morphine | 2 |
| 30 | 75/M | mCRPC | B/L orchidectomy, chemotherapy, first- and second-generation anti-androgens, radiotherapy | None | Atypical opioids and non-morphine opioids | 2 |
| 31 | 67/M | mCRPC |
B/L orchidectomy, first-generation anti-androgens Chemotherapy, radiotherapy, |
Second-generation anti-androgens | Atypical opioids and non-morphine opioids | 2 |
| 32 | 78/M | mCRPC | B/L orchidectomy, chemotherapy | Androgen synthesis inhibitor | Atypical opioids and non-morphine opioids | 2 |
| 33 | 48/F | B/L breast cancer | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 34 | 40 | Right breast cancer | Right modified radical mastectomy, chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 35 | 70/M | mCRPC | Medical castration, chemotherapy, radiotherapy | Androgen-synthesis inhibitors | Morphine | 2 |
| 36 | 43/M | Non-small cell lung cancer of the right lung | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 37 | 32/F | Right breast cancer | Right breast mastectomy chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 38 | 67/F | Right breast cancer | Right modified radical mastectomy, hormonal therapy chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 2 |
| 39 | 30/F | Small cell cancer of lung | Chemotherapy, radiotherapy | – | Atypical opioids and non-morphine opioids | 3 |
| 40 | 76/M | mCRPC | B/L orchidectomy, first-generation anti-androgens, chemotherapy, radiotherapy | Second-generation anti-androgens | Atypical opioids and non-morphine opioids | 2 |
B/L bilateral, mCRPC metastatic castration-resistant prostate cancer
Treatment cycles and efficacy assessment
The cumulative activity administered was 2.1 ± 0.6 GBq (range 1.3–2.7 GBq) (56.8 ± 17 mCi; range 35–70 mCi). A total of 65 cycles of [177Lu]Lu-DOTA-ZOL were administered in 40 patients, among whom, 25 patients received two cycles each and the remaining 15 patients received only 1 cycle. Flair phenomenon was noted in 7/40 (17.5%) of patients within 2–3 days of [177Lu]Lu-DOTA-ZOL treatment. These patients complained of persistent pain even on strong analgesics; however, it was transient and reduced in 7–10-day post-treatment. According to the VAS response criteria, complete response was demonstrated in 27.5% (11/40), partial response in 50% (20/40), the minimal response in 12.5% (5/40), and no response in 10% (4/40) of patients with an overall response rate (ORR) of 90%. Similarly, as per the global pain assessment response criteria, pain relief with [177Lu]Lu-DOTA-ZOL was 90%: 5% (2/40) CR, 62.5% (25/40) PR, and 22.5% (9/40) MR, respectively. The detailed classification of relation between, the extent of skeletal metastases, number of cycles administered, and responses according to both VAS criteria and global pain assessment criteria are explained in Table 3. Interestingly, Among 14 patients with superscan or diffuse involvement of skeletal metastases, only one patient did not respond to treatment. A similar pattern of responses was observed in patients with > 20 skeletal metastases (Table 3). Nine patients who attained minimal response after the first cycle were re-challenged with the second cycle of [177Lu]Lu-DOTA-ZOL, but found very minimal improvement despite the second cycle and remained in the minimal response category. Among the 15 patients who received only one cycle of [177Lu]Lu-DOTA-ZOL treatment, according to VAS criteria, 4 did not respond to treatment, 3 experienced only minimal response, and 8 patients responded well but did not consent for the 2nd cycle of treatment (Table 3).
Table 3.
Response and number of [177Lu]Lu-DOTA-ZOL treatment cycles administered according to the extent of disease
| Extent of skeletal metastases | Number of patients (%) | Response according to VAS assessment criteria (number of patients) | Number of cycles administered | Response according to global pain assessment criteria (number of patients) | Number of cycles administered |
|---|---|---|---|---|---|
| < 6 | 2 (5%) | CR-1 | 2C | PR-2 | 1st patient-1C |
| PR-1 | 1C | 2nd patient-2C | |||
| 6–20 | 17 (42.5%) | CR-5 | 4 patients-2C, 1 patient-1C | CR-1 | 2C |
| PR-9 | 5 patients-2C, 4 patients-1C | PR-11 | 8 patients-2C, 3 patients-1C | ||
| MR-1 | 2C | MR-3 | 2 patients-1C, 1 patient-2C | ||
| NR-2 | Both patients-1C | NR-2 | Both patients-1C | ||
| > 20 | 7 (17.5%) | CR-2 | 2 patients, 2C | CR-1 | 2C |
| PR-4 | 3 patients-2C, 1 patient-1C | PR-4 | 3 patients-2C, 1 patient-1C | ||
| MR-0 | 0 | MR-1 | 2C | ||
| NR-1 | 1C | NR-1 | 1C | ||
| Superscan/diffuse | 14 (35%) | CR-3 | All patients-2C | CR-0 | – |
| PR-6 | 5 patients-2C, 1 patient-1C | PR-8 | 6 patients-2C, 2 patients-1C | ||
| MR-4 | 1 patient-2C, 3 patients-1C | MR-5 | 3 patients-2C, 2 patients-1C | ||
| NR-1 | 1 patient 1C | NR-1 | 1C |
VAS visual analogue score, CR complete response, PR partial response, MR minimal response, NR no response, C cycles
In the post-treatment period, there was a significant decrease in VAS [(pre-therapy: 9 (IQR 8–10) vs. post-therapy: 4 (IQR 3–5), P < 0.0001). Similarly, a remarkable improvement noted in the KPS [(pre-therapy: 60 (IQR 50–70) vs. post-therapy: 80 (IQR 60–80), P < 0.0001), and the ECOG performance status [(pre-therapy: 3 (IQR 2–4) vs. post-therapy: 2 (IQR 2–3), P 0.0013) post-[177Lu]Lu-DOTA-ZOL therapy. While there was a significant reduction in the analgesic score pre- and post-treatment with [177Lu]Lu-DOTA-ZOL in the CR, PR, and the MR categories, the same was not found in the NR category (Table 4).
Table 4.
Comparison of pre- and post-treatment analgesic scores stratified according to VAS response criteria
| Response (VAS criteria) | Number of patients (N) | Baseline AS (median, IQR) | Post-treatment AS (median, IQR) | P value |
|---|---|---|---|---|
| CR | 11 | 6 (6–8) | 2 (1.2–2.7) | 0.001 |
| PR | 20 | 7 (6–8) | 3 (3–3.5) | < 0.0001 |
| MR | 5 | 8 (6–8.25) | 4.8 (4–6) | 0.006 |
| NR | 4 | 8.5 (8–12.5) | 8 (8–12) | 0.918 |
All the values are mentioned as median and inter-quartile range (IQR)
VAS visual analogue score, N number of patients, IQR interquartile range, CR complete response, PR partial response, MR minimal response, NR no response
Response according to the type of cancer revealed 100% (23/23), 82% (9/11), and 66.6% (4/6) ORR in patients with breast, prostate, and lung cancer, respectively (Table 5).
Table 5.
Response assessment according to the type of cancer
| Response criteria | Breast cancer N = 23 | Prostate cancer N = 11 | Lung cancer N = 6 |
|---|---|---|---|
| CR | 9 | 2 | 0 |
| PR | 11 | 7 | 2 |
| MR | 3 | 0 | 2 |
| NR | 0 | 2 | 2 |
N number of patients, CR complete response, PR partial response, MR minimal response, NR no response
The median time for the initiation of pain relief was ≤ 7 days (IQR 6–9 days) (Fig. 3). The median time of sustained response after the last cycle of the [177Lu]Lu-DOTA-ZOL is 3 months (IQR 2–4 months). Only one patient observed the most prolonged duration of sustained response, which was 10 months.
Fig. 3.
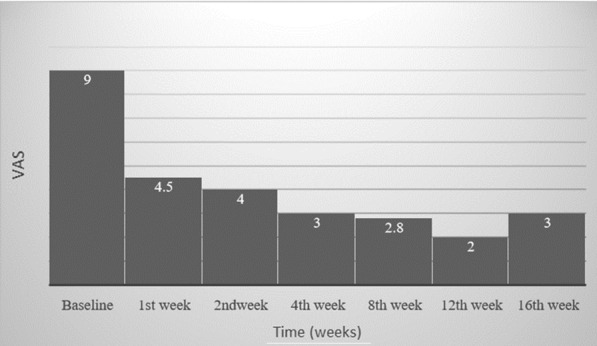
Plot showing the temporal relationship of the median value of visual analogue score (VAS) of patients from baseline up to 16 weeks of post-[177Lu]Lu-DOTA-ZOL therapy
Survival analysis
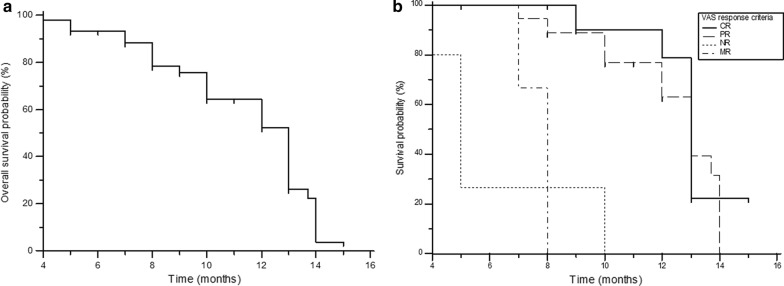
Among the entire series, 28 patients died during the follow-up. The median survival from treatment was 13 months (95% CI 10–14 months), with a 1-year survival probability of 55.4% (Fig. 4a). On subgroup analysis, in patients with CR, PR, MR, and NR, the median overall survival was 13, 13, 8, and 5 months, respectively (Fig. 4b). Sub-categorical analysis based on the type of cancer revealed patients with breast and prostate cancer to depict a similar median overall survival duration of 13 months, while patients with lung cancer demonstrated a median overall survival of 10 months. However, the log-rank test did not prove significant (P 0.1431) (Fig. 5).
Fig. 4.
a Kaplan–Meier analysis: overall survival. b Overall survival, according to the response category as per VAS response criteria
Fig. 5.
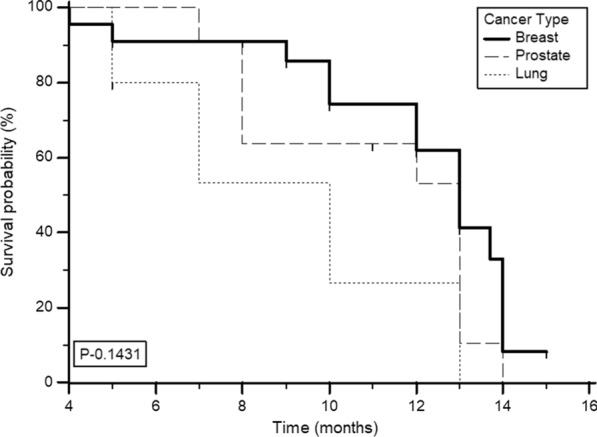
Kaplan–Meier analysis: overall survival according to the type of cancer
Toxicity
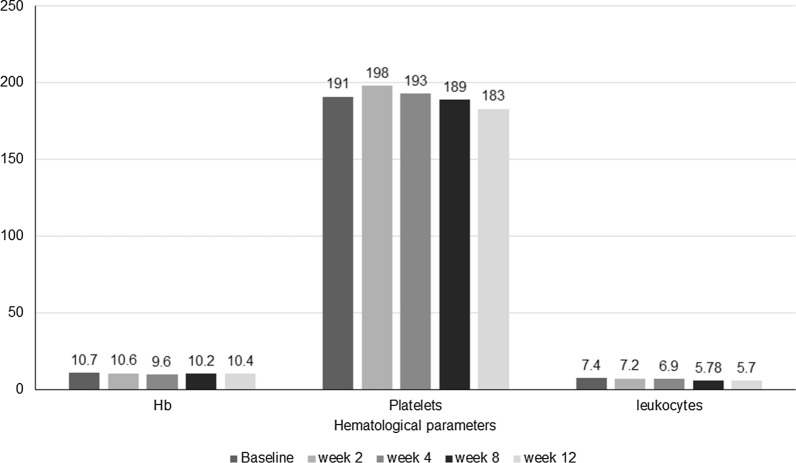
The laboratory parameters were tested and analysed for toxicity post-treatment (Table 6). Haematological serious adverse events (SAE) [grade III/IV toxicity] were not observed in any patient despite completing two cycles of [177Lu]Lu-DOTA-ZOL treatment. Though the difference in the haemoglobin levels post-second cycle was significant (P < 0.0001), only two patients in the series experienced grade II anaemia after the [177Lu]Lu-DOTA-ZOL therapy; nadir was around 4 weeks and subsequently recovered (Fig. 6). None of the patients had shown renal toxicity and other side effects like hypercalcemia.
Table 6.
Laboratory parameters at baseline and post-[177Lu]Lu-DOTA-ZOL treatment
| Parameters | Patients receiving single cycle | Patients receiving 2 cycles | ||||
|---|---|---|---|---|---|---|
| Baseline (median, IQR) | Post-treatment (median, IQR) | P value | Baseline (median, IQR) | Post-treatment (median, IQR) | P value | |
| Haemoglobin (g/dL) | 10.2 (9.6–10.6) | 10 (8.9–11) | 0.129 | 14.7 (10–12.4) | 13 (9.2–11) | < 0.0001 |
| Platelets (lakhs/µL) | 172 (140.2–198) | 143 (129.2–198.5) | 0.039 | 223 (164–284.25) | 217 (170–275.5) | 0.421 |
| Leukocytes 109/L | 6760 (4210–11,800) | 5400 (3700–10,800 | 0.291 | 6700 (4280–12,000) | 5400 (3800–10,900) | 0.298 |
| Creatinine (mg/dL) | 0.8 (0.63–0.96) | 0.79 (0.6–0.94) | 0.259 | 0.7 (0.7–0.9) | 0.8 (0.7–0.8) | 0.174 |
| ALP (IU/L) | 282 (190–480) | 189 (140–280) | 0.0001 | 278 (186–390) | 230 (142–340) | 0.003 |
ALP alkaline phosphatase, IQR interquartile range
Fig. 6.
Median haematological parameters after [177Lu]Lu-DOTA-ZOL therapy
Discussion
Three independent randomised, placebo-controlled trials on zoledronic acid in about 3000 patients demonstrating hormone-resistant metastatic prostate cancer [33], breast cancer [34], have demonstrated a reduction in skeletal-related events (SREs) and proved it clinically effective. Subsequently, zoledronate received regulatory approval for the prevention of SREs and treating bone metastases. The labelling of zoledronate to beta- and alpha-emitting radionuclides like 177Lu and 225Ac, respectively, was a logical development [19, 20].
Though EDTMP has been proved effective for bone pain palliation [8], [177Lu]Lu-DOTA-ZOL proves superior to the former agent in certain aspects. In comparison with EDTMP, zoledronate demonstrates higher osteoclastic bone affinity and excellent anti-resorptive properties. While both the agents are non-bio-transformative, rapid clearance of [177Lu]Lu-DOTA-ZOL from blood, in contrast to [177Lu]Lu-EDTMP, dosimetric studies report a higher absorbed dose to the trabecular bone surface (12.17 vs. 10.02 mSv/MBq), cortical bone surface (9.524 ± 0.803 vs. 7.839 ± 0.655), and a noticeably lesser muscle uptake [27]. Therefore, [177Lu]Lu-DOTA-ZOL theoretically would have a better treatment efficacy. Moreover, unlike EDTMP, DOTA-ZOL complexes with 68 Ga and 177Lu/225Ac facilitate [19] a theranostic pair for practicing precision oncology.
However, to date, there is no study published in the literature showing therapeutic efficacy, safety, and toxicity of [177Lu]Lu-DOTA-ZOL in bone pain palliation in metastatic cancer patients. Hence, the current research focuses on the efficacy and safety study of [177Lu]Lu-DOTA-ZOL pain palliation therapy in patients with bone metastases.
Well, in line with the literature [1], 85% of the patients treated comprised of skeletal metastases from breast and prostate cancer. Interestingly, a significant component of our patients (70%) managed had an ECOG performance state of 3 or 4, and 35% of patients presented with either diffuse or super-scan reflecting the high tumour burden and poor ECOG status, which is a real-life scenario at the clinics. These sets of patients were refractory to the ongoing therapy options, including strong analgesics.
Khawar et al. [21] reported an estimated maximum tolerable dose (MTD) of [177Lu]Lu-DOTA-ZOL to be 3.6–5.0 GBq can be administered based on the 2 Gy bone marrow limit. In the present study, we administered a mean cumulative dose of 2.1 ± 0.6 GBq and ranged 1.3–2.7 GBq over a median of 2 cycles at three-monthly intervals; these administered activities were well within the prescribed limits. According to Khawar et al. [21], if the administered activities do not exceed MTD, the threshold adsorbed doses for the critical organ, namely kidneys, shall be within the predicted limit. Patients who responded from the first cycle of [177Lu]Lu-DOTA-ZOL were counselled or self-consented for the second cycle, while patients who were not willing to undergo the next cycle of treatment or those who did not experience any relief in pain after the first cycle of [177Lu]Lu-DOTA-ZOL were not treated further. Another interesting find observed was that those patients who experienced minimal response from the 1st cycle of [177Lu]Lu-DOTA-ZOL treatment when re-challenged with the 2nd cycle did not encounter further reduction in the pain.
The current study reports an ORR of 90%, which is well within the range of response rate with other bone-seeking pain palliating agent [177Lu]Lu-EDTMP ranging between 83 and 86% [8, 9]. Interestingly, pain relief was initiated within ≤ 7 days after the [177Lu]Lu-DOTA-ZOL treatment and lasted up to 10 months. In a previous study using [177Lu]Lu-EDTMP, we had reported response durations that varied from 2 weeks to 4 months from the onset of pain relief [8]. Detailed analysis revealed a similar ORR of 82% in prostate cancer patients when compared to the historic [177Lu]Lu-EDTMP study (ORR 84%) [8]. However, a remarkably high ORR of 100% was observed in the breast cancer cohort of the present study in contrast to 92% in the historic [177Lu]Lu-EDTMP study [8]. Though data regarding the response pattern in bone metastases from lung cancer are limited, Ye et al. [35] reported a treatment efficacy rate of 75.4% in the lung cancer group, but with 2.22 MBq/kg 89SrCl2. In agreement with our results, they reported efficacy was lower in patients with bone metastases from the lung cancer sub-group than in those with bone metastasis from breast and prostate cancer [35].
The 1-year survival rate was 55.4% and was significantly higher when compared to that of the [177Lu]Lu-EDTMP historical data set [35% and 38% in the two-level dose group] [8], but drastically dropped to 26% at 13 months. This drop of overall survival is not clearly understood; however, it could be attributed to the aggressive biology of the tumour and the extent of spread. Among the cancer types, similar overall survival was noted in patients with breast and prostate cancer (13 months) and a slightly decreased survival in bone metastases patients with primary lung cancer. Attributed to the superior radiobiological properties of the alpha emitter, radium-223 chloride, Parker et al. [4] in their RCT, involving 921 patients with bone metastases from prostate cancer, outlined an OS of 14.9 months. Nilsson et al. [36] performed a survival follow-up of the phase-II RCT in which bone metastases patients from prostate cancer were treated with radium-223 chloride, and their study findings suggested a survival benefit of 65 weeks versus 46 weeks in patients treated with a single dose of radium-223 chloride and placebo group, respectively.
Analgesic consumption was recorded according to the analgesic scoring of EORTC protocol. Among our recruited patients, 15 patients were on opioid analgesic morphine before radionuclide therapy out of whom 12 responded to [177Lu]Lu-DOTA-ZOL treatment and thus were weaned off morphine. It is these set of patients whom the pain palliation was most beneficial as they have exhausted the available pain relief options. Interestingly, reassuring results were obtained with [177Lu]Lu-DOTA-ZOL in patients demonstrating extensive skeletal metastases and those presenting with superscan or diffuse involvement of the bone. The relief in pain was also reflected in their KPS and ECOG performance status. Toxicity related to [177Lu]Lu-DOTA-ZOL therapy was minimal, and no grade III/IV toxicities were documents.
Limitations
The significant limitations of this cohort study are the non-randomised study, and no parallel arm was used. Although the results of [177Lu]Lu-DOTA-ZOL were comparable to historical data of [177Lu]Lu-EDTMP, a randomised control trial to compare the efficacy and safety of two bone-seeking palliative agents is urgently desired to take this agent further.
Conclusion
[177Lu]Lu-DOTA-ZOL is safe, effective, and an ideal agent in the treatment of metastatic bone pain. Thanks to its characteristics as theranostics, it allows for patient-individual therapies and perfectly matches the expectations for precision oncology. Interestingly, [177Lu]Lu-DOTA-ZOL is not only effective in addressing bone metastases derived from breast cancer; it shows clear evidence also for prostate and lung cancers. [177Lu]Lu-DOTA-ZOL pain palliation treatment demonstrated an ORR of 90% with 27.5% of complete response and 50% of partial response.
Acknowledgements
We would like to acknowledge Department of Nuclear Chemistry, Johannes Gutenberg University, Mainz, Germany, for providing the zoledronate (DOTA-ZOL) molecule which was used in this study.
Abbreviations
- IQR
Interquartile range
- VAS
Visual analogue score
- AS
Analgesic score
- ECOG
Eastern Cooperative Oncology Group Assessment performance status
- KPS
Karnofsky performance status
- SREs
Skeletal-related events
- EBRT
External beam radiotherapy
- EDTMP
Ethylene diamine tetramethylene phosphonic acid
- BPAMD
4-{[(Bis(phosphonomethyl)) carbamoyl]methyl}-7,10-bi (carboxymethyl)-1,4,7,10-tetraazacyclododec-1-Yl) acetic acid
- DOTA-ZOL
Zoledronate
- BSA
Body surface area
- EORTC
European Organization of Research and Treatment of Cancer
- LuCl3-
Lutetium chloride
- GFR
Glomerular filtration rate
- CBC
Complete blood counts
- KFT
Kidney function tests
- ALP
Alkaline phosphatase
- CR
Complete remission
- PR
Partial remission
- MR
Minimal response
- NR
No response
- PET/CT
Positron emission tomography/computed tomography
- NCI-CTC
National Cancer Institute’s Common Toxicity Criteria
- OS
Overall survival
- SD
Standard deviation
- MTD
Maximum tolerable dose
- ORR
Overall response rate
Author contributions
MPY and SB helped in patient treatment, data collection, and manuscript writing. MM contributed to radiochemistry; synthesis of DOTA ZOL; and standardisation of radiolabelling. FR helped in radiochemistry; synthesis of DOTA ZOL; standardisation of radiolabelling; and manuscript review. CB planned the study, patient recruitment, patient treatment, follow-up, image assessment, and manuscript writing and final review. All authors read and approved the final manuscript.
Funding
No funding was received from any organisation to conduct this study.
Availability of data and materials
Data and material are available.
Ethical approval and consent to participate
Ref. No IEC/349/4/2020. Name of ethics committee is Institute Ethics committee, AIIMS. A written informed consent was obtained from all the patients before commencing the treatment.
Consent for publication
All the authors gave their consent for the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Madhav Prasad Yadav and Sanjana Ballal have contributed equally to this work
References
- 1.Roodman GD. Mechanisms of bone lesions in multiple myeloma and lymphoma. Cancer. 1997;80:1557–1563. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1557::aid-cncr5>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Geneva: World Health Organization; 2018. [PubMed]
- 3.Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the Radiation Therapy Oncology Group. Cancer. 1982;50:893–899. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Pillai MR, Chakraborty ST, Venkatesh M, Ramamoorthy N. Production logistics of 177Lu for radionuclide therapy. Appl Radiat Isot. 2003;59:109–118. doi: 10.1016/s0969-8043(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 6.Lebedev NA, Novgorodov AF, Misiak R, Brockmann J, Rösch F. Radiochemical separation of no-carrier-added 177Lu as produced via the 176Yb(n, g)177Yb–177Lu process. Appl Radiat Isot. 2000;53:421–425. doi: 10.1016/s0969-8043(99)00284-5. [DOI] [PubMed] [Google Scholar]
- 7.Mazzarri S, Guidoccio F, Mariani G. The emerging potential of 177Lu-EDTMP: an attractive novel option for radiometabolic therapy of skeletal metastases. Clin Transl Imaging. 2015;3:167–168. [Google Scholar]
- 8.Agarwal KK, Singla S, Arora G, Bal C. 177Lu-EDTMP for palliation of pain from bone metastases in patients with prostate and breast cancer: a phase II study. Eur J Nucl Med Mol Imaging. 2015;42:79–88. doi: 10.1007/s00259-014-2862-z. [DOI] [PubMed] [Google Scholar]
- 9.Alavi M, Omidvari S, Jalilian A, Mehdizadeh A, Bahrami-Samani A. Metastatic bone pain palliation using 177Lu-ethylenediamine tetramethylene phosphonic acids. World J Nucl Med. 2015;14:109–115. doi: 10.4103/1450-1147.157124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumors: on the way to THERANOSTICS. Dalton Trans. 2011;40:6104–6111. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- 11.Fellner M, Baum RP, Peters JA, Lukeš I, Hermann P, Prasad V, et al. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates—first in the human study. Eur J Nucl Med Mol Imaging. 2010;37:834. doi: 10.1007/s00259-009-1355-y. [DOI] [PubMed] [Google Scholar]
- 12.Fellner M, Riss P, Loktionova NS, Zhernosekov KP, Thews O, Geraldes CFGC, et al. Comparison of different phosphorus-containing ligands complexing 68Ga for PET-imaging of bone metabolism. Radiochim Acta. 2011;99:43–51. [Google Scholar]
- 13.Fellner M, Bisalski B, Bausbacher N, Kubícek V, Hermann P, Rösch F, et al. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol. 2012;39:993–999. doi: 10.1016/j.nucmedbio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Meckel M, Fellner M, Thieme N, Bergmann R, Kubicek V, Rösch F. In vivo comparison of DOTA based 68Ga-labelled bisphosphonates for bone imaging in non-tumor models. Nucl Med Biol. 2013;40:823–830. doi: 10.1016/j.nucmedbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Meckel M, Nauth A, Timpe J, Zhernosekov K, Puranik AD, Baum RP, et al. Development of a [177Lu]BPAMD labeling kit and an automated synthesis module for routine bone targeted endoradiotherapy. Cancer Biother Radiopharm. 2015;30:94–99. doi: 10.1089/cbr.2014.1720. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann R, Meckel M, Kubíček V, Pietzsch J, Steinbach J, Hermann P, et al. 177Lu-labeled macrocyclic bisphosphonates for targeting bone metastasis in cancer treatment. EJNMMI Res. 2016;6:5. doi: 10.1186/s13550-016-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meckel M, Kubíček V, Hermann P, Miederer M, Rösch F. A DOTA based bisphosphonate with an albumin binding moiety for delayed body clearance for bone targeting. Nucl Med Biol. 2016;43:670–678. doi: 10.1016/j.nucmedbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Meckel M, Bergmann R, Miederer M, Roesch F. Bone targeting compounds for radiotherapy and imaging: *Me(III)-DOTA conjugates of bisphosphonic acid, pamidronic acid, and zoledronic acid. EJNMMI Radiopharm Chem. 2017;1:14. doi: 10.1186/s41181-016-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfannkuchen N, Meckel M, Bergmann R, Bachmann M, Bal C, Sathekge M, et al. Novel radiolabeled bisphosphonates for PET diagnosis and endoradiotherapy of bone metastases. Pharmaceuticals (Basel) 2017;10:E45. doi: 10.3390/ph10020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfannkuchen N, Bausbacher N, Pektor S, Miederer M, Rösch F. In vivo evaluation of [225Ac]Ac-DOTAZOL for α-therapy of bone metastases. Curr Radiopharm. 2018;11:223–230. doi: 10.2174/1874471011666180604083911. [DOI] [PubMed] [Google Scholar]
- 21.Khawar A, Eppard E, Roesch F, Ahmadzadehfar H, Kürpig S, Meisenheimer M, et al. Biodistribution and post-therapy dosimetric analysis of [177Lu]Lu-DOTAZOL in patients with osteoblastic metastases: first results. EJNMMI Res. 2019;9:102. doi: 10.1186/s13550-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khawar A, Eppard E, Roesch F, Ahmadzadehfar H, Kürpig S, Meisenheimer M, et al. Preliminary results of biodistribution and dosimetric analysis of [68Ga]Ga-DOTA-ZOL: a new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann Nucl Med. 2019;6(404–13):7. doi: 10.1007/s12149-019-01348-7. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot. 2008;66:1196–1205. doi: 10.1016/j.apradiso.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 24.Das T, Shinto A, Kamaleshwaran KK, Banerjee S. Theranostic treatment of metastatic bone pain with 177Lu-DOTMP. Clin Nucl Med. 2016;41:966–967. doi: 10.1097/RLU.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 25.Russell RGG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):150–162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 26.Nikzad M, Jalilian AR, Shirvani-Arani S, Bahrami-Samani A, Golchoubian H. Production, quality control and pharmacokinetic studies of 177Lu zoledronate for bone pain palliation therapy. J Radioanal Nucl Chem. 2013;298:1273–1281. [Google Scholar]
- 27.Yousefnia H, Zolghadri S, Jalilian AR. Absorbed dose assessment of 177Lu-zoledronate and 177Lu-EDTMP for human based on biodistribution data in rats. J Med Phys. 2015;40:102–108. doi: 10.4103/0971-6203.158694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017.
- 29.McCaffery M, Pasero C. Pain: clinical manual. 2. St Louis: Mosby; 1999. [Google Scholar]
- 30.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 31.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M144. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 32.Thapa P, Nikam D, Das T, Sonawane G, Agarwal JP, Basu S. Clinical efficacy and safety comparison of 177Lu-EDTMP with 153Sm-EDTMP on an equidose basis in patients with painful skeletal metastases. J Nucl Med. 2015;56:1513–1519. doi: 10.2967/jnumed.115.155762. [DOI] [PubMed] [Google Scholar]
- 33.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 34.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 35.Ye X, Sun D, Lou C. Comparison of the efficacy of strontium-89 chloride in treating bone metastasis of lung, breast, and prostate cancers. J Cancer Res Ther. 2018;14:S36–40. doi: 10.4103/0973-1482.181172. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson S, Franzén L, Parker C, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11(1):20–26. doi: 10.1016/j.clgc.2012.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available.